The class I MHC-CD8+–T-cell immunosurveillance system constitutes a major component of the adaptive immune system in mammals and other jawed vertebrates. All cell types (except erythrocytes) constitutively express MHC class I molecules, which are comprised of a type I membrane-anchored glycoprotein (heavy chain) noncovalently bound to β2-microglobulin (β2m) and a peptide typically of 8–11 residues. Peptides derive from proteins synthesized by cells, often from defective ribosomal products (DRiPs), which are a subset of nascent proteins that are rapidly shunted to proteasomes for degradation because of either stochastic errors in gene expression (transcription, translation, protein folding or assembly) or a deliberate process of translating standard and nonstandard mRNAs for the purpose of immunosurveillance (1). Peptides are transported from the cytosol to the endoplasmic reticulum (ER) by an oligopeptide transporter (TAP) that also serves as a scaffold for the assembly of class I heavy chains with β2m (2). When a peptide binds with sufficient affinity, class I molecules release from TAP and are exported from the ER to the cell surface. Heavy chains are encoded by three genes (HLA-A, -B, and -C in humans) that are typically among the most polymorphic in their vertebrate species. Class I allomorphs differ in many properties, most prominently in their specificity for peptides, which is largely because of the interaction of two to three residues in the peptides with pockets in the class I binding groove. Consequently, individuals express a unique set of peptides on their cells depending on their class I genes (potentially six different allomorphs) and the peptides generated by the cells (3). In PNAS, Bassani-Sternberg et al. (4) report a nonintrusive method to mine patients’ peptide repertoires for discovering cancer biomarkers and peptide targets for immunotherapy.
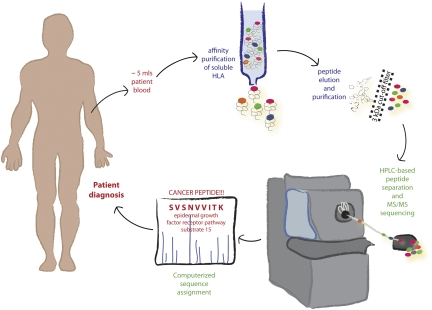
Fig. 1.
Mining the sHLA immunopeptidome. A small amount of blood is used for affinity purification of soluble MHC class I/peptide complexes. Peptides are isolated and separated from the associated heavy chains. Individual peptides are sequenced using tandem MS and in silico analysis. Sequences are mined to identify cancer biomarkers and immunotherapy targets. After appropriate biomarkers are identified, they can potentially be used for cancer diagnosis and monitoring. The technology can also be applied to other diseases, with obvious applications in infection and autoimmunity.
Cell surface class I molecules are recognized by CD8+ T cells, which express a clonally restricted receptor (TCR) that interacts in a highly specific manner with class I molecules bearing a limited set of peptides and triggers T-cell activation and secretion of potent molecules that can kill or reprogram target cells. Although it is generally considered that the class I-antigen processing system principally evolved to deal with viral infections, it clearly has other important functions, including odorant-based mate selection (5), tumor immunosurveillance (6), and tissue rejection. The latter two functions now seem to be intimately entwined with the discovery of tumors directly transmitted between animals (7, 8). Indeed, transmissible tumors may represent a major evolutionary force shaping the class I immunosurveillance system. This would help explain the extraordinary ability of CD8+ T cells to detect tumor-specific peptides from gene products translated at extremely low levels by standard and, particularly, auxillary translation mechanisms (9, 10).
Oligopeptides are extremely unstable in cells and extracellular fluids because of their lack of structure and the abundance of endoproteases and aminopeptidases (11), and, typically, they are only preserved by their binding to class I molecules (12). Bassani-Sternberg et al. (4) exploit this phenomenon to describe a method to characterize peptide repertoire bound to MHC class I molecules (the immunopeptidome) (13) present in plasma. In addition to being expressed on the plasma membrane, class I molecules are released (through membrane shedding or metalloprotease cleavage) (14) or actively secreted from cells (by generation of splice variants lacking the transmembrane domain) (15) and are present in human plasma, which was first reported in 1970 (16). The number of soluble HLA class I molecules (sHLAs) in plasma is often increased by inflammation induced by infections and cancer (17). Soluble class I molecules suppress the activities of CD8+ T cells and natural killer cells ex vivo (17, 18), but the in vivo relevance of these intriguing findings remains to be firmly established.
Insightfully, Bassani-Sternberg et al. (4) recognize that the soluble immunopeptidome (i.e., peptides bound to plasma sHLA) represents a window into ongoing disease processes that potentially offers enormous advantages over standard proteomic-based tissue analysis. The latter, although requiring ever-decreasing cell numbers with each technological advance, still necessitates tissue biopsy, precluding its routine use. Additionally, the number of tissues selected for sampling is obviously limited, whereas the soluble immunopeptidome potentially samples a patient's complete proteome. Plasma can be screened for disease-associated proteins (standard biomarkers); however, this is often compromised by the rapid clearance of these proteins and the relatively limited number of plasma proteins. In broadly monitoring gene product expression throughout an organism, sHLAs are ideally suited as a source of biomarker peptides from tumors, particularly because tumors frequently show enhanced sHLA release relative to normal tissues (17).
To identify sHLA-bound peptides, Bassani-Sternberg et al. (4) use a pan-conformational HLA class I mAb to isolate native class I molecules present in plasma. They acid release peptides from sHLA, remove high Mr material by filtration, HPLC fractionate peptides, and characterize fractions by online tandem MS. Peptide sequences are assigned to mass spectra through automated computer software. Using this method, more than 10,000 sHLA-associated peptides can be identified from only 3–4 mL of blood.
How accurately do sHLAs recapitulate the peptide repertoire of membrane HLA class I molecules (mHLA)? Initial studies using sHLA showed a tight correlation between sHLA and mHLA peptide repertoires (19); however, these sHLA were isolated from cultured cells and not plasma. Extending these findings, Bassani-Sternberg et al. (4) compare peptides eluted from sHLA shed from multiple myeloma cells in vitro with those recovered from mHLA from the same cells, finding 40% of the sHLA peptides in the mHLA repertoire. Most importantly, in patients with advanced stages of cancer, more than 85% of plasma sHLA peptides were present in mHLA-derived peptides obtained from cells. The power of this method is limited if it only identifies abundant normal cellular peptides. The authors (4), however, detect cancer-related peptides in each patient tested, and several cancer-associated peptides are identified in several acute lymphoblastic leukemia and multiple myeloma patients examined.
For optimal use as biomarkers, plasma sHLA peptides should reflect peptides present in the tumor microenvironment. For lymphoblastic leukemia and multiple myeloma, peptides derived from bone marrow vs. plasma sHLA showed 90% overlap. Whether this applies to more common tumor types (e.g., carcinomas) remains to be established as does the ability to detect sHLA peptides from small tumors, a critical issue for diagnosis and monitoring residual tumor burdens after ablative therapy.
The authors (4) freely acknowledge that precious little is known about which peptides will be useful for diagnostic or therapeutic purposes. Given the complexity of the HLA system, with hundreds of different alleles that vary subtly to dramatically in peptide-binding specificity, hundreds to thousands of patients will need to be sampled to generate comprehensive class I allomorph-specific peptide signatures for different disease states. Illustrating this point, the authors (4) show that two individuals with the most divergent HLA genotype (i.e., no shared alleles) present little more than 10% of the same peptides. However, because the most prevalent HLA alleles occur in a large percentage of many populations, it should be possible to rapidly amass significant data for those alleles. Definition of a single allele's peptide repertoire per individual might suffice to provide useful biomarkers.
sHLA peptide identification is tailored for analyzing cancer-related gene product-derived peptides for tumors with enhanced sHLA release relative to normal cells. However, tumors often suppress antigen processing and presentation to escape immunosurveillance (20). Even in these circumstances, however, sHLA-derived peptides could provide biomarkers in the form of normal peptides whose abundances are modulated by the disease process in question. Indeed, it would be of interest to characterize the sHLA immunopeptidome for novel biomarkers in other diseases (e.g., autoimmune diseases) and normal physiological conditions (aging, pregnancy, puberty, dietary changes, etc.) as a tool for understanding global changes in physiology. An extremely promising extension of the sHLA technology is its application to infectious diseases. Virtually nothing is known about pathogen-derived peptides presented to CD8+ T cells in actual humans. sHLA provides a unique window to the in vivo pathogen immunopeptidome and also, a means for monitoring pathogen persistence.
Widespread clinical application of plasma sHLA screening will require more robust instrumentation to accelerate data acquisition. HPLC separation followed by MS/MS, while rapid compared with prior technology, is still labor intensive, technically challenging, and expensive. After these technical hurdles are surmounted (as they surely will be given the rapid advances in MS hardware and software), Bassani-Sternberg et al. (4) provide an exciting approach for targeting, screening, and monitoring cancer and other common human diseases.
Footnotes
The authors declare no conflict of interest.
See companion article on page 18769.
References
- 1.Yewdell JW, Nicchitta CV. The DRiP hypothesis decennial: Support, controversy, refinement and extension. Trends Immunol. 2006;27:368–373. doi: 10.1016/j.it.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 2.Peaper DR, Cresswell P. Regulation of MHC class I assembly and peptide binding. Annu Rev Cell Dev Biol. 2008;24:343–368. doi: 10.1146/annurev.cellbio.24.110707.175347. [DOI] [PubMed] [Google Scholar]
- 3.Shastri N, Schwab S, Serwold T. Producing nature's gene-chips: The generation of peptides for display by MHC class I molecules. Annu Rev Immunol. 2002;20:463–493. doi: 10.1146/annurev.immunol.20.100301.064819. [DOI] [PubMed] [Google Scholar]
- 4.Bassani-Sternberg M, et al. Soluble plasma HLA peptidome as a potential source for cancer biomarkers. Proc Natl Acad Sci USA. 2010;107:18769–18776. doi: 10.1073/pnas.1008501107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Setchell JM, Huchard E. The hidden benefits of sex: Evidence for MHC-associated mate choice in primate societies. Bioessays. 2010 doi: 10.1002/bies.201000066. 10.1002/bies.201000066. [DOI] [PubMed] [Google Scholar]
- 6.Bui JD, Schreiber RD. Cancer immunosurveillance, immunoediting and inflammation: Independent or interdependent processes? Curr Opin Immunol. 2007;19:203–208. doi: 10.1016/j.coi.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 7.O'Neill ID. Tasmanian devil facial tumor disease: Insights into reduced tumor surveillance from an unusual malignancy. Int J Cancer. 2010;127:1637–1642. doi: 10.1002/ijc.25374. [DOI] [PubMed] [Google Scholar]
- 8.Murgia C, Pritchard JK, Kim SY, Fassati A, Weiss RA. Clonal origin and evolution of a transmissible cancer. Cell. 2006;126:477–487. doi: 10.1016/j.cell.2006.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boon T, Van Pel A. T cell-recognized antigenic peptides derived from the cellular genome are not protein degradation products but can be generated directly by transcription and translation of short subgenic regions. A hypothesis. Immunogenetics. 1989;29:75–79. doi: 10.1007/BF00395854. [DOI] [PubMed] [Google Scholar]
- 10.Schwab SR, Shugart JA, Horng T, Malarkannan S, Shastri N. Unanticipated antigens: Translation initiation at CUG with leucine. PLoS Biol. 2004;2:e366. doi: 10.1371/journal.pbio.0020366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reits E, et al. Peptide diffusion, protection, and degradation in nuclear and cytoplasmic compartments before antigen presentation by MHC class I. Immunity. 2003;18:97–108. doi: 10.1016/s1074-7613(02)00511-3. [DOI] [PubMed] [Google Scholar]
- 12.Falk K, Rötzschke O, Rammensee HG. Cellular peptide composition governed by major histocompatibility complex class I molecules. Nature. 1990;348:248–251. doi: 10.1038/348248a0. [DOI] [PubMed] [Google Scholar]
- 13.Istrail S, et al. Comparative immunopeptidomics of humans and their pathogens. Proc Natl Acad Sci USA. 2004;101:13268–13272. doi: 10.1073/pnas.0404740101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Demaria S, Bushkin Y. Soluble HLA proteins with bound peptides are released from the cell surface by the membrane metalloproteinase. Hum Immunol. 2000;61:1332–1338. doi: 10.1016/s0198-8859(00)00213-5. [DOI] [PubMed] [Google Scholar]
- 15.Krangel MS. Secretion of HLA-A and -B antigens via an alternative RNA splicing pathway. J Exp Med. 1986;163:1173–1190. doi: 10.1084/jem.163.5.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Rood JJ, van Leeuwen A, van Santen MC. Anti HL-A2 inhibitor in normal human serum. Nature. 1970;226:366–367. doi: 10.1038/226366a0. [DOI] [PubMed] [Google Scholar]
- 17.Campoli M, Ferrone S. Tumor escape mechanisms: Potential role of soluble HLA antigens and NK cells activating ligands. Tissue Antigens. 2008;72:321–334. doi: 10.1111/j.1399-0039.2008.01106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zavazava N, Krönke M. Soluble HLA class I molecules induce apoptosis in alloreactive cytotoxic T lymphocytes. Nat Med. 1996;2:1005–1010. doi: 10.1038/nm0996-1005. [DOI] [PubMed] [Google Scholar]
- 19.Hickman HD, et al. C-terminal epitope tagging facilitates comparative ligand mapping from MHC class I positive cells. Hum Immunol. 2000;61:1339–1346. doi: 10.1016/s0198-8859(00)00216-0. [DOI] [PubMed] [Google Scholar]
- 20.Chang CC, Ferrone S. Immune selective pressure and HLA class I antigen defects in malignant lesions. Cancer Immunol Immunother. 2007;56:227–236. doi: 10.1007/s00262-006-0183-1. [DOI] [PMC free article] [PubMed] [Google Scholar]