SUMMARY
Aurora A kinase localizes to centrosomes and is required for centrosome maturation and spindle assembly. Here, we describe a microtubule-independent role for aurora A and centrosomes in nuclear envelope breakdown (NEBD) during the first mitotic division of the C. elegans embryo. Aurora A depletion does not alter the onset or kinetics of chromosome condensation, but dramatically lengthens the interval between the completion of condensation and NEBD. Inhibiting centrosome assembly by other means also lengthens this interval, albeit to a lesser extent than aurora A depletion. By contrast, centrosomally-nucleated microtubules and the nuclear envelope-associated motor dynein are not required for timely NEBD. These results indicate that mitotic centrosomes generate a diffusible factor, which we propose is activated aurora A, that promotes NEBD. A positive feedback loop, in which an aurora A-dependent increase in centrosome size promotes aurora A activation, may temporally couple centrosome maturation to NEBD during mitotic entry.
INTRODUCTION
Aurora A is a mitotic kinase that choreographs events during mitotic entry. Interest in aurora A has been stimulated by its connection to tumorigenesis. Aurora A resides in a genomic region often amplified in tumors (Bar-Shira et al., 2002) and its overexpression can transform cells in culture and in vivo (Bischoff et al., 1998; Wang et al., 2006; Zhou et al., 1998). Aurora A is overexpressed in a high proportion of breast, colorectal and gastric cancers and a specific allele of aurora A, F31I, has been linked to increased cancer susceptibility in humans (Andrews, 2005; Crane et al., 2004; Marumoto et al., 2005; Meraldi et al., 2004).
Several demonstrated functions of aurora A are connected to centrosomes (Crane et al., 2004; Ducat and Zheng, 2004; Dutertre et al., 2002; Marumoto et al., 2005). Centrosomes consist of a pair of centrioles surrounded by pericentriolar material that promotes microtubule assembly. During cell division, centrosomal microtubule asters contribute to the formation and positioning of the mitotic spindle. In preparation for these functions, centrosomes “mature” during mitotic entry, recruiting additional pericentriolar material to increase ~5-fold in size and nucleating capacity (Palazzo et al., 2000). Aurora A localizes to the pericentriolar material and is required for maturation (Berdnik and Knoblich, 2002; Blagden and Glover, 2003; Brittle and Ohkura, 2005; Hannak et al., 2001). Centrosomal aurora A is in dynamic equilibrium with a cytoplasmic pool, turning over rapidly (half-life of ~3s in human cells; Stenoien et al., 2003). This rapid turnover indicates that aurora A has a signaling rather than structural role in centrosome assembly, and that events at centrosomes have the potential to influence the state of the cytoplasmic pool of aurora A.
In addition to centrosome maturation, aurora A has been implicated in regulating cell cycle progression. In cycling Xenopus extracts, depletion of aurora A delays both the activation of Cdk1 and chromosome condensation (Liu and Ruderman, 2006). A delay in Cdk1 activation has also been documented following RNAi-mediated depletion of aurora A in human cells (Hirota et al., 2003). The connection between the role of aurora A in centrosome assembly and cell cycle progression is less clear. Although postulated to be inter-connected in human cells (Hirota et al., 2003), the effect of depleting aurora A on Cdk1 activation is independent of the presence of centrosomes in Xenopus extracts (Liu and Ruderman, 2006).
Subsequent to its involvement in Cdk1 activation and centrosome maturation, both of which occur prior to NEBD, aurora A promotes spindle assembly in conjunction with its activator TPX2. TPX2 is regulated by the Ran pathway after NEBD, and inhibition of TPX2 blocks spindle assembly without apparent effects on centrosome structure or cell cycle progression (Crane et al., 2004; Ducat and Zheng, 2004; Eyers and Maller, 2003; Garrett et al., 2002; Kufer et al., 2003; Özlü et al., 2005).
Here, we capitalize on the highly stereotypical first division of the C. elegans embryo to explore the role of aurora A in the coordination of mitotic events during the period leading up to NEBD. We show that following aurora A inhibition chromosomes initiate and complete condensation with normal timing, suggesting that Cdk1 is activated normally. However, aurora A depleted embryos exhibit a specific delay between the completion of chromosome condensation and NEBD. Inhibition of centrosome assembly via other means also delays NEBD, but to a lesser extent than depletion of aurora A. By contrast, inhibition of microtubule assembly or depletion of dynein does not alter NEBD timing, indicating that the role of centrosomes and aurora A is not mediated by centrosomal microtubules. Our results demonstrate an important role for centrosomes and aurora A in NEBD, and suggest a model in which positive feedback between increasing centrosome size and aurora A activation temporally couples centrosome maturation to NEBD.
RESULTS
Aurora A Is Required for Centrosome Maturation and the Proper Timing of Events During Mitotic Entry
To explore the functions of aurora A during the period leading up to NEBD, we took advantage of the stereotypical first mitotic division of the C. elegans embryo. After fertilization, the chromosomes in the oocyte nucleus complete their meiotic segregation, generating the oocyte pronucleus and two polar bodies. Subsequently, the embryo enters its first mitotic division. During prophase, the replicated chromosomes condense as the oocyte and sperm pronuclei migrate towards each other. Concurrently, the centrosomes associated with the sperm pronucleus increase ~5-fold in size. After the pronuclei meet, the nuclear envelopes become permeable and the chromosomes interact with spindle microtubules to align and segregate. (Oegema and Hyman, 2005; Fig. 1B). Nuclear envelope permeabilization can be followed by monitoring the diffusion of free GFP:histone out of the nucleus. We refer to the timepoint when the free nuclear GFP:histone fluorescence has equilibrated with the cytoplasm as NEBD (nuclear envelope breakdown). In previous work, we established RNAi conditions that reduce aurora A protein levels by >90% and analyzed the consequences of this depletion (Hannak et al., 2001; Fig. 1A). This analysis revealed that aurora A is required for the increase in centrosome size during mitotic entry. In addition, mitotic entry appeared to take longer in aurora A-depleted embryos (Hannak et al., 2001; Fig. 1A), although the lack of quantifiable cell cycle reference points precluded further analysis of this defect.
Figure 1. Aurora A Is Required for Centrosome Maturation and for Proper Timing of Events During Mitotic Entry.
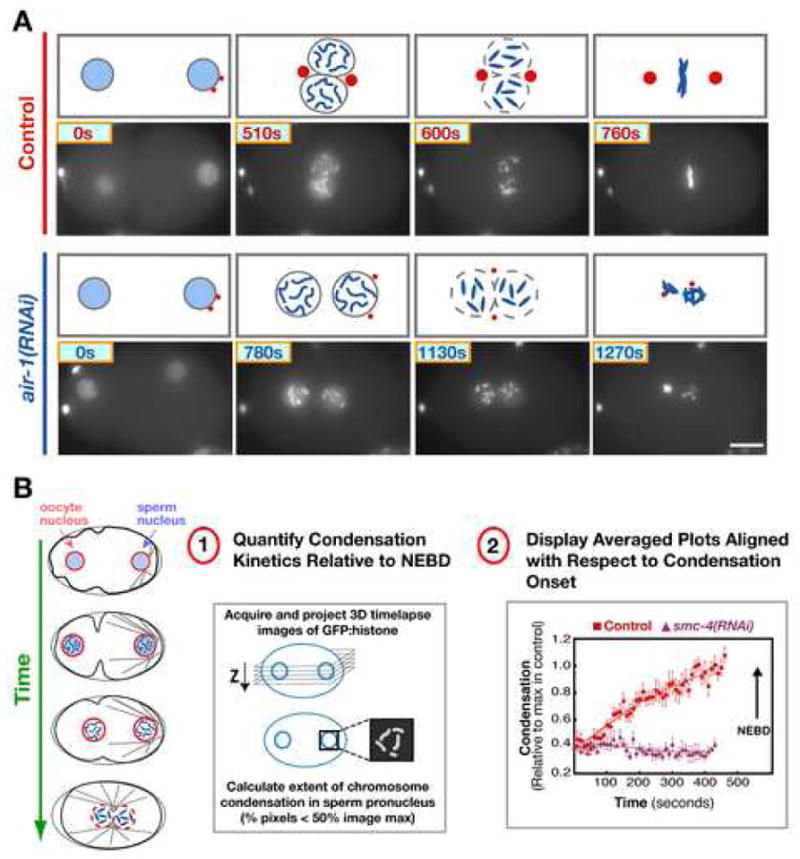
(A) Selected stills from time-lapse sequences of control (top) and aurora A depleted (air-1(RNAi); bottom) embryos expressing GFP:histone. Times are seconds after the first panel of each sequence. Schematics above each panel also illustrate the previously characterized (Hannak et al., 2001) effect of aurora A depletion on centrosome (red) maturation. Bar, 10 μm. (B) Schematics illustrate the stages between the onset of chromosome condensation and NEBD of the first mitotic division that follows fertilization. (B-1) Outline of the image analysis method (Maddox et al., 2006) used to quantitatively monitor the kinetics of chromosome condensation. (B-2) Plot of the averaged condensation parameter versus time for control (red squares; n=12 embryos) and smc-4(RNAi) (purple triangles; n=8 embryos) embryos. The average value of the condensation parameter was calculated after aligning the sequences with respect to NEBD. Traces are displayed with the onset of condensation in controls as t=0 (arrow marks NEBD in both data sets). Error bars are SEM.
Depletion of Aurora A Results in a Specific Delay between the Completion of Chromosome Condensation and Nuclear Envelope Breakdown
In Xenopus extracts, depletion of aurora A results in parallel delays in Cdk1 activation and chromosome condensation (Liu and Ruderman, 2006), a process downstream of Cdk1 activation (Liu and Ruderman, 2006; Potapova et al., 2006; Shimada et al., 1998). To test if the delay in mitotic entry in aurora A depleted C. elegans embryos is due to a defect in Cdk1 activation, we used a recently developed image analysis method (outlined in Fig. 1B; Maddox et al., 2006) to compare chromosome condensation kinetics in control and aurora A depleted embryos. In this method, the progressive shift of the fluorescence intensity distribution of the GFP:histone signal that accompanies condensation is monitored by measuring the percentage of pixels with intensities less than 50% of the image maximum (the condensation parameter) in individually scaled projections of 3D timelapse sequences. The condensation parameter increases monotonically as chromosomes condense and can be used to quantitatively compare condensation kinetics between control and perturbed embryos (Fig. 1B; Maddox et al., 2006). For every tested perturbation, the average of the condensation parameter at each time point was calculated from values measured in 6–12 embryos time-aligned with respect to NEBD. To simplify presentation, the plots of the condensation parameter are displayed aligned with the onset of condensation. No increase in the condensation parameter was detected during prophase in embryos depleted of SMC-4 (Fig. 1B), one of the ATPase subunits of the condensin complex (Hagstrom et al., 2002), and intermediate effects are clearly detected following depletions of other chromosomal proteins (Maddox et al., 2006), validating the method.
In aurora A depleted embryos, chromosomes condensed with kinetics identical to those in control embryos, attaining a state of maximum condensation over a period of ~500s (Fig. 2A). However, there was a striking delay between the end of condensation and NEBD (Fig. 2A,B,D). To determine if the onset of condensation was delayed, we used the independent timer of pronuclear size. We filmed embryos starting at the onset of anaphase of meiosis II, and found that pronuclear diameter steadily increased ~2-fold between the end of meiosis II and NEBD in both control and aurora A-depleted embryos. The average pronuclear diameter when aurora A depleted embryos initiated condensation (7.0 + 0.1 μm) was indistinguishable from controls (7.1 + 0.1 μm), indicating that the timing of condensation onset was not altered by aurora A depletion (Fig. 2C, D). However, in contrast to control embryos where NEBD occurred ~60s after chromosome condensation was complete, chromosomes remained in the condensed state for an additional 7 minutes before NEBD in aurora A depleted embryos.
Figure 2. Depletion of Aurora A Specifically Delays Nuclear Envelope Breakdown.
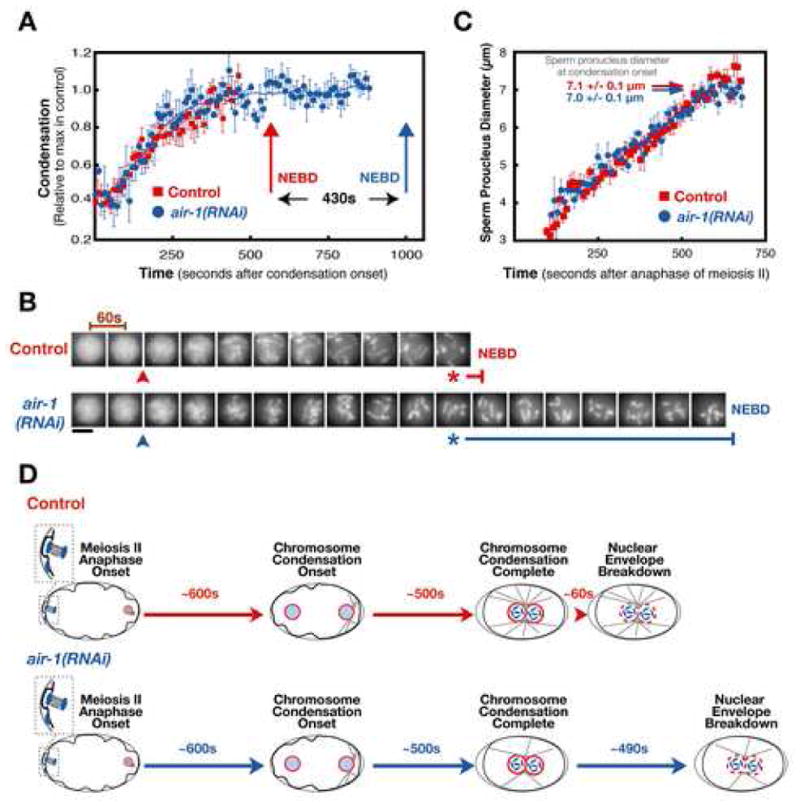
Aurora A depleted embryos condense chromosomes with normal kinetics but exhibit a dramatic delay between the completion of condensation and NEBD. (A) Plot of the average of the condensation parameter versus time for control (red squares; n=12) and aurora A depleted embryos (air-1(RNAi); blue circles; n=8). The average value of the condensation parameter was calculated after aligning the sequences with respect to NEBD. Traces are displayed aligned with respect to the onset of condensation. Error bars are SEM. (B) Representative images of the sperm pronucleus in a control and an aurora A depleted embryo. Images are at 60s intervals. The onset (arrowheads) and completion (asterisks) of condensation defined by the analysis in (A) are indicated, as is the timing of NEBD (vertical bars). Scale bar is 5 μm. (C) Plot of the average diameter of the sperm pronucleus vs. time after the onset of anaphase of meiosis II in control (red squares; n=5) and aurora A depleted (air-1(RNAi); blue circles; n=5) embryos. The average pronuclear diameter at the onset of condensation calculated from the data in (A) is indicated for control (red arrow) and aurora A depleted (blue arrow) embryos. (D) Schematic summarizing the timing of events during the first mitotic division in control and aurora A-depleted embryos. Chromosomes initiate and complete condensation with identical timing relative to anaphase of meiosis II in both types of embryos. However, nuclear envelope permeabilization is dramatically delayed by aurora A depletion.
The normal onset and kinetics of chromosome condensation indicates that aurora A depletion does not result in a global defect in Cdk1 activation. Consistent with this conclusion, the interval between the onset of meiosis II anaphase and regression of the pseudocleavage furrow, a cortical event analogous to the relaxation of surface contractile waves that accompany Cdk1 activation in Xenopus embryos (Rankin and Kirschner, 1997), was also not altered by aurora A depletion (12.9 + 0.3 min in controls vs. 13.4 + 0.5 min in aurora A depleted embryos; n=9 for both). Given that wild-type embryos take only 20 min to progress from the end of meiosis II to NEBD, and an additional 4 minutes to progress from NEBD to the onset of cytokinesis, the magnitude of the NEBD delay following aurora A depletion (~7 min) indicates that aurora A makes a major contribution to timely NEBD.
Depletion of Aurora A Slows the Progression of Nuclear Envelope Breakdown in Addition to Delaying its Onset
Permeabilization of the nuclear envelope has been shown to proceed in two phases (Lenart et al., 2003; Terasaki et al., 2001). During the first phase, the peripheral components of the nuclear pores are dismantled, rendering the envelope permeable to macromolecules up to the size of the open pore, ~40 nm in diameter. In the second phase, the nuclear pores are completely removed, concomitant with the fenestration of the nuclear envelope which allows larger particles to enter the nuclear space (Lenart et al., 2003). To determine whether depletion of aurora A slows the process of permeabilization in addition to delaying its onset, we monitored the interval between the sequential entry into the nuclear space of two different-sized cytoplasmic markers (Fig. 3A,B). As a marker for the first phase, we used a Texas-Red labeled 70 kD dextran with a predicted hydrodynamic radius of 36 nm (Lenart et al., 2003). As a marker for the second phase, we used a GFP fusion with the heavy chain of myosin II (GFP:NMY-2; Nance et al., 2003), which is expected to have a hydrodynamic radius larger than 40 nm (Citi and Kendrick-Jones, 1987). In control embryos (n=10), GFP:NMY-2 entered the nucleus 42 + 4 s after the 70kD dextran. In aurora A depleted embryos (n=7), this interval was increased to 60 ± 9 s (p<0.0004), indicating that, in addition to a dramatic delay in the onset of permeabilization, depletion of aurora A slows the progressive permeabilization of the nuclear envelope once it initiates.
Figure 3. Aurora A Depletion Slows the Progression of Nuclear Envelope Breakdown in Addition to Dramatically Delaying its Onset.
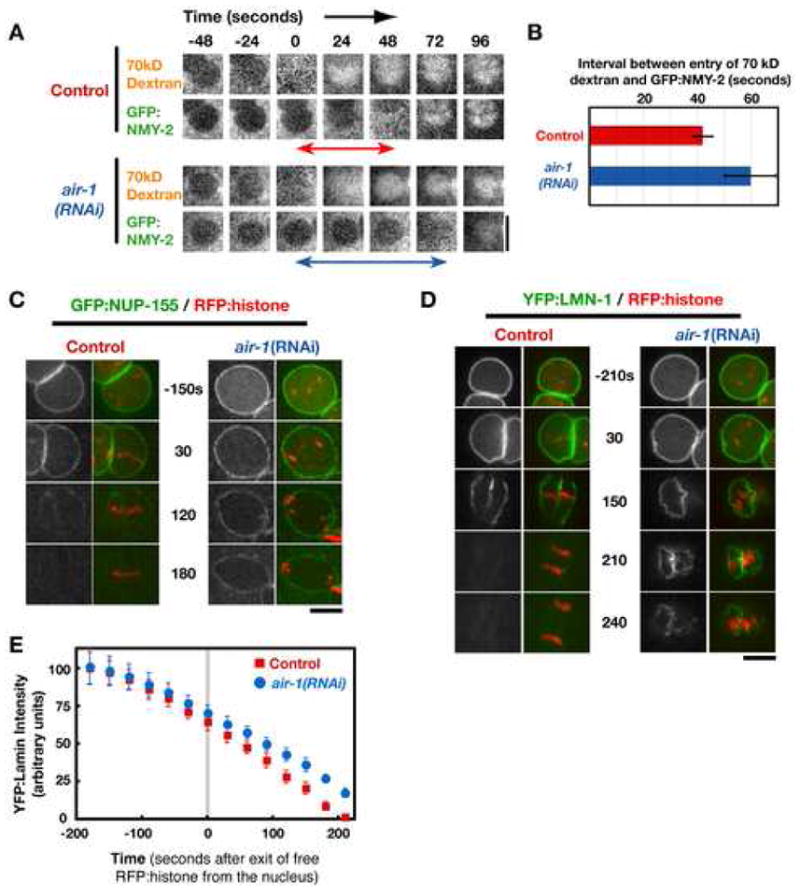
(A) Selected stills from time-lapse sequences of nuclei in control and aurora A depleted embryos that are expressing GFP:NMY-2 and contain Texas Red conjugated 70kD dextran introduced by injection. Images are shown at 24s intervals. Times are in seconds relative to entry of 70kD dextran into the nuclear space. (B) Bar graph of the average interval between the entry of 70kD dextran and GFP:NMY-2 into the nuclear space (indicated by the double-headed arrows in A, measured in 10 control and 7 air-1(RNAi) embryos. Error bars are the 95% confidence interval. (C,D) Selected stills from time-lapse sequences of nuclei in control and aurora A depleted embryos co-expressing RFPmCherry:histone and either GFP:NUP-155 (C) or YFP:LMN-1 (D). Times are in seconds relative to exit of free RFP:histone from the nucleus. Bars, 5 μm. (E) Graph plotting the average intensity of YFP:LMN-1 associated with the nuclear periphery as a function of time in seconds relative to loss of free RFP:histone from the nucleus (n=6 for each condition; error bars are the 80% confidence interval).
Next, we examined the dynamics of nuclear pores and the nuclear lamina using strains co-expressing RFP-histone and either a GFP fusion with NUP-155, a stable component of the pore wall (Franz et al., 2005), or a YFP fusion with LMN-1, the single C. elegans B-type lamin (Liu et al., 2000; Riemer et al, 1993). This analysis revealed no significant difference between control and aurora A-depleted embryos in the localization of NUP-155 or LMN-1 at the time when the nuclear envelope became permeable to free RFP-histone. This indicates that depletion of aurora A coordinately delays the onset of nuclear envelope permeabilization, nuclear pore removal, and lamin disassembly by ~ 7 minutes.
We next monitored the progressive loss of pores and the lamina following permeabilization onset. Consistent with the slowing of permeabilization observed in the dextran-myosin analysis (Fig. 3A), the progression of nuclear pore removal (Fig. 3C) and disassembly of the lamin meshwork (Fig. 3D,E) were also slowed by aurora A depletion. We conclude that after permeabilization initiates, pore removal, fenestration of the envelope, and lamin disassembly all progress more slowly in aurora A-depleted embryos than in controls.
The Aurora A Activator TPXL-1 is Not Required for Timely Nuclear Envelope Breakdown
TPX2 is a well-characterized aurora A activator that plays an important role in spindle assembly (Crane et al., 2004; Ducat and Zheng, 2004; Eyers and Maller, 2003; Kufer et al., 2003). To determine if TPX2 contributes to aurora A-dependent timely NEBD, we analyzed embryos depleted of the C. elegans TPX2-related protein, TPXL-1. Centrosomes mature and separate normally in TPXL-1 depleted embryos. However, after NEBD, the two centrosomal microtubule asters collapse into the chromosomes (Supplemental Fig. 1A; Özlü et al., 2005). There was no significant difference between control and TPXL-1 depleted embryos in the length of the interval between the completion of chromosome condensation and NEBD (Supplemental Figure 1). This result is consistent with the emerging picture of genetically separable functions for aurora A before and after NEBD (Crane et al., 2004; Ducat and Zheng, 2004; Eyers and Maller, 2003; Garrett et al., 2002; Kufer et al., 2003; Özlü et al., 2005). After NEBD, aurora A acts in concert with TPX2-like proteins and the Ran pathway to promote spindle assembly. Prior to NEBD, a different activator(s) mediates the critical functions of aurora A in centrosome maturation and nuclear envelope permeabilization.
Centrosomal Microtubules and Nuclear Envelope-Associated Dynein are Not Critical for the Timing of Nuclear Envelope Permeabilization
Previous work in human cells suggested that centrosomal microtubules interact with dynein on the nuclear envelope to generate tension that accelerates NEBD (Beaudouin et al., 2002; Salina et al., 2002). Since aurora A is required for the increase in centrosome size and nucleating capacity that normally occurs prior to NEBD, such a mechanism could explain the NEBD delay in aurora A-inhibited embryos. To investigate this possibility, we characterized embryos depleted of dynein or treated with nocodazole to depolymerize centrosomal microtubules. Depletion of dynein resulted in the expected phenotypes (Gönczy et al., 1999) —multiple oocyte pronuclei as a consequence of defects in female meiosis (Fig. 4A; arrowheads), and failure of centrosome separation and pronuclear migration. However, the interval between the completion of chromosome condensation and breakdown of the sperm pronuclear envelope was not significantly different from controls (Fig. 4B, E). Nocodazole treatment, which eliminated detectable microtubule polymers, prevented pronuclear migration, and caused the centrosomes to dissociate from the nuclei (Fig. 4A), also did not cause a significant delay (Fig. 4C, E). To more rigorously test the consequences of disrupting centrosome-nucleus attachment, we analyzed embryos depleted of ZYG-12, a hook domain-containing protein required for the association of centrosomes with the nuclear envelope (Malone et al., 2003). Prior to NEBD, the centrosomes in ZYG-12 depleted embryos are randomly positioned within the embryo (Fig. 4A). Although the interval between the completion of chromosome condensation and NEBD that we measured in ZYG-12 depleted embryos was ~50s longer than that in controls (Fig. 4D, E), this delay is an order of magnitude less than that following depletion of aurora A (Fig. 2A) and we cannot be certain if this difference is significant as it is close to the typical error for this measurement (between ~10–40s). We conclude that a defect in mechanical interactions between centrosomally-nucleated microtubules and the nuclear envelope cannot account for the dramatic delay in nuclear envelope breakdown caused by aurora A depletion.
Figure 4. Centrosomal Microtubules and Nuclear Envelope-Associated Dynein are Not Critical for the Timing of Nuclear Envelope Permeabilization.
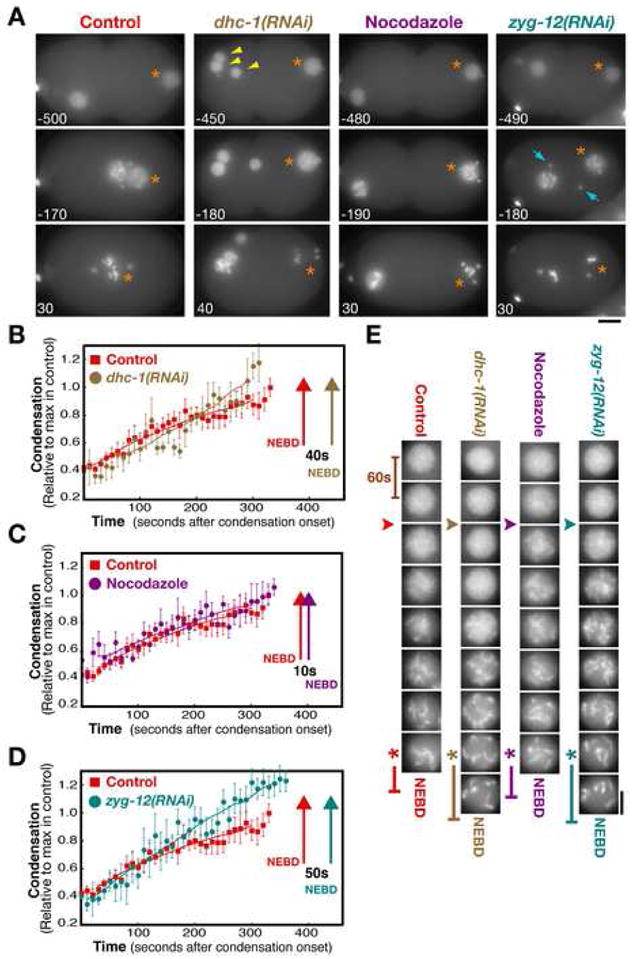
(A) Selected stills from time-lapse sequences of control, dynein depleted (dhc-1(RNAi)), nocodozole treated, and zyg-12(RNAi) embryos expressing GFP:histone and GFP:γ-tubulin. The sperm pronucleus (orange asterisks), extra maternal pronuclei resulting from failure of meiotic segregation in the DHC-1 depleted embryo (yellow arrowheads), and the centrosomes in the ZYG-12 depleted embryo (blue arrows) are indicated. Times are with respect to the exit of free GFP histone from the nucleus. Bar, 10μm. (B–D) Plots comparing condensation kinetics in control embryos (n=10; red squares) with those in dhc-1(RNAi) (B; brown circles, n=6), nocodazole treated (C; purple circles, n=7), and zyg-12(RNAi) (D; cyan circles, n=10) embryos. Arrows mark the timing of NEBD for each condition. Error bars are SEM. (E) Representative images of the sperm pronucleus in a control, a dhc-1(RNAi), a nocodazole-treated, and a zyg-12(RNAi) embryo. Images are at 60s intervals. The onset (arrowheads) and completion (asterisks) of condensation defined by the analysis in (B–D) are indicated, as is the timing of NEBD (vertical bars). Bar, 5 μm.
Inhibiting Centrosome Assembly Delays Nuclear Envelope Breakdown, but to a Lesser Extent than Depletion of Aurora A
The NEBD delay resulting from inhibition of aurora A, while independent of centrosomal microtubules, could be a consequence of its effect on centrosome structure. To explore this possibility, we analyzed embryos in which centrosome assembly was perturbed by depletion of SPD-2 or SPD-5. Like aurora A, SPD-2 localizes to centrosomes and is required for their mitotic maturation. Depletion of SPD-2 results in mitotic centrosomes that are much smaller than in wild-type (Fig. 5A,B; Kemp et al., 2004; Pelletier et al., 2004). SPD-5 is the major scaffold protein for centrosome assembly in C. elegans. Depletion of SPD-5 completely inhibits the recruitment of pericentriolar material by the centrioles; in SPD-5 depleted embryos no foci of the centrosomal marker γ-tubulin or centrosomal microtubule asters are observed at any cell cycle stage (Fig. 5A,B; Hamill et al., 2002). Chromosomes in embryos depleted of SPD-2 (Fig. 5C, E) or SPD-5 (Fig. 5D, E) condensed with kinetics similar to controls. However, both perturbations increased the interval between the completion of chromosome condensation and NEBD by ~3 min. This delay, while significant, is less than the 7 min delay observed for aurora A depletion under identical conditions. Simultaneous depletion of SPD-5 and aurora A resulted in an NEBD delay identical to that in embryos depleted of aurora A alone (Supplemental Figure 2). We conclude that centrosomes play an important role in promoting timely NEBD. This function is intrinsic to the pericentriolar material and independent of centrosomally-nucleated microtubules. In aurora A depleted embryos, the centrosomes that remain do not accelerate NEBD. However, cytoplasmic aurora A can accelerate NEBD, albeit less effectively, in the absence of centrosomes.
Figure 5. Inhibiting Centrosome Assembly Delays Nuclear Envelope Breakdown, but to a Lesser Extent than Depletion of Aurora A.
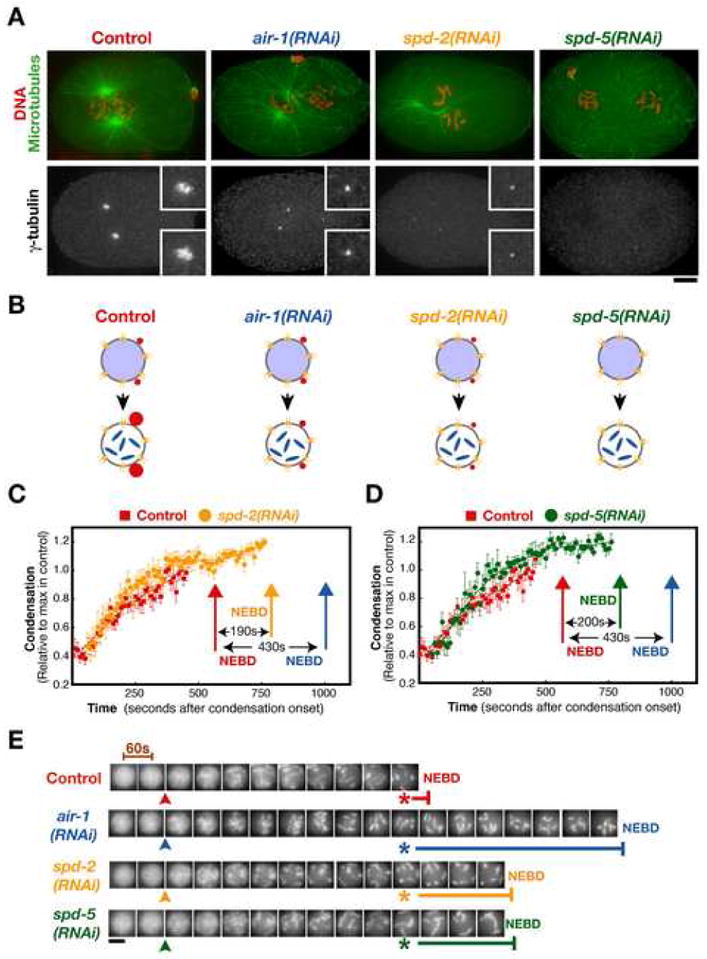
(A) Prophase control, air-1(RNAi), spd-2(RNAi) and spd-5(RNAi) embryos just prior to or after NEBD were fixed and stained for DNA and microtubules (red and green; top panels) and γ-tubulin (lower panels). Bar, 5 μm. Insets are magnified 2.5-fold. (B) Schematic illustrating the consequences of depleting each of the indicated proteins on centrosome structure. (C,D) Plots of the condensation parameter versus time comparing control (n=12) and SPD-2 depleted (C; n=10), or SPD-5 depleted (D; n=6), embryos. The timing of NEBD in control (red arrows), SPD-2 depleted (yellow arrow), and SPD-5 depleted (green arrow) embryos is indicated. For comparison, the timing of NEBD in embryos depleted of aurora A (blue arrows; replotted from Fig. 2) is also shown. Error bars are SEM. (E) Representative images of the sperm pronucleus from a control, an air-1(RNAi), a spd-2(RNAi) and a spd-5(RNAi) embryo. Images are at 60s intervals. The onset (arrowheads) and completion (asterisks) of condensation defined by the analysis in (C, D) are indicated, as is the timing of NEBD (horizontal bars). Scale bar is 5 μm.
Centrosomes Generate a Diffusible Factor that Promotes Nuclear Envelope Permeabilization
Our results demonstrate that centrosomes promote nuclear envelope permeabilization and that it is the presence of centrosomes, and not mechanical interactions between centrosomal microtubules and motor proteins on the nuclear envelope, that is critical for timely NEBD. Cumulatively, these findings suggest that mitotic centrosomes generate a diffusible factor that promotes NEBD. If this hypothesis is correct, then increasing the distance between the centrosome and the nucleus should delay nuclear envelope permeabilization by the amount of time it takes the signal to diffuse the additional distance. We tested this idea by measuring the interval between permeabilization of the oocyte-derived and sperm-derived pronuclei in embryos that fail to undergo pronuclear migration (Fig. 6A,B). Migration of the oocyte-derived pronucleus towards the sperm-pronucleus is mediated by interactions between microtubules (nucleated by the centrosomes associated with the sperm pronucleus), and dynein (on the envelope of the oocyte-derived pronucleus). Consequently, pronuclei fail to migrate in nocodazole-treated embryos as well as in embryos in which centrosome assembly (spd-5(RNAi)) or dynein is inhibited (dhc-1(RNAi)). In nocodazole-treated embryos, the sperm pronucleus, which is immediately adjacent to the centrosomes, became permeable to GFP:histone ~80s before the oocyte pronucleus, which is on the other side of the embryo (Fig. 6A,B). A similar asynchrony in the permeabilization of the sperm and oocyte pronuclei was observed in dynein-depleted embryos. This asynchrony is absent in SPD-5 depleted embryos (which lack functional centrosomes) and in ZYG-12 depleted embryos (in which functional centrosomes are present but not in preferential proximity to either pronucleus). We conclude that increasing the distance between the centrosomes and the maternal pronucleus by preventing pronuclear migration can delay its permeabilization by as much as 80s. In the absence of centrosomes, both pronuclei breakdown synchronously about ~200s after the pronuclei in controls. Cumulatively, these data strongly argue that centrosomes generate a diffusible factor that promotes NEBD.
Figure 6. Centrosomes Generate a Diffusible Factor that Promotes Nuclear Envelope Permeabilization.
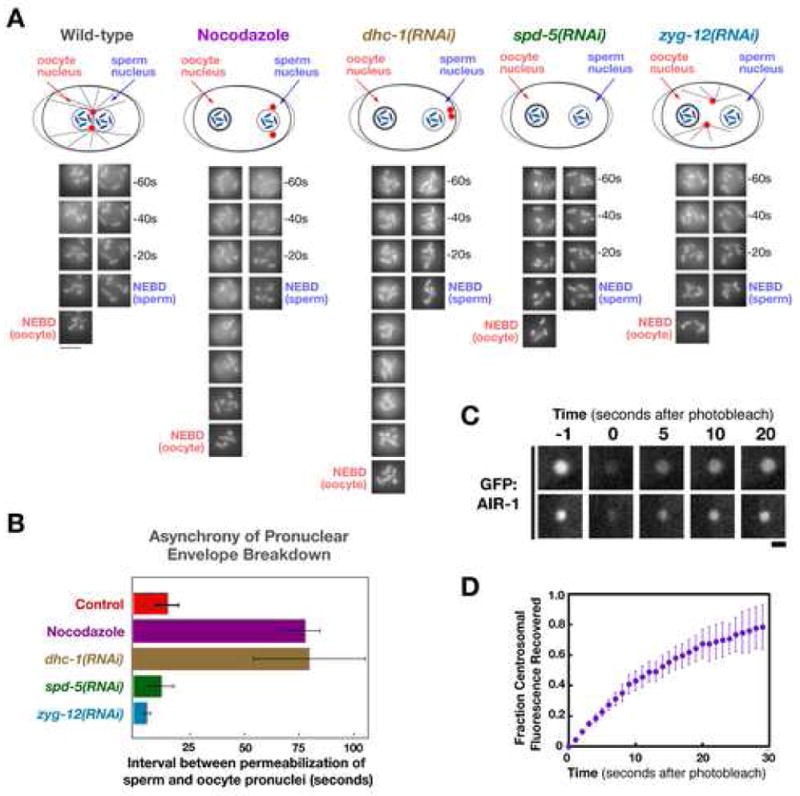
(A) Images of the oocyte and sperm pronuclei in representative embryos for each condition. Schematics above each set of images illustrate the effect of the perturbations on the relative positions of the centrosomes and nuclei. (B) Graph of the interval between permeabilization of the oocyte and sperm-derived pronuclei to GFP:histone (time of oocyte pronuclear permeabilization - time of sperm pronuclear permeabilization) in control embryos, and embryos that fail to undergo pronuclear migration. In contrast to control embryos (n=12), permeabilization of the oocyte and sperm derived pronuclei is asynchronous in nocodazole-treated (n=5) and dynein depleted (dhc-1(RNAi); n=5) embryos, with the oocyte pronucleus breaking down later than the sperm pronucleus. This asynchrony is not observed in embryos in which pronuclear migration fails due to lack of functional centrosomes (spd-5(RNAi); n=5) or when centrosomes do not remain in proximity to the sperm pronucleus (zyg-12(RNAi); n=10). Error bars are the 80% confidence interval. (C) Selected still images from two representative experiments in which the recovery of centrosomal GFP:AIR-1 fluorescence was monitored after photobleaching. Times in seconds after the bleach. Bar, 1 μm. (D) Graph shows the average fraction of GFP fluorescence recovered as a function of time in seconds after the photobleach (n=8; error bars are the 80% confidence interval).
To determine if an ~80s delay is consistent with diffusion of a cytoplasmic signal across the embryo, we introduced a 10 kD photoactivatable dextran by gonad injection into embryos. 10 kD dextran has an effective hydrodynamic diameter of 10.6 nm (Lenart et al., 2003), similar to that of a globular protein complex of ~250 kDa. A signal produced by the sperm centrosomes in the nocodazole/dynein-inhibition experiment (Fig 6A,B) was simulated by using a pulse of UV light to photoactivate the dextran on one side of the embryo (Supplemental Fig. 3A). Diffusion of the activated dextran was monitored by plotting the equilibrium ratio (Supplemental Fig. 3B, C), which declines from 1 to 0 as the activated dextran comes to diffusional equilibrium. The equilibrium ratio dropped by 50%, over ~50s. This value is similar to the ~80s asynchrony between the breakdown of the centrosome proximal and distal pronuclei, indicating that diffusion of a centrosomally-generated cytoplasmic signal is a feasible mechanism for explaining this asynchrony.
The fact that depletion of aurora A delays NEBD to a significantly greater extent than completely inhibiting centrosome assembly, leads us to speculate that the diffusible factor generated by centrosomes that promotes NEBD is activated aurora A. This idea is consistent with the rapid turnover of aurora A previously documented in human cells (half-life = 3s; Stenoien et al., 2003). To confirm that centrosomal aurora A also turns over rapidly in the C. elegans embryo, we monitored the fluorescence recovery after photobleaching of centrosomal GFP:AIR-1 prior to NEBD (Fig. 6C,D). Centrosomal GFP:AIR-1 recovered to 95 + 24% of its initial value, with a half time of 11.8 + 2.1 seconds (n=8; errors are 95% confidence interval). We conclude that the centrosomal and cytoplasmic populations of aurora A are in rapid equilibrium, making aurora A an excellent candidate for the diffusible centrosomally-generated signal that promotes NEBD.
DISCUSSION
A Role For Aurora A in Accelerating the Timing and Kinetics of NEBD
Previous work demonstrated that aurora A depletion appears to lengthen mitotic entry during the first division of the C. elegans embryo (Hannak et al., 2001). However, the lack of quantifiable cell cycle reference points prevented determination of the nature of this delay. Here, we use a recently developed method to monitor the kinetics of chromosome condensation (Maddox et al., 2006) to show that depletion of aurora A specifically lengthens the interval between the completion of chromosome condensation and NEBD. By contrast, the onset and kinetics of chromosome condensation and regression of the pseudocleavage furrow, a cortical event analogous to the Cdk1-stimulated relaxation of surface contractile waves in Xenopus embryos (Rankin and Kirschner, 1997), were not altered by aurora A depletion. These results indicate that the delay in nuclear envelope permeabilization that we describe here is distinct from the delay in global Cdk1 activation observed following depletion of aurora A in human cells and Xenopus extracts (Hirota et al., 2003; Liu and Ruderman, 2006). It is important to note that our data do not rule out the possibility that aurora A might also have a role in Cdk1 activation in C. elegans. When the dsRNA against aurora A is introduced into L4 stage hermaphrodites, embryo production ceases after ~26 hours. By contrast, control hermaphrodites (as well as hermaphrodites in which other essential cell division proteins are depleted) continue embryo production for more than 48 hours. We therefore analyzed aurora A depleted embryos produced between 22 and 26 hours after dsRNA injection. These embryos are ~90% depleted of aurora A by western blotting (Hannak et al., 2001), but are hypomorphic and not null for aurora A function. As RNAi of the C. elegans homolog of Cdk1 also leads to cessation of embryo production (Boxem et al., 1999), further work will be needed to determine if the sterility following depletion of aurora A reflects an additional role for this kinase in Cdk1 activation during oocyte maturation.
Centrosomes Generate a Diffusible Factor that Promotes NEBD
Our results provide three lines of evidence supporting the idea that centrosomes generate a diffusible factor that promotes nuclear envelope permeabilization. First, analysis of nocodazole-treated and dynein depleted embryos indicates that it is the presence of the mitotic centrosome scaffold rather than mechanical interactions between centrosomal microtubules and the nuclear envelope that is critical for timely NEBD. Second, depletion of ZYG-12, which causes the centrosomes to dissociate from the nuclear envelope and be randomly positioned within the embryo, does not delay NEBD to the same extent as completely inhibiting centrosome assembly. This result indicates that centrosomes can act at a distance to promote NEBD. Third, analysis of the asynchrony in NEBD between the sperm and oocyte nuclei when pronuclear migration is inhibited indicates that the breakdown of the oocyte nucleus, which is further from the centrosomes, is significantly delayed relative to breakdown of the sperm nucleus, which is proximal to the centrosomes. A comparison with the rate of diffusion of photoactivated 10 kD dextran indicates that the magnitude of this delay is consistent with the time required for diffusion of a centrosomally-generated factor that promotes NEBD.
As depletion of aurora A delays NEBD to a significantly greater extent than completely inhibiting centrosome assembly, we speculate that the diffusible factor generated by centrosomes that promotes NEBD is activated aurora A. We propose that the pericentriolar material of the centrosome catalyzes aurora A activation (Fig. 7A) and activated aurora A in turn, either directly or indirectly, promotes NEBD. The idea that events at mitotic centrosomes could affect the cytoplasmic pool of aurora A is supported by the rapid turnover of aurora A at centrosomes. Such a mechanism requires a centrosomally-localized aurora A activator. TPXL-1, the well-characterized aurora A activator, is important for spindle assembly after NEBD, but does not contribute to the functions of aurora A prior to NEBD (Özlü et al., 2005; this study). In human cells, the LIM protein ajuba is proposed to activate aurora A during mitotic entry (Hirota et al., 2003). However, homologs of ajuba have not been identified outside of vertebrates. A recently described aurora A activator, Bora, does not localize to centrosomes (Hutterer et al., 2006) and its inhibition in multiple genome-wide RNAi screens is reported to not affect C. elegans embryo viability (Kamath et al., 2003; Rual et al., 2004; Sönnichsen et al., 2005). We therefore suspect that an unidentified activator on the centrosome scaffold is important for aurora A function prior to NEBD.
Figure 7. Model for the Role of Aurora A in Coordinating Centrosome Maturation with NEBD.
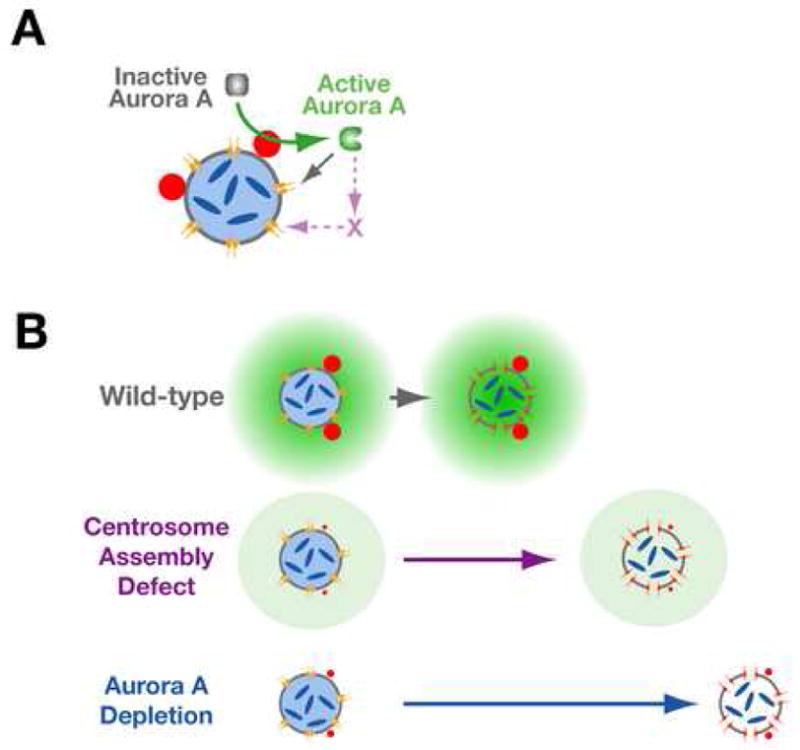
(A) We propose that the mitotic centrosome scaffold harbors an aurora A activator. Activated aurora A promotes NEBD, the first step of which is permeabilization of the envelope due to loss of peripheral components of the nuclear pores (Lenart et al., 2003; Terasaki et al., 2001). The role of aurora A in pore opening could be via direct phosphorylation of pore components or mediated by as yet unknown intermediates. (B) In wild-type embryos, a positive feedback loop in which the aurora A-dependent increase in centrosome size increases the rate of aurora A activation, generates a gradient of active aurora A with the maximum concentration near the centrosomes. When centrosomes are mature and the local concentration of active aurora A is sufficiently high, the nuclear envelope becomes permeable. In the absence of centrosomes, the activation of aurora A and permeabilization of the nuclear envelope are delayed. In aurora A depleted embryos an even greater delay is observed.
Overall, our analysis supports the emerging picture in which the functions of aurora A prior to and after NEBD are mediated by distinct sets of activators/effectors. It will be interesting to see if the same is true for the different events that require aurora A in the period leading up to NEBD: Cdk1 activation (Hirota et al., 2003; Liu and Ruderman, 2006), centrosome maturation (Berdnik and Knoblich, 2002; Blagden and Glover, 2003; Brittle and Ohkura, 2005; Hannak et al., 2001), the establishment of cell polarity (Berdnik and Knoblich, 2002; Hutterer et al., 2006; Schumacher et al., 1998), and nuclear envelope breakdown itself (this study).
Possible Roles for Aurora A in the Control of NEBD Timing
Depletion of aurora A delays the onset of nuclear envelope permeabilization, lamin disassembly, and nuclear pore removal to an essentially identical extent (Fig. 3). Although it is possible that aurora A regulates all three events independently, the coordinate delay suggests that aurora A might regulate one event that is a prerequisite for the other two. Since nuclear pore disassembly is accompanied by phosphorylation of a subset of the nucleoporins (Belgareh et al., 2001; Favreau et al., 1996; Ganeshan and Parnaik, 2000; Macaulay et al., 1995; Onischenko et al., 2005), an attractive possibility is that aurora A either directly or indirectly triggers phosphorylation of nuclear pore components, promoting envelope permeabilization and subsequent pore removal and lamin disassembly. Alternatively, the coordinate regulation by aurora A could be explained by its control of an upstream event required for all three aspects of nuclear envelope breakdown. One mechanism is suggested by the observation that cyclin B1 accumulates in the nucleus after chromosome condensation but prior to changes in nuclear envelope permeability (Hagting et al., 1999, Terasaki et al., 2003). This nuclear accumulation is regulated by phosphorylation (Hagting et al., 1998, 1999; Toyoshima et al., 1998; Yang et al., 1998) and may reflect a role for nuclear-localized Cdk1-cyclin B1 in triggering NEBD, although direct evidence for this idea is currently lacking. Assuming that the translocation of Cdk1-cyclin B1 into the nucleus is important for NEBD, aurora A could promote NEBD by regulating the balance of Cdk1-cyclin B1 import and export during prophase. Distinguishing between these and other possibilities will require future work.
Aurora A Coordinates Multiple Events During Mitotic Entry
Mitotic progression is directed by the sequential activation of cyclin dependent kinases. However, how the many events that occur during mitotic entry are coordinated remains an important question. Aurora and polo family kinases refine the broad strokes of Cdk regulation to ensure that mitotic events occur in their proper sequence (Barr et al., 2004; Crane et al., 2004; Ducat and Zheng, 2004; Dutertre et al., 2002; Lowery et al., 2005; Marumoto et al., 2005). The fact that aurora A is important for both centrosome maturation and NEBD suggests the existence of a positive feedback loop between an aurora A-dependent increase in centrosome size during mitotic entry and the cellular pool of activated Aurora A that, either directly or indirectly, promotes NEBD. This idea provides an attractive explanation for how the temporal coupling between centrosome maturation, (an event that occurs in preparation for chromosome segregation on the spindle), and NEBD, (which provides centrosomal microtubules access to the replicated chromosomes), is achieved. Our findings also lend support to the emerging idea that the centrosome acts as a signaling scaffold (Doxsey et al., 2005), coordinating the progression of mitotic events independently of its role as a microtubule nucleating and organizing center.
EXPERIMENTAL PROCEDURES
Strains and Culture Conditions
C. elegans strains expressing GFP:histone (AZ212; Praitis et al., 2001), GFP:NMY-2 (JJ1473; Nance et al., 2003), and co-expressing GFP:γ-tubulin and GFP:histone (TH32; Cheeseman et al., 2004) were maintained at 20°C. The strains OD139, co-expressing RFPmCherry:histone H2B and YFP:LMN-1 (unc-119(ed3) III; ltIs37[pAA64; unc-119(+) pie-1/RFPmCherry::HIS-58]; qals3507 [unc-119(+) pie-1/YFP::LMN-1]), and OD141, co-expressing RFPmCherry:histone H2B and GFP:NUP-155 (unc-119(ed3) III; ltIs37[pAA64; unc-119(+) pie-1/RFPmCherry::HIS-58]; [unc-119(+); pie-1/GFP::NUP-155]), were generated by mating previously described strains expressing fluorescent fusions with LMN-1 (Galy et al., 2003), NUP-155 (Franz et al., 2005), and histone H2B (McNally et al., 2006) and were maintained at 25°C. The strain OD142 expressing a GFP fusion with AIR-1 was generated by cloning the spliced AIR-1 coding sequence into the Spe I site of pIC26 (Cheeseman et al., 2004), and integrating the construct into DP38 (unc-119 (ed3)) by ballistic bombardment (Praitis et al., 2001).
RNA Interference
dsRNA was prepared as described (Oegema et al., 2001). DNA templates were generated using the following gene-specific primers: smc-4 (TAATACGACTCACTATAGGCTCCAAAACAAGCCGAACTT, AATTAACCCTCACTAAAGGTGCATCTTCTTCTTTCCCTACA), air-1 (TAATACGACTCACTATAGGGCCTCTCGGAAAAGGAAAGT, AATTAACCCTCACTAAAGGCCTTGATTCTGGCGATCAAT), spd-2 (AATTAACCCTCACTAAAGGTGCATGCGAATAAGACGAAG, TAATACGACTCACTATAGGTTGCGGACACAGAAAACAAA), spd-5 (AATTAACCCTCACTAAAGGTGTCGCAACCAGTTCTGAAT, TAATACGACTCACTATAGGATGGAGGCAAATTGTTGCTG), dhc-1 (AATTAACCCTCACTAAAGGGAAGGAAGGAGCTCAACGACA, TAATACGACTCACTATAGGCCTTTCCTTCCTGGGTCTTC), zyg-12 (AATTAACCCTCACTAAAGGGACGGCTGGCTTGAAACAATG, TAATACGACTCACTATAGGGCAACTGAGCAATCCCATTT) to amplify regions of genomic N2 DNA. For depletion of aurora A, the previously described RNAi conditions that led to >90% depletion were used (Hannak et al., 2001). For depletion of DHC-1 and ZYG-12, worms were incubated at 20°C for 24–28 hours post injection. For all other depletions, injected L4 larvae were incubated at 20°C for 48 hours prior to analysis of their embryos.
Imaging of Embryos Expressing GFP:histone and Analysis of Chromosome Condensation
Embryos expressing GFP:histone were imaged at 20°C using a Nikon E800 upright microscope (Nikon Instruments, Melville, NY) equipped with a 60x 1.4NA Plan Apo objective lens and an Orca ER CCD camera (Hamamatsu Photonics, Bridgewater, NJ) without binning. For condensation analysis, embryos were dissected and mounted in M9 (22 mM KH2PO4, 42 mM Na2HPO4, 86 mM NaCl, 1 mM MgSO4) on a 2% agarose pad under a coverslip. At 10s intervals, 5 z-sections at 2 μm steps were acquired using a 250 ms exposure and a 12.5% transmission neutral density filter. Presented images are maximum intensity projections of the z-series for the indicated time points. Condensation kinetics were analyzed as described previously (Maddox et al., 2006). For measurement of pronuclear diameter, embryos were mounted prior without compression (Monen et al., 2005). Prior to anaphase of meiosis II, 3 fluorescence z-sections at 2 μm steps were collected at 30 s intervals. After anaphase of meiosis II, 5 DIC (Differential Interference Contrast) z-sections at 2 μm steps were collected at 10s intervals until pronuclear meeting. After pronuclear meeting, 5 fluorescence z-sections at 2 μm steps were collected at 10s intervals until metaphase of mitosis.
Imaging of Embryos Expressing YFP:LMN-1 and GFP:NUP-155
Embryos from the strains OD139 and OD141 were imaged using a spinning disk confocal (McBain Instruments, Los Angles, CA) mounted on a Nikon TE2000e inverted microscope (Nikon Instruments, Melville, NY). Images were acquired using a 60x 1.4NA Plan Apo objective lens with 1.5x auxiliary magnification using an Orca ER CCD camera (Hamamatsu Photonics, Bridgewater, NJ) with 2x2 binning. Acquisition parameters, shutters and focus were controlled by MetaMorph software (Universal Imaging, Downingtown, PA). YFP:Lamin at the nuclear periphery was quantified by averaging the total fluorescence intensity measured in six separate 5x5 pixel regions at the periphery of the sperm pronucleus for each timepoint.
Monitoring Nuclear Entry of 70kDa dextran and GFP:NMY-2
Texas-Red conjugated, lysine fixable 70kD dextran (Molecular Probes) at 0.08 mg/mL in injection buffer (1 mM potassium citrate, 6.7 mM KPO4, pH 7.5, 0.67% PEG) was injected into the gonads of control and air-1(RNAi) worms expressing GFP:NMY-2. After 4 hours, dextran-containing embryos were dissected from the mothers and imaged using a spinning disk confocal as described for imaging of embryos from the strains OD139 and OD141 except 4x4 binning was used. Entry was defined as the point when the nuclear and cytoplasmic fluorescence of the probe had equilibrated.
Nocodazole Treatment
To introduce nocodazole into embryos, 3 worms were dissected in 8μL egg salts (48mM NaCl, 118 mM KCl) on a 24x60 coverslip on which a drop of 4μL polylysine (1 mg/mL) had been dried in an oven for 10 minutes. The buffer was removed with a mouth pipet and replaced with 8μL of 9:1 ddH2O:bleach by volume. After 2 minutes, the bleach solution was replaced with 8μL egg salts buffer, followed sequentially by 8μL L-15 blastomere culture medium (Edgar, 1995) and 8μL chitinase (5U/mL in L-15 blastomere culture medium). After 4 minutes, the chitinase solution was replaced with 8μL L-15 blastomere culture medium containing 10μg/mL nocodazole. Embryos were filmed as described above for measurement of pronuclear size except 5 fluorescence sections were acquired at 2μm z steps every 10s.
Measuring Turnover of Centrosomal Aurora A
For photobleaching of centrosomal GFP:AIR-1, embryos were mounted without compression (Monen et al., 2005) in L-15 blastomere culture medium containing 10μg/mL nocodazole to minimize centrosome movement. Embryos were imaged using a spinning disk confocal (McBain Instruments, Los Angles, CA) mounted on a Nikon TE2000e inverted microscope (Nikon Instruments, Melville, NY). Images were acquired by using a 60 × 1.4 N.A. Plan Apo objective lens with a Xion electron multiplication back-thinned CCD camera (Andor technologies) with no binning. Photobleaching was performed by selecting the 488nm line from a Krypton-Argon mixed gas 2.5W water cooled laser (Spectra-Physics, Mountain View, CA) which was steered into a custom modified epi-fluorescence port creating a single diffraction limited spot at the field diaphragm which is projected to the objective focal plane (full width half max at the focal point ~800nm). Exposure times for bleaching were 1–2 seconds. Acquisition parameters, shutters, and focus were controlled by MetaMorph software (Universal Imaging, Downingtown, PA). The fluorescence intensity of centrosomal GFP:AIR-1 was calculated by measuring the total intensity in a box containing the centrosome and subtracting the camera background. The signal at the first postbleach timepoint (typically 15–25% the pre-bleach value) was subtracted from all postbleach measurements and the fraction of fluorescence recovered at each timepoint was calculated by dividing by the difference between the prebleach and first postbleach measurements. Kaleidagraph software (Synergy Software, Reading PA) was used to fit the data to an equation of the form y= m(1−exp(−k*t)) where m is the maximum fractional recovery and the half time for recovery t1/2= −ln(0.5)/k.
Supplementary Material
Acknowledgments
KO is a Pew Scholar in the Biomedical Sciences; AD is the Connie and Bob Lurie Scholar of the Damon Runyon Cancer Research Foundation (DRS 38-04). AA is a fellow of the Helen Hay Whitney Foundation; PSM is the Fayez Sarofim Fellow of the Damon Runyon Cancer Research Foundation (DRG-1808-04); RAG is a fellow of the American Cancer Society. Some nematode strains used in this work were provided by the Caenorhabditis Genetics Center, which is funded by the NIH National Center for Research Resources (NCRR). KO and AD receive salary and additional support from the Ludwig Institute for Cancer Research.
ABBREVIATIONS
- NEBD
Nuclear Envelope Breakdown
- RNAi
RNA mediated interference
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrews PD. Aurora kinases: shining lights on the therapeutic horizon? Oncogene. 2005;24:5005–5015. doi: 10.1038/sj.onc.1208752. [DOI] [PubMed] [Google Scholar]
- Audhya A, Hyndman F, McLeod IX, Maddox AS, Yates JR, 3rd, Desai A, Oegema K. A complex containing the Sm protein CAR-1 and the RNA helicase CGH-1 is required for embryonic cytokinesis in Caenorhabditis elegans. J Cell Biol. 2005;171:267–79. doi: 10.1083/jcb.200506124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Shira A, Pinthus JH, Rozovsky U, Goldstein M, Sellers WR, Yaron Y, Eshhar Z, Orr-Urtreger A. Multiple genes in human 20q13 chromosomal region are involved in an advanced prostate cancer xenograft. Cancer Res. 2002;62:6803–6807. [PubMed] [Google Scholar]
- Barr FA, Sillje HH, Nigg EA. Polo-like kinases and the orchestration of cell division. Nat Rev Mol Cell Biol. 2004;5:429–440. doi: 10.1038/nrm1401. [DOI] [PubMed] [Google Scholar]
- Beaudouin J, Gerlich D, Daigle N, Eils R, Ellenberg J. Nuclear envelope breakdown proceeds by microtubule-induced tearing of the lamina. Cell. 2002;108:83–96. doi: 10.1016/s0092-8674(01)00627-4. [DOI] [PubMed] [Google Scholar]
- Belgareh N, Rabut G, Bai SW, van Overbeek M, Beaudouin J, Daigle N, Zatsepina OV, Pasteau F, Labas V, Fromont-Racine M, et al. An evolutionarily conserved NPC subcomplex, which redistributes in part to kinetochores in mammalian cells. J Cell Biol. 2001;154:1147–1160. doi: 10.1083/jcb.200101081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdnik D, Knoblich JA. Drosophila Aurora-A is required for centrosome maturation and actin-dependent asymmetric protein localization during mitosis. Curr Biol. 2002;12:640–647. doi: 10.1016/s0960-9822(02)00766-2. [DOI] [PubMed] [Google Scholar]
- Bischoff JR, Anderson L, Zhu Y, Mossie K, Ng L, Souza B, Schryver B, Flanagan P, Clairvoyant F, Ginther C, et al. A homologue of Drosophila aurora kinase is oncogenic and amplified in human colorectal cancers. Embo J. 1998;17:3052–3065. doi: 10.1093/emboj/17.11.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagden SP, Glover DM. Polar expeditions--provisioning the centrosome for mitosis. Nat Cell Biol. 2003;5:505–511. doi: 10.1038/ncb0603-505. [DOI] [PubMed] [Google Scholar]
- Boxem M, Srinivasan DG, van den Heuvel S. The Caenorhabditis elegans gene ncc-1 encodes a cdc2-related kinase required for M phase in meiotic and mitotic cell divisions, but not for S phase. Development. 1999;126:2227–2239. doi: 10.1242/dev.126.10.2227. [DOI] [PubMed] [Google Scholar]
- Brittle AL, Ohkura H. Centrosome maturation: Aurora lights the way to the poles. Curr Biol. 2005;15:R880–882. doi: 10.1016/j.cub.2005.10.022. [DOI] [PubMed] [Google Scholar]
- Cheeseman IM, Niessen S, Anderson S, Hyndman F, Yates JR, 3rd, Oegema K, Desai A. A conserved protein network controls assembly of the outer kinetochore and its ability to sustain tension. Genes Dev. 2004;18:2255–2268. doi: 10.1101/gad.1234104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citi S, Kendrick-Jones J. Regulation of non-muscle myosin structure and function. Bioessays. 1987;7:155–9. doi: 10.1002/bies.950070404. [DOI] [PubMed] [Google Scholar]
- Crane R, Gadea B, Littlepage L, Wu H, Ruderman JV. Aurora A, meiosis and mitosis. Biol Cell. 2004;96:215–229. doi: 10.1016/j.biolcel.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Doxsey S, McCollum D, Theurkauf W. Centrosomes in cellular regulation. Annu Rev Cell Dev Biol. 2005;21:411–434. doi: 10.1146/annurev.cellbio.21.122303.120418. [DOI] [PubMed] [Google Scholar]
- Ducat D, Zheng Y. Aurora kinases in spindle assembly and chromosome segregation. Exp Cell Res. 2004;301:60–67. doi: 10.1016/j.yexcr.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Dutertre S, Descamps S, Prigent C. On the role of aurora-A in centrosome function. Oncogene. 2002;21:6175–6183. doi: 10.1038/sj.onc.1205775. [DOI] [PubMed] [Google Scholar]
- Edgar LG. Methods in Cell Biolgy. 1995. Blastomere culture and analysis; pp. 303–321. [DOI] [PubMed] [Google Scholar]
- Eyers PA, Maller JL. Regulating the regulators: Aurora A activation and mitosis. Cell Cycle. 2003;2:287–289. [PubMed] [Google Scholar]
- Favreau C, Worman HJ, Wozniak RW, Frappier T, Courvalin JC. Cell cycle-dependent phosphorylation of nucleoporins and nuclear pore membrane protein Gp210. Biochemistry. 1996;35:8035–8044. doi: 10.1021/bi9600660. [DOI] [PubMed] [Google Scholar]
- Franz C, Askjaer P, Antonin W, Iglesias CL, Haselmann U, Schelder M, de Marco A, Wilm M, Antony C, Mattaj IW. Nup155 regulates nuclear envelope and nuclear pore complex formation in nematodes and vertebrates. EMBO J. 2005;24:3519–3531. doi: 10.1038/sj.emboj.7600825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galy V, Mattaj IW, Askjaer P. Caenorhabditis elegans nucleoporins Nup93 and Nup205 determine the limit of nuclear pore complex size exclusion in vivo. Mol Biol Cell. 2003;14:5104–15. doi: 10.1091/mbc.E03-04-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganeshan R, Parnaik VK. Phosphorylation of NPA58, a rat nuclear pore- associated protein, correlates with its mitotic distribution. Exp Cell Res. 2000;261:199–208. doi: 10.1006/excr.2000.5052. [DOI] [PubMed] [Google Scholar]
- Garrett S, Auer K, Compton DA, Kapoor TM. hTPX2 is required for normal spindle morphology and centrosome integrity during vertebrate cell division. Curr Biol. 2002;12:2055–2059. doi: 10.1016/s0960-9822(02)01277-0. [DOI] [PubMed] [Google Scholar]
- Gönczy P, Pichler S, Kirkham M, Hyman AA. Cytoplasmic dynein is required for distinct aspects of MTOC positioning, including centrosome separation, in the one cell stage Caenorhabditis elegans embryo. J Cell Biol. 1999;147:135–150. doi: 10.1083/jcb.147.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagstrom KA, Holmes VF, Cozzarelli NR, Meyer BJ. C. elegans condensin promotes mitotic chromosome architecture, centromere organization, and sister chromatid segregation during mitosis and meiosis. Genes Dev. 2002;16:729–742. doi: 10.1101/gad.968302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagting A, Karlsson C, Clute P, Jackman M, Pines J. MPF localization is controlled by nuclear export. EMBO J. 1998;17:4127–4138. doi: 10.1093/emboj/17.14.4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagting A, Jackman M, Simpson K, Pines J. Translocation of cyclin B1 to the nucleus at prophase requires a phosphorylation-dependent nuclear import signal. Curr Biol. 1999;9:680–689. doi: 10.1016/s0960-9822(99)80308-x. [DOI] [PubMed] [Google Scholar]
- Hamill DR, Severson AF, Carter JC, Bowerman B. Centrosome maturation and mitotic spindle assembly in C. elegans require SPD-5, a protein with multiple coiled-coil domains. Dev Cell. 2002;3:673–684. doi: 10.1016/s1534-5807(02)00327-1. [DOI] [PubMed] [Google Scholar]
- Hannak E, Kirkham M, Hyman AA, Oegema K. Aurora-A kinase is required for centrosome maturation in Caenorhabditis elegans. J Cell Biol. 2001;155:1109–1116. doi: 10.1083/jcb.200108051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota T, Kunitoku N, Sasayama T, Marumoto T, Zhang D, Nitta M, Hatakeyama K, Saya H. Aurora-A and an interacting activator, the LIM protein Ajuba, are required for mitotic commitment in human cells. Cell. 2003;114:585–598. doi: 10.1016/s0092-8674(03)00642-1. [DOI] [PubMed] [Google Scholar]
- Hutterer A, Berdnik D, Wirtz-Peitz F, Zigman M, Schleiffer A, Knoblich JA. Mitotic activation of the kinase Aurora-A requires its binding partner Bora. Dev Cell. 2006;11:147–157. doi: 10.1016/j.devcel.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Kamath RS, Fraser AG, Dong Y, Poulin G, Durbin R, Gotta M, Kanapin A, Le Bot N, Moreno S, Sohrmann M, et al. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature. 2003;421:231–237. doi: 10.1038/nature01278. [DOI] [PubMed] [Google Scholar]
- Kemp CA, Kopish KR, Zipperlen P, Ahringer J, O'Connell KF. Centrosome maturation and duplication in C. elegans require the coiled-coil protein SPD-2. Dev Cell. 2004;6:511–523. doi: 10.1016/s1534-5807(04)00066-8. [DOI] [PubMed] [Google Scholar]
- Kufer TA, Nigg EA, Sillje HH. Regulation of Aurora-A kinase on the mitotic spindle. Chromosoma. 2003;112:159–163. doi: 10.1007/s00412-003-0265-1. [DOI] [PubMed] [Google Scholar]
- Lenart P, Rabut G, Daigle N, Hand AR, Terasaki M, Ellenberg J. Nuclear envelope breakdown in starfish oocytes proceeds by partial NPC disassembly followed by a rapidly spreading fenestration of nuclear membranes. J Cell Biol. 2003;160:1055–1068. doi: 10.1083/jcb.200211076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Ben-Shahar TR, Riemer D, Treinin M, Spann P, Weber K, Fire A, Gruenbaum Y. Essential roles for Caenorhabditis elegans lamin gene in nuclear organization, cell cycle progression, and spatial organization of nuclear pore complexes. Mol Biol Cell. 2000;11:3937–47. doi: 10.1091/mbc.11.11.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Ruderman JV. Aurora A, mitotic entry, and spindle bipolarity. Proc Natl Acad Sci U S A. 2006;103:5811–5816. doi: 10.1073/pnas.0601425103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery DM, Lim D, Yaffe MB. Structure and function of Polo-like kinases. Oncogene. 2005;24:248–259. doi: 10.1038/sj.onc.1208280. [DOI] [PubMed] [Google Scholar]
- Macaulay C, Meier E, Forbes DJ. Differential mitotic phosphorylation of proteins of the nuclear pore complex. J Biol Chem. 1995;270:254–262. doi: 10.1074/jbc.270.1.254. [DOI] [PubMed] [Google Scholar]
- Maddox PS, Portier N, Desai A, Oegema K. Molecular analysis of mitotic chromosome condensation using a quantitative time-resolved fluorescence microscopy assay. Proc Natl Acad Sci U S A. 2006;103:15097–102. doi: 10.1073/pnas.0606993103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone CJ, Misner L, Le Bot N, Tsai MC, Campbell JM, Ahringer J, White JG. The C. elegans hook protein, ZYG-12, mediates the essential attachment between the centrosome and nucleus. Cell. 2003;115:825–836. doi: 10.1016/s0092-8674(03)00985-1. [DOI] [PubMed] [Google Scholar]
- Marumoto T, Zhang D, Saya H. Aurora-A - a guardian of poles. Nat Rev Cancer. 2005;5:42–50. doi: 10.1038/nrc1526. [DOI] [PubMed] [Google Scholar]
- McNally K, Audhya A, Oegema K, McNally FJ. Katanin controls mitotic and meiotic spindle length. J Cell Biol. 2006;175:881–91. doi: 10.1083/jcb.200608117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meraldi P, Honda R, Nigg EA. Aurora kinases link chromosome segregation and cell division to cancer susceptibility. Curr Opin Genet Dev. 2004;14:29–36. doi: 10.1016/j.gde.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Monen J, Maddox PS, Hyndman F, Oegema K, Desai A. Differential role of CENP-A in the segregation of holocentric C. elegans chromosomes during meiosis and mitosis. Nat Cell Biol. 2005;7:1248–1255. doi: 10.1038/ncb1331. [DOI] [PubMed] [Google Scholar]
- Nance J, Munro EM, Priess JR. C. elegans PAR-3 and PAR-6 are required for apicobasal asymmetries associated with cell adhesion and gastrulation. Development. 2003;130:5339–5350. doi: 10.1242/dev.00735. [DOI] [PubMed] [Google Scholar]
- Oegema K, Desai A, Rybina S, Kirkham M, Hyman AA. Functional analysis of kinetochore assembly in Caenorhabditis elegans. J Cell Biol. 2001;153:1209–1226. doi: 10.1083/jcb.153.6.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oegema K, Hyman AA. Cell Division. WormBook. 2005 doi: 10.1895/wormbook.1.72.1. http://wormbook.org, the C. elegans Research community, ed. [DOI] [PMC free article] [PubMed]
- Onischenko EA, Gubanova NV, Kiseleva EV, Hallberg E. Cdk1 and okadaic acid-sensitive phosphatases control assembly of nuclear pore complexes in Drosophila embryos. Mol Biol Cell. 2005;16:5152–5162. doi: 10.1091/mbc.E05-07-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özlü N, Srayko M, Kinoshita K, Habermann B, O'Toole ET, Müller-Reichert T, Schmalz N, Desai A, Hyman AA. An essential function of the C. elegans ortholog of TPX2 is to localize activated aurora A kinase to mitotic spindles. Dev Cell. 2005;9:237–248. doi: 10.1016/j.devcel.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Palazzo RE, Vogel JM, Schnackenberg BJ, Hull DR, Wu X. Centrosome maturation. Curr Top Dev Biol. 2000;49:449–470. doi: 10.1016/s0070-2153(99)49021-0. [DOI] [PubMed] [Google Scholar]
- Pelletier L, Özlü N, Hannak E, Cowan C, Habermann B, Ruer M, Muller-Reichert T, Hyman AA. The Caenorhabditis elegans centrosomal protein SPD-2 is required for both pericentriolar material recruitment and centriole duplication. Curr Biol. 2004;14:863–873. doi: 10.1016/j.cub.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Potapova TA, Daum JR, Pittman BD, Hudson JR, Jones TN, Satinover DL, Stukenberg PT, Gorbsky GJ. The reversibility of mitotic exit in vertebrate cells. Nature. 2006;440:954–958. doi: 10.1038/nature04652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praitis V, Casey E, Collar D, Austin J. Creation of low-copy integrated transgenic lines in Caenorhabditis elegans. Genetics. 2001;157:1217–1226. doi: 10.1093/genetics/157.3.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin S, Kirschner MW. The surface contraction waves of Xenopus eggs reflect the metachronous cell-cycle state of the cytoplasm. Curr Biol. 1997;7:451–454. doi: 10.1016/s0960-9822(06)00192-8. [DOI] [PubMed] [Google Scholar]
- Riemer D, Dodemont H, Weber K. A nuclear lamin of the nematode Caenorhabditis elegans with unusual structural features; cDNA cloning and gene organization. Eur J Cell Biol. 1993;62:214–23. [PubMed] [Google Scholar]
- Rual JF, Ceron J, Koreth J, Hao T, Nicot AS, Hirozane-Kishikawa T, Vandenhaute J, Orkin SH, Hill DE, van den Heuvel S, Vidal M. Toward improving Caenorhabditis elegans phenome mapping with an ORFeome-based RNAi library. Genome Res. 2004;14:2162–2168. doi: 10.1101/gr.2505604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salina D, Bodoor K, Eckley DM, Schroer TA, Rattner JB, Burke B. Cytoplasmic dynein as a facilitator of nuclear envelope breakdown. Cell. 2002;108:97–107. doi: 10.1016/s0092-8674(01)00628-6. [DOI] [PubMed] [Google Scholar]
- Schumacher JM, Ashcroft N, Donovan PJ, Golden A. A highly conserved centrosomal kinase, AIR-1, is required for accurate cell cycle progression and segregation of developmental factors in Caenorhabditis elegans embryos. Development. 1998;125:4391–4402. doi: 10.1242/dev.125.22.4391. [DOI] [PubMed] [Google Scholar]
- Shimada A, Ohsumi K, Kishimoto T. An indirect role for cyclin B-Cdc2 in inducing chromosome condensation in Xenopus egg extracts. Biol Cell. 1998;90:519–530. [PubMed] [Google Scholar]
- Sönnichsen B, Koski LB, Walsh A, Marschall P, Neumann B, Brehm M, Alleaume AM, Artelt J, Bettencourt P, Cassin E, et al. Full-genome RNAi profiling of early embryogenesis in Caenorhabditis elegans. Nature. 2005;434:462–469. doi: 10.1038/nature03353. [DOI] [PubMed] [Google Scholar]
- Stenoien DL, Sen S, Mancini MA, Brinkley BR. Dynamic association of a tumor amplified kinase, Aurora-A, with the centrosome and mitotic spindle. Cell Motil Cytoskeleton. 2003;55:134–146. doi: 10.1002/cm.10120. [DOI] [PubMed] [Google Scholar]
- Terasaki M, Campagnola P, Rolls MM, Stein PA, Ellenberg J, Hinkle B, Slepchenko B. A new model for nuclear envelope breakdown. Mol Biol Cell. 2001;12:503–510. doi: 10.1091/mbc.12.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasaki M, Okumura E, Hinkle B, Kishimoto T. Localization and dynamics of Cdc2-cyclin B during meiotic reinitiation in starfish oocytes. Mol Biol Cell. 2003;14:4685–94. doi: 10.1091/mbc.E03-04-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoshima F, Moriguchi T, Wada A, Fukuda M, Nishida E. Nuclear export of cyclin B1 and its possible role in the DNA damage-induced G2 checkpoint. EMBO J. 1998;17:2728–2735. doi: 10.1093/emboj/17.10.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhou YX, Qiao W, Tominaga Y, Ouchi M, Ouchi T, Deng CX. Overexpression of aurora kinase A in mouse mammary epithelium induces genetic instability preceding mammary tumor formation. Oncogene. 2006;25:7148–58. doi: 10.1038/sj.onc.1209707. [DOI] [PubMed] [Google Scholar]
- Yang J, Bardes ESG, Moore JD, Brennan J, Powers MA, Kornbluth S. Control of cyclin B1 localization through regulated binding of the nuclear export factor CRM1. Genes Dev. 1998;12:2131–2143. doi: 10.1101/gad.12.14.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Kuang J, Zhong L, Kuo WL, Gray JW, Sahin A, Brinkley BR, Sen S. Tumour amplified kinase STK15/BTAK induces centrosome amplification, aneuploidy and transformation. Nat Genet. 1998;20:189–193. doi: 10.1038/2496. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


