Abstract
Alzheimer's disease (AD) is a progressive and incurable neurodegenerative disorder. Early in the pathophysiology of AD, synaptic function is disrupted by soluble Aβ oligomers, possibly through Aβ-mediated internalization of NMDA receptors. Striatal-enriched phosphatase (STEP) is a tyrosine phosphatase that regulates the internalization of NMDA receptors. Recent work shows that STEP is elevated in the prefrontal cortex of human AD patients and in animal models of AD. Here, we use genetic manipulations to reduce STEP activity in a triple transgenic AD mouse model and show that a decrease in STEP levels reverses cognitive and cellular deficits observed in these mice. Our results suggest that STEP inhibitors may prove therapeutic for this devastating disorder.
Keywords: β amyloid, glutamate receptor trafficking, protein tyrosine phosphatase, long-term potentiation
Alzheimer's disease (AD) is the most common form of dementia. Considerable evidence implicates β amyloid (Aβ) peptides in the pathophysiology of AD (1). Aβ1–42 is derived from sequential cleavage of amyloid procursor protein (APP) by β- and γ-secretases. A recent hypothesis suggests that soluble Aβ oligomers disrupt synaptic and cognitive function early in the disease process (2–4). This model is supported by the findings that synaptic function is disrupted and synapses are lost early in the disorder and in animal models, even before amyloid plaques are detected (5–7). Application of soluble Aβ preparations results in synapse loss, blocks long-term potentiation (LTP), and impairs cognitive function in animals (8–11). Furthermore, animals that express high levels of Aβ show impaired synaptic plasticity and learning (6, 12, 13).
Striatal-enriched phosphatase 61 (STEP61; protein tyrosine phosphatase non-receptor 5 [PTPN5]) is a brain-specific tyrosine phosphatase targeted to synaptic compartments in striatum, hippocampus, cortex, and related brain regions (14–16). STEP61 associates with the NMDA subclass of glutamate receptors, decreases NMDA receptor (NMDAR) activity, and opposes the induction of LTP through dephosphorylation of Y1472 on the NR2B receptor subunit, leading to endocytosis of NR1/NR2B receptors (17, 18). Acute reduction in STEP expression by RNAi leads to increased surface expression and function of NR1/NR2B receptors (19), and STEP knockout (STEP−/−) mice have enhanced hippocampal LTP (SI Appendix, Fig. S1). In addition, STEP61 dephosphorylates a regulatory tyrosine within the activation loop of two enzymes critical for the development of synaptic strengthening, ERK1/2 and Fyn, thereby inactivating them (20, 21). Together, these findings support the current model that STEP activity opposes the development of synaptic strengthening (22).
Elevated levels of STEP61 are found in several transgenic AD mouse models as well as human AD prefrontal cortex (Tg-2576 and J20) (23, 24). The increase in STEP61 levels and activity contributes to the removal of NR1/NR2B complexes from synapses (24). Given that STEP61 regulates Aβ-induced internalization of NR1/NR2B, we hypothesized that lowering STEP61 levels might reduce cognitive deficits in a triple transgenic AD mouse model (3xTg-AD) (13). To test this, we have crossed STEP−/− mice (25) with 3xTg-AD mice to produce progeny null for STEP but with elevated Aβ levels [3xTg-AD/STEP−/−; double mutant (DM)]. The results indicate that a genetic reduction of STEP in AD mouse models reverses both cognitive deficits as well as the loss of glutamate receptors from synaptosomal membranes.
Results
Reduction of STEP Levels Improves Cognitive Function in 3xTg-AD Mice.
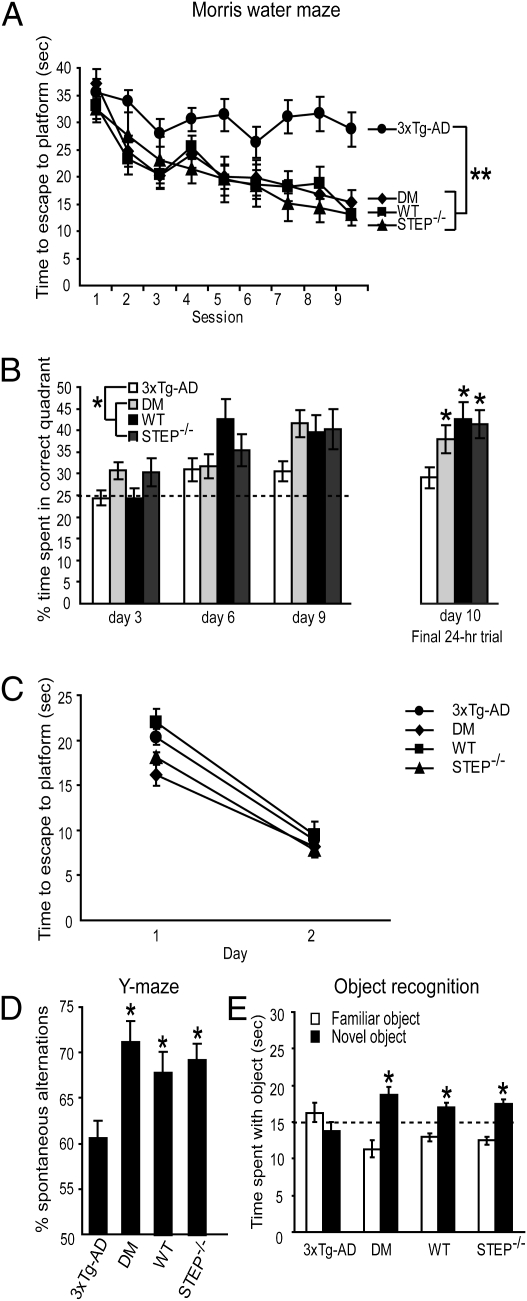
We first tested 6-mo-old mice in a Morris water maze task to assess spatial reference memory. 3xTg-AD mice are impaired in this task at 6 mo (26). The absence of STEP ameliorated this deficit. In hidden platform trials (Fig. 1A), the main effect of genotype on latency to escape to the platform was significant (P < 0.001), and post hoc tests revealed that 3xTg-AD mice exhibit longer escape latencies compared with all other groups (P < 0.002). There were no differences among the DM, wild-type (WT), and STEP−/− mice (P > 0.05), and the session by genotype interaction was not significant (P > 0.05).
Fig. 1.
Reduction of STEP levels improves cognitive function in 3xTg-AD mice. (A) In the hidden platform trials of the Morris water maze, 6-mo-old wild-type (WT; n = 11), STEP−/− (n = 9), and 3xTg-AD/STEP−/− [n = 17; double mutant (DM)] mice significantly outperformed 3xTg-AD mice (n = 25) in escape latencies [one-way (genotype) repeated measures (session) ANOVA with Fisher's least significant difference post hoc tests; **P < 0.002]. DM mice did not significantly differ from WT or STEP−/− mice (P > 0.05). (B) In probe trials performed 90 min after the final training trial on days 3, 6, and 9, WT, STEP−/−, and DM mice spent more time in the target quadrant than 3xTg-AD [one-way (genotype) repeated measures (day) ANOVA with Fisher's LSD post hoc tests; *P < 0.05]. The same effect was seen in a final probe trial 24 h after the last training trial (one-way ANOVA with Fisher's post hoc; *P < 0.05). (C) No significant differences were detected among groups in the visible platform trials [one-way (genotype) repeated measures (day) ANOVA with Fisher's LSD post hoc tests; P > 0.05]. (D) 3xTg-AD mice (n = 31) exhibited an impairment in spontaneous alternation in a Y maze compared with all other groups (one-way ANOVA with Fisher's post hoc; *P < 0.02). DM mice (n = 20) did not differ in spontaneous alternation compared with WT (n = 18) and STEP−/− (n = 18) mice (P > 0.05). (E) In an object recognition task, 3xTg-AD mice (n = 10) were outperformed by all other groups (one-way ANOVA with Fisher's post hoc tests; P < 0.05) and did not exhibit a preference for the novel object relative to chance (dashed line at 15 s; one-sample t test; P > 0.05). In contrast, WT (n = 10), STEP−/− (n = 8), and DM (n = 7) mice spent significantly more time than chance with the novel object (one-sample t test; *P < 0.02).
DM mice also performed better than 3xTg-AD mice when memory was assessed in probe trials (Fig. 1B). Probe trials were performed 90 min after the last hidden platform training trial on days 3, 6, and 9. Across these trials, the main effect of genotype was significant (P < 0.05), and post hoc tests revealed that 3xTg-AD mice spent significantly less time in the target quadrant than other groups (P < 0.05). There were no differences between the DM, WT, and STEP−/− mice (P > 0.05), and the genotype by day interaction was not significant (P > 0.05). On a final probe trial, completed 24 h after the last hidden platform session, the main effect of genotype was again significant (P < 0.05), and post hoc tests revealed that 3xTg-AD mice spent significantly less time in the target quadrant than all other groups (P < 0.05).
In visible platform trials (Fig. 1C), the main effect of day on latency was significant (P < 0.05), but the main effect of genotype was not (P > 0.05), indicating that all groups improved over 2 d. The visual platform task controls for sensory function, swimming ability, motivation, and other learning-independent factors. These results support the conclusion that the deficit in the 3xTg-AD mice, which was rescued by STEP knockout, was specifically related to spatial learning.
We next tested spontaneous alternation performance in a Y maze, a test of spatial working memory in which 3xTg-AD mice are impaired (27). The main effect of genotype on percent alternation was significant (P < 0.01), and post hoc tests revealed that 3xTg-AD mice were impaired relative to all other genotypes (P < 0.02) (Fig. 1D). There were no significant differences among the DM, WT, and STEP−/− groups (P > 0.05) and no significant differences among all groups in the total numbers of arm entries (P > 0.05).
We next tested object recognition (OR), which assesses nonspatial hippocampal-dependent memory (28). Mice were exposed to two objects, and recognition memory was tested 24 h later (Fig. 1E). The main effect of genotype was significant (P < 0.05), and post hoc tests revealed that 3xTg-AD mice spent less time with the novel object than all other groups (P < 0.05). No differences were observed in the time spent with the novel object among DM, WT, and STEP−/− mice (P > 0.05). Furthermore, 3xTg-AD mice showed no preference for the novel object relative to the chance value of 15 s (P > 0.05). In contrast, DM, WT, and STEP−/− mice spent significantly more time than chance (15 s) with the novel object (P < 0.02), indicating intact object memory retention.
To investigate locomotor activity and exploratory behavior, we tested these animals in an open field task. No significant differences were observed among the groups in total distance traveled in 10 min or in time spent in the box center (P > 0.05), indicating that all genotypes have the same degree of exploratory behavior. Taken together, these results show that loss of STEP in 3xTg-AD mice significantly improves cognitive function in the Morris water maze, the Y maze, and the object recognition task, without evident effects on sensory or motor abilities, exploratory drive, or other nonspecific factors.
STEP Knockout Restores Synaptosomal Membrane NR1/NR2B Levels in 3xTg-AD and Tg2576 Mice.
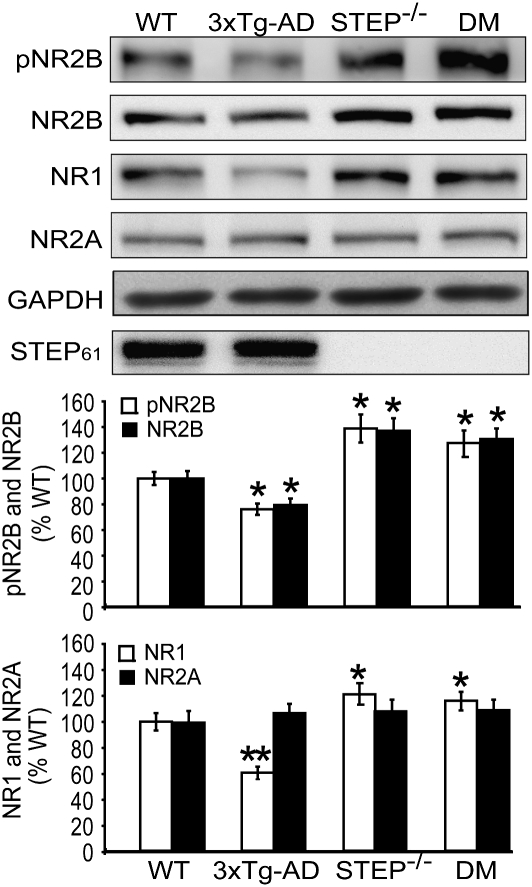
We next examined hippocampal synaptosomal membrane levels of phospho-NR2B Y1472, total NR2B, NR1, and NR2A in 6-mo-old 3xTg-AD compared with WT, STEP−/−, and DM mice (Fig. 2). Notably, like other AD mouse models, STEP61 levels were elevated in the 3xTg-AD samples (STEP61: 128.4% ± 6.2%) compared with WT. Genotype significantly affected the surface levels of NR1 and NR2B (P < 0.05) but not NR2A, which is not thought to be a STEP target. Post hoc tests revealed that basal expression of pNR2B Y1472, NR2B, and NR1 was higher in synaptosomal fractions derived from STEP−/− compared with WT mice, with no significant changes in NR2A levels (pNR2B: 141.3% ± 11.2%, P < 0.05; NR2B: 139.3% ± 8.8%, P < 0.05; NR1: 120.9% ± 6.0%, P < 0.05; NR2A: 109.9% ± 8.9%, P > 0.05; n = 8).
Fig. 2.
STEP knockout restores synaptosomal membrane NR1/NR2B levels in 3xTg-AD mice. NMDAR levels were analyzed in hippocampal synaptosomal fractions (LP1) of WT, 3xTg-AD, STEP−/−, and DM mice. Representative immunoblots from 6-mo-old mice show pNR2B, NR2B, NR1, and NR2A. Histograms are shown in Lower panel. 3xTg-AD mice showed significantly lower levels of synaptosomal pNR2B Y1472, NR2B, and NR1 compared with WT (pNR2B Y1472, NR2B: *P < 0.05; NR1: **P < 0.01). No differences were found in NR2A levels (P > 0.05; n = 8). STEP−/− had significantly higher levels of synaptosomal pNR2B Y1472, NR2B, and NR1 compared with WT (*P < 0.05) but not NR2A (P > 0.05; n = 8). In DM mice, pNR2B Y1472, NR2B, and NR1 synaptosomal levels were increased compared with WT (*P < 0.05; n = 8) and 3xTg-AD mice (P < 0.05; n = 8) but were not significantly different from STEP−/− (P > 0.05; n = 8). Normalization was to GAPDH. STEP61 levels were significantly increased in 3xTg-AD brain membrane fractions compared with WT littermates (*P < 0.05; n = 4).
pNR2B Y1472, NR2B, and NR1 synaptosomal levels were significantly reduced in 3xTg-AD mice relative to WT, with no change in NR2A (pNR2B: 78.6% ± 4.5%, P < 0.05; NR2B: 79.5% ± 5.0%, P < 0.05; NR1: 62.1% ± 4.8%, P < 0.01; NR2A: 110.5% ± 7.3%, P > 0.05; n = 8). Conversely, DM mice had significantly higher synaptosomal levels of pNR2B Y1472, NR2B, and NR1 compared with 3xTg-AD mice (pNR2B: 129.4% ± 9.8%; NR2B: 132.8% ± 8.3%; NR1: 120.8% ± 7.2%; NR2A: 113.6% ± 8.4%; P > 0.05 compared with STEP−/−; n = 8).
Biochemical analyses were conducted in a second AD mouse model of Tg2576 mice with the APPSWE mutation (29) (SI Appendix, Fig. S2). We examined the cortices of 9-mo-old mice, because Tg2576 mice have elevated levels of Aβ and memory deficits at this age (29). Mice with the APP transgene and the WT STEP gene showed a significant decrease in pNR2B Y1472, NR2B, and NR1 levels, with no significant change to NR2A levels (SI Appendix, Fig. S2) (pNR2B: 65.6% ± 5.6%, P < 0.05; NR2B: 57.5% ± 13.1%, P < 0.05; NR1: 63.5% ± 5.9%, P < 0.01; NR2A: 113.6% ± 5.7%, P > 0.05; n = 6).
The decreased levels of the receptors observed in Tg2576 mice were reversed in Tg2576 mice null for STEP. Synaptosomal pNR2B Y1472, NR2B, and NR1 levels in Tg2576/STEP−/− mice resembled STEP−/− mice (SI Appendix, Fig. S2) (pNR2B: 131.4% ± 6.3%; NR2B: 139.5% ± 13.4%; NR1: 154.7% ± 16.5%, P < 0.05; NR2A: 118.5% ± 6.6%, P > 0.05 compared with STEP−/−; n = 6). These results indicate that genetic reduction of STEP in two different models of AD significantly raises levels of NR1/NR2B receptors in synaptosomal fractions compared with littermate AD controls.
Loss of STEP in 3xTg-AD Mice Increases Phospho-ERK and Phospho-Fyn but Does Not Alter Aβ or Phospho-tau Levels.
Both pFyn and pERK1/2 are STEP substrates (20, 21) and are required for synaptic strengthening and memory consolidation (30, 31). We, therefore, examined whether the absence of STEP in 6-mo-old 3xTg-AD mice led to an increase in pFyn and pERK1/2 by using phospho-tyrosine antibodies specific to Fyn (pY416) and ERK1/2 (pY204), the sites dephosphorylated by STEP. We observed a significant increase in pFyn and pERK1/2 (SI Appendix, Fig. S3) in DM and STEP−/− compared with WT controls (pFyn: DM = 132.6% ± 8.6%, STEP−/− = 137.5% ± 13.8%, P < 0.05; n = 3; pERK1/2: DM = 143.0% ± 7.2%, STEP−/− = 138.0% ± 9.6%, P < 0.05; n = 4). There were no significant differences between the 3xTg-AD mice and WT controls (pFyn: 3xTg-AD = 96.6% ± 7.2%; pERK1/2: 3xTg-AD = 81.6% ± 9.3%; P > 0.05). These results indicate that pFyn and pERK1/2 are also increased in 3xTg-AD mice null for STEP.
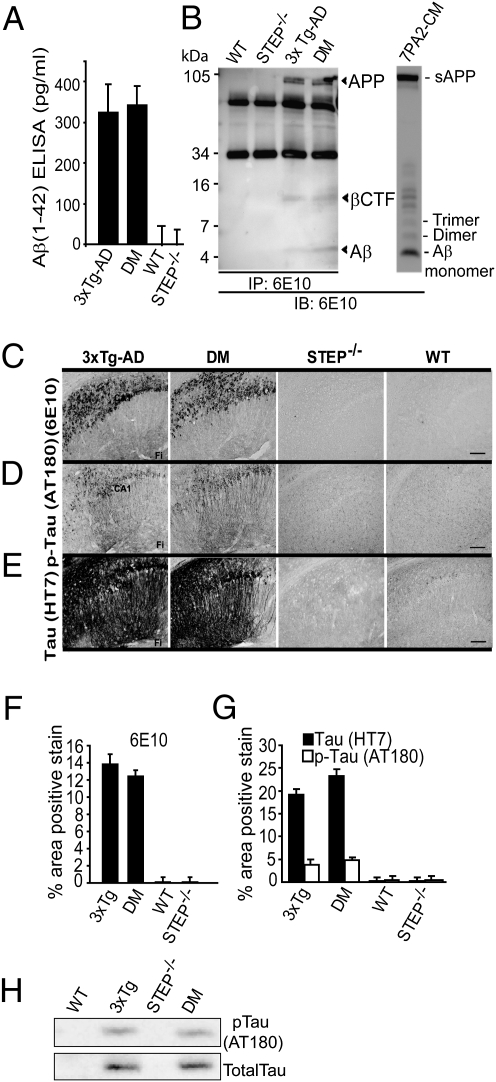
Total Aβ1–42 levels in 3xTg-AD and DM cohorts at 6 mo showed no significant difference as measured by ELISA using human Aβ (1–42) antibody (Fig. 3A) (3xTg-AD = 324 ± 69 pg/mL; DM = 343 ± 47 pg/mL, P > 0.05; n = 4). Aβ from SDS soluble brain extracts were quantified by using immunoprecipitation and Western blotting with 6E10 antibody, and no significant differences between 3xTg-AD and DM were detected for APP, βC-terminal fragment, or Aβ (Fig. 3B) (P > 0.05; n = 3). We also analyzed total APP levels and βCTF levels using antibodies specific to either the N or C terminal of APP. Both 3xTg-AD and DM mouse brains showed no significant difference in total APP and βCTF levels (SI Appendix, Fig. S4), suggesting that the absence of STEP in DM mice has no detectable effect on these proteins at 6 mo of age.
Fig. 3.
Absence of STEP in 3xTg-AD does not alter Aβ or tau levels. (A) 3xTg-AD and DM had no significant differences in total Aβ1–42 levels as measured by ELISA (P > 0.05; n = 4). (B) Immunoprecipitation from mouse brain homogenates used 6E10 antibody, and samples were also blotted with 6E10 antibody. CTFs and Aβ are indicated by arrowheads. Representative Aβ-enriched conditioned medium (7PA2-CM) immunoreactivity showing Aβ-monomer, dimer, and trimers (Right). 3xTg-AD and DM brain samples showed no significant difference in Aβ levels (P > 0.05). (C) In hippocampus, 3xTg-AD and DM 6-mo-old mice have similar patterns of intraneuronal and extracellular staining with 6E10. STEP−/− and WT mice showed no detectable staining with 6E10. (D) tau pathology was evaluated with an antibody that recognizes p-Ser235 and p-Thr231 (AT180) and (E) a human-specific tau antibody (HT7). 3xTg-AD or DM mice had no significant differences in immunoreactivity staining with either tau antibody. STEP−/− and WT mice showed no detectable staining with these antibodies. (Magnification: 10×; scale bar: 200 μm.) CA, cornu ammonis; Fi, fissure. (F) Quantification of 6E10 staining revealed no significant difference between 3xTg-AD and DM mice (one-way ANOVA with Fisher's LSD post hoc tests; P > 0.05). (G) Quantification of p-tau (AT180) and total tau (HT7) staining also revealed no significant differences between 3xTg-AD and DM mice (one-way ANOVA with Fisher's LSD post hoc tests; P > 0.05). (H) Mouse brain membrane fractions were probed with p-tau antibody and total tau antibody (P > 0.05; n = 4).
3xTg-AD and DM mice displayed similar 6E10 immunostaining patterns in hippocampus and cortex. 6E10 immunoreactivity revealed primarily intraneuronal staining, with intensely stained pyramidal neurons in the CA1 region of the hippocampus and the subiculum (Fig. 3C). Some faint staining was also observed in the CA3 region, whereas no positive staining was observed in the dentate gyrus. Consistent with previously published findings (13), at this age, very few extracellular diffuse plaque deposits were observed in either cohort throughout the hippocampal or cortical regions. Quantification of 6E10 staining showed no statistical difference between the 3xTg-AD and DM mice in the hippocampus (Fig. 3F) (P > 0.05; n = 4) or cortex (P > 0.05; n = 4). As expected, WT and STEP−/− mice showed no 6E10 staining in either region (Fig. 3C).
Immunostaining for phospho-tau (p-tau) and tau was also performed on fixed brain tissue using a paired helical filament-tau specific antibody (AT180) that recognizes p-Thr231 and p-Ser235 and an antihuman τ-specific antibody (HT7). AT180 positive immunostaining was limited to the cell bodies and axons of pyramidal cells in the hippocampal CA1 region for both 3xTg-AD and DM mice (Fig. 3 D and G). Furthermore, HT7 immunostaining, which recognizes normal human tau as well as p-tau, appeared darker in intensity in neuronal cell bodies and axons, indicating the presence and expression of the human tau-transgene and p-tau. Positive staining was limited primarily to the CA1 in both 3xTg-AD and DM mice (Fig. 3 E and G). A few HT7 positively stained pyramidal cells were also observed in the CA3 region and mossy cells in the hilus of the dentate gyrus. In addition, Western blotting with the AT180 antibody showed no significant difference in p-tau levels in 3xTg-AD and DM mouse brains (P > 0.05; n = 4). (Fig. 3H). Taken together, these results indicate that the cognitive improvements observed in the DM mice did not correlate with changes in APP, βCTF, Aβ, tau, or p-tau levels, which remain similarly elevated in both 3xTg-AD and DM mice.
Hippocampal Synaptic Plasticity Is Enhanced in 3xTg-AD Mice Lacking STEP.
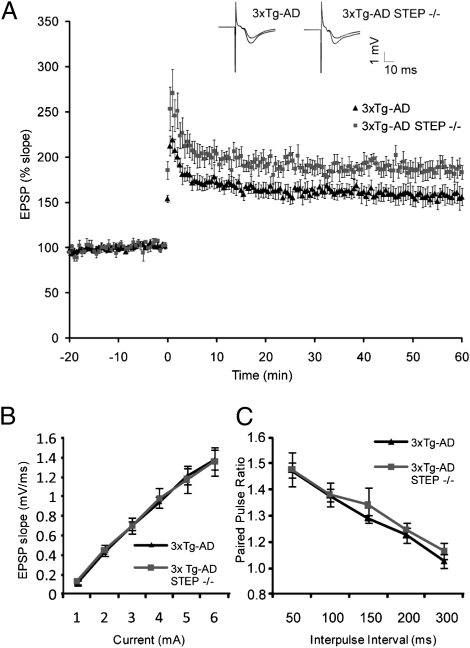
Impairments in synaptic plasticity have been shown in various AD mouse models, including 3xTg-AD mice, and are correlated with behavioral dysfunction (12, 13). We reasoned that enhanced LTP in mice lacking STEP might be associated with the reversal of cognitive deficits in DM mice. Consistent with this hypothesis, CA1-LTP was significantly enhanced in hippocampal slices from 10-mo-old DM mice compared with 3xTg-AD mice (Fig. 4A) (DM: 187% ± 8.8%; 3xTg-AD: 159% ± 8.5% between 40 and 60 min post-θ burst stimulation; P < 0.05). Basal synaptic transmission (Fig. 4B) and paired pulse responses (Fig. 4C) were similar between these genotypes. The data show that loss of STEP facilitates the expression of CA1-LTP in 3xTg-AD mice, consistent with improved memory performance.
Fig. 4.
Hippocampal synaptic plasticity is enhanced in 3xTg-AD mice lacking STEP. (A) Field potentials were recorded in the CA1 region of hippocampal slices from 10-mo-old 3xTg-AD mice with or without STEP. A stable baseline was recorded for at least 20 min before LTP induction by θ-burst stimulation (TBS). Inset shows traces before and after TBS. The slope of the EPSP relative to the pre-TBS level is plotted as a function of time. For 3xTg-AD, seven slices from three animals were used; for DM, six slices from three animals were used. Data are means ± SEM from separate slices. The increase in EPSP between 30 and 60 min post-TBS was greater in DM slices than 3xTg-AD slices (repeated measures ANOVA; P < 0.05). (B and C) Basal synaptic transmission and paired pulse response were indistinguishable between 3xTg-AD and DM mice.
Discussion
These results extend our recent finding that STEP61 levels are increased in prefrontal cortex of AD patients and the cortex of several AD mouse models (23, 24). The increase in STEP61 in these models is likely because of an inhibition of the proteasome by Aβ and a consequent loss of the normal degradation of STEP61. If elevated levels of STEP61 contribute to the pathophysiology of AD, we reasoned that reducing STEP levels might prove beneficial.
The data presented here show that a genetic reduction of STEP reverses cognitive deficits normally present in a mouse model of AD. Six-month-old DM mice were improved, relative to 3xTg-AD littermates, when tested for spatial reference memory in the Morris water maze, spontaneous alteration performance in the Y maze, and nonspatial hippocampus-dependent memory in the object recognition task. Importantly, all groups tested displayed similar locomotor activity and exploratory behavior in the open field task.
The behavioral improvements that were observed in the DM mice are consistent with the biochemical data on NMDAR trafficking. NR1/NR2B receptor levels are significantly lowered in hippocampal synaptosomal membrane fractions from both Tg2576 and the triple transgenic mice. Similar findings between these two different AD mouse models suggest that the effect is likely caused by increases of Aβ or overexpression of the APP mutant itself. Reducing STEP levels restored NR1/NR2B levels on membrane surfaces in both strains of mice.
Additional substrates of STEP could also be contributing to the improved cognitive function of the DM mice. Previous studies have shown that the loss of STEP increases the tyrosine phosphorylation of ERK1/2 (25). This proved to be the case in mouse models of AD as well; cortical tissue from DM mice had increased levels of both p-ERK and p-Fyn. p-Fyn activation phosphorylates NR2B at Y1472 and leads to exocytosis of NR1/NR2B receptors, whereas increased activation of pERK is required both for maintenance of LTP and the consolidation of memories (30).
One of the more interesting findings in this study was the rescue of AD-like cognitive deficits in DM mice, despite the unchanged levels of both Aβ and p-tau. These findings suggest that the improved cognitive function was because of the decrease in STEP levels and that these improvements could be achieved without diminishing Aβ and p-tau levels in the earlier stages of the disease tested here. Studies are underway to determine whether the improved cognitive functioning persists over time in older AD mice null for STEP.
The electrophysiology results are generally consistent with the behavioral and biochemical findings. Loss of STEP facilitated the expression of CA1-LTP, although facilitated LTP occurred both in the presence and absence of the triple transgenes. Note that CA1-LTP was present in 3xTg-AD slices and was similar in magnitude to the level of CA1-LTP observed in WT mice in a separate cohort, which contrasts a previously published report (13). This distinction may be attributable to differences in strain background, LTP induction protocols, or age.
In conclusion, we tested the hypothesis that elevated STEP61 levels in human patients and mouse models of AD contribute to the cognitive deficits in this disorder. We genetically reduced STEP in 3xTg-AD mice and found significant improvements in three cognitive tasks as well as increased levels of NR1/NR2B on synaptosomal membranes in progeny null for STEP. CA1-LTP in 3xTg-AD mice was increased in the absence of STEP. The Tyr phosphorylation of three STEP substrates, NR2B, ERK1/2, and Fyn, was elevated in DM mice relative to 3xTg-AD mice. We propose that blocking internalization of NR1/NR2B receptors by removal of STEP contributes to the improved cognitive functioning in 3xTg-AD mice, possibly in conjunction with the increased Tyr phosphorylation and activation of ERK1/2 and Fyn. These results identify STEP61 as a promising target for drug discovery in the search for more effective therapeutic treatments for AD.
Materials and Methods
Materials.
Tris·Tricine gradient acrylamide gels (10–20%) were from Bio-Rad. The human Aβ (1–42) ELISA kit was from Invitrogen. All primary and secondary antibodies and their dilutions are listed in SI Appendix, SI Methods and Table S1.
Animals.
The Yale Institutional Animal Care and Use Committee approved these experiments. Only male mice were used. Three strains of mice were included: Tg2576 mice in C57BL/6/SJL background (29), STEP−/− mice in C57BL/6 background (25), and triple transgenic mice in C57BL/6/SV129 background (13). An extended section on breeding and genotyping is provided in SI Appendix, SI Methods.
Morris Water Maze.
All behavioral tests were performed blind to genotype. Mice were handled for 2 min/d for 5 d before training. The Morris water maze apparatus consisted of a 1.2-m circular pool, and water was made opaque with white nontoxic tempura paint. First, mice completed the cued task, in which they were trained to escape from the pool using a visible platform. Cued task training consisted of 4 trials/d for 2 d. Time spent to platform escape (latency) was recorded. Next, mice completed the spatial version of the task in which they were trained to find a submerged platform (10 cm in diameter) by use of extramaze cues. For each mouse, the platform remained in the same quadrant throughout spatial training; starting positions (north, south, east, and west) were varied pseudorandomly. Platform location was counterbalanced across animals. Four trials per mouse were conducted for 9 consecutive d. Each mouse was allowed 60 s to locate the platform; if the mouse did not escape within 60 s, the experimenter manually placed the mouse on the platform where it remained for 15 s. Latency to locate the hidden platform and escape the water was recorded. To assess the development of a spatial bias, probe trials were conducted 90 min and 24 h after spatial training sessions on days 3, 6, and 9. For the probe trials, the platform was removed from the pool, and the mouse was allowed to swim freely for 60 s, at which point the mouse was removed from the pool. Percent time spent in each quadrant was recorded. Percent time was determined using AnyMaze software (Stoelting).
Data Analysis.
All data were presented as means ± SEM, and P values less than 0.05 were considered significant. For biochemical analyses, one-way ANOVAs were performed with Fisher's post hoc tests. For the Morris water maze, latency measures and percent time spent in the target quadrant were analyzed using a one-way (genotype) repeated measures (day or session) ANOVA with Fisher's least significant difference post hoc tests. For the Y maze, percent spontaneous alternations were analyzed using a one-way ANOVA with Fisher's LSD post hoc tests. For the object recognition task, a one-way ANOVA with Fisher's LSD post hoc tests was performed on novel object exploration time, and separate one-sample t tests were performed for each group to determine whether time spent with the novel object differed from the chance value of 15 s. One-sample t tests were conducted, because the exploration times with the two objects are not independent of one another; time spent with one object leads to a reduction of time spent with the other object.
Electrophysiology.
All measurements and analyses were completed blind to genotype. Hippocampal slices (400 μm) were prepared from adult mice (7–10 mo of age) and bathed in oxygenated artificial cerebrospinal fluid (aCSF; containing Mg2+ and Ca2+), as previously described (32). The Schaffer collateral pathway was stimulated with a bipolar tungsten electrode at 0.033 Hz at levels that evoked less than 50% of maximal field excitatory postsynaptic potentials. Evoked CA1 field potentials were recorded through a 3-MΩ micropipette filled with aCSF, and the slope of the EPSP was determined (Clampfit; Molecular Devices). After continuous perfusion for 20–40 min, long-term potentiation was induced by stimulation with 10 100-Hz trains at five pulses delivered at 5 Hz.
Supplementary Material
Acknowledgments
We thank laboratory members for helpful discussions and critical reading of the manuscript. We thank Drs. Marina Picciotto and Deepa Venkitaramani for assistance with the mice crosses. The work was funded by The American Health Assistance Foundation and National Institutes of Health Grants MH081190 (to C.P.), AG09464 (to P.G. and A.C.N.), NS42304 (to S.M.S.), NS39962 (to S.M.S.), MH01527 (to P.J.L.), and MH52711 (to P.J.L.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1013543107/-/DCSupplemental.
References
- 1.Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: Lessons from the Alzheimer's amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 2.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: Progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 3.Venkitaramani DV, et al. Beta-amyloid modulation of synaptic transmission and plasticity. J Neurosci. 2007;27:11832–11837. doi: 10.1523/JNEUROSCI.3478-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Selkoe DJ. Soluble oligomers of the amyloid beta-protein impair synaptic plasticity and behavior. Behav Brain Res. 2008;192:106–113. doi: 10.1016/j.bbr.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mucke L, et al. High-level neuronal expression of abeta 1-42 in wild-type human amyloid protein precursor transgenic mice: Synaptotoxicity without plaque formation. J Neurosci. 2000;20:4050–4058. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacobsen JS, et al. Early-onset behavioral and synaptic deficits in a mouse model of Alzheimer's disease. Proc Natl Acad Sci USA. 2006;103:5161–5166. doi: 10.1073/pnas.0600948103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Terry RD, et al. Physical basis of cognitive alterations in Alzheimer's disease: Synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 8.Walsh DM, et al. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- 9.Lacor PN, et al. Abeta oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer's disease. J Neurosci. 2007;27:796–807. doi: 10.1523/JNEUROSCI.3501-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shankar GM, et al. Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J Neurosci. 2007;27:2866–2875. doi: 10.1523/JNEUROSCI.4970-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shankar GM, et al. Amyloid-beta protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chapman PF, et al. Impaired synaptic plasticity and learning in aged amyloid precursor protein transgenic mice. Nat Neurosci. 1999;2:271–276. doi: 10.1038/6374. [DOI] [PubMed] [Google Scholar]
- 13.Oddo S, et al. Triple-transgenic model of Alzheimer's disease with plaques and tangles: Intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 14.Boulanger LM, et al. Cellular and molecular characterization of a brain-enriched protein tyrosine phosphatase. J Neurosci. 1995;15:1532–1544. doi: 10.1523/JNEUROSCI.15-02-01532.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goebel-Goody SM, Davies KD, Alvestad Linger RM, Freund RK, Browning MD. Phospho-regulation of synaptic and extrasynaptic N-methyl-d-aspartate receptors in adult hippocampal slices. Neuroscience. 2009;158:1446–1459. doi: 10.1016/j.neuroscience.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 16.Xu J, et al. Extrasynaptic NMDA receptors couple preferentially to excitotoxicity via calpain-mediated cleavage of STEP. J Neurosci. 2009;29:9330–9343. doi: 10.1523/JNEUROSCI.2212-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Snyder EM, et al. Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci. 2005;8:1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- 18.Pelkey KA, et al. Tyrosine phosphatase STEP is a tonic brake on induction of long-term potentiation. Neuron. 2002;34:127–138. doi: 10.1016/s0896-6273(02)00633-5. [DOI] [PubMed] [Google Scholar]
- 19.Braithwaite SP, et al. Regulation of NMDA receptor trafficking and function by striatal-enriched tyrosine phosphatase (STEP) Eur J Neurosci. 2006;23:2847–2856. doi: 10.1111/j.1460-9568.2006.04837.x. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen TH, Liu J, Lombroso PJ. Striatal enriched phosphatase 61 dephosphorylates Fyn at phosphotyrosine 420. J Biol Chem. 2002;277:24274–24279. doi: 10.1074/jbc.M111683200. [DOI] [PubMed] [Google Scholar]
- 21.Paul S, Nairn AC, Wang P, Lombroso PJ. NMDA-mediated activation of the tyrosine phosphatase STEP regulates the duration of ERK signaling. Nat Neurosci. 2003;6:34–42. doi: 10.1038/nn989. [DOI] [PubMed] [Google Scholar]
- 22.Braithwaite SP, Paul S, Nairn AC, Lombroso PJ. Synaptic plasticity: One STEP at a time. Trends Neurosci. 2006;29:452–458. doi: 10.1016/j.tins.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chin J, et al. Fyn kinase induces synaptic and cognitive impairments in a transgenic mouse model of Alzheimer's disease. J Neurosci. 2005;25:9694–9703. doi: 10.1523/JNEUROSCI.2980-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurup P, et al. Abeta-mediated NMDA receptor endocytosis in Alzheimer's disease involves ubiquitination of the tyrosine phosphatase STEP61. J Neurosci. 2010;30:5948–5957. doi: 10.1523/JNEUROSCI.0157-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Venkitaramani DV, et al. Knockout of striatal enriched protein tyrosine phosphatase in mice results in increased ERK1/2 phosphorylation. Synapse. 2009;63:69–81. doi: 10.1002/syn.20608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Billings LM, Oddo S, Green KN, McGaugh JL, LaFerla FM. Intraneuronal Abeta causes the onset of early Alzheimer's disease-related cognitive deficits in transgenic mice. Neuron. 2005;45:675–688. doi: 10.1016/j.neuron.2005.01.040. [DOI] [PubMed] [Google Scholar]
- 27.Carroll JC, et al. Progesterone and estrogen regulate Alzheimer-like neuropathology in female 3xTg-AD mice. J Neurosci. 2007;27:13357–13365. doi: 10.1523/JNEUROSCI.2718-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fernandez SM, et al. Estradiol-induced enhancement of object memory consolidation involves hippocampal extracellular signal-regulated kinase activation and membrane-bound estrogen receptors. J Neurosci. 2008;28:8660–8667. doi: 10.1523/JNEUROSCI.1968-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsiao K, et al. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 30.Satoh Y, et al. Extracellular signal-regulated kinase 2 (ERK2) knockdown mice show deficits in long-term memory; ERK2 has a specific function in learning and memory. J Neurosci. 2007;27:10765–10776. doi: 10.1523/JNEUROSCI.0117-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grant SG, et al. Impaired long-term potentiation, spatial learning, and hippocampal development in fyn mutant mice. Science. 1992;258:1903–1910. doi: 10.1126/science.1361685. [DOI] [PubMed] [Google Scholar]
- 32.Laurén J, Gimbel DA, Nygaard HB, Gilbert JW, Strittmatter SM. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers. Nature. 2009;457:1128–1132. doi: 10.1038/nature07761. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.