Abstract
The molecular mechanisms underlying the developmental processes that shape living organisms provide a basis to understand the evolution of biological complexity. Gene duplication allows biological functions to become separated, leading to increased complexity through subfunctionalization. Recently, the relative contributions to morphological evolution of changes to the regulatory and/or coding regions of duplicated genes have been the subject of debate. Duplication generated multiple copies of the MADS-box transcription factor genes that play essential roles in specifying organ identity in the flower, making this evolutionary novelty a good model to investigate the nature of the changes necessary to drive subfunctionalization. Here, we show that naturally occurring variation at a single amino acid in a MADS-box transcription factor switches its ability to specify male and female reproductive organs by altering its repertoire of protein–protein interactions. However, these different developmental fates are only manifest because of an underlying variation in the expression pattern of interacting proteins. This shows that the morphological outcomes of changes to protein sequence and gene expression must be interpreted in the context of the wider regulatory network. It also suggests an explanation for the surprisingly widespread duplications of some of the floral transcription factors.
Keywords: evolution, flower development, gene duplication, transcription factors
In a relatively short period of around five million years of the late Cretaceous, flowering plants diversified massively to eclipse the extant flora. Darwin referred to this rapid radiation as an abominable mystery (1). Duplication and subfunctionalization of genes, especially those encoding MADS-box transcription factors, has been specifically implicated in the evolution of the flower (2–4), making this an ideal system in which to study the factors that have driven subfunctionalization in evolution. The role of gene duplication in the evolution of novel features through sub- and neofunctionalization has long been recognized (5). More recently, the relative influence on morphological evolution of changes to coding sequences and changes to cis elements regulating gene expression has been debated (6–8).
Male and female reproductive organs form in the center of flowers because of the spatially restricted activity of classes of MADS-box transcription factors known as the B- and C-functions (9). Expression of C-function genes in the central whorl causes female organs to develop, and the overlapping expression of B- and C-function genes, exclusively in the third whorl, specifies male organs. The activity of the B- and C-function factors is mediated by direct interactions with another class of MADS-box factor, the E function or SEPALLATA factors (SEPs), according to the quartet model (10–12). Duplications have resulted in extant angiosperms carrying multiple copies of many of the B-, C-, and particularly, E-function genes (2). In Arabidopsis, a single C-function gene, AGAMOUS (AG), specifies both male and female organ development. In contrast, in Antirrhinum, the C-function genes PLENA (PLE) and FARINELLI (FAR) make an unequal contribution to the specification of male and female organs (13, 14).
As predicted by the ABC model (9), ectopic expression of the Arabidopsis C-function gene, AG, specifies homeotic conversion of the first and second whorl organs, causing sepals to develop as female organs (carpels) and petals to develop as male organs (stamens) (35S::AG in Fig. 1A). Ectopic expression of the Antirrhinum C-function gene PLE in Antirrhinum has the same effect (13). In contrast, ectopic expression of the Antirrhinum FAR gene in Antirrhinum converts petals to stamens but does not alter sepal identity (13). Although reproductive organ identity in Arabidopsis is specified by a single C-function gene (AG), it responds to ectopic expression of the heterologous FAR and PLE genes in the same way as Antirrhinum (35S::FAR in Fig. 1A) (13). In summary, expression of PLE or AG in the outer whorls of Antirrhinum or Arabidopsis promotes both male and female organs, whereas FAR can only specify male organs. The difference in the ability of a homeotic protein to specify male and female reproductive organ development can be used to study the mechanism of organ specification and the influence of evolutionary changes on developmental processes.
Fig. 1.
Ectopic expression of forms of AG and FAR in Arabidopsis using the 35S promoter. (A) Expression of intact AG and FAR. In 35S::AG, stigmatic papillae and ovules develop on first whorl-converted carpeloid sepals (white arrows), and in both 35S::AG and 35S::FAR, petals are converted into stamens. (B) Alignment of AG, FAR, and PLE protein sequences around the K3 region. The red arrow points to the critical glutamine residue (Q173) in FAR that is absent in AG and PLE. (C) Ectopic expression of modified forms of FAR and AG. 35S::FAR-Q indicates a full-length FAR protein, lacking only Q173. The white arrow points to representative carpeloid structures. 35S::AG+Q and 35S::AG+R indicate a full-length AG protein with an insertion of either a glutamine (Q) or an arginine (R) at the position in K3 equivalent to Q173. Note that neither of these flowers shows the characteristic conversion of sepals to carpeloidy.
Results
A Single Amino Acid Influences Reproductive Organ Identity.
AG, PLE, and FAR show their different abilities to induce stamen and carpel development when expressed under the same constitutive promoter, clearly suggesting that their differences are driven by changes in their protein sequence. To determine the regions of the proteins responsible for discriminating between the male and female organ identity pathways, we created domain swaps between AG, PLE, and FAR and tested the consequences of their expression in the outer whorls of Arabidopsis (Fig. S1).
These experiments implicated a single glutamine amino acid residue (Q173), present in FAR but absent from both PLE and AG, as the decisive difference (arrowed in Fig. 1B), suggesting that absence of Q173 correlates with the induction of male and female organ development, whereas its presence limits the protein to the induction of male organs.
We tested this hypothesis by removing Q173 from FAR (35S::FAR-Q in Fig. 1C), which was then switched to become capable of activating both the male and female developmental pathways. Conversely, adding a Q at the appropriate position in AG (35S::AG+Q in Fig. 1C) resulted in the specific loss of its ability to induce female organs in the first whorl. These experiments confirmed that the presence or absence of Q173 defines the developmental outcome of C-function gene expression. To determine whether the identity of the amino acid insertion was critical to its function, we created a variant of AG where an additional arginine (R) is inserted (35S::AG+R in Fig. 1C). The flowers of these plants were identical to the AG+Q flowers, indicating that the effect is not specific to glutamine.
Reproductive Organ Identity Relies on Protein–Protein Interaction Specificity.
Q173 is located in a predicted α-helical region that spans the junction of two domains known as the K and C domains (15, 16). Since this region of MADS-box proteins has been shown to be involved in protein–protein interactions, we speculated that the insertion of an amino acid might alter the range of possible protein–protein interactions. C-function MADS-box proteins interact with SEP (or E-function) MADS-box proteins to drive stamen and carpel development (10–12). We used yeast three-hybrid experiments to test the influence of the presence or absence of Q173 on complex formation between C-function and SEP proteins (17, 18). In agreement with published data (18), we found that AG, which lacks Q173, can form complexes with SEP1, SEP2, and SEP3. However, FAR, which contains Q173, is restricted to interacting with SEP3 (Fig. 2A and Table S1). We did not detect any interactions involving SEP4 in this system (Table S1). This finding highlights the importance of the K3 helix in determining protein–protein interactions and shows that the SEP proteins are likely to have biologically significant interaction preferences that could explain why they are present in multiple copies in angiosperm plants (2).
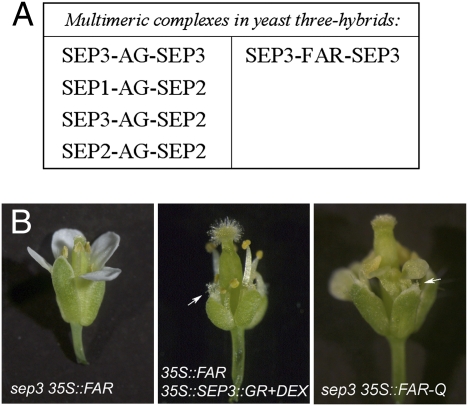
Fig. 2.
Interactions between FAR and SEP. (A) Multimeric complexes identified in yeast three-hybrid experiments. AG shows an ability to form multimeric complexes with SEP1, SEP2, and SEP3, whereas FAR can only interact with SEP3. (B) Genetic interaction evidence. 35S::FAR flowers in a sep3 mutant background show no conversion of petals into stamens. Conversely, 35S::FAR in an induced 35S::SEP3::GR background shows both stamenoid petals and carpelloid sepals. 35S::FAR-Q in a sep3 mutant background also shows both stamenoid petals and carpelloid sepals. First-whorl stigmatic papillae are indicated by white arrows.
Morphological Development Is Determined by Expression and Interactions of the Regulatory Network.
SEP3 is not expressed at early developmental stages in the first whorl of Arabidopsis flowers. Therefore, the failure of FAR to induce female organ development in the first whorl could be because of the lack of SEP3, its sole interaction partner, in this whorl. If this model is correct, ectopic expression of FAR in a sep3 mutant background should be unable to induce the homeotic transformation of petals to stamens observed on expression of FAR in wild-type plants. Fig. 2B shows that this is the case; expression of FAR in a sep3 mutant background produces a flower with no homeotic transformations. We confirmed the specificity of this result by ectopically expressing FAR-Q in the sep3 mutant background, and as expected, this produces carpeloid and stamenoid transformations in whorls 1 and 2, respectively (Fig. 2B). In contrast, the typical petal to stamen transformation associated with ectopic expression of FAR is unaffected in a sep1 sep2 mutant background (Fig. S2A), confirming the importance of SEP3 alone for FAR activity. Furthermore, ectopic expression of AG in a sep3 mutant background produces both carpels and stamens, showing that SEP3 is not absolutely required to make these organs in the outer whorls (Fig. S2A). We also tested the effect of artificially expressing SEP3 in the first whorl of plants ectopically expressing FAR. In contrast to the wild-type background, carpelloid organs were now produced in the first whorls, indicating that the inability of FAR to induce carpels in the first whorl of wild-type plants is because of the lack of SEP3 expression there (Fig. 2B). This result is confirmed by the expression of FAR in an ag-3 mutant background (ag-3 35S::FAR in Fig. S2B). In these plants, the fourth whorl organs develop carpelloid identity, confirming that concomitant expression of SEP3 and FAR can specify carpels. Despite this, coexpression of FAR and SEP3 does not restore floral determinacy in the fourth whorl.
Origin of the Amino Acid Insertion in FAR.
The additional glutamine in the FAR protein of Antirrhinum results from a three-base duplication of the splice acceptor (CAG) at the 5′ end of exon seven (Fig. 3A). This mutation is likely to have occurred after the duplication that produced the PLE/FAR (and SHATTERPROOF/AG) lineages (13, 19), because it is found only in FAR orthologs. The three-base addition is conserved in the FAR orthologs of Torenia fournieri (TfFAR) and Ipomoea nil [DUPLICATED (DP)] (Fig. 3B). The two putative C-function genes in Ipomoea, DP, and PEONY (PN) have been previously described (20), although a mutant phenotype has only been reported for DP (20). Surprisingly, dp mutants lack stamens and carpels, resembling the ag and ple mutants. Thus, despite the presence of an additional Q, DP function is not restricted to male organ development in Ipomoea. Nevertheless, as shown in Fig. 3C, when expressed in Arabidopsis, the presence of the additional Q in DP restricts it to the specification of male organs, whereas PN, which lacks the additional Q, is capable of specifying both male and female organs.
Fig. 3.
Evolutionary conservation of the inserted glutamine residue. (A) Genomic structure and partial transcript map of AG and FAR. The DNA sequence surrounding the triplet that encodes the additional glutamine (CAG) is magnified in FAR, and the corresponding position is also shown in AG. The two CAG triplets that represent the splice acceptor consensus sequences are underlined in black. In FAR, the CAG duplication slides the acceptor forward, thereby introducing the critical Q173 residue. (B) Phylogenetic tree of species with known FAR orthologs. Those species lacking Q173 are shown in blue, and those known to carry Q173 are shown in red. The presence of Q173 is confined to, but not present in all Lamiids. Among the Solanales, Solanum Lycopersicon, Petunia, and Nicotiana tabacum do not have the additional glutamine. Dotted lines represent the part of the tree that remains uncertain (34). (C) Ectopic expression of the Ipomoea PLE and FAR orthologs, PN and DP, in Arabidopsis. The organ conversions of 35S::PN are identical to those present in 35S::AG, whereas 35S::DP is identical to 35S::FAR.
Discussion
Determinants of Interaction Specificity.
Although the determinants of interaction specificity among the ABC and SEP proteins are not yet known, the finding that a single amino acid insertion in one of the proposed α-helices of the K domain (K3) abolishes some protein–protein interactions and retains others implicates this region as a determinant of protein–protein interaction specificity. The importance of this domain for the formation of multimeric complexes has previously been shown for SEP3 and the B-function MADS-box protein PISTILLATA (PI) (15, 18). A mutation in K3 helix (S145P) of the PI protein compromised the formation of the AP3/PI/SEP1 complex, and PI carrying this mutation is also unable to complement the pi mutant (21). In the case of SEP3, the K3 domain is important to promote cooperative binding to DNA (22) and form the complex SEP3-SEP3/AP3-PI (12). In addition, SEP3 lacking the C-terminal end of the K3 helix is unable to form multimeric complexes with AG and SEEDSTICK (STK) (18). The finding that the K3 helix plays an important role in the interaction and function of E-, B-, and C-function proteins supports the idea that determinants of protein–protein interaction specificity are likely to lie in this area. Protein sequence changes in this region could be capable of altering the specificity and affinity of interactions, leading to subfunctionalization and neofunctionalization.
Protein Evolution Through Splice Site Modification.
We have shown that an altered splice donor site has resulted in some species acquiring an additional amino acid in the K3 domain of the C-function protein FAR. In FAR, the splice acceptor site CAG is followed by another CAG that introduces an additional glutamine. The presence of two consecutive CAG codons could lead to alternatively spliced products and the presence of two versions of FAR. This is unlikely to be the case in Antirrhinum, because extensive cDNA and expressed sequence tag (EST) sequencing has never identified a FAR transcript lacking the additional glutamine codon (14). The additional glutamine codon is most likely to have been acquired before the most recent common ancestor of the Lamiales and Solanales, because it is present in FAR orthologs in both Lamiales and Solanales. Surprisingly, the FAR orthologs in the Solanales species Nicotiana tabacum and in Petunia hybrida do not include the additional glutamine codon, suggesting that it could have been lost in these species.
Although yeast three-hybrid experiments to examine the interactions between putative C-function and SEP proteins have not been carried out in these species, differential interaction between Antirrhinum C-function proteins and SEP proteins has been reported at the level of yeast two-hybrid analysis (14). This supports the hypothesis that the splice change that affects the K3 domain of FAR is responsible for its reduced organ identity potential in the endogenous system. However, the full effects of loss of FAR function are obscured in Antirrhinum mutants because of the cross-regulation between PLE and FAR. PLE shows increased expression in far mutants, whereas FAR shows reduced expression in ple mutants, and this likely to account for the relatively mild far phenotype (14). Nevertheless, the restriction of interaction potential in the case of FAR potentially represents a step to independent regulation of male and female reproductive organs.
Outside Antirrhinum, functional analysis of FAR orthologs has been restricted to the DP gene of Ipomoea (20). As expected, we have shown that DP, which, like FAR, contains the additional glutamine residue, is restricted to the specification of male reproductive organs when ectopically expressed in Arabidopsis. However, this result contrasts with the characterization of the dp mutant, which, unlike Antirrhinum far mutants, lacks both male and female organs. There are several possible explanations for this. First, the regulatory relationship between the two Ipomoea genes DP and PN has not been established. As discussed above, the cross-regulation between PLE and FAR in Antirrhinum is complex and affects the phenotypic consequences of the single mutants (14). If such a regulatory system is not present or if it works differently in Ipomoea, a more severe outcome could be expected in dp than far mutants. Furthermore, neither the protein–protein specificities between Ipomoea C- and E-class factors nor their exact spatial and temporal expression patterns have yet been determined. It will be interesting to compare the expression, interaction, and regulation of these genes in the future.
Developmental Outcomes Are Defined at the Network Level.
The finding that the presence or absence of a single amino acid in a critical protein–protein interaction domain can switch the capacity of a homeotic protein to specify reproductive organ fate seems to support the significance of evolutionary changes to coding regions (6–8). We have shown that an altered splice acceptor site can produce a protein with novel interaction specificities, providing an obvious way to facilitate subfunctionalization after gene duplication. The ability to differentiate between proteins after gene duplication provides a means to separately regulate processes originally controlled by a single protein, paving the way for independent regulation and increased complexity. In this case, an evolutionarily conserved modification to the K3 helix restricts FAR proteins to interaction with a single SEP protein, SEP3.
However, these results also highlight the importance of considering the regulatory network as a whole. Although the change in interaction specificity is driven by a change in the coding sequence of FAR, the morphological outcome is also critically dependent on the differences in the expression patterns of the individual SEP proteins. These differences presumably rely on changes to the cis-regulatory elements of the SEP genes. Therefore, although this seems to be a case of a localized change in the amino acid sequence of a protein leading to an altered developmental outcome by changing the protein–protein interaction spectrum, the realization of that altered outcome relies on the presence of an expanded and diversified E function where both protein and regulatory changes are significant. It is, therefore, advisable to consider an evolutionary change in relation to the regulatory network where both coding and regulatory changes are likely to influence the network properties.
Duplication of E-Function Genes.
Multiple E-function genes are a puzzling characteristic of flowering plants. The presence of duplicated E-function genes in basal angiosperms suggests that duplication of these genes was an early and perhaps, critical event in floral evolution (2). E-function duplicates have been retained in different species, suggesting that they have undergone neo- and/or subfunctionalization. Molecular and genetic studies have shown that the E-function proteins provide the floral environment for the homeotic ABC functions to regulate their target genes and that they do this by complex formation (10, 17, 18, 23, 24). However, it has been unclear why the retention of multiple duplicated SEP genes, which superficially seem redundant, should be common to flowering plants. Here, we show that differences in the interaction domains of the floral organ identity proteins can highlight differences between the E-function proteins in terms of multimeric complex formation. We propose that the reproducibility and robustness of flower development is underpinned by a complex regulatory network involving differential interaction specificities, protein–protein affinities, and dynamic expression patterns of ABC and E-function proteins. Systems approaches will be necessary to model and understand such a network.
Materials and Methods
Domain Swaps and Point Mutation Plasmid Construction.
Domain fusions between AG, PLE, and FAR were obtained by amplifying separately two DNA fragments with primers that have a 20-bp overlap (see primer list) (25). The product from the two PCRs was then annealed at 54 °C, and five PCR cycles of amplification were used to obtain a single fragment. Subsequently, the forward and reverse primers were added to amplify the full fragment with 30 additional cycles. The PCR fragments were cloned into pDH51 using the restriction sites BamHI/XbaI and subsequently, in pGREEN0229 using EcoRI.
Primers used in assembly PCR:
CA13 ATGGCGTACCAATCGGAGCTAG
CA16 CCTTACCAGCTTTTGTCAAAATG
CA15 CCAGCTAAAAACAAATAAATG
CA86 GCTGAAAGTGAGAGAGTGCAGCCGAGTATAAGTCTAATGCCAG
CA87 CTGGCATTAGACTTATACTCGGCTGCACTCTCTCACTTTCAGC
FARc TCAGACTAATTGAAGAGGGAG
AGc2 ATTACACTAACTGGAGAGCGG
CA93 ATCAAATTCATATGCTGCCCATTGTTCCTCTCATTTTCAGC
CA92 CTGAAAATGAGAGGAACAATGGGCAGCATATGAATTTGATGC
CA96 GCAATCTCAACCGTTTGATGCTAGAAACTATCTTCAAGTC
PLEc TCAAACAAGCTGGAGAGCCG
CA11 TCGGTGGCAGAAATTAATGCACAGTATTACCAGCAAGAAGC
CA12 GCTTCTTGCTGGTAATACTGTGCATTAATTTCTGCCACCGA
OLA TTTGCTGAAATTGAGTACATGCAGAAGAGGGAGCTTGAGCTG
OLB CAGCTCAAGCTCCCTCTTCTGCATGTACTCAATTTCAGCAAA
OLC TTTGCTGAAATTGAGTACATGCAGAAAAGAGAAGTTGATTTG
OLD CAAATCAACTTCTCTTTTCTGCATGTACTCAATTTCAGCAAA
OLE TATGAATATGCTAACAACAGTGTTAAAGCAACCATTGATAGA
OLIGOF TCTATCAATGGTTGCTTTAACACTGTTGTTAGCATATTCATA
OLIGOG TATGAGTACTCTAACAACAGTGTTAAAGCAACCATTGATAGA
OLIGOH TCTATCAATGGTTGCTTTAACACTGTTGTTAGAGTACTCATA
Point mutations were obtained using site-directed mutagenesis using the Stratagene QuikChange method. The following primers were used to mutate FAR and AG cloned into pDH51 plasmid.
FAR-QF GAGTACATGCAAAAGAGGGAGATTGACTTGCACCAC
FAR-QRGTGGTGCAAGTCAATCTCCCTCTTTTGCATGTACTC
AG+QF CGACTACATGCAGAAAAGACAGGAAGTTGATTTGCATAACG
AG+QR CGTTATGCAAATCAACTTCCTGTCTTTTCTGCATGTAGTCG
AG+RF CGACTACATGCAGAAAAGGAGAGAAGTTGATTTGCATAACG
AG+RR CGTTATGCAAATCAACTTCTCTCCTTTTCTGCATGTAGTCG
Arabidopsis Transformation.
Agrobacterium strain GV301 prepared for electroporation (26) was used to transform Arabidopsis. Arabidopsis transformation was performed using the floral dip protocol (27). Transgene expression levels were constant between transgenic lines (Fig. S3).
Dexamethasone Treatment.
35S::SEP3::GR was obtained from the group of Gerco Angenet (28). Dexamethasone induction of 35S::SEP3::GR was performed as in ref. 29.
Genotyping.
PCR for genotyping of SEP1 SEP2 SEP3 was performed as described in ref. 30 Supporting Information. Ag-3 genotyping was performed as in ref. 31.
Yeast Three-Hybrids.
The yeast three-hybrids were performed using the vectors pDEST22 (pAD), pDEST32 (pBD), and pTFT1. pDEST22 and pDEST32 vectors containing AG, SEP1, SEP2, and SEP3 were obtained from Gerco Angenent (17). Other AG and FAR yeast vectors were cloned using Topo cloning and Gateway recombination (Invitrogen). AG and FAR were amplified using the primers:
FARforCACCATGGCGTCTCTAAGCGATC FARrevTCAGACTAATTGAAGAGGGAG
AGforCACCATGGCGTACCAATCGGAGCTAG
AGrevATTACACTAACTGGAGAGCGG
The yeast strains PJ69-4α and PJ69-4a were used for yeast two- and three-hybrid experiments.
Yeast transformation was performed as in ref. 32 with an additional incubation in LiAc 100 mM at 30 °C for 5 min before the poly ethylene glycol (PEG)/LiAc incubation. Three-hybrid experiments were performed as described in refs. 18 and 31. Combinations of variants SEP1–4 were initially tested in the pAD and pBD vectors in PJ69-4a. Those combinations not activating the HIS3 reporter gene were subsequently tested for interaction with AG and FAR by mating to the PJ69-4α strain carrying those genes in pTFT. After mating selection on -L-W-A, the yeasts were grown at room temperature on control plates (-L-W-A) and selective plates (-L-W-A-H). The experiments were repeated four times, and growth of the colonies on control and selective plates was assessed. The interaction data are presented in Table S1.
Identification of FAR Orthologs.
The identification of FAR orthologs was performed by an initial BLAST search using tBLASTn; this search was followed by a phylogenetic analysis performed as in ref. 33. A figure to clarify the phylogeny of the genes used in this study is presented in Fig. S4.
Supplementary Material
Acknowledgments
We thank Gerco Angenent and Richard Immink (Plant Research International, Wageningen, The Netherlands) for sharing vectors for yeast three-hybrid analysis and for the 35S::SEP3-GR construct. We thank Barry Causier for his support during the first stages of the project and the helpful comments on the manuscript. We are indebted to Enrico Coen, Jane Langdale, and Stefan Kepinski for valuable suggestions. C.A.A. was supported by funding from the European Union Marie Curie Research Training Program and the Biotechnology and Biological Sciences Research Council.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1009050107/-/DCSupplemental.
References
- 1.Darwin F, Seward AC. More Letters of Charles Darwin. London: John Murray; 1903. [Google Scholar]
- 2.Zahn LM, et al. The evolution of the SEPALLATA subfamily of MADS-box genes: A preangiosperm origin with multiple duplications throughout angiosperm history. Genetics. 2005;169:2209–2223. doi: 10.1534/genetics.104.037770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Irish VF. The evolution of floral homeotic gene function. Bioessays. 2003;25:637–646. doi: 10.1002/bies.10292. [DOI] [PubMed] [Google Scholar]
- 4.Soltis DE, et al. The floral genome: An evolutionary history of gene duplication and shifting patterns of gene expression. Trends Plant Sci. 2007;12:358–367. doi: 10.1016/j.tplants.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 5.Ohno S. Evolution by Gene Duplication. New York: Springer; 1970. [Google Scholar]
- 6.Wray GA. The evolutionary significance of cis-regulatory mutations. Nat Rev Genet. 2007;8:206–216. doi: 10.1038/nrg2063. [DOI] [PubMed] [Google Scholar]
- 7.Carroll SB. Evo-devo and an expanding evolutionary synthesis: A genetic theory of morphological evolution. Cell. 2008;134:25–36. doi: 10.1016/j.cell.2008.06.030. [DOI] [PubMed] [Google Scholar]
- 8.Hoekstra HE, Coyne JA. The locus of evolution: Evo devo and the genetics of adaptation. Evolution. 2007;61:995–1016. doi: 10.1111/j.1558-5646.2007.00105.x. [DOI] [PubMed] [Google Scholar]
- 9.Coen ES, Meyerowitz EM. The war of the whorls: Genetic interactions controlling flower development. Nature. 1991;353:31–37. doi: 10.1038/353031a0. [DOI] [PubMed] [Google Scholar]
- 10.Honma T, Goto K. Complexes of MADS-box proteins are sufficient to convert leaves into floral organs. Nature. 2001;409:525–529. doi: 10.1038/35054083. [DOI] [PubMed] [Google Scholar]
- 11.Theissen G. Development of floral organ identity: Stories from the MADS house. Curr Opin Plant Biol. 2001;4:75–85. doi: 10.1016/s1369-5266(00)00139-4. [DOI] [PubMed] [Google Scholar]
- 12.Melzer R, Theissen G. Reconstitution of ‘floral quartets’ in vitro involving class B and class E floral homeotic proteins. Nucleic Acids Res. 2009;37:2723–2736. doi: 10.1093/nar/gkp129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Causier B, et al. Evolution in action: Following function in duplicated floral homeotic genes. Curr Biol. 2005;15:1508–1512. doi: 10.1016/j.cub.2005.07.063. [DOI] [PubMed] [Google Scholar]
- 14.Davies B, et al. PLENA and FARINELLI: Redundancy and regulatory interactions between two Antirrhinum MADS-box factors controlling flower development. EMBO J. 1999;18:4023–4034. doi: 10.1093/emboj/18.14.4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang Y, Jack T. Defining subdomains of the K domain important for protein–protein interactions of plant MADS proteins. Plant Mol Biol. 2004;55:45–59. doi: 10.1007/s11103-004-0416-7. [DOI] [PubMed] [Google Scholar]
- 16.Ma H, Yanofsky MF, Meyerowitz EM. AGL1-AGL6, an Arabidopsis gene family with similarity to floral homeotic and transcription factor genes. Genes Dev. 1991;5:484–495. doi: 10.1101/gad.5.3.484. [DOI] [PubMed] [Google Scholar]
- 17.de Folter S, et al. Comprehensive interaction map of the Arabidopsis MADS Box transcription factors. Plant Cell. 2005;17:1424–1433. doi: 10.1105/tpc.105.031831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Immink RG, et al. SEPALLATA3: The ‘glue’ for MADS box transcription factor complex formation. Genome Biol. 2009;10:article R24. doi: 10.1186/gb-2009-10-2-r24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liljegren SJ, et al. SHATTERPROOF MADS-box genes control seed dispersal in Arabidopsis. Nature. 2000;404:766–770. doi: 10.1038/35008089. [DOI] [PubMed] [Google Scholar]
- 20.Nitasaka E. Insertion of an En/Spm-related transposable element into a floral homeotic gene DUPLICATED causes a double flower phenotype in the Japanese morning glory. Plant J. 2003;36:522–531. doi: 10.1046/j.1365-313x.2003.01896.x. [DOI] [PubMed] [Google Scholar]
- 21.Yang Y, Fanning L, Jack T. The K domain mediates heterodimerization of the Arabidopsis floral organ identity proteins, APETALA3 and PISTILLATA. Plant J. 2003;33:47–59. doi: 10.1046/j.0960-7412.2003.01473.x. [DOI] [PubMed] [Google Scholar]
- 22.Melzer R, Verelst W, Theissen G. The class E floral homeotic protein SEPALLATA3 is sufficient to loop DNA in ‘floral quartet’-like complexes in vitro. Nucleic Acids Res. 2009;37:144–157. doi: 10.1093/nar/gkn900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davies B, Egea-Cortines M, de Andrade Silva E, Saedler H, Sommer H. Multiple interactions amongst floral homeotic MADS box proteins. EMBO J. 1996;15:4330–4343. [PMC free article] [PubMed] [Google Scholar]
- 24.Pelaz S, Ditta GS, Baumann E, Wisman E, Yanofsky MF. B and C floral organ identity functions require SEPALLATA MADS-box genes. Nature. 2000;405:200–203. doi: 10.1038/35012103. [DOI] [PubMed] [Google Scholar]
- 25.Dillon PJ, Rosen CA. A rapid method for the construction of synthetic genes using the polymerase chain reaction. Biotechniques. 1990;9:298–300. [PubMed] [Google Scholar]
- 26.Mattanovich D, et al. Efficient transformation of Agrobacterium spp. by electroporation. Nucleic Acids Res. 1989;17:6747. doi: 10.1093/nar/17.16.6747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clough SJ, Bent AF. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- 28.Kaufmann K, et al. Target genes of the MADS transcription factor SEPALLATA3: Integration of developmental and hormonal pathways in the Arabidopsis flower. PLoS Biol. 2009;7:e1000090. doi: 10.1371/journal.pbio.1000090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kieffer M, et al. Analysis of the transcription factor WUSCHEL and its functional homologue in Antirrhinum reveals a potential mechanism for their roles in meristem maintenance. Plant Cell. 2006;18:560–573. doi: 10.1105/tpc.105.039107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ditta G, Pinyopich A, Robles P, Pelaz S, Yanofsky MF. The SEP4 gene of Arabidopsis thaliana functions in floral organ and meristem identity. Curr Biol. 2004;14:1935–1940. doi: 10.1016/j.cub.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 31.Favaro R, et al. MADS-box protein complexes control carpel and ovule development in Arabidopsis. Plant Cell. 2003;15:2603–2611. doi: 10.1105/tpc.015123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gietz RD, Woods RA. Transformation of yeast by lithium acetate/single-stranded carrier DNA/polyethylene glycol method. Methods Enzymol. 2002;350:87–96. doi: 10.1016/s0076-6879(02)50957-5. [DOI] [PubMed] [Google Scholar]
- 33.Pelucchi N, et al. Comparative analysis of rice MADS-box genes expressed during flower development. Sex Plant Reprod. 2002;15:113–122. [Google Scholar]
- 34.Forest F, Chase MW. The Timetree of Life. London: Oxford University Press; 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.