Abstract
Seed dormancy is an ecologically important adaptive trait in plants whereby germination is repressed even under favorable germination conditions such as imbibition with water. In Arabidopsis and most plant species, dormancy absolutely requires an unidentified seed coat germination-repressive activity and constitutively higher abscisic acid (ABA) levels upon seed imbibition. The mechanisms underlying these processes and their possible relationship are incompletely understood. We developed a “seed coat bedding” assay monitoring the growth of dissected embryos cultured on a layer of seed coats, allowing combinatorial experiments using dormant, nondormant, and various genetically modified seed coat and embryonic materials. This assay, combined with direct ABA measurements, revealed that, upon imbibition, dormant coats, unlike nondormant coats, actively produce and release ABA to repress embryo germination, whatever the embryo origin, i.e., from dormant, nondormant, or never dormant aba seeds, unable to synthesize ABA. The persistent high ABA levels in imbibed dormant seeds requires the permanent expression of the DELLA gene RGL2, where it remains insensitive to gibberellins (GA) unlike in nondormant seeds. These findings present the seed coat as an organ actively controlling germination upon seed imbibition and provide a framework to investigate how environmental factors break seed dormancy.
Keywords: gibberellins, seed dormancy, DELLA, seed germination, ABI5
In plants, mature dry seeds are the endpoint of embryogenesis and consist of a seed coat surrounding a desiccated and highly resistant embryo. Upon termination of embryogenesis, many seeds are dormant; i.e., they will not germinate even under favorable germination conditions (1, 2). Seed dormancy can be broken after a period of seed after-ripening or upon seed stratification (i.e., exposure to cold and moist conditions) (3, 4).
Dormancy is a trait of considerable agronomical and ecological importance, enhancing plant fitness by keeping the embryo protected and avoiding germination out of season. Previous work in Arabidopsis has established that repression of germination upon dormant seed imbibition is an active process requiring de novo and constitutive production of the phytohormone abscisic acid (ABA) upon seed imbibition (5–7). Thus, treating imbibed dormant WT seeds with Norflurazon, an inhibitor of ABA synthesis, will trigger their germination (6). The importance of the role of de novo ABA synthesis to repress germination is further supported by the observation that dormant and nondormant dry seeds contain similarly high amounts of ABA, which played an essential and well-established developmental role during seed maturation (5) (see also below). However, upon seed imbibition, the high ABA levels present in both dormant and nondormant seeds drop drastically by about 10-fold within the first 12 h (5, 8) (see below). What indeed distinguishes dormant seeds from nondormant seeds in what concerns their ABA content is the capacity of dormant seeds to synthesize de novo and constitutively ABA once the initial drop of ABA levels has occurred upon seed imbibition. How dormant seeds achieve this characteristic de novo constitutive ABA production upon imbibition is unknown.
Furthermore, in numerous agronomically relevant species and in the model plant Arabidopsis, researchers have also established that the seed coat is also essential to maintain the active repression of seed germination that is occurring in imbibed dormant seeds. This conclusion is based on the observation that seed coat removal triggers plant embryo growth and greening (i.e., germination) (3, 9, 10).
In Arabidopsis, the seed coat consists of an outer layer of dead tissue, the testa, and a single cell layer of endosperm live tissue surrounding the embryo (9). Dissection experiments removing the external testa layer while leaving the endosperm layer have shown that the endosperm provides most if not all of the germination repressive activity (11). How the endosperm prevents seed germination and whether there is a relationship between the processes of coat-imposed seed dormancy and de novo ABA production in imbibed dormant seeds is not clearly understood.
Previous reports using different plant species (but not Arabidopsis) have shown that seed structures surrounding the embryo contain compounds possessing germination-inhibitory activities that include ABA (12). Evidence suggests that the Arabidopsis endosperm in the seed coat can biosynthesize ABA. Indeed, gene expression studies in isolated Arabidopsis seed coat tissue have shown that the endosperm expresses genes involved in ABA metabolism (13). Furthermore, a previous report has provided indirect genetic evidence that the endosperm may participate to produce a substantial proportion of the final pool of ABA present in dry seeds (14). Finally, Barrero et al. recently demonstrated that the Arabidopsis seed coat contains ABA (15). On the other hand, Bassel et al. proposed a model where gibberellins (GA) or a GA-derived signal passing from the endosperm to the embryo may maintain dormancy by promoting the expression of ABA-dependent germination repressors in the embryo (16). However, Bassel et al. could not rule out that an unidentified factor X is released by the endosperm to promote dormancy by inhibiting GA synthesis and/or signaling in the embryo (16).
Thus, it remains unknown whether the germination-inhibitory compounds present in seed coats, including ABA, directly participate in the germination-repressive activity provided by the seed coat in imbibed dormant seeds. Furthermore, it is also unknown whether the seed coats of dormant seeds actively synthesize germination inhibitory products such as ABA or assist somehow the de novo ABA synthesis that prevents germination upon dormant seed imbibition. Hence, it remains to be established what are the mechanisms controlling in imbibed dormant seeds the two key properties of de novo ABA synthesis and of the germination-repressive activity of the seed coat as well as whether they are related.
When seeds are nondormant, environmental factors control germination upon seed imbibition by determining the relative levels of the phytohormones ABA and GA (17, 18). Concerning ABA, unfavorable germination conditions, such as an osmotic stress or unfavorable light (e.g., light enriched in far-red, as under the canopy) lead to de novo endogenous ABA synthesis upon seed imbibition (19). In turn, ABA represses germination by stimulating de novo and upon imbibition the expression of specific germination repressors such as the ABA-insensitive 3 and -5 (ABI3 and ABI5) genes (20, 21), encoding transcription factors. Thus, abi mutant seeds are able to germinate under unfavorable germination conditions or in presence of exogenous ABA (22, 23).
Concerning GA, nondormant seeds are able to germinate because they synthesize GA upon imbibition (17, 18). In turn, GA synthesis ensures that RGL2 accumulation is restricted to the first 24 h upon seed imbibition by enhancing RGL2 proteasomal destruction after binding to a GA receptor (GID1), which enhances RGL2 protein interaction with the F-box protein SLY1 (24–26). If GA synthesis is prevented upon seed imbibition, as in imbibed ga1 seeds, RGL2 protein accumulates persistently in imbibed seeds and promotes persistent high endogenous ABA accumulation after the initial drop in dry seed ABA levels has taken place upon imbibition (8, 27). In turn, high ABA levels in imbibed ga1 seeds repress germination by stimulating de novo the expression of ABI3 and ABI5 (8, 20, 21, 27).
In this present work, the two key properties of dormant seeds, namely the germination-restrictive activity provided by the seed coat and their ability to accumulate high ABA levels after their initial drop upon imbibition, were further explored. An assay was devised where the seed coat is physically separated (i.e., dissected) from the embryo so that embryos are laid on a bed of seed coats. Strikingly, this assay maintains the germination-repressive activity of the seed coat on the embryo. The “seed coat bedding” assay allows exploring the function of the seed coat by performing combinatorial experiments using dormant, nondormant, and various genetically modified seed coat and embryonic materials. The analysis using this method suggested that the endosperm of dormant seeds releases ABA in an RGL2-dependent manner to prevent the germination of the embryo, thus sustaining its dormancy. We show that dormant seeds sustain high ABA accumulation because they specifically fail to down-regulate RGL2 expression in response to GA upon imbibition, unlike nondormant seeds.
Results
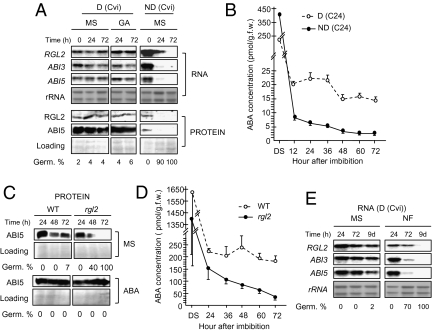
Dormant WT seeds of the highly dormant Cvi and C24 ecotypes persistently maintained RGL2 mRNA and protein upon seed imbibition (Fig. 1A and Fig. S1A). Similarly, dormant Ler seeds persistently accumulated RGL2 protein upon imbibition (Fig. S1B). In contrast, upon imbibition, nondormant WT Cvi, C24, or Ler seeds expressed RGL2 only transiently and germinated (Fig. 1A and Fig. S1 A and B). Dormant seeds also maintained high ABI3 and ABI5 expression relative to nondormant seeds, consistent with their higher endogenous ABA levels (measured for C24) and their failure to germinate (Fig. 1 A and B and Fig. S1A).
Fig. 1.
RGL2 is constitutively expressed in dormant seeds and is necessary to maintain high ABA levels. (A) Time course of RGL2, ABI3, and ABI5 expression in dormant (D) and stratified, i.e., nondormant (ND), Cvi seeds in absence (MS) or presence of 100 μM GA (GA). (B) Time course of endogenous ABA levels in freshly harvested WT (C24) D and ND seeds upon imbibition in MS medium. Error bars indicate SD (n = 3). (C) Time course of ABI5 protein levels in freshly harvested WT (Ler), i.e., dormant and rgl2-1 (rgl2) mutant seeds imbibed in absence (MS) or presence of 5 μM ABA (ABA). (D) Time course of endogenous ABA levels in freshly harvested WT (Ler) and rgl2-1 (rgl2) mutant seeds upon imbibition in MS medium. Error bars indicate SD (n = 3). (E) Same as A but seeds were harvested in absence (MS) or presence of Norflurazon (NF). Germination rates (Germ. %) at each time point are indicated in A, C, and E.
We next investigated the capacity of rgl2 mutants to sustain de novo ABA synthesis. rgl2 mutants lacked dormancy as they germinated precociously, consistent with previous reports (28–30) (Fig. S2). Furthermore, when compared with freshly harvested (i.e., dormant) WT (Ler) seeds, freshly harvested rgl2-1 (Ler) mutant seeds failed to sustain high ABA levels and high ABI5 expression upon seed imbibition (Fig. 1 C and D). Exposure to ABA blocked rgl2-1 germination and indeed restored high ABI5 expression (Fig. 1C).
Among the five DELLA factors present in Arabidopsis, RGL2 plays a unique and central role among DELLAs to promote ABA synthesis in seeds. Indeed, ABA strongly stimulates RGL2 mRNA levels, but not those of the other DELLA genes, in a positive feedback loop because ABA promotes the expression of RGL2, which enhances ABA synthesis (8, 31). This ensures predominant RGL2 protein accumulation relative to other DELLAs when GA levels are low (8, 27). Consistent with the notion of a positive feedback loop sustaining RGL2 and ABI expression, treating dormant seeds with Norflurazon, an inhibitor of ABA synthesis, down-regulated RGL2, ABI3, and ABI5 expression and triggered germination (Fig. 1E and Fig. S1C).
Taken together, these observations strongly suggest a model describing how RGL2 promotes dormancy whereby RGL2 is necessary to produce de novo ABA in dormant seeds upon imbibition. In turn, ABA is necessary to sustain RGL2, ABI3, and ABI5 expression over time by virtue of the positive feedback loop described above.
Role of the Seed Coat.
Previous reports showed that the endosperm expresses RGL2 as well as ABA biosynthetic genes (8, 13, 14). Furthermore, seed coats isolated from dormant Ler seeds isolated 72 h after imbibition accumulated RGL2 protein (Fig. S3A). This led us to hypothesize that the seed coat releases ABA to repress embryo germination and therefore promote dormancy.
Consistent with this hypothesis, seed coat removal of dormant seeds triggered germination and led to ABI5 protein disappearance in coatless embryos left for 96 h in the imbibition medium but not when ABA was present in the medium (Fig. S4A). In contrast, embryos dissected at various times from imbibed dormant seeds retained high ABI5 protein accumulation over time (Fig. S4B).
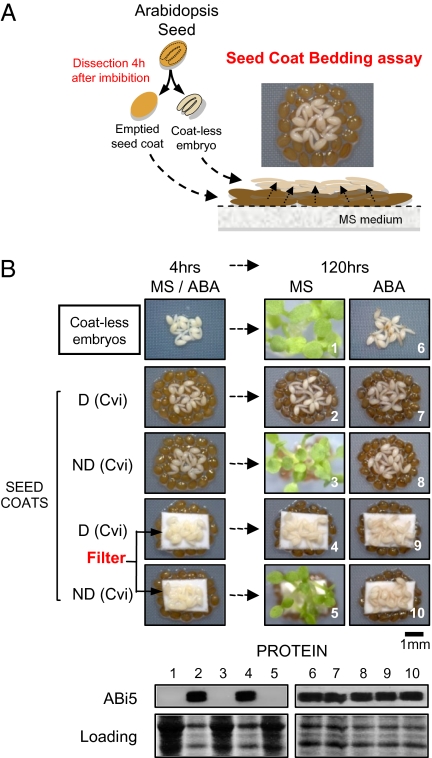
To test this hypothesis more directly, we devised a “seed coat bedding” assay physically separating seed coats from embryos to study how the seed coat controls embryo germination (Fig. 2A). A bed of seed coats dissected from dormant Cvi seeds (thereafter “dormant seed coats”) repressed the germination of coatless embryos dissected from dormant Cvi seeds and stimulated their ABI5 protein levels (Fig. 2B). Similar results were obtained when a nylon filter further separated the dormant seed coats from embryos while keeping embryos wet, thus maintaining the exchange of diffusible molecules (Fig. 2B). In contrast, seed coats dissected from nondormant Cvi seeds did not repress the germination of embryos dissected from dormant Cvi seeds nor stimulated their ABI5 protein levels (Fig. 2B). Presence of ABA in the imbibition medium blocked germination and restored high ABI5 levels, confirming that these two features of dormancy are ABA mediated (Fig. 2B).
Fig. 2.
Dormant seed coats produce diffusible signals with germination-repressive activities. (A) Diagram describing the procedure of a seed coat bedding assay. Embryos and seed coats are dissected 4 h after seed imbibition and coatless embryos are laid on a layer of dissected seed coats. (B) Seed coat bedding assays using seed coats dissected from dormant (D) or nondormant (ND) Cvi seeds. Pictures show embryos upon their dissection from dormant seeds (4 h) and 120 h thereafter in absence (MS) or presence of 0.5 μM ABA (ABA). Seed coats and coatless embryos were further physically separated by a 0.45-μm pore-size nylon transfer membrane (filter). Proteins were extracted from the embryo material shown.
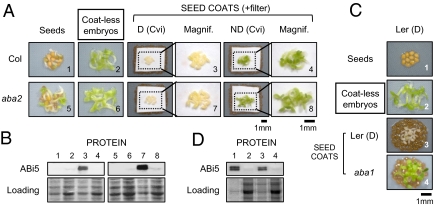
In addition, dormant Cvi seed coats, unlike nondormant Cvi seed coats, were sufficient to block the germination of embryos dissected from nondormant Col seeds, stimulating their ABI5 protein accumulation (Fig. 3 A and B). Furthermore, dormant Cvi seed coats, unlike nondormant Cvi seed coats, blocked the germination of embryos dissected from nondormant seeds carrying a β-glucuronidase blue marker transgene fused to an ABA-responsive promoter (pRD29B-GUS) (32) (Fig. S5). Absence of germination was associated with strong blue marker staining in cotyledons (Fig. S5). Dormant Cvi seed coats, unlike nondormant Cvi seed coats, could also block the germination of aba2 embryos dissected from aba2 seeds, unable to synthesize ABA and therefore never dormant, and stimulated their ABI5 protein levels (Fig. 3 A and B). On the other hand, aba1 seed coats, unlike Ler dormant seed coats, could not block the germination of embryos dissected from dormant WT (Ler) seeds nor stimulate their ABI5 protein levels (Fig. 3 C and D). Furthermore, extracts obtained from Cvi dormant seed coats isolated 24 h and 48 h after seed imbibition markedly delayed the germination of Col embryos and stimulated their ABI5 proteins levels, unlike extracts obtained from nondormant seed coats (Fig. S6A). Taken together, these observations show that ABA coming from dormant seed coats stimulated diffusely ABA-dependent genes in the overlying embryos and repressed their germination. We observed that germination arrest did not take place when embryos dissected from nondormant Col seeds were laid on a layer of intact dormant Cvi seeds (Fig. S6B). Thus, it appears that ABA released by the seed coat is retained within the intact dormant seed (Discussion).
Fig. 3.
Seed coat-dependent repression of germination involves ABA production in endosperm cells. (A) Seed coat bedding assays using embryos dissected from nondormant WT (Col) and aba2-1 (aba2) seeds and seed coats dissected from dormant (D) or nondormant (ND) Cvi seeds. Coats are hidden by the filter in magnified pictures. Pictures were taken 57 h after imbibition. (B) Proteins were extracted from the embryo material shown in A. (C) Seed coat bedding assays using embryos dissected from freshly harvested, i.e., dormant (D), Ler seeds laid on a layer of seed coats dissected from freshly harvested Ler seeds [Ler (D)], or aba1-1 (aba1) mutant seeds. Pictures were taken 86 h after imbibition. (D) Proteins were extracted from the embryo material shown in C.
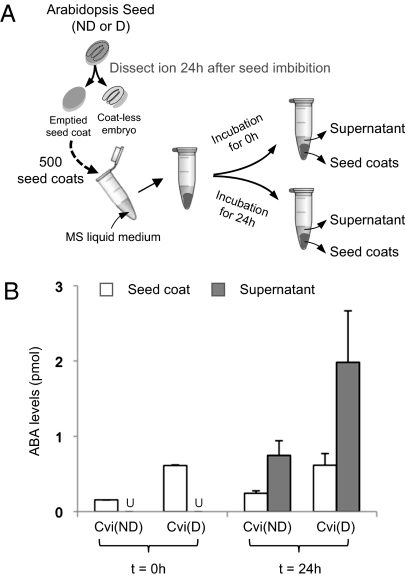
To directly detect ABA released from seed coats, dormant and nondormant Cvi seed coats dissected 24 h (t = 0 h) upon imbibition (i.e., a time when the large drop of ABA levels present in dry seeds has occurred) were incubated in MS medium for 24 h (t = 24 h) (Fig. 4A). ABA levels remained constant in both dormant and nondormant seed coat material for each time point (t < 0.05; Fig. 4B). However, dormant seed coat material contained three- to fourfold higher ABA levels than nondormant seed coat material (t < 0.05; Fig. 4B). ABA levels 2.6-fold higher were found in the incubation MS medium containing dormant seed coats relative to that containing nondormant seed coats after 24 h of incubation (t < 0.05; Fig. 4B). More absolute ABA levels were found in the incubation medium than in the seed coat material itself, consistent with the notion that ABA is produced de novo in the endosperm of dormant seeds and released toward the embryo to repress germination (t < 0.05; Fig. 4B).
Fig. 4.
Dissected dormant seed coats release higher ABA levels than nondormant seed coats. (A) Diagram describing the procedure to prepare samples for ABA levels measurement. A total of 500 seed coats from dormant (D) and nondormant (ND) Cvi seeds were dissected 24 h after imbibition (t = 0 h). Seed coats were then incubated in MS liquid medium for 24 h (t = 24 h) under continuous light before ABA measurements. (B) Histograms show ABA levels (pmol) present in dissected seed coat material and seed coat incubation liquid medium at t = 0 h and t = 24 h of incubation. U, ABA is undetected.
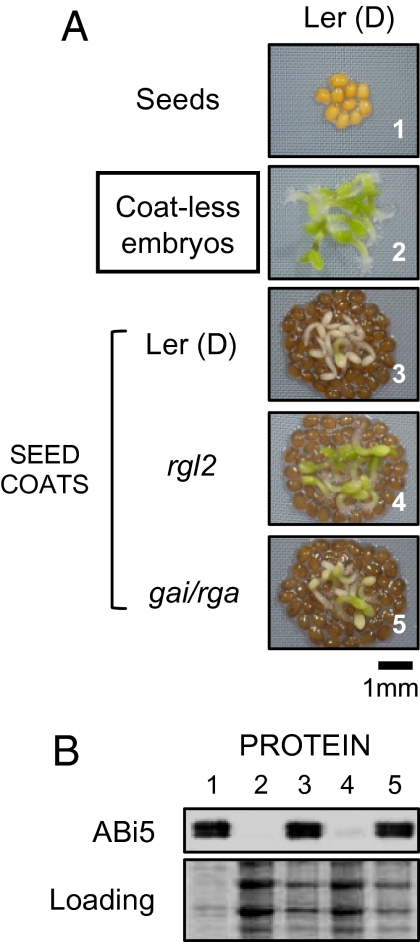
Finally and consistent with our findings described above showing that RGL2 promotes ABA synthesis, we found that rgl2 mutant seed coats, unlike dormant seed coats (Ler ecotype), were unable to arrest the germination of dormant embryos (Ler) and to stimulate ABI5 protein levels (Fig. 5 A and B). As expected, dormant Ler seed coats used in the seed coat bedding assay accumulated RGL2 protein when analyzed 72 h after imbibition (Fig. S3B). We wished to test whether other DELLA factors are active in the seed coat to repress germination. The DELLA factors GAI and RGA are known to promote dormancy and to promote ABA accumulation in seeds under far-red light illumination (27, 28, 30). gai/rga double mutant seed coats, unlike rgl2 mutant seed coats, were able to arrest the germination of dormant embryos (Ler) and to stimulate ABI5 protein levels (Fig. 5 A and B). These results strongly indicate that release of ABA by the seed coat is specifically dependent on the DELLA factor RGL2.
Fig. 5.
Seed coat-dependent repression of germination necessitates a functional RGL2 gene in endosperm cells. (A) Seed coat bedding assays using embryos dissected from freshly harvested, i.e., dormant (D), Ler seeds laid on a layer of seed coats dissected from freshly harvested, Ler seeds [Ler (D)], rgl2-1(rgl2), or gai-t6/rga-t2 (gai/rga) mutant seeds. Pictures were taken 86 h after imbibition. (B) Proteins were extracted from the embryo material shown in A.
Discussion
In summary, our observations show that (i) constitutively higher ABA levels in imbibed dormant seeds necessitate a functional RGL2 gene whose expression remains high and irresponsive to GA, unlike nondormant seeds (Fig. 1 and Fig. S1); (ii) dormant seed coats sustain higher ABA release than nondormant seed coats and in a seed coat bedding assay elicit the same embryonic responses as those observed with exogenous ABA, including in aba2 embryos (Fig. 3); and (iii) repression of germination and stimulation of ABA-responsive gene expression in embryos requires functional RGL2 and ABA1 genes in the seed coat (Figs. 3 and 5). Altogether, these observations strongly support a model in which the seed coat maintains seed dormancy by releasing ABA to repress embryo germination, with RGL2 being a contributing factor to promote ABA synthesis in the endosperm (see model in Fig. 6).
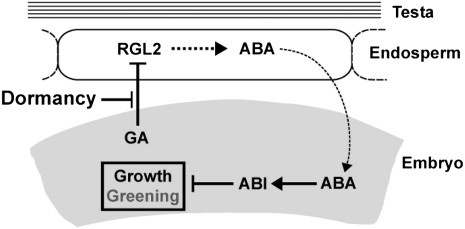
Fig. 6.
Model for seed coat- and ABA-dependent repression of dormant seed germination. Dormancy is a state where GA-dependent down-regulation of RGL2 mRNA and protein expression is blocked. As a result, constitutive RGL2-dependent production of ABA in the endosperm maintains ABI-dependent repression of embryo germination.
We found that intact dormant seeds are unable to repress embryo germination, suggesting that ABA released by the seed coat is retained within seeds (Fig. S6B). This could be due to a specific transmembrane ABA flux between endosperm cells and embryonic cells possibly involving recently identified members of the ABCG subfamily of ABC transporters (33, 34). Furthermore, the reported observations should not be meant to imply that the ABA derived from the endosperm is the major and only cause of dormancy. The strength of dormancy in a given seed, i.e., its capacity to restrain from germinating upon imbibition, is most likely related to the seed's capacity to synthesize ABA as a whole: in both the endosperm and the embryo. Rather, we wish to propose that the endosperm is an essential contributor to sustain dormancy, i.e., to prevent germination over time upon imbibition. That ABA synthesized in embryos also contributes to overall seed dormancy is most likely reflected by the observation that dormant embryos green and germinate slower than nondormant embryos upon dissection (Fig. S7A). In agreement with this view, we found that coatless embryos dissected from isolated dormant seeds 24 h after imbibition contained 2.5-fold more ABA than coatless embryos isolated from nondormant seeds (Fig. S7B).
It should be pointed out that dormancy-promoting function of the seed coat is not strictly restricted to the endosperm. Structural and pigmentation testa defects in Arabidopsis transparent testa (tt) mutant are associated with decreased dormancy in a maternally inherited manner (6, 9). This suggested that the testa layer, a tissue of maternal origin, has a dormancy-promoting effect that appears to be independent of endosperm function. The low dormancy phenotype of tt seeds is unlikely related to ABA present in the testa or ABA synthesized in the endosperm. Indeed, Karssen et al. used reciprocal crosses to show that maternal ABA does not contribute to dormancy and tt germination is stimulated by Norflurazon. Rather, it was proposed that the testa may inhibit germination by decreasing permeability to water and/or oxygen or by exerting a mechanical restraint to the growing embryo (6, 9, 35).
This work focused on the role of the DELLA factor RGL2 to promote higher ABA levels in dormant seeds upon imbibition. Indeed, RGL2 is central among DELLAs to repress seed germination because its mRNA expression is positively regulated by ABA, thus establishing the positive feedback loop that sustains RGL2 expression (8, 27). However, genetic studies have shown that other DELLA factors, such as GAI and RGA, promote dormancy although their contribution was revealed in a rgl2 mutant background (30). We previously reported that GAI and RGA also promote, together with RGL2, higher endogenous ABA levels in the context of far-red light-dependent repression of seed germination (27). One would therefore anticipate that GAI and RGA also participate to sustain higher ABA levels upon imbibition of dormant seeds but only in the embryo because gai/rga seed coats could arrest germination in a seed coat bedding assay (Fig. 5).
The model proposed here to describe seed dormancy (Fig. 6) leaves unanswered an important question, that of the control of RGL2 expression: how do dormancy-breaking factors abolish constitutive RGL2 expression in dormant seeds, and, to begin with, how do dormant seeds achieve constitutive expression of RGL2 upon imbibition. It is unlikely that constitutive RGL2 expression reflects low GA synthesis because exogenous GA does not significantly alter RGL2 expression in dormant seeds (Fig. 1A and Fig. S1A). In contrast, stratification or after-ripening treatments indeed down-regulate RGL2 expression (Fig. 1A and Fig. S1A), in agreement with previous reports showing that GA does not stimulate germination of highly dormant seeds, unlike stratification or after-ripening treatments (36). Thus, dormant seeds may fail to sufficiently express essential GA signaling components necessary to the proteolytic degradation of RGL2 in response to GA, such as SLY1 or GID1 receptors (Fig. S8). Consistent with this possibility, we observed that 24 h upon imbibition, dormant seeds failed to increase SLY1 mRNA levels, unlike nondormant seeds (Fig. S8). In contrast, GID1a mRNA levels were similar in both dormant and nondormant seeds (GID1b and GID1c are not significantly expressed in seeds) (Fig. S8). Thus, low expression of SLY1, an essential component of the GA-dependent DELLA degradation machinery, in dormant seeds may be a contributing factor leading to persistent RGL2 accumulation upon dormant seed imbibition. However, sly1 mutant seeds may show various levels of dormancy, and after-ripened sly1 seeds are nondormant, i.e., are able to germinate, despite expressing high RGL2 protein levels comparable to those found in freshly harvested dormant sly1 seeds (8, 37). This suggests the existence of proteolysis-independent down-regulating pathways of DELLA germination repressor activity, operative during the after-ripening time period, which may involve protein interactions or posttranslational modifications (37, 38). It has recently been proposed that natural variations of dormancy in Arabidopsis seeds are controlled by several additive but independent molecular and genetic pathways (39).
Materials and Methods
Plant Material.
The rgl2-1 and gai-t6/rga-t2 mutant (Ler ecotype; kindly provided by Jinrong Peng, National University of Singapore, Singapore) described in ref. 40 and pRD29B-GUS transgenic line (Col ecotype; kindly provided by Erwin Grill, TU Munich, Munich, Germany) described in ref. 32 and aba2-1 mutant (Col ecotype; kindly provided by Maarten Koornneef, Max Planck Institute, Cologne, Germany) described in ref. 41.
Seed Coat Bedding Assays.
Arabidopsis seeds were dissected into embryos and seed coats at 4 h after seed imbibition by using a very fine syringe needle (BD Micro-Fine) on Whatman 3MM paper laid on the surface of MS agar medium. Ten coatless embryos were laid on a layer of different numbers [30 (Fig. 2B), 100 (Fig. 3A), 50 (Figs. 3D and 5A)] of seed coats on the surface of MS agar medium in presence or absence of 0.5 μM ABA and grown under continuous light (40 μmol m−2 s−1) at 20–21 °C. Seed coats and coatless embryos were further physically separated by a 0.45-μm pore-size nylon transfer membrane (Schleicher and Schuell BioScience).
ABA Measurements.
To measure ABA levels, several different plant materials were used. In Fig. 1 B and D about 50 mg of Arabidopsis seeds was used and in Fig. S7B, 500 coatless embryos and 500 seed coats from dormant and nondormant Cvi seeds were obtained at 24 h after seed imbibition on MS agar medium. In Fig. 4, 500 seed coats from Cvi (ND) and Cvi (D) seeds were dissected at 24 h after seed imbibition on MS agar medium. The seed coats were incubated in 150 μL MS liquid medium for 0 h and 24 h under continuous light (40 μmol m−2 s−1) at 20–21 °C. Thereafter, absolute ABA levels in seeds coats and in the MS incubation liquid medium (without the seed coats) were measured. The ABA levels were determined as described in ref. 27. All experiment was repeated three times.
RNA Extraction and Analysis.
Details of procedures are provided as SI Materials and Methods.
Protein Extraction and Analysis.
Plant materials were collected and frozen under liquid nitrogen and homogenized. Total protein was extracted with homogenization buffer [0.0625 M Tris-HCl (pH 6.8), 1% (wt/vol) SDS, 10% (vol/vol) glycerin, 0.01% (vol/vol) 2-mercaptoethanol]. For ABi5 and RGL2 protein detection in plant materials delivered from intact seed, 20 μg of total protein was separated by SDS/PAGE gel and blotted onto Immobilon-P transfer membrane (Millipore). For the detection of ABi5 protein in coatless embryo, 10 embryos were used and for the detection of RGL2 protein in seed coat, 100 seed coats were used. ABI5 and RGL2 proteins were immunologically detected as described (8).
Germination Assays and GUS Assay.
Details of procedures are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We are greatly indebted to the following colleagues for their generous gifts: Jingrong Peng for rgl2-1 and gai-t6/rga-t2 mutants, Erwin Grill for pRD29B-GUS transgenic line, and Maarten Koornneef for aba2-1 mutant. We thank Richard Chappuis and Christian Megies for technical assistance. We thank Maryline Freyre and Victor Fell for generating seed material. We thank Pierre Vassalli, Christophe Belin, and Michel Goldschmidt-Clermont for critical reading of the manuscript. This work was supported by the Swiss National Science Foundation and the state of Geneva (L.L.-M.) and MSM6198959216 and Internal Grant Agency Grant 913104081/31 (to V.T. and M.S.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1012896107/-/DCSupplemental.
References
- 1.Bentsink L, Koornneef M. Seed Dormancy and Germination. The Arabidopsis Book. Rockville, MD: American Society of Plant Biologists; 2009. pp. 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finkelstein R, Reeves W, Ariizumi T, Steber C. Molecular aspects of seed dormancy. Annu Rev Plant Biol. 2008;59:387–415. doi: 10.1146/annurev.arplant.59.032607.092740. [DOI] [PubMed] [Google Scholar]
- 3.Finch-Savage WE, Leubner-Metzger G. Seed dormancy and the control of germination. New Phytol. 2006;171:501–523. doi: 10.1111/j.1469-8137.2006.01787.x. [DOI] [PubMed] [Google Scholar]
- 4.Holdsworth MJ, Bentsink L, Soppe WJ. Molecular networks regulating Arabidopsis seed maturation, after-ripening, dormancy and germination. New Phytol. 2008;179:33–54. doi: 10.1111/j.1469-8137.2008.02437.x. [DOI] [PubMed] [Google Scholar]
- 5.Ali-Rachedi S, et al. Changes in endogenous abscisic acid levels during dormancy release and maintenance of mature seeds: Studies with the Cape Verde Islands ecotype, the dormant model of Arabidopsis thaliana. Planta. 2004;219:479–488. doi: 10.1007/s00425-004-1251-4. [DOI] [PubMed] [Google Scholar]
- 6.Debeaujon I, Koornneef M. Gibberellin requirement for Arabidopsis seed germination is determined both by testa characteristics and embryonic abscisic acid. Plant Physiol. 2000;122:415–424. doi: 10.1104/pp.122.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Millar AA, et al. Seed dormancy and ABA metabolism in Arabidopsis and barley: The role of ABA 8′-hydroxylase. Plant J. 2006;45:942–954. doi: 10.1111/j.1365-313X.2006.02659.x. [DOI] [PubMed] [Google Scholar]
- 8.Piskurewicz U, et al. The gibberellic acid signaling repressor RGL2 inhibits Arabidopsis seed germination by stimulating abscisic acid synthesis and ABI5 activity. Plant Cell. 2008;20:2729–2745. doi: 10.1105/tpc.108.061515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Debeaujon I, Léon-Kloosterziel KM, Koornneef M. Influence of the testa on seed dormancy, germination, and longevity in Arabidopsis. Plant Physiol. 2000;122:403–414. doi: 10.1104/pp.122.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Debeaujon I, Lepiniec L, Pourcel L, Routaboul J-M. Seed coat development and dormancy. In: Bradford K, Nonogaki H, editors. Seed Development, Dormancy and Germination. Oxford, UK: Blackwell; 2007. pp. 25–43. [Google Scholar]
- 11.Bethke PC, et al. The Arabidopsis aleurone layer responds to nitric oxide, gibberellin, and abscisic acid and is sufficient and necessary for seed dormancy. Plant Physiol. 2007;143:1173–1188. doi: 10.1104/pp.106.093435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bewley JD, Black M. Seeds: Physiology of Development and Germination. New York: Plenum; 1994. [Google Scholar]
- 13.Penfield S, Li Y, Gilday AD, Graham S, Graham IA. Arabidopsis ABA INSENSITIVE4 regulates lipid mobilization in the embryo and reveals repression of seed germination by the endosperm. Plant Cell. 2006;18:1887–1899. doi: 10.1105/tpc.106.041277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lefebvre V, et al. Functional analysis of Arabidopsis NCED6 and NCED9 genes indicates that ABA synthesized in the endosperm is involved in the induction of seed dormancy. Plant J. 2006;45:309–319. doi: 10.1111/j.1365-313X.2005.02622.x. [DOI] [PubMed] [Google Scholar]
- 15.Barrero JM, et al. Gene expression profiling identifies two regulatory genes controlling dormancy and ABA sensitivity in Arabidopsis seeds. Plant J. 2010;61:611–622. doi: 10.1111/j.1365-313X.2009.04088.x. [DOI] [PubMed] [Google Scholar]
- 16.Bassel GW, Mullen RT, Bewley JD. ABI3 expression ceases following, but not during, germination of tomato and Arabidopsis seeds. J Exp Bot. 2006;57:1291–1297. doi: 10.1093/jxb/erj101. [DOI] [PubMed] [Google Scholar]
- 17.Nambara E, Marion-Poll A. Abscisic acid biosynthesis and catabolism. Annu Rev Plant Biol. 2005;56:165–185. doi: 10.1146/annurev.arplant.56.032604.144046. [DOI] [PubMed] [Google Scholar]
- 18.Olszewski N, Sun TP, Gubler F. Gibberellin signaling: Biosynthesis, catabolism, and response pathways. Plant Cell. 2002;14(Suppl):S61–S80. doi: 10.1105/tpc.010476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seo M, et al. Regulation of hormone metabolism in Arabidopsis seeds: Phytochrome regulation of abscisic acid metabolism and abscisic acid regulation of gibberellin metabolism. Plant J. 2006;48:354–366. doi: 10.1111/j.1365-313X.2006.02881.x. [DOI] [PubMed] [Google Scholar]
- 20.Lopez-Molina L, Mongrand S, Chua NH. A postgermination developmental arrest checkpoint is mediated by abscisic acid and requires the ABI5 transcription factor in Arabidopsis. Proc Natl Acad Sci USA. 2001;98:4782–4787. doi: 10.1073/pnas.081594298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lopez-Molina L, Mongrand S, McLachlin DT, Chait BT, Chua NH. ABI5 acts downstream of ABI3 to execute an ABA-dependent growth arrest during germination. Plant J. 2002;32:317–328. doi: 10.1046/j.1365-313x.2002.01430.x. [DOI] [PubMed] [Google Scholar]
- 22.Koornneef M, Reuling G, Karssen CM. The isolation and characterization of abscisic acid-insensitive mutants of Arabidopsis thaliana. Physiol Plant. 1984;61:377–383. [Google Scholar]
- 23.Finkelstein RR. Abscisic acid-insensitive mutations provide evidence for stage-specific signal pathways regulating expression of an Arabidopsis late embryogenesis-abundant (lea) gene. Mol Gen Genet. 1993;238:401–408. doi: 10.1007/BF00291999. [DOI] [PubMed] [Google Scholar]
- 24.Feng S, et al. Coordinated regulation of Arabidopsis thaliana development by light and gibberellins. Nature. 2008;451:475–479. doi: 10.1038/nature06448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harberd NP, Belfield E, Yasumura Y. The angiosperm gibberellin-GID1-DELLA growth regulatory mechanism: How an “inhibitor of an inhibitor” enables flexible response to fluctuating environments. Plant Cell. 2009;21:1328–1339. doi: 10.1105/tpc.109.066969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwechheimer C, Willige BC. Shedding light on gibberellic acid signalling. Curr Opin Plant Biol. 2009;12:57–62. doi: 10.1016/j.pbi.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 27.Piskurewicz U, Turecková V, Lacombe E, Lopez-Molina L. Far-red light inhibits germination through DELLA-dependent stimulation of ABA synthesis and ABI3 activity. EMBO J. 2009;28:2259–2271. doi: 10.1038/emboj.2009.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carrera E, et al. Gene expression profiling reveals defined functions of the ATP-binding cassette transporter COMATOSE late in phase II of germination. Plant Physiol. 2007;143:1669–1679. doi: 10.1104/pp.107.096057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holman TJ, et al. The N-end rule pathway promotes seed germination and establishment through removal of ABA sensitivity in Arabidopsis. Proc Natl Acad Sci USA. 2009;106:4549–4554. doi: 10.1073/pnas.0810280106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Penfield S, Gilday AD, Halliday KJ, Graham IA. DELLA-mediated cotyledon expansion breaks coat-imposed seed dormancy. Curr Biol. 2006;16:2366–2370. doi: 10.1016/j.cub.2006.10.057. [DOI] [PubMed] [Google Scholar]
- 31.Piskurewicz U, Lopez-Molina L. The GA-signaling repressor RGL3 represses testa rupture in response to changes in GA and ABA levels. Plant Signal Behav. 2009;4:63–65. doi: 10.4161/psb.4.1.7331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Christmann A, Hoffmann T, Teplova I, Grill E, Müller A. Generation of active pools of abscisic acid revealed by in vivo imaging of water-stressed Arabidopsis. Plant Physiol. 2005;137:209–219. doi: 10.1104/pp.104.053082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kang J, et al. PDR-type ABC transporter mediates cellular uptake of the phytohormone abscisic acid. Proc Natl Acad Sci USA. 2010;107:2355–2360. doi: 10.1073/pnas.0909222107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuromori T, et al. ABC transporter AtABCG25 is involved in abscisic acid transport and responses. Proc Natl Acad Sci USA. 2010;107:2361–2366. doi: 10.1073/pnas.0912516107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karssen CM, Brinkhorst-van der Swan DLC, Breekland AE, Koornneef M. Induction of dormancy during seed development by endogenous abscisic acid: Studies on abscisic acid deficient genotypes of Arabidopsis thaliana (L.) Heynh. Planta. 1983;157:158–165. doi: 10.1007/BF00393650. [DOI] [PubMed] [Google Scholar]
- 36.Alonso-Blanco C, Bentsink L, Hanhart CJ, Blankestijn-de Vries H, Koornneef M. Analysis of natural allelic variation at seed dormancy loci of Arabidopsis thaliana. Genetics. 2003;164:711–729. doi: 10.1093/genetics/164.2.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ariizumi T, Steber CM. Seed germination of GA-insensitive sleepy1 mutants does not require RGL2 protein disappearance in Arabidopsis. Plant Cell. 2007;19:791–804. doi: 10.1105/tpc.106.048009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Silverstone AL, et al. Functional analysis of SPINDLY in gibberellin signaling in Arabidopsis. Plant Physiol. 2007;143:987–1000. doi: 10.1104/pp.106.091025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bentsink L, et al. Natural variation for seed dormancy in Arabidopsis is regulated by additive genetic and molecular pathways. Proc Natl Acad Sci USA. 2010;107:4264–4269. doi: 10.1073/pnas.1000410107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee S, et al. Gibberellin regulates Arabidopsis seed germination via RGL2, a GAI/RGA-like gene whose expression is up-regulated following imbibition. Genes Dev. 2002;16:646–658. doi: 10.1101/gad.969002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Léon-Kloosterziel KM, et al. Isolation and characterization of abscisic acid-deficient Arabidopsis mutants at two new loci. Plant J. 1996;10:655–661. doi: 10.1046/j.1365-313x.1996.10040655.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.