Abstract
Recent studies suggest that the human Abelson helper integration site-1 (AHI1) gene on chromosome 6 is associated with susceptibility to schizophrenia and autism, two common neuropsychological disorders with depression symptoms. Mouse Ahi1 protein is abundant in the hypothalamus and amygdala, which are important brain regions for controlling emotion. However, the neuronal function of Ahi1 remains unclear. With the Cre–loxP system, we created a mouse model that selectively reduces Ahi1 expression in neuronal cells. Mice with neuronal Ahi1 deficiency show reduced TrkB level in the brain and depressive phenotypes, which can be alleviated by antidepressant drugs or by overexpression of TrkB in the amygdala. Ahi1 deficiency promotes the degradation of endocytic TrkB and reduces TrkB signaling in neuronal cells. Our findings suggest that impaired endocytic sorting and increased degradation of TrkB can induce depression and that this impaired pathway may serve as a previously uncharacterized therapeutic target for depression.
Keywords: neuropathology, neuropsychiatric, neurotrophic, endocytosis, trafficking
The Abelson helper integration site-1 (AHI1) locus was initially identified as a common helper provirus integration site for murine leukemias and lymphomas (1). Later studies revealed that nonsense or frame-shift mutations in AHI1 are associated with Joubert syndrome, a rare autosomal recessive disorder characterized by abnormal brain development and mental retardation (2, 3). Recently, fine mapping (4, 5), association (6), and replication (7, 8) studies have identified AHI1 as a susceptibility gene for schizophrenia, a major neuropsychiatric disorder associated with depression. The AHI1 gene locus has also been linked with autism, which overlaps with a schizophrenia haplotype (9) and is also seen in some patients with Joubert syndrome (10). Despite the genetic association of the AHI1 gene with susceptibility to neuropsychiatric disorders, the function of Ahi1 in the brain remains unknown, and there has been no evidence that a functional deficiency of AHI1 is involved in neurological phenotypes.
In the mouse brain, Ahi1 is abundant in the hypothalamus and amygdala (11, 12), two important brain regions whose dysfunction can lead to emotional and depression phenotypes (13). Ahi1 contains seven WD40 repeats, an SH3 domain, potential SH3 binding sites, and an N-terminal coiled–coiled domain (14). Ahi1 is a cytoplasmic protein and forms a stable complex with Hap1, a huntingtin-associated protein (11). Hap1 binds more tightly to mutant huntingtin containing an expanded polyglutamine repeat (15, 16), which causes Huntington's disease, another important neuropsychiatric disorder with a depression phenotype. Increasing evidence shows that Hap1 is associated with microtubule-dependent transporters (16–19) and is involved in the internalization and trafficking of membrane receptors (20–24). Given the genetic association of the AHI1 locus with the susceptibility to schizophrenia and autism, it is important to investigate whether and how Ahi1 dysfunction in the mouse brain dysfunction can lead to the neurological phenotypes similar to those in neuropsychiatric disorders.
In the current study, we used the Cre–loxP system to generate conditional Ahi1 knockout mice in which the expression of Ahi1 is reduced in neuronal cells. Suppression of Ahi1 level in the mouse brain results in a depressive phenotype and reduced levels of TrkB caused by impaired endocytosis of TrkB. Because TrkB signaling plays a critical role in the depression associated with neuropsychiatric disorders (25, 26), we overexpressed TrkB in the mouse amygdala and found that this overexpression could ameliorate the depressive phenotype of Ahi1 mutant mice. Our findings also suggest that impaired endocytic TrkB sorting and trafficking can be a unique therapeutic target for depression and that Ahi1 mutant mice will be useful for investigating both the pathogenesis and possible therapeutics of depression.
Results
Ahi1 Deficiency Reduces the Expression Level of Hap1.
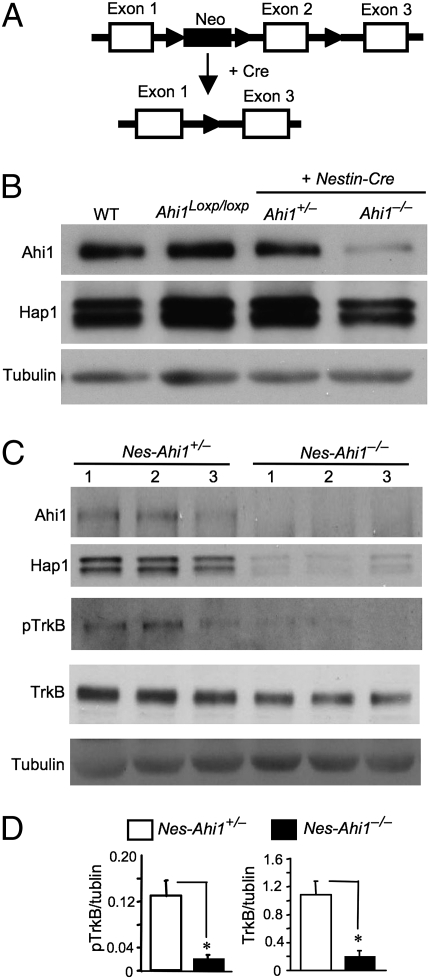
To investigate the role of Ahi1 in neuronal function, we generated conditional Ahi1 knockout mice by inserting loxP sites flanking exon 2 of the mouse Ahi1 gene (Fig. 1A). The floxed mice (Ahi1loxp/loxp) were crossed with transgenic mice (nes-Cre) expressing Cre recombinase in neuronal cells under the neuronal nestin promoter (27). Genotyping confirmed the removal of exon 2 of the Ahi1 gene in the crossed mouse (nesCre+/Ahi1loxp/loxp, abbreviated to nes-Ahi1−/−) brains (Fig. S1A). Nes-Ahi1−/− mice (C57BL/6), which expressed Cre under the Nestin promoter, were born at the same Mendelian ratios and live normally as control mice of other genotypes. Western blotting verified a reduction of Ahi1 and Hap1 at the protein level in the hypothalamus and brainstem of nes-Ahi1−/− mice (Fig. 1B and Fig. S1B). RT-PCR analysis revealed that the level of Hap1 transcripts is unchanged in Ahi1-deficient brains despite the decreased Ahi1 transcripts (Fig. S1C), supporting the notion that Ahi1 and Hap1 stabilize each other at the protein level (11).
Fig. 1.
Ahi1 deficiency diminishes Hap1 and TrkB. (A) Generation of conditional Ahi1 knockout mice. Ahi1 exon 2 was removed by the Cre–loxP system to suppress Ahi1 expression. (B) Western blot analysis of Ahi1 and Hap1 expression in the hypothalamic tissue from wild-type (WT), floxed Ahi1 (Ahi1loxp/loxp), heterozygous (nes-Ahi1+/−), and homozygous conditional knockout (nes-Ahi1−/−) mice. (C) Total TrkB and pTrkB in the hypothalamus of control (nes-Ahi1+/−) and Ahi1-deficient (nes-Ahi1−/−) mice were examined by Western blotting. The blots were also probed with antibodies to Ahi1, Hap1, and tubulin. (D) Densitometric analysis of the ratios of TrkB or pTrkB to tubulin showing a decrease of total TrkB and pTrkB in Ahi1-deficient (nes-Ahi1−/−) mice (n = 3 each group, *P < 0.05).
Western blotting showed that mice with other genotypes, such as wild-type, floxed mice (Ahi1loxp/loxp), and heterozygous floxed C57BL/6 mice with Cre (nesCre+/Ahi1+/loxp, abbreviated to nes-Ahi1+/−), have the same levels of Ahi1 and Hap1 in their brains (Fig. 1B). Immunostaining of the mouse hypothalamus, which is enriched in both Hap1 and Ahi1, confirmed that there were fewer neurons expressing Ahi1 and Hap1 in nes-Ahi1−/− mice than in the control nes-Ahi1+/− mice (Fig. S2). Because heterozygous Ahi1 conditional knockout mice (nes-Ahi1+/−) also carry the Cre transgene and are closer to homozygous Ahi1 conditional knockout mice (nes-Ahi1−/−), but show no significant difference in the expression of Ahi1 and Hap1 compared with wild-type mice, we used nes-Ahi1+/− mice as a control for comparison with homozygous Ahi1 knockout mice (nes-Ahi1−/−) to identify biochemical or behavioral changes related specifically to Ahi1 deficiency.
Ahi1 Deficiency Decreases TrkB and Its Signaling.
A recent study showed that Ahi1 deficiency in kidney tissue can impair Wnt-β-catenin signaling (28). Examining nes-Ahi1−/− mouse brains did not reveal any significant change in the levels of β-catenin in their brains (Fig. S3), suggesting that the effects of loss of Ahi1 may be cell type-dependent and prompting us to examine other targets in the brains of Ahi1-deficient mice. Because Ahi1 deficiency reduces Hap1 that is involved in the intracellular trafficking and endocytosis of neurotrophin and membrane receptors (11, 22–24), we examined TrkB, which is important for neuronal development and functions. Ahi1 deficiency reduced the levels of both TrkB and its phosphorylated form (pTrkB) in the mouse hypothalamus (Fig. 1 C and D). We also examined the levels of phosphorylated Akt and Erk, two molecules that are activated in TrkB-mediated pathways, and found a decrease in the phosphorylation of these signaling molecules in the nes-Ahi1−/− mouse hypothalamus (Fig. S4).
Ahi1 Deficiency Causes Depressive Phenotypes in Mice.
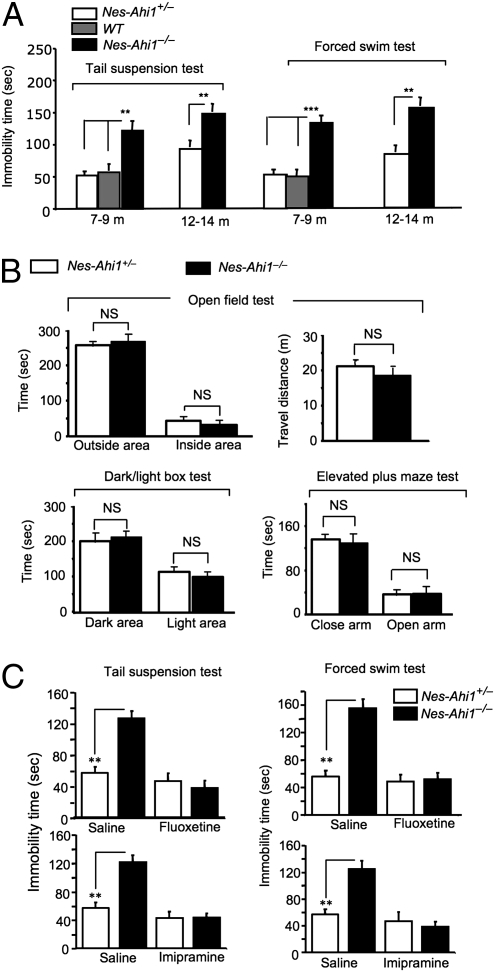
Unlike Hap1 null mice that show postnatal death (29), conditional depletion of Ahi1 in nes-Ahi1−/− mice does not result in abnormal survival or growth compared with mice of other genotypes. However, Ahi1-deficient mice allowed us to examine the consequences of loss of Ahi1/Hap1 in adult mice. The motor function of Ahi1-deficient mice assessed by the rotarod and locomotor activity tests did not differ significantly from that of controls (Fig. S5). We saw no obvious abnormal brain structures or neuronal degeneration in Ahi1-deficient mice. Because brain-derived neurotrophic factor (BDNF)/TrkB dysfunction can lead to depressive phenotypes (25, 26), we chose to focus our analysis on mouse behaviors. Classical analyses of depressive phenotypes include the forced swim test (FST), which is based on a measurement of the rodents’ immobility time after they are placed in a tank filled with water and from which they cannot escape. Another assay is the tail suspension test (TST), which is considered as efficient as the FST for detecting depressive phenotypes in mice. We focused on nes-Ahi1−/− mice to assess the specific effect of Ahi1 deficiency in neuronal cells. Nes-Ahi1−/− mice at the age of 7–9 mo were immobile for a longer time than nes-Ahi1+/− and wild-type mice in the FST and TST (Fig. 2A). Ahi1-deficient (nes-Ahi1−/−) mice show much less escape activity in the water tank (Movies S1 and S2) and manifested a longer immobility time than the corresponding control mice in TST (Movie S3) (representative videos were taken from different male mice at the age of 4 mo). Although there was an increase in immobility of TST and FST in older control mice, old Ahi1−/− mice aged at 12–15 mo also showed a longer immobility time than the age-matched control mice in TST and FST (Fig. 2A). Because depressive phenotypes often overlap with anxiety behavior, we performed three types of classic anxiety tests: the open field, dark/light box, and elevated plus maze tests. None of these tests revealed any significant difference between Ahi1-deficient (nes-Ahi1−/−) and control (nes-Ahi1+/−) mice (Fig. 2B), suggesting that Ahi1 deficiency selectively induces the depressive phenotype.
Fig. 2.
Ahi1 deficiency leads to depressive behaviors. (A) Depressive phenotypes of nes-Ahi1−/− mice at 7–9 and 12–15 mo of age. *P < 0.05, **P < 0.01 between age-matched nes-Ahi1−/− and nes-Ahi1+/− or wild-type (WT) groups. Each group contains 8–16 mice. (B) Anxiety tests of conditional Ahi1 knockout mice. The open field test was performed, and time spent in the outside area and inside area of the cage (Upper Left) as well as distance traveled (Upper Right) were calculated. The times spent in the dark area and light area (Lower Left) and in the closed arm and open arm in the elevated plus maze test (Lower Right) were also recorded. NS, not significant or P > 0.05, n = 10. (C) The increased immobility time in TSTs and FSTs in the Ahi1-deficient mice (nes-Ahi1−/−) was reversed by the antidepressants imipramine (30 mg/kg) or fluoxetine (20 mg/kg), which was i.p. injected into mice for 30 min. The control is the injection of saline. *P < 0.05 or **P < 0.01.
The i.p. injection of imipramine (30 mg/kg) and fluoxetine (20 mg/kg) into mice can rapidly increase TrkB phosphorylation and reduce depressive phenotypes in mice (30, 31). By using the same drug treatments, we found that these antidepressants significantly improved the mobility of nes-Ahi1−/− mice in the FST and TST (Fig. 2C). In addition, fluoxetine i.p. injection increased TrkB phosphorylation in the amygdala in both control and nes-Ahi1−/− mice (Fig. S6). All these results combined support the idea that Ahi1 deficiency in mice can cause a depressive phenotype.
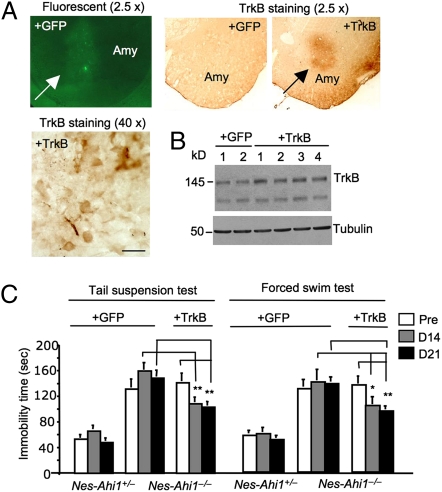
If the decreased TrkB in Ahi1-deficient mice is responsible for the depressive phenotype, overexpression of TrkB should ameliorate this phenotype. The amygdala is an important brain region to control emotion and is also important for forced swim and tail suspension behaviors in mice (32–34), providing a good target for us to examine whether overexpression of TrkB in this region can alter depressive phenotypes. We performed stereotaxic injection of lentiviral TrkB into the amygdala and verified the expression of control lentiviral GFP and TrkB in the mouse basolateral amygdala via fluorescent imaging or TrkB immunohistochemistry (Fig. 3A). Western blot analysis also showed the increased level of TrkB in the amygdala tissue extracts from lentiviral-TrkB-injected nes-Ahi1−/− mice compared with the lentiviral GFP-injected control mice (Fig. 3B). Nes-Ahi1+/− and nes-Ahi1−/− mice that were bilaterally injected with lentiviral GFP or TrkB into the amygdala were examined via TST and FST. Although lentiviral TrkB was expressed only in the amygdala, this overexpression could partially and significantly reduce the immobility of nes-Ahi1−/− mice in TST and FST (Fig. 3C). This finding strongly supports the idea that impaired TrkB signaling is involved in the depressive phenotype seen in nes-Ahi1−/− mice.
Fig. 3.
Overexpression of TrkB in the amygdala of Ahi1-deficient mice partially rescues their depressive phenotype. (A) Stereotaxic injection of lentiviral GFP or TrkB into the amygdala (Amy) of mice resulted in the expression of GFP or TrkB in the injected area, which was revealed by fluorescent or TrkB immunostained (2.5× and 40×) micrographs. The antibody to TrkB (80G2, Cell Signaling Technology) did not react with endogenous mouse TrkB but was able to recognize overexpressed rat TrkB via lentiviral vector expression. (B) Western blotting showing the increased level of TrkB in four different amygdala tissues that had been injected with lentiviral TrkB for 2 weeks compared with two lentiviral GFP-injected amygdala tissues. (C) Stereotaxic injection of lentiviral TrkB reduced the immobility of Ahi1-deficient (nes-Ahi1−/−) mice in TSTs and FSTs, compared with those injected with lentiviral GFP. *P < 0.05 or **P < 0.01. Each group contains 8–12 mice. Mice were examined before (pre) and at 14 (D14) or 21 (D21) d after the lentiviral injection.
Neuronal Ahi1 Deficiency Impairs TrkB Internalization and Promotes Its Degradation.
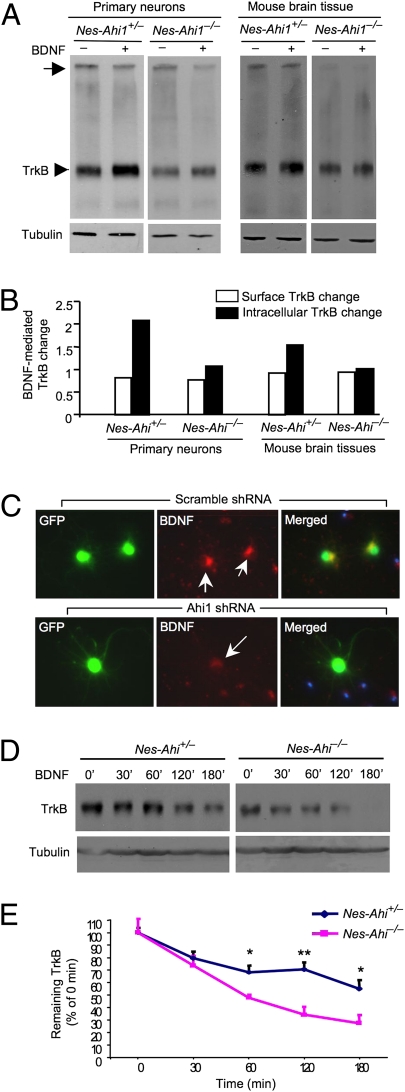
Although Hap1 participates in intracellular trafficking and endocytosis of membrane receptors (15, 22–24), it remains to be investigated whether the decreased TrkB level in the mouse brain with Ahi1 deficiency results from the impaired internalization and endocytic sorting of TrkB in neuronal cells. Internalization of TrkB in the brain is triggered by BDNF and can be assessed by examining brain slices by using bis(sulfosuccinimidyl) substrate (BS3), a reagent that selectively crosslinks surface-membrane-bound receptors to form high-molecular-weight aggregates (35). We then used BS3 to treat cultured brainstem cells, which express abundant Ahi1/Hap1 and can grow better in vitro than neurons from the hypothalamus or amygdala when Ahi1 is absent. To verify the results from cultured brainstem cells, we also used brainstem sections from nes-Ahi1−/− mice. TrkB Western blotting revealed a reduction of membrane-bound and intracellular TrkB receptors in cultured Ahi1-deficient cells or brainstem sections from Ahi1-deficiency mice (Fig. 4 A and B). To verify this finding, we cultured brainstem cells from wild-type mouse brains and treated them with adenoviral Ahi1 shRNA. Only Ahi1 shRNA, but not the scrambled control shRNA, could selectively reduce Ahi1 expression in cultured neurons (Fig. S7). We then examined the internalized TrkB–BDNF complex in cultured neurons by treating these cells with BDNF. Cultured neuronal cells showed positive intracellular BDNF, indicating that this assay does reflect neuronal internalization of TrkB–BDNF (Fig. 4C). Importantly, the level of internalized BDNF was reduced in Ahi1 shRNA-treated, but not in the scrambled control shRNA-treated, neurons (Fig. 4C).
Fig. 4.
Ahi1 deficiency reduces the internalized TrkB and facilitates TrkB degradation. (A) Western blotting and BS3 crosslinking of membrane-bound receptors showing a reduction in membrane TrkB (arrow) and intracellular TrkB (arrowhead) in cultured primary brainstem cells and brainstem sections from nes-Ahi1−/− mice. BDNF (100 ng/mL for 1 h) was used to trigger TrkB internalization. (B) Quantifying BDNF-mediated changes of TrkB relative to that before BDNF treatment. (C) Decreased internalization of BDNF in cultured mouse brainstem cells treated with adenoviral Ahi1 shRNA. Arrows indicate neurons that coexpress adenoviral Ahi1 shRNA and GFP. (D) Cultured brainstem cells from control (nes-Ahi1+/−) mice and Ahi1-deficient (nes-Ahi1−/−) mice were treated with BDNF to induce TrkB internalization. TrkB Western blotting was then performed to assess the levels of TrkB at different times. (E) Quantitative analysis of the degradation of TrkB in brainstem slices of nes-Ahi1+/− and nes-Ahi1−/− mice. The ratios of TrkB to tubulin were measured via densitometry. The relative TrkB expression level at different times is expressed as percentage of the TrkB/tubulin ratio at 0 min before BDNF-induced internalization. The data were obtained from three independent experiments. *P < 0.05.
Upon binding neurotrophins, Trk receptors are rapidly endocytosed to lysosomes for degradation, recycled back to the plasma membrane, or retrogradely transported. Because TrkB levels are lower in Ahi1-deficient cells and the reduced levels of internalized TrkB can be attributable to increased degradation, it is important to examine whether the degradation of TrkB is enhanced when Ahi1 is deficient. We isolated brainstem slices from the control and Ahi1-deficient mice and examined the half-life of TrkB after BDNF triggers TrkB internalization and recycling. The levels of TrkB were assessed by measuring the ratios of TrkB at different times to TrkB before BDNF treatment. The results showed that TrkB is more rapidly degraded in Ahi1-deficient neurons (Fig. 4 D and E).
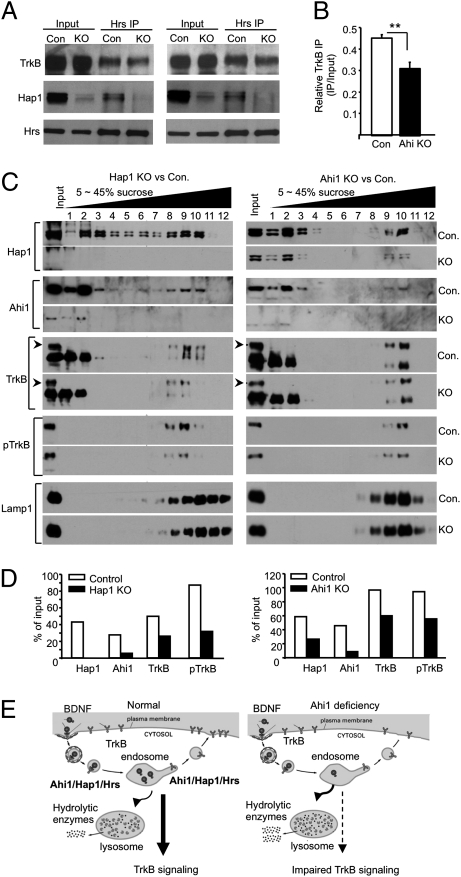
The increased degradation of TrkB in Ahi1-deficient mouse brain is consistent with our previous finding that lack of Hap1 decreases TrkB level in neuronal cells (11). However, the mechanism underlying this phenomenon remains elusive. The rapid degradation of TrkB could be caused by a fast degradation of TrkB by lysosomes. Hepatocyte growth factor-regulated tyrosine kinase substrate (Hrs) binds TrkB upon BDNF stimulation to regulate the sorting of TrkB to lysosomes, and overexpression of Hrs can reduce TrkB degradation (36, 37). Hrs also interacts with Hap1 to stabilize EGF receptor (20). Based on these previous findings, we examined the association of Hrs with TrkB in brain slices from nes-Ahi1−/− mice after BDNF stimulation. Hrs immunoprecipitation could coprecipitate Hap1 and TrkB. However, when Hap1 is reduced in nes-Ahi1−/− tissues, there is a significant reduction of TrkB coprecipitated with Hrs (Fig. 5A). Because TrkB level is lower in Ahi1-deficient samples, we also increased the input from Ahi1-deficient samples to have the equal amounts of TrkB from both control and Ahi1-deficient samples for Hrs immunoprecipitation. The result also shows a decreased precipitation of TrkB from Ahi1-deficient sample (Fig. 5A). The ratios of precipitated TrkB to input were then quantified, and the findings suggest that Ahi1/Hap1 enhance the association of TrkB with Hrs (Fig. 5B).
Fig. 5.
Ahi1 deficiency decreases the association of Hrs with TrkB and levels of TrkB in lysosomal fractions. (A) Hrs immunoprecipitation of brainstem tissues treated with BDNF (100 ng/mL for 30 min) and isolated from control (nes-Ahi1+/− or Con.) mice and Ahi1-deficient (nes-Ahi1−/− or KO) mice. Two immunoprecipitation results are presented with the one (Right) that used more input from nes-Ahi1−/− (KO) sample. Note that in the KO samples, there is reduced Hap1 in the input and precipitate samples, as well as decreased TrkB in the precipitate. (B) Quantification of the relative immunoprecipitated TrkB (ratios of precipitated to input TrkB) from three immunoprecipitation experiments. *P < 0.05. (C) Distribution of Hap1, Ahi1, TrkB, pTrkB, and lysosomal marker protein Lamp1 in 5–45% sucrose gradient fractions. Brain cortex tissues from Hap1 null (KO) and wild-type (Con.) mouse at P1–P2 (Left) or from (nes-Ahi1+/− or Con.) and Ahi1-deficient (nes-Ahi1−/− or KO) mice at 3 mo of age were analyzed. (D) Quantification of the amounts of proteins relative to input (% of input) in the lysosomal fractions (fraction-9 for Hap1 KO vs. control and fraction-10 for Ahi1 KO vs. control) isolated via sucrose gradient density centrifugation. (E) Proposed molecular mechanism for the depressive phenotype in Ahi1-deficient mice. BDNF triggers the internalization of TrkB receptor and signaling mediated by endocytic BDNF/TrkB. Ahi1/Hap1/Hrs complex is involved in intracellular trafficking of endocytic TrkB, prevents its degradation by lysosomes, and promotes its recycling to the plasma surface. When Ahi1 is reduced, more endocytic TrkB is transferred into lysosomes for degradation, resulting in the decreased level of TrkB and impaired TrkB signaling.
Sucrose gradient fractionation further shows a decrease in the amounts of TrkB and pTrkB in the fractions that are enriched in lysosomes and are from either nes-Ahi1−/− (Fig. 5 C Left and D Left) or Hap1 null (Fig. 5 C Right and D Right) mouse-brain cortex tissues, also suggesting a rapid degradation of TrkB by lysosomes when Ahi1 or Hap1 is reduced or absent. Because there is residual HAP1 due to incomplete elimination of Ahi1 in the nes-Ahi1−/− mouse brain, the levels of TrkB in the fractions enriched in lysosomes are decreased to a lesser extent in Ahi1-deficient brains than in Hap1 null brains. Thus, the association of TrkB with Hrs/Hap1/Ahi1 or their complex is likely to stabilize endocytic TrkB by preventing its rapid degradation by lysosomes. All these data suggest that Ahi1 deficiency affects the sorting and stability of endocytic TrkB, resulting in the faster degradation of TrkB and decreased surface and intracellular TrkB, which together disrupt intracellular TrkB signaling (Fig. 5E).
Discussion
Although genetic studies have demonstrated the association of the AHI1 gene with Joubert syndrome and other neuropsychiatric disorders, the function of AHI1 remains to be characterized. The loss of human AHI1 or mouse Ahi1 in distinct types of cells appears to affect different cellular signaling pathways or function (28, 38, 39,). How the loss or dysfunction of AHI1 in the brain impacts neuronal cells is still unknown, although we do know that the recessive mutations of the AHI1 gene lead to abnormal brain development in Joubert syndrome. Recent studies have shown that Ahi1 and Hap1 form a protein complex in the mouse brain (11, 12) and participate in intracellular trafficking (15, 23, 40). Our early study has revealed that lack of Hap1 decreases Ahi1 at the protein level (11). The findings of our current study show that Ahi1 deficiency also reduces the level of Hap1 in the mouse brain. Thus, both Ahi1 and Hap1 stabilize each other and are likely to be in a protein complex participating in intracellular trafficking. However, it remains unclear whether and how Ahi1 deficiency causes neuronal dysfunction and related neurological symptoms. By analyzing conditional Ahi1 knockout mice, in which Ahi1 expression is reduced in neurons via the Cre–loxP system, we demonstrate that neuronal Ahi1 deficiency can cause depressive phenotypes in mice.
Depression is a devastating mental illness with a lifetime prevalence of up to 20% and covers a wide range of pathologies caused by many different factors. We know that Ahi1 and Hap1 are abundant in the hypothalamus and amygdala; these brain regions are critical for stress response, and their dysfunction can lead to emotional and depressive phenotypes (13). In Joubert syndrome, however, the major pathological changes are the abnormal development of the cerebellar vermis and the brainstem. Because Joubert syndrome is a rare genetic disease that can cause young children to die and is caused by mutations in multiple genes, it remains to be investigated whether the loss of Ahi1 can mediate any psychiatric or emotional symptoms in this disease. In addition, Ahi1 deficiency in mice could lead to phenotypes that are different from those caused by the complete loss of AHI1 function in patients with Joubert syndrome.
Although the neurological phenotypes and pathology of conditional Ahi1 knockout mice may not be the same as those of mutant mice that lack Ahi1 or Hap1 completely in all types of cells via germ-line mutation, the significant reduction of Ahi1 and Hap1 in the brains of our mosaic Ahi1 mutant mice nevertheless allowed us to uncover depressive phenotypes that are linked to decreased TrkB, a membrane receptor for the neurotrophic factor BDNF. BDNF/TrkB signaling impairment is associated with depression in schizophrenia and other neuropsychiatric disorders (25, 26, 41). The rescuing effects of overexpression of TrkB in the amygdala and of antidepressant drugs that can increase TrkB phosphorylation and signaling (30, 31) further suggest that BDNF/TrkB signaling impairment can contribute to depressive phenotypes in Ahi1-deficient mice.
Our findings provide evidence for the involvement of Ahi1 deficiency in depression, which occurs in schizophrenia and autism, two important psychiatric diseases that have been found to associate with the AHI1 locus (4–10). These studies will promote further research into the involvement of AHI1 in neuropsychiatric disorders that include a depression phenotype. It is known that depressive phenotypes can be caused by multiple genetic and environment factors. For example, MK801, a noncompetitive NMDA receptor antagonist, can induce schizophrenia-like psychosis via insufficient glutamate neurotransmission (42). Because Ahi1 is involved in the endocytosis and trafficking of TrkB, our finding suggests that impaired endocytic TrkB trafficking and sorting in Ahi1-deficient mice can also cause depressive phenotypes, providing new therapeutic targets for depression. Because the successful development of mouse models with depression phenotypes that are responsive to treatment is still in its infancy (43), Ahi1-deficient mice will also provide us with a genetic mouse model to facilitate studies into the pathogenesis of depression and spur the search for new antidepressant drugs.
Materials and Methods
Animals.
All animal procedures were approved by the Institutional Animal Care and Use Committee of Emory University. The mouse Ahi1 genomic DNA (12.6 kb) was obtained from a C57BL/6 BAC clone (RPC123). The targeting vector was then generated by flanking exon 2 of the mouse Ahi1 gene with two loxP sites to allow Cre-mediated deletion of exon 2. The phosphoglycerate kinase-neomycin resistance cassette (neo) was inserted together with the first loxP sequence in the 3′ flanking intron of exon 2. All loxP sequences were in the same orientation to allow Cre-mediated simultaneous excision of exon 2 and the neo cassette. The targeting vector was linearized before electroporation into B6/129-derived hybrid ES cells. Positive ES cells containing targeted vectors were identified by genomic DNA PCR and Southern blotting and then injected into C57B1/6J blastocysts to generate chimeric mice by inGenious Targeting Laboratory. Chimeric males were crossed with wild-type mice (C57BL/6) to generate heterozygous floxed Ahi1 (Ahi1+/loxp) mice, and homozygous mice (Ahi1loxp/loxp) were produced by mating of the heterozygous mice. Mice homozygous for the floxed Ahi1 allele were crossed with mice carrying a Nestin promoter-driven Cre transgene (Jackson Laboratory, B6.Cg (SJL)-TgN(NesCre)1Kln), which expresses Cre primarily in the central and peripheral nervous system under the control of the rat nestin promoter and enhancer (27). The resulting heterozygous mice were used to generate homozygous conditional knockout (nesCre+/Ahi1loxp/loxp or nes-Ahi1−/−) or heterozygous (nesCre+/Ahi1+/loxp or nes-Ahi1+/−) mice. Expression analysis of Ahi1 and behavioral tests of heterozygous mice (nesCre+/Ahi1+/loxp) and wild-type mice revealed no differences. Because homozygous (nes-Ahi1−/−) and heterozygous (nes-Ahi1+/−) mice share the same mixed genetic background, mice of these two genotypes were mainly used to reveal differences related to Ahi1 deficiency.
Drug Treatment.
Imipramine (30 mg/kg, Sigma) and fluoxetine (20 mg/kg, Sigma) were freshly prepared before use and injected i.p. 30 min before the behavioral tests (30, 31). Both drugs were dissolved in saline.
Stereotaxic Injection of Lentivirus.
Lentiviral vectors for expressing GFP and full-length rat TrkB under the control of the CMV promoter were described previously (44). Lentiviruses were produced at The Viral Vector Core at Emory University at the titer of 1 × 109 infectious particles per milliliter. Stereotaxic injection of lentiviral vectors into the amygdala was performed by using similar methods to those described in our previous studies (44, 45). In brief, mice were anesthetized by injections of Avertin (0.5 mg/g i.p.), then mounted in a stereotaxic apparatus. Mice received bilateral amygdala microinjections of either the control lentiviral GFP or lentiviral TrkB. Small holes were drilled in the skull above the injection site, and a 30-gauge Hamilton microsyringe was lowered to the following coordinates from bregma: anterior–posterior = –1.4, medial–lateral = –3.3, and dorsal–ventral = –5.0. The microsyringe was left in place 10 min before and after each injection, and a total volume of 1.0 μl of lentivirus was administered at a rate of 0.05 μl/min at each site. At 2 or 3 wk after the injection, the amygdala was isolated for examining the expression of GFP or TrkB via immunostaining or Western blotting. Mice with or without injection of lentiviral GFP or TrkB were also examined for their performance in TSTs and FSTs.
Statistical Analysis.
All data were expressed as mean ± SEM. Statistical results were analyzed by ANOVA followed when appropriate by a post hoc analysis with Dunnett's test, two-way ANOVA, paired t tests (within-group comparisons), or t tests for independent samples (between-group comparisons). For all comparisons, the criterion for significance was P < 0.05.
Additional materials and methods (antibodies, Western blotting, fluorescent immunostaining, immunoprecipitation, receptor internalization, sucrose gradient fractionation, and mouse behavioral tests) are described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Jason Schroeder (Emory Rodent Behavioral Core) for assisting with the mouse behavioral analysis, Dr. Xinping Huang (The Viral Vector Core at Emory University) (Grant P30NS055077-03 to K.J.R.) for preparation of lentiviruses, and Cheryl Strauss for critical reading of the manuscript. This work was supported by National Institutes of Health Grants NS036232 and NS041669 (to X.-J.L.) and NS045016 (to S.L.) and National Natural Science Foundation of China Grants 30700245 and 81051836 (to X.X.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1013032107/-/DCSupplemental.
References
- 1.Poirier Y, Jolicoeur P. Distinct helper virus requirements for Abelson murine leukemia virus-induced pre-B- and T-cell lymphomas. J Virol. 1989;63:2088–2098. doi: 10.1128/jvi.63.5.2088-2098.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferland RJ, et al. Abnormal cerebellar development and axonal decussation due to mutations in AHI1 in Joubert syndrome. Nat Genet. 2004;36:1008–1013. doi: 10.1038/ng1419. [DOI] [PubMed] [Google Scholar]
- 3.Dixon-Salazar T, et al. Mutations in the AHI1 gene, encoding jouberin, cause Joubert syndrome with cortical polymicrogyria. Am J Hum Genet. 2004;75:979–987. doi: 10.1086/425985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levi A, et al. Fine mapping of a schizophrenia susceptibility locus at chromosome 6q23: Increased evidence for linkage and reduced linkage interval. Eur J Hum Genet. 2005;13:763–771. doi: 10.1038/sj.ejhg.5201406. [DOI] [PubMed] [Google Scholar]
- 5.Torri F, et al. Fine mapping of AHI1 as a schizophrenia susceptibility gene: From association to evolutionary evidence. FASEB J. 2010;24:3066–3082. doi: 10.1096/fj.09-152611. [DOI] [PubMed] [Google Scholar]
- 6.Amann-Zalcenstein D, et al. AHI1, a pivotal neurodevelopmental gene, and C6orf217 are associated with susceptibility to schizophrenia. Eur J Hum Genet. 2006;14:1111–1119. doi: 10.1038/sj.ejhg.5201675. [DOI] [PubMed] [Google Scholar]
- 7.Ingason A, et al. Support for involvement of the AHI1 locus in schizophrenia. Eur J Hum Genet. 2007;15:988–991. doi: 10.1038/sj.ejhg.5201848. [DOI] [PubMed] [Google Scholar]
- 8.Ingason A, et al. GROUP Investigators A large replication study and meta-analysis in European samples provides further support for association of AHI1 markers with schizophrenia. Hum Mol Genet. 2010;19:1379–1386. doi: 10.1093/hmg/ddq009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alvarez Retuerto AI, et al. Association of common variants in the Joubert syndrome gene (AHI1) with autism. Hum Mol Genet. 2008;17:3887–3896. doi: 10.1093/hmg/ddn291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holroyd S, Reiss AL, Bryan RN. Autistic features in Joubert syndrome: A genetic disorder with agenesis of the cerebellar vermis. Biol Psychiatry. 1991;29:287–294. doi: 10.1016/0006-3223(91)91291-x. [DOI] [PubMed] [Google Scholar]
- 11.Sheng G, et al. Huntingtin-associated protein 1 interacts with Ahi1 to regulate cerebellar and brainstem development in mice. J Clin Invest. 2008;118:2785–2795. doi: 10.1172/JCI35339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doering JE, et al. Species differences in the expression of Ahi1, a protein implicated in the neurodevelopmental disorder Joubert syndrome, with preferential accumulation to stigmoid bodies. J Comp Neurol. 2008;511:238–256. doi: 10.1002/cne.21824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.David DJ, et al. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron. 2009;62:479–493. doi: 10.1016/j.neuron.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang X, Hanna Z, Kaouass M, Girard L, Jolicoeur P. Ahi-1, a novel gene encoding a modular protein with WD40-repeat and SH3 domains, is targeted by the Ahi-1 and Mis-2 provirus integrations. J Virol. 2002;76:9046–9059. doi: 10.1128/JVI.76.18.9046-9059.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gauthier LR, et al. Huntingtin controls neurotrophic support and survival of neurons by enhancing BDNF vesicular transport along microtubules. Cell. 2004;118:127–138. doi: 10.1016/j.cell.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 16.Li XJ, Li SH. HAP1 and intracellular trafficking. Trends Pharmacol Sci. 2005;26:1–3. doi: 10.1016/j.tips.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Engelender S, et al. Huntingtin-associated protein 1 (HAP1) interacts with the p150Glued subunit of dynactin. Hum Mol Genet. 1997;6:2205–2212. doi: 10.1093/hmg/6.13.2205. [DOI] [PubMed] [Google Scholar]
- 18.Li SH, Gutekunst CA, Hersch SM, Li XJ. Interaction of huntingtin-associated protein with dynactin P150Glued. J Neurosci. 1998;18:1261–1269. doi: 10.1523/JNEUROSCI.18-04-01261.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGuire JR, Rong J, Li SH, Li XJ. Interaction of Huntingtin-associated protein-1 with kinesin light chain: Implications in intracellular trafficking in neurons. J Biol Chem. 2006;281:3552–3559. doi: 10.1074/jbc.M509806200. [DOI] [PubMed] [Google Scholar]
- 20.Li Y, Chin LS, Levey AI, Li L. Huntingtin-associated protein 1 interacts with hepatocyte growth factor-regulated tyrosine kinase substrate and functions in endosomal trafficking. J Biol Chem. 2002;277:28212–28221. doi: 10.1074/jbc.M111612200. [DOI] [PubMed] [Google Scholar]
- 21.Tang TS, et al. Huntingtin and huntingtin-associated protein 1 influence neuronal calcium signaling mediated by inositol-(1,4,5) triphosphate receptor type 1. Neuron. 2003;39:227–239. doi: 10.1016/s0896-6273(03)00366-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kittler JT, et al. Huntingtin-associated protein 1 regulates inhibitory synaptic transmission by modulating gamma-aminobutyric acid type A receptor membrane trafficking. Proc Natl Acad Sci USA. 2004;101:12736–12741. doi: 10.1073/pnas.0401860101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rong J, et al. Regulation of intracellular trafficking of huntingtin-associated protein-1 is critical for TrkA protein levels and neurite outgrowth. J Neurosci. 2006;26:6019–6030. doi: 10.1523/JNEUROSCI.1251-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Twelvetrees AE, et al. Delivery of GABAARs to synapses is mediated by HAP1-KIF5 and disrupted by mutant huntingtin. Neuron. 2010;65:53–65. doi: 10.1016/j.neuron.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Angelucci F, Brenè S, Mathé AA. BDNF in schizophrenia, depression and corresponding animal models. Mol Psychiatry. 2005;10:345–352. doi: 10.1038/sj.mp.4001637. [DOI] [PubMed] [Google Scholar]
- 26.Martinowich K, Manji H, Lu B. New insights into BDNF function in depression and anxiety. Nat Neurosci. 2007;10:1089–1093. doi: 10.1038/nn1971. [DOI] [PubMed] [Google Scholar]
- 27.Lakso M, et al. Efficient in vivo manipulation of mouse genomic sequences at the zygote stage. Proc Natl Acad Sci USA. 1996;93:5860–5865. doi: 10.1073/pnas.93.12.5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lancaster MA, et al. Impaired Wnt-beta-catenin signaling disrupts adult renal homeostasis and leads to cystic kidney ciliopathy. Nat Med. 2009;15:1046–1054. doi: 10.1038/nm.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li SH, et al. Lack of huntingtin-associated protein-1 causes neuronal death resembling hypothalamic degeneration in Huntington's disease. J Neurosci. 2003;23:6956–6964. doi: 10.1523/JNEUROSCI.23-17-06956.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saarelainen T, et al. Activation of the TrkB neurotrophin receptor is induced by antidepressant drugs and is required for antidepressant-induced behavioral effects. J Neurosci. 2003;23:349–357. doi: 10.1523/JNEUROSCI.23-01-00349.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rantamäki T, et al. Pharmacologically diverse antidepressants rapidly activate brain-derived neurotrophic factor receptor TrkB and induce phospholipase-Cgamma signaling pathways in mouse brain. Neuropsychopharmacology. 2007;32:2152–2162. doi: 10.1038/sj.npp.1301345. [DOI] [PubMed] [Google Scholar]
- 32.Bahi A, Mineur YS, Picciotto MR. Blockade of protein phosphatase 2B activity in the amygdala increases anxiety- and depression-like behaviors in mice. Biol Psychiatry. 2009;66:1139–1146. doi: 10.1016/j.biopsych.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Coryell MW, et al. Acid-sensing ion channel-1a in the amygdala, a novel therapeutic target in depression-related behavior. J Neurosci. 2009;29:5381–5388. doi: 10.1523/JNEUROSCI.0360-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Han F, et al. Improvement of depressive behaviors by nefiracetam is associated with activation of CaM kinases in olfactory bulbectomized mice. Brain Res. 2009;1265:205–214. doi: 10.1016/j.brainres.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 35.Mao LM, et al. Stability of surface NMDA receptors controls synaptic and behavioral adaptations to amphetamine. Nat Neurosci. 2009;12:602–610. doi: 10.1038/nn.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spellman DS, Deinhardt K, Darie CC, Chao MV, Neubert TA. Stable isotopic labeling by amino acids in cultured primary neurons: application to brain-derived neurotrophic factor-dependent phosphotyrosine-associated signaling. Mol Cell Proteomics. 2008;7:1067–1076. doi: 10.1074/mcp.M700387-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang SH, et al. Essential role of Hrs in endocytic recycling of full-length TrkB receptor but not its isoform TrkB.T1. J Biol Chem. 2009;284:15126–15136. doi: 10.1074/jbc.M809763200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Louie CM, et al. AHI1 is required for photoreceptor outer segment development and is a modifier for retinal degeneration in nephronophthisis. Nat Genet. 2010;42:175–180. doi: 10.1038/ng.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Westfall JE, et al. Retinal degeneration and failure of photoreceptor outer segment formation in mice with targeted deletion of the Joubert syndrome gene, Ahi1. J Neurosci. 2010;30:8759–8768. doi: 10.1523/JNEUROSCI.5229-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hsiao YC, et al. Ahi1, whose human ortholog is mutated in Joubert syndrome, is required for Rab8a localization, ciliogenesis and vesicle trafficking. Hum Mol Genet. 2009;18:3926–3941. doi: 10.1093/hmg/ddp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rantamäki T, Castrén E. Targeting TrkB neurotrophin receptor to treat depression. Expert Opin Ther Targets. 2008;12:705–715. doi: 10.1517/14728222.12.6.705. [DOI] [PubMed] [Google Scholar]
- 42.Errico F, et al. D-aspartate prevents corticostriatal long-term depression and attenuates schizophrenia-like symptoms induced by amphetamine and MK-801. J Neurosci. 2008;28:10404–10414. doi: 10.1523/JNEUROSCI.1618-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.El Yacoubi M, Vaugeois JM. Genetic rodent models of depression. Curr Opin Pharmacol. 2007;7:3–7. doi: 10.1016/j.coph.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 44.Rattiner LM, Davis M, French CT, Ressler KJ. Brain-derived neurotrophic factor and tyrosine kinase receptor B involvement in amygdala-dependent fear conditioning. J Neurosci. 2004;24:4796–4806. doi: 10.1523/JNEUROSCI.5654-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sheng G, et al. Hypothalamic huntingtin-associated protein 1 as a mediator of feeding behavior. Nat Med. 2006;12:526–533. doi: 10.1038/nm1382. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.