Abstract
Synaptic rules that may determine the interaction between coexisting forms of long-term potentiation (LTP) at glutamatergic central synapses remain unknown. Here, we show that two mechanistically distinct forms of LTP could be induced in thalamic input to the lateral nucleus of the amygdala (LA) with an identical presynaptic stimulation protocol, depending on the level of postsynaptic membrane polarization. One form of LTP, resulting from pairing of postsynaptic depolarization and low-frequency presynaptic stimulation, was both induced and expressed postsynaptically (“post-LTP”). The same stimulation in the absence of postsynaptic depolarization led to LTP, which was induced and expressed presynaptically (“pre-LTP”). The inducibility of coexisting pre- and postsynaptic forms of LTP at synapses in thalamic input followed a well-defined hierarchical order, such that pre-LTP was suppressed when post-LTP was induced. This interaction was mediated by activation of cannabinoid type 1 receptors by endogenous cannabinoids released in the lateral nucleus of the amygdala in response to activation of the type 1 metabotropic glutamate receptor. These results suggest a previously unknown mechanism by which the hierarchy of coexisting forms of long-term synaptic plasticity in the neural circuits of learned fear could be established, possibly reflecting the hierarchy of memories for the previously experienced fearful events according to their aversiveness level.
Keywords: synaptic transmission, glutamate, plasticity, endocannabinoids
Fear conditioning is one of the best experimental models of associative learning, which results from memorizing the temporal association between biologically neutral conditioned stimuli (CS) and aversive unconditioned stimuli (US) during behavioral training (1, 2). In the course of auditory fear conditioning, signals produced by the acoustic conditioned stimulus enter the lateral nucleus of the amygdala (LA) through projections originating in the auditory thalamus (thalamic input) and indirect projections from the auditory cortex (cortical input) (3). The acquisition of fear memory to auditory stimulation is mediated by long-term potentiation (LTP)-like synaptic enhancements in the CS pathways, including both cortical and thalamic inputs to the LA (4–9). Different forms of LTP could be observed, however, at synapses in the amygdala (7, 8, 10–12) as well as in other regions of the brain (13, 14), depending on the presynaptic activity levels and degree of postsynaptic depolarization. Thus, conventional pairing-induced LTP and spike timing-dependent LTP in thalamic projections to the LA are expressed postsynaptically and may implicate trafficking of AMPA receptors at stimulated synapses (“post-LTP”) (8, 15), whereas LTP in cortical input to the LA is expressed presynaptically, resulting from an increase in the probability of neurotransmitter release (“pre-LTP”) (7). Little is known, however, about whether the coexisting forms of LTP at glutamatergic synapses interact with each other during the induction process, and if they do, how such interactions could be mediated. It prompted us to ask which synaptic mechanisms determine the order in which the coexisting forms of LTP in the CS projections to the LA are induced.
Here, we report that the induction of LTP in thalamic input to the LA, which is both induced and expressed postsynaptically, suppresses the mechanisms of pre-LTP coexisting at the same synapses, thus potentially preventing situations where different forms of synaptic plasticity are simultaneously expressed.
Results
GluR5 Kainate Receptor-Dependent Pre-LTP Is Readily Induced in Thalamic Input to the LA.
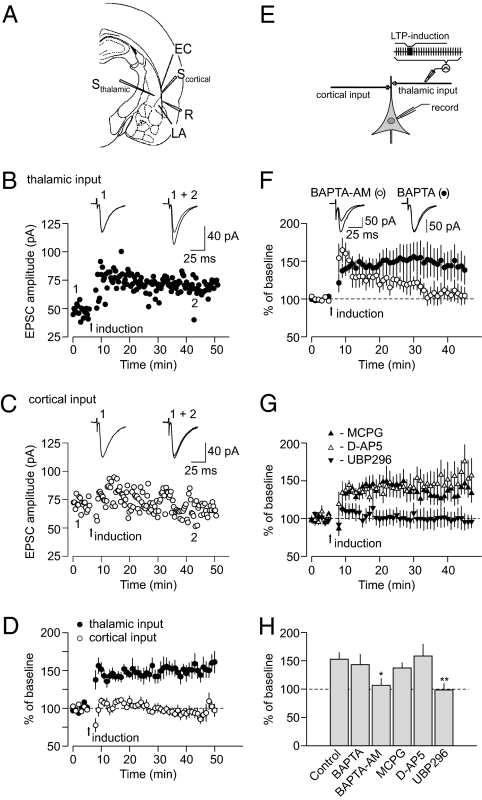
To explore the interactions between different forms of LTP in the CS pathways, we recorded excitatory postsynaptic currents (EPSCs) in LA neurons evoked by stimulation of either cortical or thalamic inputs to the LA (1, 16). Stimulation of thalamic input for 2 min with paired pulses (50-ms interpulse interval) at 2 Hz frequency and a holding potential of −70 mV led to LTP of the thalamo-amygdala EPSC (Fig. 1 A, B, D, and E and Figs. S1A and S2 A–E), whereas the same induction protocol failed to induce LTP in the cortico-amygdala pathway (Fig. 1 A, C, and D), indicating that this form of LTP was pathway-specific. The inducibility of LTP under these conditions was insensitive to changes in GABA-mediated inhibition (Fig. S3). Unlike conventional pairing-induced and spike timing-dependent LTP (7, 17), this form of synaptic potentiation was not blocked by the Ca2+ chelator 1,2-Bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA, 20 mM) in the recording pipette solution, and therefore, it did not require postsynaptic Ca2+ influx for its induction (Fig. 1 F and H). Pretreatment of slices for 30 min with the cell-permeable Ca2+ chelator 1,2-Bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis(acetoxymethyl ester) (BAPTA-AM) blocked the induction of LTP (Fig. 1 F and H and Fig. S4), indicating that presynaptic Ca2+ influx might be implicated in the induction process. This form of LTP was insensitive to both the metabotropic glutamate receptors (mGluR) antagonist (RS)-α-Methyl-4-carboxyphenylglycine (MCPG, 500 μM) and the NMDA receptor antagonist D-(-)-2-Amino-5-phosphonopentanoic acid (D-APV, 50 μM) (Fig. 1 G and H). LTP was completely blocked, however, by the selective antagonists of the GluR5 subunit-containing kainate receptors, (RS)-1-(2-Amino-2-carboxyethyl)-3-(2-carboxybenzyl)pyrimidine-2,4-dione (UBP296, 1 μM) (Fig. 1 G and H) or (S)-1-(2-Amino-2-carboxyethyl)-3-(2-carboxy-5-phenylthiophene-3-yl-methyl)-5-methylpyrimidine-2,4-dione (ACET, 0.5 μM) (Fig. S1 B and C). GluR5 subunit-containing kainate receptors are highly expressed in the amygdala (18). Together, these findings indicate that this form of LTP in thalamic input to the LA required activation of GluR5-containing kainate receptors and presynaptic Ca2+ influx.
Fig. 1.
Properties of pre-LTP in thalamic input to the LA. (A) Position of the stimulation (Scortical and Sthalamic) and recording (R) electrodes. EC, external capsule. (B) LTP of the EPSC in thalamic input to the LA was induced by the protocol consisting of a 2-min period of presynaptic stimulation with paired pulses (50-ms interpulse interval) delivered at the arrow (at −70 mV throughout the experiment). (Insets) Averaged EPSCs before (1) and after (2) the induction of LTP. (C) The same protocol did not induce LTP in cortical input. (D) LTP experiments as in B and C in cortical (n = 6) and thalamic (n = 8) inputs to the LA (t test, P = 0.001 between inputs). (E) The experimental design. (F) Normal LTP in thalamic input was observed when BAPTA (20 mM) was included in the pipette solution (n = 10), but LTP was blocked by membrane-permeable BAPTA-AM (50 μM; n = 6). Slices were incubated in BAPTA-AM–containing solution for >30 min before the recording. (Insets) Averaged EPSCs recorded before and 35 min after the delivery of LTP-inducing stimulation. (G) LTP in thalamic input in the presence of MCPG (500 μM; n = 4), D-AP5 (50 μM; n = 6), or UBP296 (1 μM; n = 6). (H) Summary of LTP experiments at thalamo-amygdala synapses (control, n = 8; BAPTA, n = 10, P = 0.68 vs. control LTP; BAPTA-AM, n = 6, P = 0.019 vs. control; MCPG, n = 4, P = 0.41 vs. control; D-AP5, n = 6, P = 0.81; UBP296, n = 6, P = 0.007). Error bars indicate SEM.
The observed kainate (KA) receptor-dependent form of LTP in the LA was associated with decreased paired-pulse facilitation (PPF), which is indicative of presynaptic enhancements (increased probability of neurotransmitter release) (Fig. S5 A and B) (19). Moreover, KA receptor-dependent LTP at the level of unitary EPSCs (7) resulted from the increased probability of successes (when a quantal synaptic event could be detected), without changes in the mean size of unitary EPSCs (potency) (7), also indicating a presynaptic site of expression (Fig. S5 C–F). Thus, similar to the mossy fiber LTP in the hippocampus, which also implicates activation of KA receptors in the induction process (20), the expression of the newly described form of LTP in thalamic input to the LA had a significant presynaptic component (pre-LTP).
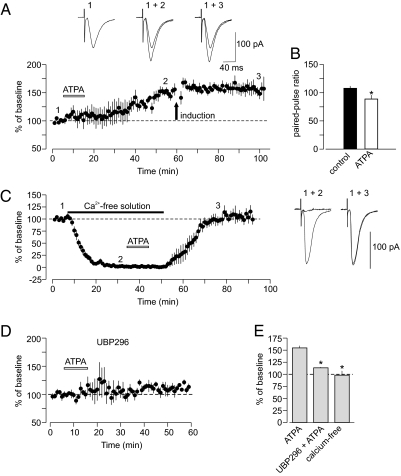
Pre-LTP could be induced by activating GluR5 kainate receptors exogenously, because GluR5 subunit-specific agonist (RS)-2-Amino-3-(3-hydroxy-5-tert-butylisoxazol-4-yl)propanoic acid (ATPA, 1 μM) has produced potentiation of synaptic transmission in thalamic input when added to the external medium (Fig. 2A). ATPA-induced potentiation was expressed presynaptically, because it was associated with decreased paired-pulse facilitation (Fig. 2B). Moreover, ATPA-induced potentiation occluded LTP induced by electrical stimulation (Fig. 2A), indicating that these processes may share common mechanisms. Similar to electrically induced LTP, ATPA-induced potentiation also needed Ca2+ influx for its induction, because it could not be induced in a Ca2+-free external solution; also, it was blocked by the GluR5 KA receptor antagonist UBP296 (Fig. 2 C–E). Thus, ATPA-induced potentiation and pre-LTP in thalamic input are mechanistically similar, and both require activation of presynaptic GluR5 subunit-containing KA receptors for their induction.
Fig. 2.
ATPA-induced potentiation and pre-LTP in thalamic input are mechanistically similar. (A) The GluR5-specific agonist ATPA (1 μM) in the external solution potentiated the EPSC in thalamic input (n = 4; P < 0.01 vs. baseline). Potentiation induced by ATPA has occluded LTP in response to electrical stimulation (delivered at arrow). (Insets) Averaged thalamo-amygdala EPSCs recorded under control conditions (1), during ATPA-induced potentiation (2), and after the delivery of LTP protocol (3). (B) Paired-pulse facilitation (PPF; 50-ms interpulse interval) was decreased during ATPA-induced potentiation (n = 4, P = 0.017). (C) Potentiation was prevented when ATPA (1 μM) was applied in Ca2+-free external solution (n = 6, P = 0.75 for post-ATPA vs. baseline). Traces (to the right) are averaged EPSCs recorded at different time points (shown as 1, 2, and 3) during the experiment. (D) UBP296 (1 μM) blocked potentiation of thalamo-amygdala EPSCs by ATPA (n = 3). (E) Summary of experiments with ATPA (ATPA, n = 4; UBP296 + ATPA, n = 3, P = 0.014 vs. ATPA alone; ATPA in Ca2+-free solution, n = 6, P = 0.01 vs. ATPA alone). Error bars indicate SEM.
Pre-LTP in Thalamic Input to the LA Is Suppressed by the Induction of Post-LTP at the Same Synapses.
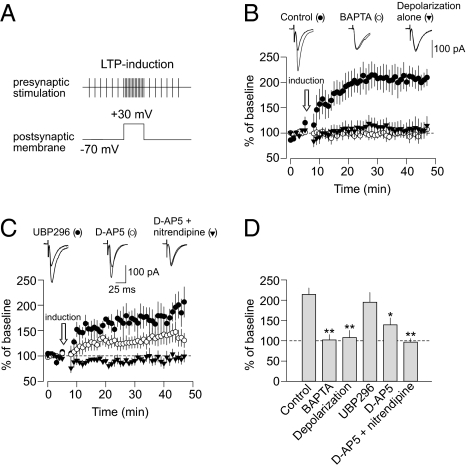
In contrast, both conventional pairing-induced LTP and spike timing-dependent LTP in thalamic projections to the LA are expressed postsynaptically (post-LTP) (8, 15). We induced post-LTP in thalamic input to the LA by paring postsynaptic depolarization to +30 mV during the induction with the presynaptic stimulation, which was identical to the stimulation delivered for the induction of KA receptor-dependent pre-LTP (240 paired pulses at 2 Hz frequency) (Fig. 3A). Under these conditions, the magnitude of PPF was unaffected by the induction of LTP (Fig. S6 A and B). At the level of unitary synaptic responses, LTP was associated with a significant increase in potency, whereas the rate of failures was unchanged (Fig. S6 C and D). This confirms that the expression of the pairing-induced form of LTP in thalamic input is postsynaptic and not associated with increases in probability of release. The resulting pairing-induced LTP was blocked by BAPTA in the recording pipette and depended on postsynaptic Ca2+ influx through NMDA receptors and L-type Ca2+ channels (Fig. 3 B–D). Unlike LTP induced with the same stimulation protocol but without postsynaptic depolarization (Fig. 1), it was not blocked by the GluR5 antagonist UBP296 (Fig. 3 C and D). Therefore, two different forms of LTP coexist in thalamic input to the LA, and their inducibility may follow a certain order. Thus, the pre-LTP, which is induced in the absence of depolarization, was suppressed when post-LTP was induced. Consistent with this notion, we found that, when post-LTP is blocked by the Ca2+ chelator BAPTA in the recording pipette solution, pre-LTP (induced by the identical stimulation but without postsynaptic depolarization) could be observed at the same synapses (Fig. 4 A and B). These findings suggest that suppression of pre-LTP does not require a rise in postsynaptic Ca2+ concentration, unlike the induction of post-LTP.
Fig. 3.
Requirements for the induction of post-LTP at thalamo-amygdala synapses. (A) LTP in thalamic input was induced by presynaptic stimulation for 2 min with paired pulses (50-ms interpulse interval). Unlike pre-LTP, the recorded LA neuron here was voltage-clamped at +30 mV during the induction. (B) Post-LTP, observed under control conditions (n = 6), was blocked by 20 mM BAPTA in the recording pipette solution (n = 10). Postsynaptic depolarization to +30 mV without presynaptic stimulation did not result in LTP (n = 5). (Insets) Averaged EPSCs recorded before and 35 min after the time point when LTP-inducing stimulation was delivered. (C) Post-LTP in thalamic input in the presence of UBP296 (1 μM; n = 4), D-AP5 (50 μM; n = 5), or D-AP5 + nitrendipine (nitrendipine, 10 μM; n = 5). (Insets) Averaged EPSCs recorded before and 35 min after the delivery of LTP-inducing stimulation. (D) Summary of LTP experiments at thalamo-amygdala synapses (control, n = 6; BAPTA, n = 10, P < 0.01 vs. control LTP; depolarization alone, n = 5, P < 0.01 vs. control LTP; UBP296, n = 4, P = 0.51 vs. control; D-AP5, n = 5, P = 0.01 vs. control; D-AP5 + nitrendipine, n = 5, P < 0.01). Error bars indicate SEM.
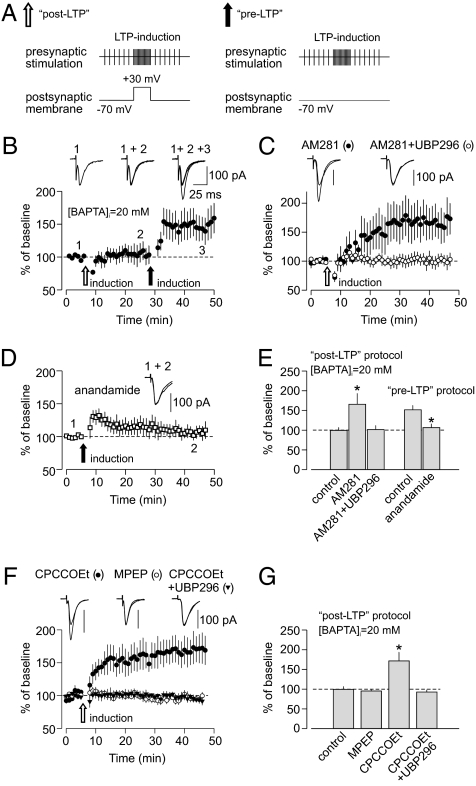
Fig. 4.
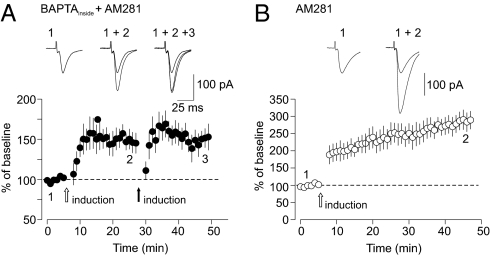
Induction of post-LTP suppresses pre-LTP at thalamo-amygdala synapses through CB1 receptor activation. (A) Stimulation protocols for the induction of post-LTP (Left) and pre-LTP (Right). (B) When post-LTP in thalamic input was blocked by 20 mM BAPTA in pipette solution (empty arrow), pre-LTP could be induced in the same pathway (filled arrow; n = 7). (Insets) Averaged EPSCs recorded before (1), after the delivery of post-LTP protocol (2), and after the delivery of pre-LTP protocol (3). (C) Post-LTP protocol, delivered in the presence of 20 mM BAPTA in pipette solution, resulted in LTP when CB1 receptors were blocked by AM281 (0.5 μM; n = 6). The induction of this unmasked LTP was suppressed in the presence of GluR5 antagonist UBP296 (1 μM; n = 7). (D) In the presence of CB1 receptor agonist anandamide (0.5 μM), the induction of pre-LTP was blocked (n = 4). (E) Summary of LTP experiments with post- and pre-induction protocols. Post-LTP protocol in the presence of 20 mM BAPTA in the pipette solution (control, n = 10; AM281, n = 6, P = 0.01 vs. control; AM281 + UBP296, n = 7, P = 0.038 vs. control). Pre-LTP protocol (control, n = 8; anandamide, n = 4, P = 0.025 vs. control). (F) Post-LTP protocol, delivered in the presence of 20 mM BAPTA in the pipette solution, resulted in LTP when mGluR1 was blocked with CPCCOEt (50 μM; n = 11). LTP was not rescued in the presence of the mGluR5 antagonist MPEP (100 μM; n = 7). No LTP was observed when CPCCOEt was applied together with the GluR5 kainate receptor antagonist UBP296 (1 μM; n = 6). (G) Summary of LTP experiments with post-LTP induction protocol delivered in the presence of BAPTA in the pipette solution as in F (control, n = 10; MPEP, n = 7, P = 0.57 vs. control; CPCCOEt, n = 11, P = 0.012 vs. control; CPCCOEt + UBP296, n = 6, P = 0.7 vs. control). Error bars indicate SEM.
Suppression of Pre-LTP Is Mediated by Activation of Cannabinoid Type 1 Receptors by Endogenous Cannabinoids.
How could the induction of post-LTP suppress pre-LTP? The only difference between the two induction protocols, leading to mechanistically distinct forms of LTP at thalamo-amygdala synapses, is postsynaptic depolarization during the induction of post-LTP. Depolarization alone, however, preceding pre-LTP–inducing stimulation in the absence of BAPTA in the recording pipette solution had no effect on the magnitude of pre-LTP in thalamic input (Fig. S7). Depolarization in combination with activation of mGluRs could lead to the production of diffusible factors (e.g., endogenous cannabinoids) capable of affecting synaptic function (21). Importantly, there is evidence that endocannabinoids could be released in the amygdala through activation of mGluRs in a Ca2+-independent manner (22). Therefore, we tested whether endocannabinoid signaling is implicated in the interaction between two forms of LTP in thalamic input to the LA. Consistent with the role of endogenous cannabinoids in such an interaction, we found that the post-LTP induction protocol led to LTP even with the intrapipette solution containing a high concentration of BAPTA (20 mM), which efficiently blocked LTP under control conditions (Fig. 3B), when delivered in the presence of the antagonist of cannabinoid type 1 (CB1) receptor, AM281 (0.5 μM) (Fig. 4 C and E). Similar to pre-LTP, LTP induced with the post-LTP induction protocol in the presence of AM281 in the external solution and 20 mM BAPTA in the pipette solution was expressed presynaptically (Fig. S8 A and B). The unmasked LTP was GluR5 kainate receptor-dependent, because it was suppressed by UBP296. Moreover, it occluded pre-LTP, providing evidence that two forms of plasticity might be mechanistically related (Fig. 5A). These findings indicate that blocking CB1 receptors, which are expressed in the amygdala (23), unmasked pre-LTP, despite postsynaptic depolarization, which would suppress it in the absence of AM281 in the external solution. It has been shown previously that the functional effects of mGluRs activation might be membrane potential-dependent (24, 25). This could explain the need for depolarization of a recorded neuron for the blocking effect on pre-LTP to occur.
Fig. 5.
Properties of LTP induced with post-LTP protocol in the presence of BAPTA in the pipette solution and AM281 in the external medium. (A) First, LTP was induced in thalamic input with the post-LTP protocol in the presence of AM281 (0.5 μM) in the bath and 20 mM BAPTA in the pipette solution. Subsequent delivery of the pre-LTP–inducing protocol (thalamic input was stimulated for 2 min with paired pulses at 2 Hz frequency at a holding potential of −70 mV) did not result in additional potentiation (n = 6, P = 0.56 vs. LTP with the post-LTP protocol). This was not caused by washout of pre-LTP, because it could be induced at ∼30 min after beginning the whole-cell recording (Fig. 4B). The observed occlusion of pre-LTP suggests that two forms of plasticity might be mechanistically similar. (Insets) Averaged EPSCs before (1) and after delivery of the first (2) and the second (3) LTP-inducing stimulation protocols. (B) Delivery of the post-LTP induction protocol in the presence of AM281 (0.5 μM) but without BAPTA in the pipette solution resulted in the very large LTP. The EPSC was potentiated to 289 ± 26% of its baseline amplitude (n = 6, P = 0.0002 vs. pre-LTP alone), suggesting that both pre-LTP and post-LTP might be induced simultaneously under conditions where the endogenous cannabionoids (eCB) cascade is blocked. (Insets) Averaged EPCS before (1) and after (2) the induction of LTP. Error bars indicate SEM.
If the production of endogenous cannabinoids, perhaps activating presynaptic CB1 receptors, during the induction of post-LTP leads to suppression of pre-LTP, then pre-LTP in thalamic input should be blocked after activation of CB1 receptors by specific agonists. Consistent with this prediction, we found that the pre-LTP–inducing stimulation (not involving postsynaptic depolarization) did not result in LTP of the thalamo-amygdala EPSCs when delivered in the presence of the endogenous agonist of CB1 receptors, anandamide (500 nM) (Fig. 4 D and E). Anandamide in this concentration had practically no effect on baseline synaptic transmission in thalamic input (Fig. S9). The endocannabinoid production during the induction of post-LTP, leading to suppression of the pre-LTP, was likely mediated by activation of the type 1 mGlu receptor (mGluR1). Thus, the KA receptor-dependent pre-LTP (as evidenced by its sensitivity to UBP296) could be induced with the post-LTP induction protocol when it was delivered in the presence of the mGluR1 antagonist CPCCOEt (50 μM), whereas the mGluR5 antagonist 2-Methyl-6-(phenylethynyl)pyridine hydrochloride (MPEP, 100 μM) had no effect (Fig. 4 F and G). In these experiments, we prevented the induction of post-LTP, including a high concentration of BAPTA in pipette solution.
Therefore, CB1 receptor activation may mediate the interaction between pre- and post- forms of LTP in thalamic input to the LA, preventing situations when the different forms of LTP coexisting at the same synapses are simultaneously expressed (Fig. 6). Interestingly, pre-LTP and post-LTP could be induced simultaneously under certain conditions. Thus, the delivery of the post-LTP induction protocol in the presence of AM281 but without BAPTA in the pipette solution resulted in nearly doubled LTP (Fig. 5B), suggesting that pre-LTP and post-LTP might be additive if the endogenous cannabinoid cascade is inactivated.
Fig. 6.
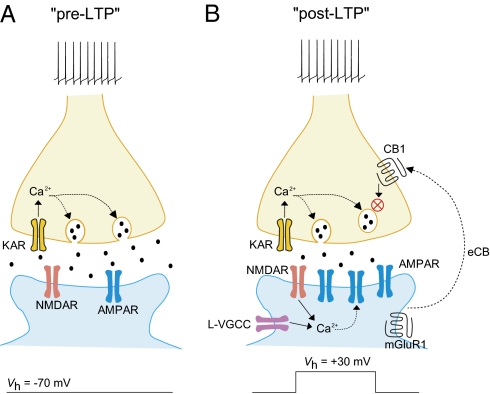
Potential mechanisms of suppression of pre-LTP by the induction of post-LTP at thalamo-LA synapses. (A) Stimulation of thalamic input to the LA with paired pulses at 2 Hz frequency for 2 min without postsynaptic depolarization leads to the induction of LTP, which is both induced and expressed presynaptically (pre-LTP). This form of LTP depends on activation of presynaptic GluR5 subunit-containing kainate receptors and presynaptic Ca2+ influx. (B) Delivery of the identical presynaptic stimulation, but under conditions of postsynaptic depolarization to +30 mV, results in a form of LTP that is both induced and expressed postsynaptically (post-LTP). The induction of post-LTP depends on postsynaptic Ca2+ influx through NMDA receptors and L-type voltage-gated Ca2+ channels. Therefore, two mechanistically distinct forms of LTP coexist in thalamic input, but pre-LTP is suppressed by the induction of post-LTP at the same synapses. The observed interaction between coexisting forms of LTP in inputs to the LA may be mediated by activation of presynaptic CB1 receptors by eCB released in response to activation of the mGluR1, thus preventing the long-term enhancements of neurotransmitter release observed during pre-LTP.
Discussion
Our findings show that postsynaptically released cannabinoids may suppress a form of LTP in thalamic input to the LA, which is both induced and expressed presynaptically (pre-LTP). Moreover, this form of LTP required presynaptic Ca2+ influx for its induction. These results indicate that cannabinoid might be acting presynaptically to suppress pre-LTP. The mechanistic explanation of the observed interaction between different forms of LTP at the same synapses would require a detailed characterization of the molecular events in nerve terminals associated with the induction and expression of pre-LTP in the thalamo-amygdala pathway. Although the processes underlying postsynaptically expressed forms of LTP at central synapses are reasonably well-characterized, relatively little is still known about the molecular mechanisms of presynaptic forms of LTP at glutamatergic synapses. Nevertheless, previous experiments suggested some interesting possibilities that might be tested in future studies. Thus, it has been proposed recently that persistent changes in the machinery of neurotransmitter release through cAMP/PKA signaling and the active zone protein RIM1α could represent a general mechanism underlying presynaptic forms of long-term plasticity at central synapses (26). For example, RIM1α was implicated in the induction of protein kinase A (PKA)-dependent forms of LTP at the mossy fiber synapses and CA3–CA1 synapses in the hippocampus (27, 28) and the parallel fiber to Purkinje cell synapses (27). Moreover, phosphorylation of RIM1α by PKA enhances neurotransmitter release (29). Because CB1 receptors are Gi/o-coupled and therefore, their activation reduces cAMP production, activation of CB1 receptors could affect the release machinery in an RIM1α-dependent manner, perhaps because of a decrease in RIM1α phosphorylation by PKA (26). Interestingly, it was shown that the expression of presynaptic LTP at cortico-LA synapses, induced by direct activation of cAMP/PKA-dependent pathways by the adenylyl cyclase activator forskolin, may implicate lasting increases in L-type Ca2+ channel-mediated glutamate release (30). Therefore, if activation of presynaptic CB1 receptors on thalamic terminals suppresses up-regulation of the cAMP/PKA pathway- and RIM1α-dependent modifications of the release machinery required for the induction of presynaptically expressed LTP, pre-LTP would be suppressed.
What might be the functional significance of mechanistically distinct forms of synaptic plasticity coexisting in thalamic input to the LA? Differences in the patterns of neuronal activity in behaving animals could produce different levels of postsynaptic depolarization, and thus, they recruit different forms of synaptic plasticity in inputs to the amygdala needed to encode conditioned fear memory and then, retrieve it. This situation is modeled in our slice experiments, where different levels of postsynaptic depolarization led to the induction of different forms of LTP. The form of LTP corresponding to the potentially more aversive US (associated with more depolarization; post-LTP) dominates. This might reflect the hierarchy of memories for the events having different biological significance, where more aversive events produce stronger memories. Our findings correspond well with the fact that the stronger US (foot shock) is often associated with the stronger freezing reactions in experimental animals at times when fear memory is tested. A role of cannabinoid release in fear-related behavior has been shown directly in a recent study describing the endocannabinoid CB1 receptor-dependent modulation of amygdala-dependent Pavlovian fear conditioning (31). The observed interaction between mechanistically distinct forms of LTP could potentially help in maintaining the link between specific forms of synaptic plasticity and the acquisition of memories for the specific types of behavioral experiences. It would be interesting to explore whether various forms of aversive learning are similar in their ability to implicate the described mechanism of interaction between coexisting forms of LTP at synapses in the neuronal circuits of learned behavioral responses.
Materials and Methods
Coronal brain slices containing the amygdala (250–300 μm) were prepared from 3- to 5-wk-old Sprague–Dawley rats with a vibratome. Slices were continuously superfused in solution containing (in mM) 119 NaCl, 2.5 KCl, 2.5 CaCl2, 1.0 MgSO4, 1.25 NaH2PO4, 26.0 NaHCO3, 10 glucose, and 0.1 picrotoxin (unless noted otherwise) and equilibrated with 95% O2 and 5% CO2 (pH 7.3–7.4) at room temperature (22–24 °C). Whole-cell recordings of compound or unitary EPSCs were obtained from pyramidal neurons in the lateral amygdala under visual guidance (differential interference contrast/infrared optics) with an EPC-9 amplifier and Pulse v8.40 software (HEKA Elektronik). The cells were classified as principal neurons based on their appearance and their ability to show spike frequency adaptation to the prolonged depolarizing current injection. Synaptic responses were evoked by field stimulation of the fibers in either the external capsule (cortical input) or the internal capsule (thalamic input) at 0.05 Hz. The patch electrodes (3–5 MΩ resistance) contained (in mM) 120 K-methane-sulfonate, 5 NaCl, 1 MgCl2, 0.2 EGTA, 10 Hepes, 2 MgATP, and 0.1 NaGTP (adjusted to pH 7.2 with KOH). Currents were filtered at 1 kHz and digitized at 5 kHz. Unitary EPSCs were evoked by low-intensity current pulses (20–40 μA; 100 μs duration) applied through a fine-tipped (∼2 μM), concentric stimulating electrode consisting of a patch pipette that was coated with silver paint. The two leads of the stimulus isolation unit (ISO-Flex, Master-8 stimulator; AMPI) were connected to the inside of the pipette and the external silver coat. The stimulating pipettes were positioned to activate either cortical or thalamic input to the LA (Fig. 1A). The recording was used if the mean EPSC amplitude showed a steep all-or-none threshold as a function of stimulating current intensity and if there was no change in potency (the mean size of responses, excluding failures of synaptic transmission) during double-pulse stimulation with a 50-ms interpulse interval, indicating stimulation of a single presynaptic input. The EPSC amplitude was measured as the difference between the mean current during a prestimulus baseline and the peak current over a 1- to 2-ms window. In all LTP experiments, the stimulus intensity was adjusted to produce synaptic responses with an amplitude that was ∼20–25% of maximum amplitude EPSC. For induction of pre-LTP, 240 paired presynaptic stimuli (with 50-ms interpulse intervals) were delivered at 2 Hz to the presynaptic fibers at a holding potential of −70 mV. For induction of post-LTP, the same stimulation was delivered at a holding potential of +30 mV. Summary LTP graphs were constructed by normalizing data in 60s epochs to the mean value of the baseline EPSP.
Supplementary Material
Acknowledgments
Support was provided by Grant-in-Aid for Scientific Research 19390309 from the Japan Society for the Promotion of Science (to R.-M.S.), Grant-in-Aid for the Molecular Imaging Program from the Ministry of Education, Culture, Sports, Science, and Technology, Japan (to R.-M.S); National Institutes of Health Grants MH083011 and MH090464 (to V.Y.B.); United States Army Medical Research Acquisition Activity (USAMRAA) Grant W81XWH-08-2-0126 (to V.Y.B.); and the Whitehall Foundation (V.Y.B.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1009803107/-/DCSupplemental.
References
- 1.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 2.Davis M, Whalen PJ. The amygdala: Vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- 3.Pitkänen A, Savander V, LeDoux JE. Organization of intra-amygdaloid circuitries in the rat: An emerging framework for understanding functions of the amygdala. Trends Neurosci. 1997;20:517–523. doi: 10.1016/s0166-2236(97)01125-9. [DOI] [PubMed] [Google Scholar]
- 4.Quirk GJ, Repa C, LeDoux JE. Fear conditioning enhances short-latency auditory responses of lateral amygdala neurons: Parallel recordings in the freely behaving rat. Neuron. 1995;15:1029–1039. doi: 10.1016/0896-6273(95)90092-6. [DOI] [PubMed] [Google Scholar]
- 5.McKernan MG, Shinnick-Gallagher P. Fear conditioning induces a lasting potentiation of synaptic currents in vitro. Nature. 1997;390:607–611. doi: 10.1038/37605. [DOI] [PubMed] [Google Scholar]
- 6.Rogan MT, Stäubli UV, LeDoux JE. Fear conditioning induces associative long-term potentiation in the amygdala. Nature. 1997;390:604–607. doi: 10.1038/37601. [DOI] [PubMed] [Google Scholar]
- 7.Tsvetkov E, Carlezon WA, Benes FM, Kandel ER, Bolshakov VY. Fear conditioning occludes LTP-induced presynaptic enhancement of synaptic transmission in the cortical pathway to the lateral amygdala. Neuron. 2002;34:289–300. doi: 10.1016/s0896-6273(02)00645-1. [DOI] [PubMed] [Google Scholar]
- 8.Rumpel S, LeDoux J, Zador A, Malinow R. Postsynaptic receptor trafficking underlying a form of associative learning. Science. 2005;308:83–88. doi: 10.1126/science.1103944. [DOI] [PubMed] [Google Scholar]
- 9.Shumyatsky GP, et al. Stathmin, a gene enriched in the amygdala, controls both learned and innate fear. Cell. 2005;123:697–709. doi: 10.1016/j.cell.2005.08.038. [DOI] [PubMed] [Google Scholar]
- 10.Weisskopf MG, Bauer EP, LeDoux JE. L-type voltage-gated calcium channels mediate NMDA-independent associative long-term potentiation at thalamic input synapses to the amygdala. J Neurosci. 1999;19:10512–10519. doi: 10.1523/JNEUROSCI.19-23-10512.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang YY, Kandel ER. Postsynaptic induction and PKA-dependent expression of LTP in the lateral amygdala. Neuron. 1998;21:169–178. doi: 10.1016/s0896-6273(00)80524-3. [DOI] [PubMed] [Google Scholar]
- 12.Humeau Y, Shaban H, Bissière S, Lüthi A. Presynaptic induction of heterosynaptic associative plasticity in the mammalian brain. Nature. 2003;426:841–845. doi: 10.1038/nature02194. [DOI] [PubMed] [Google Scholar]
- 13.Zakharenko SS, Zablow L, Siegelbaum SA. Visualization of changes in presynaptic function during long-term synaptic plasticity. Nat Neurosci. 2001;4:711–717. doi: 10.1038/89498. [DOI] [PubMed] [Google Scholar]
- 14.Nelson SB, Turrigiano GG. Strength through diversity. Neuron. 2008;60:477–482. doi: 10.1016/j.neuron.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Humeau Y, et al. Dendritic spine heterogeneity determines afferent-specific Hebbian plasticity in the amygdala. Neuron. 2005;45:119–131. doi: 10.1016/j.neuron.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 16.Li XF, Stutzmann GE, LeDoux JE. Convergent but temporally separated inputs to lateral amygdala neurons from the auditory thalamus and auditory cortex use different postsynaptic receptors: In vivo intracellular and extracellular recordings in fear conditioning pathways. Learn Mem. 1996;3:229–242. doi: 10.1101/lm.3.2-3.229. [DOI] [PubMed] [Google Scholar]
- 17.Shin RM, Tsvetkov E, Bolshakov VY. Spatiotemporal asymmetry of associative synaptic plasticity in fear conditioning pathways. Neuron. 2006;52:883–896. doi: 10.1016/j.neuron.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li H, Chen A, Xing G, Wei ML, Rogawski MA. Kainate receptor-mediated heterosynaptic facilitation in the amygdala. Nat Neurosci. 2001;4:612–620. doi: 10.1038/88432. [DOI] [PubMed] [Google Scholar]
- 19.Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]
- 20.Bortolotto ZA, et al. Kainate receptors are involved in synaptic plasticity. Nature. 1999;402:297–301. doi: 10.1038/46290. [DOI] [PubMed] [Google Scholar]
- 21.Malenka RC, Bear MF. LTP and LTD: An embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 22.Azad SC, et al. Circuitry for associative plasticity in the amygdala involves endocannabinoid signaling. J Neurosci. 2004;24:9953–9961. doi: 10.1523/JNEUROSCI.2134-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marsicano G, Lutz B. Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse brain. Eur J Neurosci. 1999;11:4213–4225. doi: 10.1046/j.1460-9568.1999.00847.x. [DOI] [PubMed] [Google Scholar]
- 24.Bianchi R, Young SR, Wong RKS. Group I mGluR activation causes voltage-dependent and -independent Ca2+ rises in hippocampal pyramidal cells. J Neurophysiol. 1999;81:2903–2913. doi: 10.1152/jn.1999.81.6.2903. [DOI] [PubMed] [Google Scholar]
- 25.Power JM, Sah P. Intracellular calcium store filling by an L-type calcium current in the basolateral amygdala at subthreshold membrane potentials. J Physiol. 2005;562:439–453. doi: 10.1113/jphysiol.2004.076711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chevaleyre V, et al. Endocannabinoid-mediated long-term plasticity requires cAMP/PKA signaling and RIM1alpha. Neuron. 2007;54:801–812. doi: 10.1016/j.neuron.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Castillo PE, Schoch S, Schmitz F, Südhof TC, Malenka RC. RIM1alpha is required for presynaptic long-term potentiation. Nature. 2002;415:327–330. doi: 10.1038/415327a. [DOI] [PubMed] [Google Scholar]
- 28.Huang YY, et al. Genetic evidence for a protein-kinase-A-mediated presynaptic component in NMDA-receptor-dependent forms of long-term synaptic potentiation. Proc Natl Acad Sci USA. 2005;102:9365–9370. doi: 10.1073/pnas.0503777102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lonart G, et al. Phosphorylation of RIM1alpha by PKA triggers presynaptic long-term potentiation at cerebellar parallel fiber synapses. Cell. 2003;115:49–60. doi: 10.1016/s0092-8674(03)00727-x. [DOI] [PubMed] [Google Scholar]
- 30.Fourcaudot E, et al. L-type voltage-dependent Ca(2+) channels mediate expression of presynaptic LTP in amygdala. Nat Neurosci. 2009;12:1093–1095. doi: 10.1038/nn.2378. [DOI] [PubMed] [Google Scholar]
- 31.Reich CG, Mohammadi MH, Alger BE. Endocannabinoid modulation of fear responses: Learning and state-dependent performance effects. J Psychopharmacol. 2008;22:769–777. doi: 10.1177/0269881107083999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.