Abstract
Cell proliferation is accompanied by an increase in the utilization of glucose and glutamine. The proliferative response is dependent on a decrease in the activity of the ubiquitin ligase anaphase-promoting complex/cyclosome (APC/C)-Cdh1 which controls G1-to-S-phase transition by targeting degradation motifs, notably the KEN box. This occurs not only in cell cycle proteins but also in the glycolysis-promoting enzyme 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase isoform 3 (PFKFB3), as we have recently demonstrated in cells in culture. We now show that APC/C-Cdh1 controls the proliferative response of human T lymphocytes. Moreover, we have found that glutaminase 1 is a substrate for this ubiquitin ligase and appears at the same time as PFKFB3 in proliferating T lymphocytes. Glutaminase 1 is the first enzyme in glutaminolysis, which converts glutamine to lactate, yielding intermediates for cell proliferation. Thus APC/C-Cdh1 is responsible for the provision not only of glucose but also of glutamine and, as such, accounts for the critical step that links the cell cycle with the metabolic substrates essential for its progression.
Keywords: cell cycle; glutaminase; 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase isoform 3; proliferation
Human blood T lymphocytes have been used for many years in studies of cell proliferation (1, 2). These cells can be obtained directly from the circulation and therefore avoid the pitfalls associated with the use of cells in culture, which acquire confounding characteristics as a result of their in vitro environment (3). Interest has recently been rekindled in the metabolic changes that underpin cell proliferation in cancer to identify potential targets for chemotherapy (4, 5). This has highlighted the need to carry out comparative studies on proliferating normal and tumor cells to ascertain whether an antimetabolic approach to cancer is possible without side effects related to the mechanism of action.
The E3 ubiquitin ligase anaphase-promoting complex/cyclosome (APC/C) attached to the activator protein Cdh1 plays a crucial role in controlling G1- to S-phase transition, and therefore proliferation, through the breakdown of cell cycle proteins (6, 7). APC/C-Cdh1 substrates are targeted for degradation through specific recognition motifs, including one known as the KEN box (8). Inactivation of APC/C-Cdh1 in G1 of the cell cycle is necessary for initiation of S phase, in which DNA is replicated and chromosomes are duplicated. We have recently found that APC/C-Cdh1 links cell cycle activity with that of the enzyme 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase isoform 3 (PFKFB3) (9, 10). PFKFB3—a key regulator of glycolysis (11)—contains a KEN box motif and is thus also broken down by APC/C-Cdh1. Inactivation of APC/C-Cdh1 enables PFKFB3 to up-regulate glycolysis, thus providing the cell with the glucose essential for the subsequent biosynthesis of macromolecules. Our previous studies (9) were carried out in two cell lines; we therefore decided to investigate whether the same mechanism operates in human proliferating primary cells.
We now show that APC/C-Cdh1 also links the metabolic supply of glucose with cell cycle progression in human freshly isolated peripheral T lymphocytes. Furthermore, we show an additional APC/C-Cdh1–mediated point of control via glutaminase; this enzyme metabolizes glutamine to glutamate and is the first step in its conversion into lactate through the process called glutaminolysis. By this process, which uses part of the tricarboxylic acid cycle, glutamine, together with glucose, provides the raw materials for cell proliferation (12, 13). The present results confirm and extend our understanding of the central role of APC/C-Cdh1 in the coordination of metabolism with cell proliferation.
Results
Correlation of T-Lymphocyte Proliferation with the Generation of Lactate.
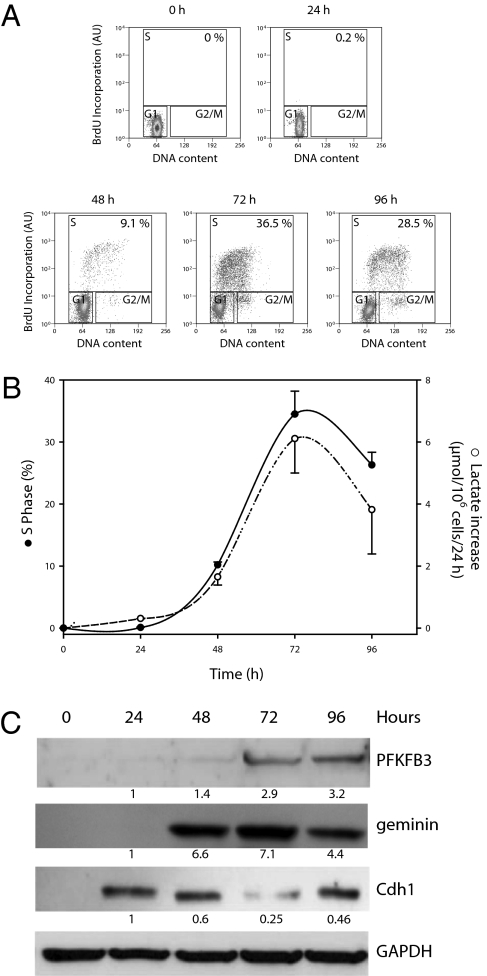
Human freshly isolated peripheral T lymphocytes were induced to proliferate by ligation of the T-lymphocyte receptor with anti-CD3 and costimulation with anti-CD28 antibodies. The percentage of cells in S phase and the generation of lactate were then monitored for as long as 96 h. Fig. 1A shows the percentage of cells in S phase at different times after initiation of proliferation. These results are shown together with the increase in lactate release by the cells at these times in Fig. 1B. Fig. S1A shows that the proportion of cells in S phase correlated positively with the rate of generation of lactate (r2 = 0.91). T-lymphocyte proliferation was accompanied by an increase in the amount of the most abundant splice variant of PFKFB3 in T lymphocytes (splice variant 5; Fig. S1B) and of the cell cycle protein geminin (a known substrate of APC/C-Cdh1); these increases were associated with a decrease in Cdh1 protein (Fig. 1C). Interestingly, we did not observe expression of Cdh1 in freshly isolated, nonactivated T lymphocytes (Fig. 1C), probably indicating their quiescent state.
Fig. 1.
Proliferation and generation of lactate in human T lymphocytes. (A) The proportion of cells in S phase [bromodeoxyuridine (BrdU) incorporation and DNA content analysis by flow cytometry; details provided in SI Materials and Methods] at different times after initiation of proliferation. Result representative of three independent experiments. (B) Percentage of cells in S phase and production of lactate per 24 h at different times after initiation of proliferation; n = 3, mean ± SEM. (C) The appearance of PFKFB3 and geminin, and the disappearance of Cdh1, at different times after initiation of proliferation. Proteins from a Western blot representative of three independent experiments were quantified by densitometry. AU, arbitrary units.
Effect of Overexpression of Cdh1 on T-Lymphocyte Proliferation and Generation of Lactate.
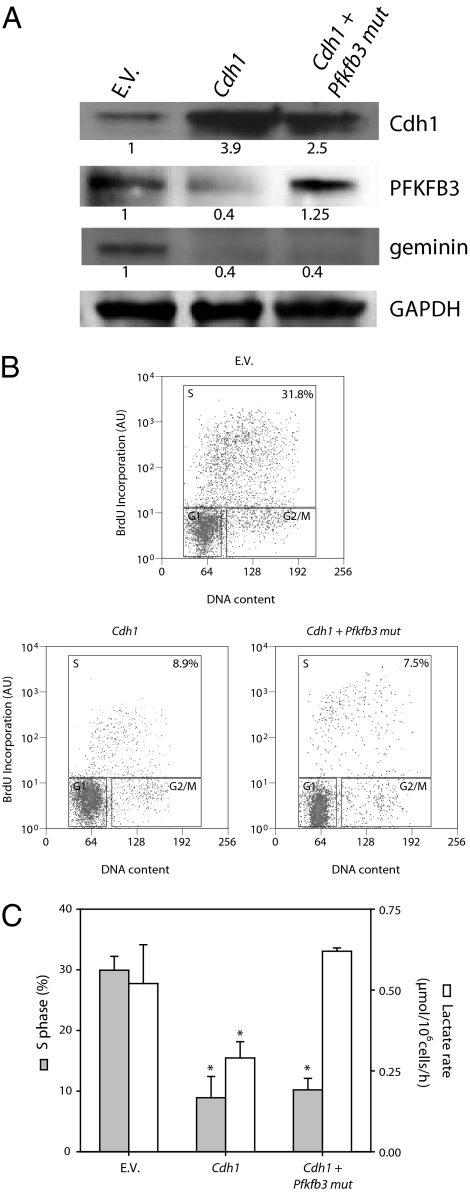
The percentage of cells in S phase, lactate production, and the appearance of PFKFB3 were compared in proliferating T lymphocytes transfected with an empty vector (EV) and in those in which Cdh1 had been overexpressed (Fig. 2A). The increase in the percentage of cells in S phase and in the rate of lactate generation previously observed at 72 h after the initiation of proliferation was unaffected in EV cells but was prevented in cells overexpressing Cdh1 (Fig. 2 B and C). These effects of Cdh1 were associated with a significant reduction in PFKFB3; the presence of geminin was also greatly reduced in cells overexpressing Cdh1 (Fig. 2A). The increase in generation of lactate, but not the proportion of cells in S phase, could be completely restored in these cells by coexpressing a PFKFB3 form in which the KEN box amino acid sequence was mutated to AAA (Pfkfb3 mut; Fig. 2 B and C), and was therefore not targeted for destruction by APC/C-Cdh1 (Fig. 2A).
Fig. 2.
Effect of overexpression of Cdh1 on PFKFB3, lactate generation, and T-lymphocyte proliferation. All data shown in this figure were obtained 72 h after initiation of proliferation. (A) Overexpression of Cdh1 promoted the degradation of PFKFB3 and geminin, but did not affect the KEN box-mutant form of PFKFB3. Proteins from a Western blot representative of three independent experiments were quantified by densitometry. (B) The proportion of cells in S phase was greatly reduced in cells overexpressing Cdh1 compared with those expressing EV. Coexpression of PFKFB3 mut did not prevent the Cdh1-induced reduction in proliferation. Result representative of three experiments. (C) In Cdh1-overexpressing cells, both proliferation and lactate generation were significantly reduced. In cells cotransfected with Pfkfb3 mut, the generation of lactate was similar to EV values but proliferation was still reduced; n = 3, mean ± SEM; *P < 0.05 versus cells containing EV. AU, arbitrary units.
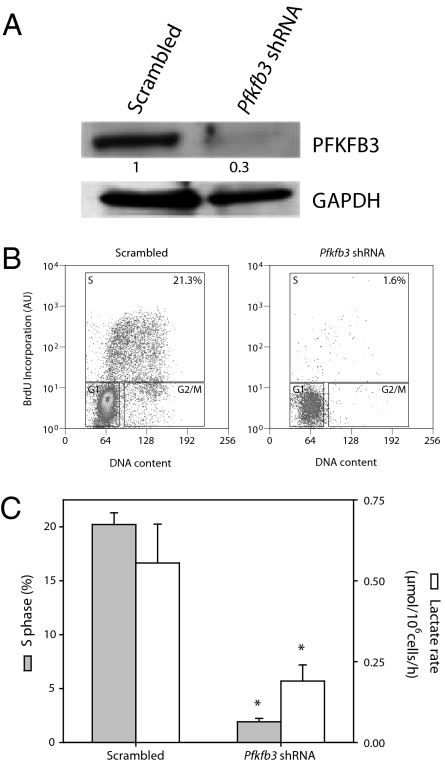
Effect of Silencing Pfkfb3 on T-Lymphocyte Proliferation and Generation of Lactate.
Pfkfb3 was silenced in T lymphocytes using small hairpin RNA (Pfkfb3 shRNA; Fig. 3A). The percentage of cells in S phase and lactate production at 72 h after initiation of proliferation in these cells were compared with those in T lymphocytes transfected with scrambled shRNA. The percentage of cells in S phase was always slightly less in cells containing scrambled shRNA than in cells transfected with an EV at this time (Figs. 2B and 3B), although the rate of lactate production was not significantly different (Figs. 2C and 3C). In contrast, silencing Pfkfb3 dramatically reduced both proliferation and generation of lactate (Fig. 3C).
Fig. 3.
Effect of silencing Pfkfb3 on lactate generation and proliferation. (A) Pfkfb3 was silenced in T lymphocytes using shRNA (Pfkfb3 shRNA). Cell lysates were analyzed by Western blotting 72 h after initiation of proliferation. Proteins from a Western blot representative of three independent experiments were quantified by densitometry. (B) The proportion of T lymphocytes in S phase 72 h after transfection with Pfkfb3 shRNA was far less than that of T lymphocytes transfected with scrambled shRNA. Result representative of three experiments. (C) Both proliferation and lactate generation were significantly reduced in T lymphocytes 72 h after transfection with Pfkfb3 shRNA compared with those transfected with scrambled shRNA; n = 3, mean ± SEM; *P < 0.05 versus cells containing scrambled shRNA. AU, arbitrary units.
Pfkfb3 Gene Expression and the Activity of APC/C-Cdh1.
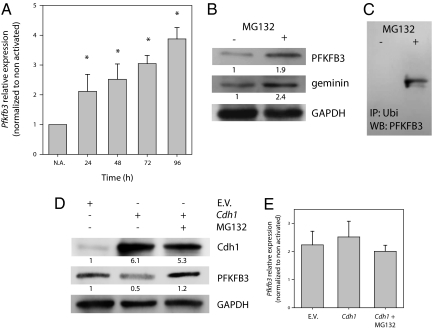
Expression of Pfkfb3 mRNA increased during proliferation so that, by 96 h, there was approximately four times the control amount (Fig. 4A). There was dissociation between gene expression, which was evident after 24 h and continued to increase throughout the observation period, and the appearance of PFKFB3 (Fig. 1D). The expression of the protein could be greatly enhanced when T lymphocytes induced to proliferate for 48 h were treated with the proteasome inhibitor MG132 (10 μM, 2 h; Fig. 4B), a treatment that also increased the amount of geminin present, suggesting that both proteins are actively degraded through the same mechanism (Fig. 4B). Addition of MG132 also resulted in accumulation of ubiquitinated PFKFB3, the tagged form recognized by the proteasome (Fig. 4C). Fig. 4D shows that overexpression of Cdh1 resulted in a significant reduction in the appearance of PFKFB3 72 h after initiation of proliferation. Under these conditions, incubation with MG132 (10 μM, 2 h) significantly enhanced the appearance of the protein. This effect was not related to gene expression, as Pfkfb3 mRNA was expressed to a similar extent in cells transfected with EV, with Cdh1, and with Cdh1 in the presence of MG132 (Fig. 4E). These results indicate that, in T lymphocytes, the induction of the gene for PFKFB3 may be necessary but is not sufficient for proliferation, as the protein is degraded through the ubiquitin–proteasome pathway as long as APC/C-Cdh1 is active.
Fig. 4.
Pfkfb3 gene expression and the activity of APC/C-Cdh1. (A) The expression of Pfkfb3 mRNA in T lymphocytes increased with time after activation. The relative expression of Pfkfb3 was determined by RT-qPCR as described in SI Materials and Methods. Results are the mean ± SEM of three independent experiments; *P < 0.05 versus nonactivated (N.A.) T lymphocytes. (B) Incubation of proliferating T lymphocytes (48 h) with the proteasome inhibitor MG132 significantly enhanced the amounts of PFKFB3 and geminin detected. Proteins from a Western blot representative of three independent experiments were quantified by densitometry. (C) PFKFB3 protein was immunoprecipitated with anti-ubiquitin antibodies from lysates of T lymphocytes activated to proliferate for 48 h and treated with DMSO (−) or MG132 (10 μM) for 2 h. The immunoprecipitated proteins were subjected to Western blotting and detected with anti-PFKFB3 as detailed in SI Materials and Methods. Figure representative of three experiments. (D) Overexpression of Cdh1 markedly reduced the appearance of PFKFB3 72 h after initiation of proliferation. However incubation with MG132 prevented degradation of the protein. Proteins from a Western blot representative of three independent experiments were quantified by densitometry. (E) The expression of Pfkfb3 mRNA at 72 h after initiation of proliferation was similar in cells transfected with EV, with Cdh1, and with Cdh1 in the presence of MG132. Pfkfb3 mRNA was quantified by RT-qPCR as described in SI Materials and Methods. Values are mean ± SEM of three independent experiments.
Glutaminase: An Additional Control Point.
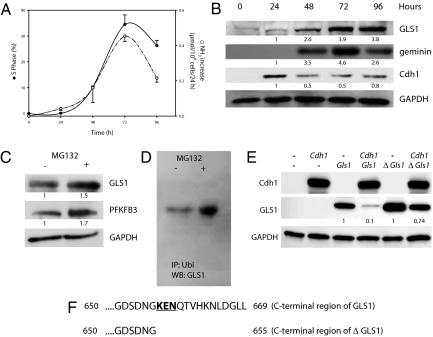
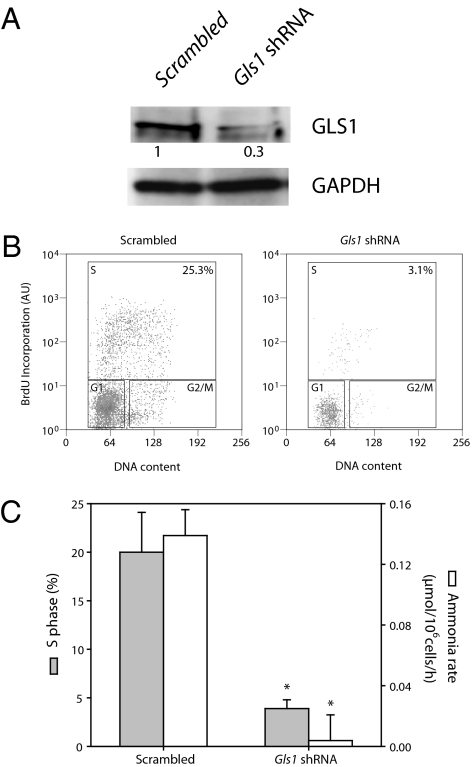
Fig. 5A shows that the release of ammonia (a coproduct of the conversion of glutamine to glutamate) from T lymphocytes activated to proliferate follows a pattern similar to that of lactate (Fig. 1B), with a maximum increase at 72 h coinciding with the highest entry of cells into S phase. This was accompanied by an increase in the presence of glutaminase 1 (GLS1; Fig. 5B), the isoform of the enzyme that we established to be the most abundant in these cells (Fig. S2A). The increase in GLS1 occurred concomitantly with the increase in geminin and in PFKFB3 (Fig. 1D) and the decrease in Cdh1 (Fig. 5B). Expression of the gene for GLS1 also increased during proliferation and was apparent after 24 h, reaching a maximum at 72 h (Fig. S2B). When T lymphocytes induced to proliferate for 48 h were treated with MG132 (10 μM, 2 h), there was also an increased accumulation of GLS1, similar to that of PFKFB3 (Fig. 5C). Moreover, GLS1 was ubiquitinated under these conditions, as shown in Fig. 5D. To establish whether GLS1 is also a substrate for APC/C-Cdh1, we transfected a Gls1-encoding plasmid into HEK 293 cells. Overexpression of Cdh1 in these transfected cells resulted in the almost complete disappearance of GLS1 (Fig. 5E). In contrast, the enzyme β-gal, which does not contain a KEN box, was largely unaffected by overexpression of Cdh1 (Fig. S2C). Furthermore, overexpression of Cdh1 had little effect on GLS1 when Gls1 was mutated to a form in which the KEN box was removed from the resulting protein (ΔGLS1; Fig. 5 E and F). When GLS1 expression in T lymphocytes was reduced by silencing Gls1 (Fig. 6A), proliferation was virtually abolished (Fig. 6 B and C) and the generation of ammonia was dramatically reduced (Fig. 6C). Similar results were obtained in T lymphocytes that were treated with 6-diazo-5-oxo-l-norleucine—a well characterized inhibitor of glutaminase activity (14)—at the time they were activated to proliferate (Fig. S2 D and E).
Fig. 5.
GLS1 activity in T-lymphocyte proliferation. (A) Percentage of cells in S phase and generation of ammonia at different times after initiation of proliferation; n = 3, mean ± SEM. (B) Time course of GLS1, geminin, and Cdh1 expression after initiation of proliferation. Proteins from a Western blot representative of three independent experiments were quantified by densitometry. (C) Incubation of proliferating T lymphocytes (48 h) with MG132 significantly enhanced the amounts of GLS1 and PFKFB3 detected. Proteins from a Western blot representative of three independent experiments were quantified by densitometry. (D) GLS1 protein was immunoprecipitated with anti-ubiquitin antibodies from lysates of T lymphocytes activated to proliferate for 48 h and treated with DMSO (−) or MG132 (10 μM) for 2 h. The immunoprecipitated proteins were subjected to Western blotting and detected with anti-GLS1. Figure representative of three experiments. (E) Overexpression of Cdh1 in HEK 293 cells promoted degradation of overexpressed GLS1 but this effect was markedly reduced in cells cotransfected with ΔGls1. Proteins from a Western blot representative of thre independent experiments were quantified by densitometry. (F) The C-terminal region of human GLS1 and of a truncated form in which the KEN box has been deleted (ΔGLS1).
Fig. 6.
Effect of silencing Gls1 on ammonia generation and proliferation. (A) Gls1 was silenced in T lymphocytes using shRNA (Gls1 shRNA). Cell lysates were analyzed by Western blotting 72 h after initiation of proliferation. Proteins from a Western blot representative of three independent experiments were quantified by densitometry. (B) The proportion of T lymphocytes in S phase 72 h after transfection with Gls1 shRNA was significantly lower than that of T lymphocytes transfected with scrambled shRNA. Result representative of three experiments. (C) Both proliferation and rate of ammonia generation were significantly reduced in T lymphocytes 72 h after transfection with Gls1 shRNA compared with those transfected with scrambled shRNA; n = 3, mean ± SEM; *P < 0.05 versus cells containing scrambled shRNA. AU, arbitrary units.
Discussion
The present results demonstrate that proliferation of human peripheral T lymphocytes is dependent on changes in the activity of the ubiquitin ligase APC/C-Cdh1. Following activation of the CD3 and CD28 receptors on these cells (15), there is a response, characterized by a progressive increase in the number of cells entering S phase, which reaches a peak at 72 h and correlates closely with an increased accumulation of lactate in the cell culture medium. This response is accompanied by an increase in the expression of PFKFB3 and geminin, coinciding with a decrease in the activity of APC/C-Cdh1, for which both proteins are substrates (16, 17). We further show that overexpression of Cdh1 decreases both the proliferative response and the accumulation of lactate, and that the concomitant overexpression of a PFKFB3, mutated in the KEN box so that it is no longer a substrate for APC/C-Cdh1, restores the production of lactate but not the proliferative response. This confirms previous results using cell lines that show that restoration of glycolysis alone is not enough to initiate proliferation (9), as the overexpressed Cdh1 continues to metabolize key proteins involved in cell cycle progression, such as geminin. Thus, as previously shown (9), there is a strict coordination between cell cycle progression and the metabolic response that underpins it. This is further supported by our experiments in which silencing Pfkfb3 rendered the activated T lymphocytes unable to proliferate.
Our results also show that, following activation, there is a progressive increase in expression of the gene for Pfkfb3. However, the protein is constantly ubiquitinated by APC/C-Cdh1 until its activity is permitted by the decrease in activity of the ubiquitin ligase. This is apparent in the experiments in which, 72 h after initiation of proliferation, cells overexpressing Cdh1 show up-regulation of Pfkfb3 mRNA but little protein, unless the proteasome inhibitor MG132 is added to prevent its destruction.
It has been known for many years that glutamine is required for cell proliferation (12, 13). Through its conversion to lactate, glutamine is an essential partner of glucose in the generation of energy, of intermediates in the tricarboxylic acid cycle required for anaplerosis, and of NADPH (13, 18). Moreover, it has been shown that the rate of utilization of glutamine in proliferating T lymphocytes is even higher than that of glucose (19). Glutaminase is a critical enzyme in glutaminolysis (13, 20), and its activity is known to be increased in proliferating T lymphocytes (13) and in cancer cells (21, 22). The fact that this enzyme contains a KEN box and, as our experiments show, is a substrate for APC/C-Cdh1 indicates that its activity is regulated in a similar manner to that of PFKFB3. Experiments in which the expression of GLS1 was reduced by silencing Gls1, or its activity inhibited pharmacologically using 6-diazo-5-oxo-l-norleucine, confirmed the relevance of this enzyme for the proliferative response of these cells.
We have therefore demonstrated that the activity of APC/C-Cdh1 directly regulates two of the most important metabolic enzymes in cell proliferation. The synchronous up-regulation of glycolysis and glutaminolysis highlights the issue of the relative contribution of each pathway to the components of the new cell (23) and to the amount of lactate that is excreted during proliferation. Indeed, the two pathways are known to be functional and complementary in proliferating lymphocytes (24) and in cancer cells (25), and are further modulated by regulatory mechanisms that might alter their relative contribution in different circumstances.
Interestingly, bioinformatic analysis of the human proteome (26) shows that there are other KEN box-containing enzymes in the metabolic pathways involved in the synthesis of macromolecules for cell proliferation. These are further potential targets of APC/C-Cdh1 and include pyruvate carboxylase, malate dehydrogenase 1, and acetyl-CoA carboxylase-α. Furthermore, two mitochondrial proteins, OPA1 and Tfam, involved in mitochondrial fusion and transcription, respectively (27, 28), contain a KEN box. If some or all of these proteins are regulated by the activity of APC/C-Cdh1, this ubiquitin ligase has a wider role than the one we have so far uncovered. Whether this system is substantially changed or simply dysregulated during neoplastic transformation now requires clarification.
Materials and Methods
Isolation of Peripheral Blood T Lymphocytes.
Fresh blood was defibrinated and centrifuged at 700 × g for 15 min. The cells were diluted in two volumes of PBS solution. Peripheral blood mononuclear cells (PBMCs) were separated by Ficoll/Paque gradient centrifugation (GE Healthcare) from diluted fresh blood cells or from the buffy coat of healthy donors (National Blood Transfusion Service, Brentwood Centre, Essex, United Kingdom). Platelets in PBMC from the buffy coat were removed by resuspending cells in PBS solution containing 2 mM EDTA and 0.5% FBS and centrifuging at 300 × g for 10 min. Purification of T lymphocytes from PBMCs was carried out using the Pan T cell isolation kit II (Miltenyi Biotec). More than 95% of the resulting eluted cells were CD3+ when analyzed by flow cytometry. T lymphocytes were then resuspended at 2 × 106 cells/mL in DMEM (Invitrogen) supplemented with 10% heat-inactivated FBS (or autologous serum when using fresh blood) and penicillin (100 U/mL)/streptomycin (100 μg/mL). Cells were cultured overnight in T-175 flasks at 37 °C in a 5% CO2 humidified incubator.
T-Lymphocyte Proliferation Assay.
T lymphocytes were harvested and plated at 106 cells/mL in six- or 12-well plates that had been coated overnight with an anti-CD3 monoclonal antibody (anti-human CD3, clone OKT3, 5 μg/106 cells; eBioscience). Soluble anti-CD28 antibody (anti-human CD28, costimulatory clone CD28.2, 5 μg/mL; eBioscience) was added to the culture medium described earlier and the cells were replaced in the incubator. In some experiments, proliferating cells were incubated with 250 μM 6-diazo-5-oxo-l-norleucine (Sigma) for 72 h to inhibit glutaminase.
Lactate and Ammonia Accumulation in the Cell Supernatant.
Cell-free supernatant samples were analyzed in triplicate using a lactate kit (Trinity Biotech) adapted for a 96-well plate reader. Briefly, 10 μL of sample or standard were incubated with 100 μL lactate reagent solution for 10 min, after which the absorbance was measured at 540 nm. The rate of lactate generation was determined from the increase in lactate production per million cells in the last 24 h or the last 1 h of each end point. These experiments were carried out using autologous serum or dialyzed heat-inactivated FBS in the medium to minimize background readings. For determination of ammonia, cell-free supernatant was analyzed using an Ammonia Assay Kit (Sigma-Aldrich) according to the manufacturer's instructions. In brief, 50 μL supernatant was mixed with 1 mL ammonia assay reagent and 10 μL l-glutamate dehydrogenase solution in a quartz cuvette for 5 min, after which absorbance at 340 nm was measured (SpectraMax M2; Molecular Devices). The background concentration of ammonia in the culture medium at the relevant time points was subtracted, and the results are expressed as the increase in ammonia released per million cells in the last 24 h or the last 1 h of each end point.
Protocols for cell transfection, Western blotting, quantitative RT-PCR (RT-qPCR), and other protocols are described in detail in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Annie Higgs for helpful discussion in preparation of the manuscript and Steven Grover for technical assistance. This work was supported by Wellcome Trust Grant 086729 (to S.L.C. and S.M.); a Scientific Collaboration Agreement with the Pharmaceutical Biotechnology Centre of the University of Rome Tor Vergata (N.D.); an Erwin Schrödinger postdoctoral fellowship, Fonds zur Förderung der wissenschaftlichen Forschung Project J2957-B11 (to C.A.S.); and UK Biotechnology and Biological Sciences Research Council Collaborative Award in Science and Engineering 36801 (to L.B.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1012362107/-/DCSupplemental.
References
- 1.Sasaki MS, Norman A. Proliferation of human lymphocytes in culture. Nature. 1966;210:913–914. doi: 10.1038/210913a0. [DOI] [PubMed] [Google Scholar]
- 2.Michalowski A. Phytohaemagglutinin-stimulated division and growth rate of human lymphocytes. Bull Acad Pol Sci Biol. 1967;15:577–581. [PubMed] [Google Scholar]
- 3.Halliwell B. Oxidative stress in cell culture: An under-appreciated problem? FEBS Lett. 2003;540:3–6. doi: 10.1016/s0014-5793(03)00235-7. [DOI] [PubMed] [Google Scholar]
- 4.Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;4:988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- 5.Jones RG, Thompson CB. Tumor suppressors and cell metabolism: A recipe for cancer growth. Genes Dev. 2009;23:537–548. doi: 10.1101/gad.1756509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peters JM. The anaphase-promoting complex: Proteolysis in mitosis and beyond. Mol Cell. 2002;9:931–943. doi: 10.1016/s1097-2765(02)00540-3. [DOI] [PubMed] [Google Scholar]
- 7.Li M, Zhang P. The function of APC/CCdh1 in cell cycle and beyond. Cell Div. 2009;4:2. doi: 10.1186/1747-1028-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pfleger CM, Kirschner MW. The KEN box: an APC recognition signal distinct from the D box targeted by Cdh1. Genes Dev. 2000;14:655–665. [PMC free article] [PubMed] [Google Scholar]
- 9.Almeida A, Bolaños JP, Moncada S. E3 ubiquitin ligase APC/C-Cdh1 accounts for the Warburg effect by linking glycolysis to cell proliferation. Proc Natl Acad Sci USA. 2010;107:738–741. doi: 10.1073/pnas.0913668107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bolaños JP, Almeida A, Moncada S. Glycolysis: A bioenergetic or a survival pathway? Trends Biochem Sci. 2010;35:145–149. doi: 10.1016/j.tibs.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 11.Okar DA, Lange AJ. Fructose-2,6-bisphosphate and control of carbohydrate metabolism in eukaryotes. Biofactors. 1999;10:1–14. doi: 10.1002/biof.5520100101. [DOI] [PubMed] [Google Scholar]
- 12.Krebs HA. Glutamine metabolism in the animal body. In: Mora J, Palacios R, editors. Glutamine Metabolism, Enzymology and Regulation. London: Academic Press; 1980. pp. 319–329. [Google Scholar]
- 13.Newsholme EA, Crabtree B, Ardawi MS. Glutamine metabolism in lymphocytes: Its biochemical, physiological and clinical importance. Q J Exp Physiol. 1985;70:473–489. doi: 10.1113/expphysiol.1985.sp002935. [DOI] [PubMed] [Google Scholar]
- 14.Ardawi MS, Newsholme EA. Intracellular localization and properties of phosphate-dependent glutaminase in rat mesenteric lymph nodes. Biochem J. 1984;217:289–296. doi: 10.1042/bj2170289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vyakarnam A, et al. Human CD4 culture. In: Rowland-Jones SL, McMichael AJ, editors. Lymphocytes: A Practical Approach. New York: Oxford Univ Press; 2000. pp. 135–159. [Google Scholar]
- 16.McGarry TJ, Kirschner MW. Geminin, an inhibitor of DNA replication, is degraded during mitosis. Cell. 1998;93:1043–1053. doi: 10.1016/s0092-8674(00)81209-x. [DOI] [PubMed] [Google Scholar]
- 17.Herrero-Mendez A, et al. The bioenergetic and antioxidant status of neurons is controlled by continuous degradation of a key glycolytic enzyme by APC/C-Cdh1. Nat Cell Biol. 2009;11:747–752. doi: 10.1038/ncb1881. [DOI] [PubMed] [Google Scholar]
- 18.DeBerardinis RJ, et al. Beyond aerobic glycolysis: Transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci USA. 2007;104:19345–19350. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ardawi MS, Newsholme EA. Glutamine metabolism in lymphocytes of the rat. Biochem J. 1983;212:835–842. doi: 10.1042/bj2120835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li C, et al. Regulation of leucine-stimulated insulin secretion and glutamine metabolism in isolated rat islets. J Biol Chem. 2003;278:2853–2858. doi: 10.1074/jbc.M210577200. [DOI] [PubMed] [Google Scholar]
- 21.Knox WE, Horowitz ML, Friedell GH. The proportionality of glutaminase content to growth rate and morphology of rat neoplasms. Cancer Res. 1969;29:669–680. [PubMed] [Google Scholar]
- 22.Matsuno T. Bioenergetics of tumor cells: Glutamine metabolism in tumor cell mitochondria. Int J Biochem. 1987;19:303–307. doi: 10.1016/0020-711x(87)90002-4. [DOI] [PubMed] [Google Scholar]
- 23.Reitzer LJ, Wice BM, Kennell D. Evidence that glutamine, not sugar, is the major energy source for cultured HeLa cells. J Biol Chem. 1979;254:2669–2676. [PubMed] [Google Scholar]
- 24.Carr EL, et al. Glutamine uptake and metabolism are coordinately regulated by ERK/MAPK during T lymphocyte activation. J Immunol. 2010;185:1037–1044. doi: 10.4049/jimmunol.0903586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang C, et al. Glioblastoma cells require glutamate dehydrogenase to survive impairments of glucose metabolism or Akt signaling. Cancer Res. 2009;69:7986–7993. doi: 10.1158/0008-5472.CAN-09-2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gasteiger E, et al. ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003;31:3784–3788. doi: 10.1093/nar/gkg563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gottlieb E. OPA1 and PARL keep a lid on apoptosis. Cell. 2006;126:27–29. doi: 10.1016/j.cell.2006.06.030. [DOI] [PubMed] [Google Scholar]
- 28.Scarpulla RC. Nuclear control of respiratory gene expression in mammalian cells. J Cell Biochem. 2006;97:673–683. doi: 10.1002/jcb.20743. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.