Abstract
Herpes simplex virus-2 (HSV-2) shedding episodes in humans vary markedly in duration and virologic titer within an infected person over time, an observation that is unexplained. To evaluate whether host or virological factors more closely accounted for this variability, we combined measures of viral replication and CD8+ lymphocyte density in genital biopsies, with a stochastic mathematical model of HSV-2 infection. Model simulations reproduced quantities of virus and duration of shedding detected in 1,003 episodes among 386 persons. In the simulations, local CD8+ lymphocyte density in the mucosa at episode onset predicted peak HSV DNA copy number and whether genital lesions or subclinical shedding occurred. High density of CD8+ T cells in the mucosa correlated with decreased infected cell lifespan and fewer infected epithelial cells before episode clearance. If infected cell lifespan increased by 15 min because of CD8+ lymphocyte decay, then there was potential for a thousandfold increase in the number of infected cells. The model suggests that the rate of containment of infected cells by the peripheral mucosal immune system is the major driver of duration and severity of HSV-2 reactivation in the immunocompetent host.
Keywords: infectious diseases, mucosal immunology, virology, mathematical modeling
Genital herpes infection is characterized by frequent episodes of clinical and subclinical viral shedding (1–3). Clinical episodes or “recurrences” result from epithelial cell loss that manifests as vesicles or erosions. “Subclinical episodes” are associated with detectable HSV DNA without a visible lesion (1–3). Clinical recurrences have greater and longer viral production than subclinical episodes (2–4). Host immunological control limits episode frequency (5–9) and the spread of lesions during genital tract recurrences (10–12). Yet, episode severity varies considerably within a person even over short time spans (weeks to months) (4), and variability in episode clearance is poorly understood.
We developed a mathematical model of genital herpes infection to better understand parameters controlling episode expansion and clearance, and to evaluate patterns of HSV-2 release from neurons in humans (13). The model predicted strong associations between numbers of epithelial cells infected and viruses produced, and episode duration. Simulated episodes with high viral production resulted in recurrences based on more infected cells before clearance; many asymptomatic simulated episodes involved infection of a single epithelial cell with peaks of <104 HSV DNA copies per milliliter (4, 13).
In this report, we expanded the model to better understand host and virological predictors of episode severity. For these analyses, we used detailed virological data from a large cohort of immunocompetent patients who underwent genital tract sampling to define heterogeneity of HSV-2 episodes, along with quantitative data on CD8+ T-cell density in the genital skin from biopsies during and after recurrences, to inform the stochastic model. Model simulations reproduced known features of empirical data closely and indicated that CD8+ lymphocyte levels in genital skin at episode onset, correlated inversely with episode peak HSV copy number and lesion diameter. Because of rapid spread of HSV between epithelial cells, containment of episodes needed to occur within a very short time frame (1–6 h). This analysis predicts a critical role for host immune cells in the periphery that control clinical expression of genital herpes.
Results
HSV-2 Shedding Episode Characteristics in Patients.
Study participants performed daily genital swabs for a minimum of 30 d. Swabs were analyzed for HSV DNA by quantitative PCR. We defined shedding episodes as any positive swabs (>102 HSV DNA copies per milliliter) preceded and followed by two negative swabs (<102 HSV DNA copies per milliliter) (14). We characterized 1,003 shedding episodes from 386 persons according to viral production: 41% (408/1,003) were low- (102 to 104 peak HSV DNA copies per milliliter), 24% (245/1,003) were medium- (104 to 106 peak HSV DNA copies per milliliter), and 35% (350/1,003) were high-copy (>106 peak HSV DNA copies per milliliter). Nine percent of low-, 27% of medium-, and 67% of high-copy episodes were associated with genital lesions.
Genital Skin CD8+ Lymphocyte Kinetics During and After Genital Recurrence.
We identified that HSV-specific CD8+ lymphocytes infiltrate into epidermis, dermis, and the dermal–epidermal junction at the ulcer site during genital recurrences and then undergo exponential decline at the healed site for months thereafter (10, 12). To enumerate CD8+ T cells in close proximity to HSV-infected cells before, during, and 2, 4, 6, and 8 wk after a recurrence, we performed in situ staining of biopsy specimens from the recurrence site, followed by confocal microscopy of immunofluorescent tissue (10, 12). We defined CD8+ T cells in a 1-mm3 area as CD8+ lymphocyte density.
Mathematical Model of HSV-2 Shedding Episodes.
We next solved a mathematical model of HSV-2 mucosal pathogenesis (Table S1) numerically to recreate patterns of shedding episodes and CD8+ T-cell response documented in clinical studies (13). The model's stochastic differential equations simulated viral shedding in a 2-cm–diameter circular area (surface area = 314 mm2) of ano-genital skin containing 5.42e6 HSV susceptible epidermal cells. The maximum size for a single HSV lesion is ≈270 mm2 (1). The model space therefore contained enough epidermal cells to accommodate even the largest possible herpetic lesions.
The model was structured according to several biological assumptions (Table S1) and populated with parameters describing viral replication and spread, epidermal cell infection and death, and clearance of infected cells by CD8+ lymphocytes. The model assumed that HSV-2 periodically bypasses ganglionic latency and is released from neuronal endings at the dermal–epidermal junction at a fixed rate ϕ. Viruses were assigned an infectivity value β that determined the number of viruses required to infect one epithelial cell per day. We defined p as the rate of viruses produced by an infected epidermal cell per day and assumed that virions survived for a duration 1/c during which time they could infect other epithelial cells.
Once an epithelial cell was infected in a simulation, it lysed after becoming packed with viruses after time 1/a, or died because of local CD8+ T-cell activity. Because CD8+ T cells localize precisely to the site of HSV-2 reactivation and are crucial mediators of cytolytic immune response (Fig. S1 A–F), we made the simplifying assumption that all host control is due to these cells. We recognize that CD4+ T cells, dendritic cells, and natural killer cells are likely to play important roles in limiting reactivation, and promoting CD8+ T-cell activation and expansion, through signaling and antigen presentation (11, 12, 15–19). However, to avoid creating an intractable model due to overparameterization, we did not include these cell lines as model variables.
The model captured accumulation of CD8+ T lymphocytes into the lesion area at a peak rate θ and assumed that CD8+ T cells contact and terminate infected epithelial cells in the basal epidermal layer at a rate f. To assess a possible immunosurveillance role for CD8+ T cells that persist for weeks at a healed lesion site (Fig. S1 G–J), we fit CD8+ T-cell decay data derived from in situ staining studies (10, 12) to an exponential decay equation to derive a mucosal decay rate δ (13). We allowed for a possible delay in antigen recognition by CD8+ T cells by including a parameter, r, which describes the number of infected cells at which rate of CD8+ T-cell replication is half maximal.
In a previous study, we arrived at 89 sets of parameter values by separately fitting the model to quantitative HSV DNA data from 89 shedding episodes (13). In the present study, we used approximate median values of each parameter from these 89 parameter sets for simulations. We defined the basic reproductive number (R0) for an HSV-2 infected cell in the model as the average number of cells that an infected cell infects, assuming no immunity. The reproductive number (R) in the model was the number of cells that a productive cell infected, assuming continual immune pressure. We arrived at values for R0 and R for each simulation based on parameter values (SI Methods).
Model Simulations and Episode Frequency.
We performed 10 1-yr stochastic model realizations and showed an association between sampling frequency and quantity of virus detected during episodes. When we solved the model assuming continuous sampling (1,000 samples per day), there were 424 total episodes (42.4/y) of which 70.5% were low, 17.7% medium, and 11.8% high copy. We subjected the model output to daily sampling and excluded episodes with less than two concurrently infected cells, because the virus probably does not reach the skin surface during these episodes (10, 13): 78% (232/299) of low-copy episodes were missed and 10 of 50 high-copy episodes were classified as medium-copy, resulting in 192 episodes (35% low, 44% medium, and 21% high copy) or 19.2/y; this estimate is consistent with a variety of empirically defined subclinical and clinical shedding studies among HSV-2 immunocompetent persons (6.0, 21, and 27.7 episodes per year) (4, 20, 21). We next performed 10,000 simulations to display that episode frequency was consistent for this particular parameter set despite model stochasticity (Tables S2 and S3). We finally ran the model by using parameter values derived from 10 different HSV-2–infected persons with daily versus continuous sampling, revealing heterogeneity of episode severity, and reconfirming an association between sampling frequency and episode frequency under different parameter regimes (Table S4).
Detailed Analysis of a 1-y Model Simulation.
During a 365-d simulation with median parameter values, the model randomly generated 54 episodes and produced estimates for shedding frequency (16.7%), recurrence frequency (5/y), and lesion diameter (1.8–5.0 mm) comparable with values derived from large databases of persons with genital HSV-2 (1, 4, 20–22). In a sensitivity analysis, we performed additional 365-d simulations with 20% adjustments in each of the included model parameters to confirm that the model produces realistic outcomes with parameter variability (Table S5).
Because our simulation with median values reproduced the key clinical outcomes described above, we pursued a more detailed analysis of the episodes from this simulation (Table 1 and Figs. 1 and 2). The 54 episodes differed by peak HSV copy number, total HSV produced, peak infected cells, total infected cells, lesion diameter, and duration (Table 1). Thirty-five of 42 low-copy episodes involved infection of no more than one epithelial cell at a single point in time. These 35 episodes lasted 1.0–21.6 h (median 3.4 h, mean 5.5 h). Thirty-five of 54 episodes (64%, all low-copy) would have been missed with daily rather than continuous surveillance. This result is consistent with a study in which 66% of shedding episodes detected with every 6-h swabs would have been missed had swabbing occurred every 24 h (4).
Table 1.
Distinguishing virologic and immunologic features of low-, medium-, and high-copy HSV-2 shedding episodes from a 365-d model simulation
| Low-copy episodes (n = 42) |
Medium-copy episodes (n = 7) |
High-copy episodes (n = 5) |
|||||
| Measurement | Median | Range | Median | Range | Median | Range | |
| Peak viral load, log10 | 2.1 | 1.9–3.5 | 5.1 | 4.3–5.9 | 6.6 | 6.1–7.1 | |
| No. of virions shed per episode, log10 | 2.3 | 1.9–4.4 | 6.3 | 5.9–6.8 | 7.5 | 7.1–8.0 | |
| Peak infected cells | 1 | 1–5 | 179 | 33–948 | 5,156 | 1,659–14,650 | |
| No. of infected cells per episode | 2 | 1–115 | 8,364 | 3,199–31,773 | 154,963 | 54,116–420,837 | |
| Peak diameter, mm | 0 | 0 | 0.7 | 0.6–1.2 | 3 | 1.9–5 | |
| CD8+ lymphocyte expansion needed for clearance (%) | 0/42 (0) | 7/7 (100) | 5/5 (100) | ||||
| CD8+ lymphocyte density at episode onset, per mm3 | 4,856 | 1,448–11,672 | 3077 | 2,130–4,016 | 1,540 | 1,327–1,894 | |
| Average infected cell lifespan at episode onset, min | 30 | 12–99 | 47 | 36–68 | 94 | 76–109 | |
| Viruses produced per infected cell at episode onset | 206 | 86–691 | 325 | 249–469 | 649 | 528–754 | |
| Reproductive number (R) at episode onset | 0.9 | 0.4–2.9 | 1.4 | 1.1–2.0 | 2.7 | 2.2–3.1 | |
| Infected cells killed by CD8+ T cells, % | 100 | 50–100 | 97.3 | 97.0–97.9 | 97.3 | 97.3–97.4 | |
Low-, medium-, and high-copy episodes are respectively defined as having a peak HSV-2 DNA copy number <104/mL, 104–106/mL, and >106/mL. Population parameter values are averages for all variables in the model: infectivity: β = 10−8/d; viral infectivity duration: 1/c = 2 h; burst phase: p = 10,000 HSV DNA copies per infected cell per day; infected cell lifespan: 1/a = 20 h; rate of HSV DNA released from neurons: ϕ = 50 HSV DNA copies per day; rate of CD8+ lymphocyte expansion: θ = 1.5/d; rate of clearance of infected cells by CD8+ lymphocytes: f = 0.01 infected cells per CD8+ T cell per day; CD8+ lymphocyte lifespan: 1/δ = 20 d; and CD8+ lymphocyte antigen recognition: r = 200 infected cells needed before θ is half-maximal.
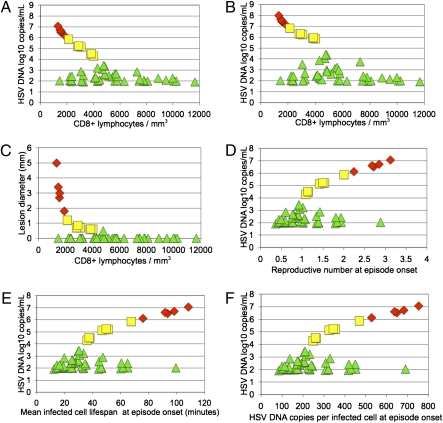
Fig. 1.
In a 365-d model simulation, CD8+ lymphocyte density (per mm3), infected cell lifespan, HSV copies produced per infected cell, and reproductive number at episode onset were determinants of shedding episode peak HSV DNA copy number and lesion diameter. Green triangles are low-copy (102 to 104 peak HSV DNA copies per milliliter), yellow squares are medium-copy (<104 to 106 peak HSV DNA copies per milliliter), and red diamonds are high-copy episodes (>106 peak HSV DNA copies per milliliter) (A) HSV-2 DNA peak copy number per episode (y axis) and CD8+ lymphocyte density at episode onset (x axis) for the 54 shedding episodes from the 365-d simulation outlined in Table 1. (B) HSV-2 DNA total copy number per episode (y axis) and CD8+ lymphocyte density at episode onset (x axis) for the 54 shedding episodes. Total HSV copy number exceeds peak HSV copy number in medium- and high-copy episodes by ≈1 log. (C) Peak lesion diameter (y axis) and CD8+ lymphocyte density (x axis) at episode onset for the 54 shedding episodes. (D) HSV-2 DNA peak copy number (y axis) and reproductive number at episode onset (x axis) for the 54 shedding episodes. (E) HSV-2 DNA peak copy number (y axis) and average infected cell lifespan at episode onset (x axis) for the 54 shedding episodes. F) HSV-2 DNA peak copy number (y axis) and mean HSV-2 production per infected cell at episode onset (x axis) for the 54 shedding episodes.
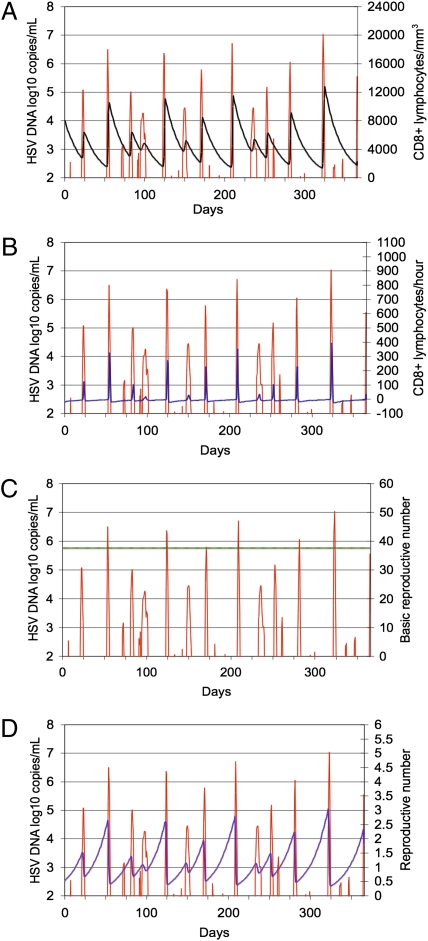
Fig. 2.
Stochastic model simulation (365 d) outlined in Table 1. (A) In this simulation, CD8+ lymphocyte replenishment randomly occurred 12 times during the course of a year. The simulation also illustrates the heterogeneity of genital HSV-2 episodes during the course of the year. Red line, log10 HSV-2 DNA/mL transport medium (left y axis). (A) Black line, CD8+ lymphocytes per mm3 (right y axis). (B) Blue line, CD8+ T-cell rate of change per hour (right y axis). Green line, basic reproductive number (R0, right y axis) (C); and violet line, reproductive number (R, right y axis) (D).
We multiplied the number of viruses introduced from neurons per year by viral lifespan and viral infectivity [365 × ϕ × (1/c) × (β × S0)] to arrive at an estimate of 83 episode initiations during the 365-d simulation period. Therefore, ≈35% (29/83) of episode initiations went undetected because they occurred while another episode was already in progress. Although initiation of shedding episodes in model simulations was a stochastic event that occurred when a virus released from neuron termini infected one epithelial cell, subsequent spread to surrounding epithelial cells determined progression to a low-, medium-, or high-copy episode, the latter being clinically evident based on geometric calculations of plaque diameter of >2 mm (13). Rapid spread of virus among epidermal cells, rather than multiple infections of epidermal cells by particles released from neurons during a single episode, accounted for the high number of infected epidermal cells present during certain simulated episodes.
CD8+ Lymphocyte Density at the Moment of Shedding Onset Predicts Episode Severity.
Episode clearance in model simulations was influenced by CD8+ lymphocyte density surrounding the initially infected epithelial cell at reactivation onset (Table 1). Above a certain density, only low-copy episodes ensued (Fig. 1A). If an epithelial cell became infected when density was low, then progression to a medium- or high-copy episode was possible (Fig. 1A). Rapid clearance of infected cells when CD8+ T-cell density was low occurred according to stochastic chance. During actual infection, this rapid containment may relate to whether there is spatial proximity of CD8+ T cells to the initially infected cell. Low-copy episodes occurred at all CD8+ lymphocyte densities, presumably reflecting almost constant leak of virus from sensory neuron endings, resulting in frequent epithelial cell infection (13).
Once concurrently infected epithelial cells exceeded ≈5 cells, a medium- or high-copy episode became inevitable and the number of infected cells increased substantially before clearance by replicating lymphocytes (Table 1). When we evaluated only medium- and high-copy episodes, CD8+ T-cell density at episode initiation inversely correlated with peak copy number (R = −0.99, P < 0.001) (Fig. 1A), total HSV produced (R = −0.97, P < 0.001) (Fig. 1B), infected cells (R = −0.72, P < 0.001), and lesion diameter (R = −0.84, P < 0.001) (Fig. 1C). If there was prolonged CD8+ T-cell decay between successive medium-/high-copy episodes, then the ensuing episode was more severe. CD8+ lymphocyte density influenced whether infection spread to enough cells for a recurrence (Fig. 1C). A small decline in density predicted substantial increases in lesion diameter (Fig. 1C).
Stochastic simulations reproduced the observed cycle of CD8+ lymphocyte expansion during a lesion, and contraction after healing, while making further predictions regarding response to asymptomatic episodes. High- and medium-copy episodes were associated with local expansion of CD8+ T cells at the reactivation site, which was temporally associated with lesion clearance (Table 1 and Fig. 2A). CD8+ lymphocyte decay progressed after clearance of these 12 episodes until the next medium- or high-copy episode. The model predicted that CD8+ T cells did not locally expand during low-copy episodes (Fig. 2B), because existing CD8+ T cells rapidly cleared low-copy episodes before no more than 5 cells were concurrently infected (Table 1).
Competing Model That Does Not Assume Local Replication of CD8+ T Cells Does Not Reproduce the Empirical Pattern of Shedding Episodes.
The original model of infection (Table S1) included a maximal proliferation rate θ for CD8+ T cells whose value depended on CD8+ T-cell density. This model assumed local replication of CD8+ T cells in reaction to infected cells. The birth term in the original model also required a low threshold of infected cells r, at which rate of CD8+ T-cell growth was half-maximal. To assess whether HSV-2–targeted CD8+ T cells replicate locally in genital skin, we constructed a competing model that assumed CD8+ T cells were trafficked from an external location according to infected cell density only (rather than local CD8+ T-cell density) at a maximal rate θI with a threshold of infected cells ri at which this rate was half-maximal (Table S6). Simulations with the competing model did not reproduce the full heterogeneity of shedding episode peak HSV DNA copy number, but instead resulted in medium and high episodes with less variable peaks, with a range of peaks that depended on maximal CD8+ T-cell infusion rate (Fig. S2). CD8+ T-cell density above a specific threshold continued to correlate with low peak HSV DNA copy number, and the inverse relationship between CD8+ T-cell density and peak HSV DNA copy number persisted with the alternative model (Fig. S3).
Effects of CD8+ Lymphocyte Density on Reproductive Number.
Based on median parameter values, R0 was 37.7 in the above simulations, implying explosive spread among epidermal cells in the absence of immunity (Fig. 2C). During simulations in which CD8+ lymphocytes could not eliminate infected cells, the spread of virus to more than five cells invariably resulted in rapid and persistent infection of nearly all cells, followed by eternal high-copy shedding (13). This pattern is not consistent with infection in the immunocompetent host, which is characterized by successively cleared episodes. The model predicted that dynamic CD8+ T-cell levels lead to fluctuations of R ≈ 1. When density was high, R was <1 and localized CD8+ T cells rapidly cleared outbreaks. At lower densities, R was >1, allowing for medium-copy episodes. R values >2.0 predicted high-copy episodes (Figs. 1D and 2D).
Effects of CD8+ Lymphocyte Density on Infected Cell Lifespan and Viral Production Capacity.
In model simulations, the average lifespan of infected cells increased as CD8+ lymphocyte density decreased (Fig. S4A), leading to higher average viral production per infected cell (Fig. S4B) and R (Fig. S4C), and more severe episodes (Table 1). When density was high after clearance, lifespan and production capacity of infected cells were low. As such, subsequent episodes did not progress beyond 104 HSV DNA copies per milliliter and were asymptomatic. Medium- and high-copy episodes were accompanied by CD8+ T-cell local expansion, which rapidly decreased lifespan and production capacity of infected cells leading to clearance. A decrease in average infected cell lifespan of 20 min was associated with a decrease in HSV production per infected cell of ≈130 copies and a 0.5–1.0 log decrease in peak copy number; a decrease in average infected cell lifespan of 40 min was associated with a decrease in viral production per infected cell of ≈260 HSV copies and a 1.0–2.0 log decrease in peak copy number (Fig. 1 E and F). Ranges of CD8+ T-cell density and average infected cell lifespan were relatively consistent across 10,000 simulations (Table S3).
Proportion of Infected Cells Killed by CD8+ Lymphocytes in Model Simulations.
During low-copy episodes, CD8+ lymphocytes eliminated almost every infected cell. During medium- and high-copy episodes, a small proportion of infected cells avoided CD8+ T cell-mediated elimination and died after ≈20 h of viral replication because of lysis, thereby producing ≈8,300 HSV DNA copies (Table 1). For the 10 fixed-parameter simulations, we tracked percentage of infected cells eliminated by CD8+ lymphocytes over 365-d (median: 97.0, range: 94.2, 98.8) (Table S4). Lower percentages occurred during simulations with high-diameter lesions because more infected cells evaded cell-mediated immunity during severe episodes.
Effect of Changes in CD8+ Lymphocyte Function and Viral Replication on Episode Diameter in Model Simulations.
If parameter values remained unchanged over time in an infected person, then CD8+ T-cell density at episode onset impacted episode severity. We solved the model for individual episodes with different starting parameter conditions to assess the effect on episode diameter. First, we used fixed parameter values to generate an “average” ulcer size at a particular CD8+ lymphocyte density. We chose an average lesion size of ≈2 mm diameter as this diameter approximates the clinical limit of genital lesion detection (1). We individually adjusted parameters to 20% below and 20% above the fixed parameter values to measure the effect on subsequent lesion diameter. We adjusted the rate of neuronal virus introduction to four different levels: 100, 1,000, 10,000, and 100,000 virions per day. For each of the 15 sets of adjusted parameters, the model was solved stochastically 10 times.
Abrupt decreases in CD8+ T-cell expansion rate and killing efficiency predicted episodes with more infected cells and greater lesion diameter (Fig. S5A). Abrupt increases in epithelial burst phase, viral infectivity, and viral lifespan predicted lesions with greater diameter. Decreases in rates of viral replication and spread predicted smaller diameter (Fig. S5B). Massive abrupt variations of neuronal rate of HSV-2 release had only a miniscule effect on diameter (Fig. S5C). Thus, the modeling data suggest that immunologic events in the periphery rather than the neurons predict genital lesion severity.
Discussion
Our findings, which are based, to our knowledge, on the largest available virological and immunological database of immunocompetent subjects with HSV-2 infection (386 persons and 1,003 episodes), offer several unique hypotheses regarding HSV-2 pathogenesis. First, the model suggests that episode rate is high (approximately weekly) but is underestimated by daily sampling because very brief episodes are rapidly cleared by peripheral CD8+ lymphocytes that perform immunosurveillance. Indeed, there may be a threshold of CD8+ lymphocyte density that is associated with short infected cell lifespan, limited viral spread, and episode containment before genital lesion formation. Second, the model predicts that local CD8+ T-cell expansion at the reactivation site is important for shedding episode clearance. Third, in model simulations, the quantity of CD8+ lymphocytes at the reactivation site at the time of episode initiation is inversely correlated with the number of infected cells, peak HSV DNA copy number, and likelihood of genital lesions. An increase of ≈40 min in the average infected cell lifespan is associated with a 2-log increase in peak copy number, indicating rapid host–viral interaction in the periphery. Finally, even a thousandfold increase in quantity of HSV-2 released from neurons into skin had little effect on episode severity in our modeling experiments, suggesting that immune responses in the periphery control clinical expression of infection.
Recent studies quantifying CD8+ T-cell density after healing of lesions showed that these cells persist for extended time periods. Our model suggests that these cells are functional in vivo, and when present at high densities, ensure that infected cell lifespan remains too low for a high number of cells to become infected after neuronal seeding of an epithelial cell. In humans, localized CD8+ lymphocyte levels decline slowly over weeks (10). The model predicts that infected cell lifespan increases accordingly. Eventually the biological system's reproductive number exceeds one and potential for a medium-copy episode is realized. Once a threshold of approximately five epithelial cells is infected during a modeled episode, a medium- or high-copy episode ensues. This threshold necessitates local cytotoxic T-cell enrichment (19) to reset infected cell lifespan to low levels that ensure rapid clearance. These predictions are consistent with a mouse model in which flank reinfection was unsuccessful because of persistence of skin-resident, HSV-specific memory T cells 100 d after infection. Infection proceeded with normal kinetics when HSV was inoculated on the opposing flank where exposure had yet to occur (23).
An important model prediction is a narrow margin between episode containment and recurrence. At a CD8+ lymphocyte density of 5,000/mm3, the average lifespan of an infected cell approximates 30 min with 200 HSV copies produced per cell. (This average probably reflects a broad, skewed range of lifespans with most infected cells producing little virus before clearance, whereas a minority evade CD8+ mediated lysis and produce a full complement of ≈8,000 virions.) R at this density is 0.8. Conditions are not suitable for efficient spread to contiguous epithelial cells or for lesion development. However, if average infected cell lifespan increases by 5 min and HSV DNA production per cell increases to 250 because of a decrease in CD8+ lymphocyte density to 4,000 per mm3, then R is 1.2 and an episode with several thousand infected cells may occur. If infected cell lifespan increases by 30 min and average HSV DNA production per cell increases to 450 because of a decrease in density to 2,000 per mm3, then R approximates 2 and a recurrence with 105 infected cells is possible.
Although CD8+ lymphocyte density at medium- or high-copy episode onset may be a determinant of episode severity, it is probable that CD8+ T-cell function also has an important effect. It is not known whether parameters of immunologic function are stable or whether they vary according to age, immunologic memory, acute physiologic stress, tissue insult, or time. We performed simulations with abrupt changes in parameter values: If CD8+ lymphocytes replicate less rapidly or eliminate infected cells less efficiently, then a more severe episode will ensue. An abrupt increase in viral infectivity or replication rate, which might occur if innate immune functions are transiently inactivated, would also predispose to larger subsequent episodes.
Decreases in viral replication rate and infectivity lead to smaller lesions in repeated realizations of the stochastic system, which may explain potent inhibitory effects of IFN-regulated gene products on viral replication (24–26). It is a limitation of the current model that we do not measure antiviral effects that are mediated by the innate immune system in a dynamic fashion: Decay rates of cytokines in tissue are not standardized adequately to model at this time. The antiviral effect may be a complementary mechanism that renders R < 1. Similarly, quantitative data on other host responses such as CD4+ lymphocytes, natural killer cells, and dendritic cells are not included in the model; levels of these cell types increase rapidly during recurrences and decline afterward (10, 12). Therefore, their density and functionality at episode onset may also be critical in determining episode severity.
In model simulations, exponential growth of number of infected epidermal cells occurs because of the rapid spread of virus among epithelial cells rather than multiple plaques of infection. Even a thousandfold increase in the amount of HSV introduced from neurons at episode initiation had little effect on lesion diameter. In mice, HSV reactivation frequency is predicted by ganglionic viral load, which correlates inversely with ganglionic CD8+ T-cell levels (7). In a previous modeling study, we estimated that only trace amounts of HSV are released from sensory neuron termini to initiate shedding episodes; even small adjustment in the daily amount released had a large influence on episode frequency (13). Taken together, these studies suggest a compartmentalized host response in which ganglionic immune control determines the frequency of virion release in a localized area (8, 9, 27), whereas peripheral immunity determines whether neuronal infection of epithelial cells results in a contained or uncontained reactivation (19, 23).
A stochastic model assuming CD8+ T-cell local expansion rather than trafficking from another site produced a more realistic spectrum of heterogeneous episodes over time. This finding complements recent animal model studies, which demonstrated that virus-specific CD8+ and CD4+ T cells in peripheral tissue can immediately replicate and demonstrate effector function in the presence of an infectious agent (12, 23, 28–30). We have documented rapid clearance of low-copy HSV shedding episodes (4, 13), as well as persistence of CD8+ lymphocytes at recurrence sites for at least 6 mo. The findings from this model suggest that these specialized cells may quickly recognize and kill infected cells, leading to prompt termination of many shedding episodes. This model prediction will need further experimental verification in humans.
The modeling does not rule out a central role for nonresident central memory CD8+ T cells in the clearance of infected cells (31). Circulating HSV-2–specific CD8 T cells overexpress high levels of cutaneous lymphocyte-associated antigen, an adhesion molecule involved in T-cell trafficking from blood into skin (32). There is T-cell receptor clonotypic identity between circulation and skin-derived HSV-2–specific CD8 T cells (33). Expansion of central memory cells is likely to be important for responses to reactivations at sites spatially removed from prior reactivations, where few peripheral immune cells reside (23), or perhaps for particularly severe recurrences.
We emphasize that lack of inclusion of the full complement of immunologic cell lines is a major limitation of our model. However, even though our model only has a limited number of parameters, incomplete parameter identifiability is a potential issue with all mathematical models of biology. Further research is needed to assess the accuracy of our current parameter value estimates. Our calculations of lesion diameter are based on histologic review, and a spatial model would allow for more accurate estimates.
In conclusion, these data suggest that a vaccine or immunotherapy that promotes persistent high densities of functional immune cells in the periphery may control reactivation and clinical disease (34). Control of a rapidly replicating virus at mucosal portals of entry may have to occur within minutes rather than hours.
Methods
We solved the model numerically in a stochastic stage-structured form to generate successive episodes (13, 35). We defined modeled episodes as starting when HSV DNA exceeded 100 HSV DNA copies per milliliter and ending when levels declined below 100 HSV DNA copies per milliliter. We estimated diameter of an ulcer by estimating epidermal cell size and numbers on review of histologic slides, and calculating the volume of missing cells due to excess cell death before regrowth during a lesion (SI Methods).
Supplementary Material
Acknowledgments
We thank our study clinicians, study participants, Rachel Tompa for manuscript edits, and David Swan for programming assistance. This work was supported by National Institutes of Health (NIH) Grants P01 AI030731, R37 AI042528, T32 CA80416, and T32 AI07140, the Qatar National Research Fund (NPRP 08-068-3-024), and NIH-supported HIV Vaccine Trials Network.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1006614107/-/DCSupplemental.
References
- 1.Corey L, Adams HG, Brown ZA, Holmes KK. Genital herpes simplex virus infections: Clinical manifestations, course, and complications. Ann Intern Med. 1983;98:958–972. doi: 10.7326/0003-4819-98-6-958. [DOI] [PubMed] [Google Scholar]
- 2.Wald A, Zeh J, Selke S, Ashley RL, Corey L. Virologic characteristics of subclinical and symptomatic genital herpes infections. N Engl J Med. 1995;333:770–775. doi: 10.1056/NEJM199509213331205. [DOI] [PubMed] [Google Scholar]
- 3.Wald A, et al. Reactivation of genital herpes simplex virus type 2 infection in asymptomatic seropositive persons. N Engl J Med. 2000;342:844–850. doi: 10.1056/NEJM200003233421203. [DOI] [PubMed] [Google Scholar]
- 4.Mark KE, et al. Rapidly cleared episodes of herpes simplex virus reactivation in immunocompetent adults. J Infect Dis. 2008;198:1141–1149. doi: 10.1086/591913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cherpes TL, Busch JL, Sheridan BS, Harvey SA, Hendricks RL. Medroxyprogesterone acetate inhibits CD8+ T cell viral-specific effector function and induces herpes simplex virus type 1 reactivation. J Immunol. 2008;181:969–975. doi: 10.4049/jimmunol.181.2.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Decman V, Kinchington PR, Harvey SA, Hendricks RL. Gamma interferon can block herpes simplex virus type 1 reactivation from latency, even in the presence of late gene expression. J Virol. 2005;79:10339–10347. doi: 10.1128/JVI.79.16.10339-10347.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoshino Y, Pesnicak L, Cohen JI, Straus SE. Rates of reactivation of latent herpes simplex virus from mouse trigeminal ganglia ex vivo correlate directly with viral load and inversely with number of infiltrating CD8+ T cells. J Virol. 2007;81:8157–8164. doi: 10.1128/JVI.00474-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knickelbein JE, et al. Noncytotoxic lytic granule-mediated CD8+ T cell inhibition of HSV-1 reactivation from neuronal latency. Science. 2008;322:268–271. doi: 10.1126/science.1164164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu T, Khanna KM, Chen X, Fink DJ, Hendricks RL. CD8(+) T cells can block herpes simplex virus type 1 (HSV-1) reactivation from latency in sensory neurons. J Exp Med. 2000;191:1459–1466. doi: 10.1084/jem.191.9.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu J, et al. Virus-specific CD8+ T cells accumulate near sensory nerve endings in genital skin during subclinical HSV-2 reactivation. J Exp Med. 2007;204:595–603. doi: 10.1084/jem.20061792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koelle DM, et al. Clearance of HSV-2 from recurrent genital lesions correlates with infiltration of HSV-specific cytotoxic T lymphocytes. J Clin Invest. 1998;101:1500–1508. doi: 10.1172/JCI1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu J, et al. Persistence of HIV-1 receptor-positive cells after HSV-2 reactivation is a potential mechanism for increased HIV-1 acquisition. Nat Med. 2009;15:886–892. doi: 10.1038/nm.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schiffer JT, et al. Frequent release of low amounts of herpes simplex virus from neurons: Results of a mathematical model. Sci Transl Med. 2009;1:7ra16. doi: 10.1126/scitranslmed.3000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Magaret AS, Wald A, Huang ML, Selke S, Corey L. Optimizing PCR positivity criterion for detection of herpes simplex virus DNA on skin and mucosa. J Clin Microbiol. 2007;45:1618–1620. doi: 10.1128/JCM.01405-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith CM, et al. Cognate CD4(+) T cell licensing of dendritic cells in CD8(+) T cell immunity. Nat Immunol. 2004;5:1143–1148. doi: 10.1038/ni1129. [DOI] [PubMed] [Google Scholar]
- 16.Milligan GN, Bernstein DI, Bourne N. T lymphocytes are required for protection of the vaginal mucosae and sensory ganglia of immune mice against reinfection with herpes simplex virus type 2. J Immunol. 1998;160:6093–6100. [PubMed] [Google Scholar]
- 17.Cunningham AL, Turner RR, Miller AC, Para MF, Merigan TC. Evolution of recurrent herpes simplex lesions. An immunohistologic study. J Clin Invest. 1985;75:226–233. doi: 10.1172/JCI111678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakanishi Y, Lu B, Gerard C, Iwasaki A. CD8(+) T lymphocyte mobilization to virus-infected tissue requires CD4(+) T-cell help. Nature. 2009;462:510–513. doi: 10.1038/nature08511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wakim LM, Waithman J, van Rooijen N, Heath WR, Carbone FR. Dendritic cell-induced memory T cell activation in nonlymphoid tissues. Science. 2008;319:198–202. doi: 10.1126/science.1151869. [DOI] [PubMed] [Google Scholar]
- 20.Wald A, et al. Frequent genital herpes simplex virus 2 shedding in immunocompetent women. Effect of acyclovir treatment. J Clin Invest. 1997;99:1092–1097. doi: 10.1172/JCI119237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crespi CM, Cumberland WG, Wald A, Corey L, Blower S. Longitudinal study of herpes simplex virus type 2 infection using viral dynamic modelling. Sex Transm Infect. 2007;83:359–364. doi: 10.1136/sti.2006.022020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benedetti J, Corey L, Ashley R. Recurrence rates in genital herpes after symptomatic first-episode infection. Ann Intern Med. 1994;121:847–854. doi: 10.7326/0003-4819-121-11-199412010-00004. [DOI] [PubMed] [Google Scholar]
- 23.Gebhardt T, et al. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol. 2009;10:524–530. doi: 10.1038/ni.1718. [DOI] [PubMed] [Google Scholar]
- 24.Peng T, et al. Evasion of the mucosal innate immune system by herpes simplex virus type 2. J Virol. 2009;83:12559–12568. doi: 10.1128/JVI.00939-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donaghy H, et al. Role for plasmacytoid dendritic cells in the immune control of recurrent human herpes simplex virus infection. J Virol. 2009;83:1952–1961. doi: 10.1128/JVI.01578-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peng T, Zhu J, Hwangbo Y, Corey L, Bumgarner RE. Independent and cooperative antiviral actions of beta interferon and gamma interferon against herpes simplex virus replication in primary human fibroblasts. J Virol. 2008;82:1934–1945. doi: 10.1128/JVI.01649-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu T, Khanna KM, Carriere BN, Hendricks RL. Gamma interferon can prevent herpes simplex virus type 1 reactivation from latency in sensory neurons. J Virol. 2001;75:11178–11184. doi: 10.1128/JVI.75.22.11178-11184.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Masopust D, Vezys V, Marzo AL, Lefrançois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 29.Hogan RJ, et al. Protection from respiratory virus infections can be mediated by antigen-specific CD4(+) T cells that persist in the lungs. J Exp Med. 2001;193:981–986. doi: 10.1084/jem.193.8.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hogan RJ, et al. Activated antigen-specific CD8+ T cells persist in the lungs following recovery from respiratory virus infections. J Immunol. 2001;166:1813–1822. doi: 10.4049/jimmunol.166.3.1813. [DOI] [PubMed] [Google Scholar]
- 31.Wherry EJ, Ahmed R. Memory CD8 T-cell differentiation during viral infection. J Virol. 2004;78:5535–5545. doi: 10.1128/JVI.78.11.5535-5545.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koelle DM, et al. Expression of cutaneous lymphocyte-associated antigen by CD8(+) T cells specific for a skin-tropic virus. J Clin Invest. 2002;110:537–548. doi: 10.1172/JCI15537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Posavad CM, Huang ML, Barcy S, Koelle DM, Corey L. Long term persistence of herpes simplex virus-specific CD8+ CTL in persons with frequently recurring genital herpes. J Immunol. 2000;165:1146–1152. doi: 10.4049/jimmunol.165.2.1146. [DOI] [PubMed] [Google Scholar]
- 34.Straus SE, et al. Immunotherapy of recurrent genital herpes with recombinant herpes simplex virus type 2 glycoproteins D and B: Results of a placebo-controlled vaccine trial. J Infect Dis. 1997;176:1129–1134. doi: 10.1086/514103. [DOI] [PubMed] [Google Scholar]
- 35.Chao DL, Davenport MP, Forrest S, Perelson AS. A stochastic model of cytotoxic T cell responses. J Theor Biol. 2004;228:227–240. doi: 10.1016/j.jtbi.2003.12.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.