Abstract
We construct a verbal and graphical theory (the “fecundity-limitation hypothesis”) about how constraints on the brooding space for embryos probably truncate individual fecundity in male-pregnant and female-pregnant species in ways that should differentially influence selection pressures for multiple mating by males or by females. We then review the empirical literature on genetically deduced rates of multiple mating by the embryo-brooding parent in various fish species with three alternative categories of pregnancy: internal gestation by males, internal gestation by females, and external gestation (in nests) by males. Multiple mating by the brooding gender was common in all three forms of pregnancy. However, rates of multiple mating as well as mate numbers for the pregnant parent averaged higher in species with external as compared with internal male pregnancy, and also for dams in female-pregnant species versus sires in male-pregnant species. These outcomes are all consistent with the theory that different types of pregnancy have predictable consequences for a parent's brood space, its effective fecundity, its opportunities and rewards for producing half-sib clutches, and thereby its exposure to selection pressures for seeking multiple mates. Overall, we try to fit these fecundity-limitation phenomena into a broader conceptual framework for mating-system evolution that also includes anisogamy, sexual-selection gradients, parental investment, and other selective factors that can influence the relative proclivities of males versus females to seek multiple sexual partners.
Keywords: genetic parentage, mating systems, microsatellites, pregnancy, sexual selection
Many selective factors can influence the evolution of differences in mating tactics between males and females. At the ultimate level of explanation, anisogamy (the pronounced size difference between male and female gametes) helps to set the evolutionary stage by making females intrinsically more fecundity-limited than males (1, 2). At a penultimate level of explanation, this potential fertility difference between the sexes often translates into steeper sexual-selection gradients (3) for males than for females, meaning that males in many species tend to profit more than females (in terms of genetic fitness) from having multiple mates (4). Because a male's reproductive success can increase greatly with mate count whereas a female's reproductive success is limited mostly by her fecundity regardless of mate number, males in many animal species presumably are under stronger selection pressure than females to seek multiple sexual partners. Finally, selection pressures on male versus female mating behaviors can further be impacted by numerous more proximate considerations such as operational sex ratios in local populations (5, 6), relative levels of parental investment in offspring (7, 8), and other species-specific ecological and genetic factors that can differentially impact the two sexes’ potential reproductive rates (9, 10) or their variances in reproductive success (11, 12).
In taxa such as Syngnathidae (pipefishes and seahorses) that display the phenomenon of male pregnancy, some of the evolutionary ground rules described above can shift dramatically (reviewed in ref. 13). In the ≈200 extant syngnathid species, a male in effect is fecundity-limited (sometimes even more so than a typical female) due to the finite size of the brood pouch within which he must incubate the embryos that he has sired with one or more mates (14–17). This inversion from the familiar situation in female-pregnant animals apparently has translated in some but not all syngnathid species into mating systems characterized by “sex-role reversal” (18, 19): a higher intensity of sexual selection on females than on males and an elaboration of sexual secondary traits mostly in females. For one such pipefish species, researchers also have documented that the sexual-selection gradient for females is steeper than that for males (20).
More generally, fishes should be excellent subjects for assessing how alternative types of pregnancy might influence the evolution of genetic mating systems and sexual selection on males versus females, because most fish have high fecundities (many embryos per brood) and because both internal male pregnancy and female pregnancy are displayed by various taxa. Furthermore, males in many other taxonomic families of fishes display what can be interpreted as “external pregnancy” wherein each “bourgeois male” (21) builds a nest in which he tends the embryos from one or more females whose eggs he himself has mostly fertilized (22–25). Both internal and external pregnancy in fishes imply a substantial energetic investment in offspring care by the brooding sex.
For any type of pregnancy, multiple successful mating by the adult caregiver is relatively straightforward to detect in nature via molecular parentage analyses because each resulting brood of half-sib embryos is physically associated with its pregnant sire or pregnant dam. By contrast, documenting the frequency of multiple mating by members of the nonpregnant sex is much more difficult because each such individual may have parented additional broods that did not happen to be included in the genetic assays, and thus remain undetected. Thus arises another sexual asymmetry relevant to the current discussion: For purely logistical reasons, molecular parentage analyses usually are best-suited for assessing rates of multiple paternity within the broods of female-pregnant species (i.e., multiple mating by the females) and multiple maternity within the broods of male-pregnant species (i.e., multiple mating by the males), rather than vice versa (26, 27).
Here we take advantage of all these facts by reviewing genetic data on multiple mating by the brooding sex in fish species with several alternative forms of pregnancy. We define an individual's fecundity (or maximum reproductive potential) as the number of gametes it produces that stand a reasonably good prospect, given the species’ biology, of contributing to successful embryos during a breeding season or episode. Thus, in effect, both anisogamy and pregnancy can be thought of as fecundity-truncating phenomena for the gender in question, all else being equal. In other words, brood space constrains the number of embryos that a pregnant individual can parent, and the effect probably is more extreme for internal brooders than external brooders. However, for any kind of pregnancy, each individual's maximum fecundity also depends on its mating habits. For example, in species with female pregnancy, a polygynous male clearly has a much higher reproductive potential than a monogamous male or a monogamous or polyandrous female. [Here we define a polygamous specimen (a polygynous male or a polyandrous female) as an individual that has two or more successful mates during a breeding season or episode, whereas a monogamous specimen has only one such mate]. In some but not all cases, an individual's maximum fecundity also depends upon that of its pregnant mate(s). For example, in any species with internal pregnancy, the maximum fecundity of a monogamous individual (but not a polygamous one) is physically limited by the size of its mate's brood chamber, which places a ceiling on the number of embryos that can be brooded successfully. Furthermore, that number of embryos is likely to be lower than the number of eggs a female can produce, because embryos take up more space than do unfertilized eggs. The broader point is that each type of pregnancy in effect can truncate the fecundity component of individual fitness in a predictable way and thereby have profound consequences with respect to selective pressures on both male and female mating behaviors.
Another general category of explanations for multiple mating by pregnant males or pregnant females mostly sidesteps the fecundity issue per se and focuses instead on other potential fitness benefits from having multiple sexual partners. Regardless of its number of mates, each male or female in any sexual species presumably can enhance its genetic fitness by choosing one or more mates of the highest possible genetic quality. Thus, for example, despite any inherent fecundity limitations imposed by pregnancy, any sire in a male-pregnant species or any dam in a female-pregnant species might in theory seek multiple mates for any of the following reasons: more “nuptial gifts”; fertilization insurance against the risk that a mate is sterile; higher genetic diversity among the resulting half-sib progeny in the brood [sometimes called genetic “bet-hedging” (28, 29)]; better chances of finding a genetically compatible mate for the brood; or more opportunities to imbue at least some of the offspring with “good genes” for viability (30, 31). For current purposes, we will interpret all such factors to be “bonus effects” that might elevate a polygamous individual's fitness above the fecundity plateaus otherwise imposed by pregnancy or anisogamy. However, none of these bonus effects seems likely to boost the genetic fitness of a polygamous individual nearly as much as does the greater access to opposite-sex gametes that multiple mating provides. Of course, multiple mating by either sex can incur costs as well, such as the time and energy required to secure mates and the increased chance of contracting a sexually transmitted disease.
In any event, to distinguish between fecundity limitations per se and other hypotheses for any disparity between rates of multiple mating by males versus females, it should be helpful to compare the incidences of multiple mating in numerous related species in which an individual's maximum fecundity varies predictably as a function of the type of pregnancy. Here we introduce this approach by comparing genetically determined mate numbers and rates of multiple mating for (i) males in fish species with internal versus external male pregnancy, and (ii) males in male-pregnant fish species vis-à-vis females in female-pregnant fishes. We review the genetic literature on rates of multiple maternity within broods of male-pregnant fish species and rates of multiple paternity within broods of female-pregnant fishes. We then discuss the results in the framework of traditional mating-system theory as amended for the special circumstance in which brood space itself imposes specifiable limits on the fecundity of pregnant individuals and their mates.
Results
Fecundity-Limitation Scenario: Pregnancy's Potential Impacts on Multiple Mating.
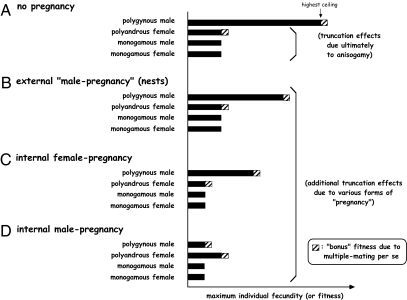
Fig. 1 encapsulates our current theoretical scenario for how alternative forms of pregnancy might limit each individual's fecundity in ways that should influence the selective pressures on males or females to seek multiple mates. In general, the phenomenon of pregnancy physically constrains an individual's potential fecundity (and genetic fitness) by placing an upper limit (available brood space for embryos) on the number of progeny that a pregnant individual and its partner(s) can produce during each reproductive season or episode. For a species in which the pregnant gender is female (Fig. 1C), one effect of this truncation is to modestly lower each dam's fecundity below the relatively low value (compared with a male; Fig. 1A) that already existed due to anisogamy per se. However, for a species in which the pregnant gender is male (Fig. 1 B and D), a male's maximum fecundity can fall dramatically below what it otherwise might be in the absence of the male-pregnancy phenomenon (Fig. 1A). This basic asymmetry between the sexes in the fitness-truncating effects of internal gestation is presumably one reason (among many) why male pregnancy is less common than female pregnancy in the biological world. However, the truncation effect on a male's fecundity is probably less severe under external pregnancy (Fig. 1B) than it is under internal male pregnancy (Fig. 1D), due to the likelihood that more brooding space for embryos is available within a nest than within a male's internal brood chamber. Nevertheless, internal male pregnancy is ubiquitous in the Syngnathidae.
Fig. 1.
General expectations about the maximum potential fecundities (potential reproductive rates) of monogamous and polygamous males and females in species displaying each of four different categories of pregnancy: (A) no pregnancy (i.e., external fertilization with no brooding chamber for the embryos); (B) external male pregnancy (e.g., embryos brooded in male-tended nests); (C) internal female pregnancy (gestation inside the dam's body); and (D) internal male pregnancy (gestation inside the sire's body). In each case, anisogamy operates either alone (A) or in conjunction with pregnancy-imposed limitations on brooding space (B–D) to truncate an individual's maximum fecundity well below the level that could otherwise be attained in the absence of these effects. The hatched bars indicate additional or bonus fitness effects (see text) that might apply to polygamous versus monogamous individuals in any of the four pregnancy categories.
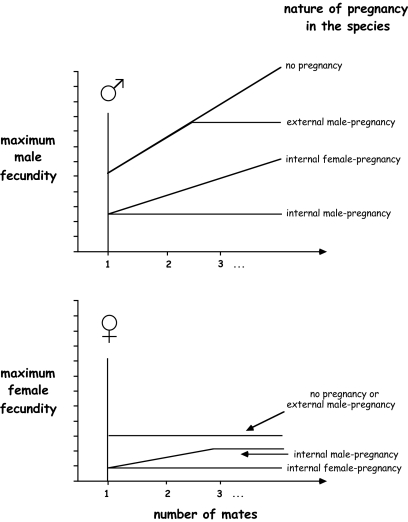
Fig. 2 summarizes all of these theoretical expectations in another format: as sexual-selection gradients for males (Upper) versus females (Lower). For each line in each graph, the left-hand intercept at “one mate” indicates a focal individual's maximum fecundity (as dictated by the various types of pregnancy within a species) if he or she has paired with only one sexual partner, and each slope shows the focal individual's potential increase in fecundity as he or she acquires additional mates. Thus, the topmost lines in the two graphs show the steeper Bateman gradients generally expected for males than for females in many nonpregnant species, and the intercepts and slopes of the other lines predict how brood-space restrictions imposed by the three different types of pregnancy should lower these maximum fecundity potentials for particular males and females that are monogamous or polygamous.
Fig. 2.
Theoretical sexual-selection gradients (or Bateman gradients) for males (Upper) and females (Lower) in species displaying each of four different categories of pregnancy: no pregnancy (i.e., external fertilization with no brooding chamber for the embryos); external male pregnancy (e.g., embryos brooded in male-tended nests); internal female pregnancy (gestation inside the dam's body); and internal male pregnancy (gestation inside the sire's body). The generally lower maximum fertilities for females than for males reflect mostly the effects of anisogamy. The “no-pregnancy” lines in the two graphs reflect the standard situation for many species in which Bateman gradients are expected to be steeper for males than females. The relative heights and slopes of various other lines in the graphs depart from these no-pregnancy baselines due to brood-space limitations for the developing embryos in the various forms of pregnancy (see also text and Fig. 1). The positive initial slope in the Bateman gradient for females in species with internal male pregnancy further assumes (as is true in many syngnathid fishes) that a female can produce more eggs than a male can fertilize and incubate.
To make the predictions in Fig. 2 somewhat more concrete, assume for example that the internal brood pouch of a pregnant male or a pregnant female can hold at most 25 embryos whereas an external nest can accommodate hundreds or more embryos (such values are typical for many pregnant fish species in nature). Thus, as shown in the upper half of Fig. 2, the left-hand intercept (a monogamous male's maximum fitness) for species with either form of internal pregnancy is lower than that for external pregnancy. As also shown in the top half of Fig. 2, the flat slope of the fecundity line for species with internal male pregnancy reflects the fact that a pregnant male's reproductive potential is truncated at 25 (in this case), regardless of how many mates he acquires; the moderate but similar initial slopes in the lines for internal female pregnancy and external male pregnancy reflect the fact that a polygynous male can expect substantial but proportionately similar gains in reproductive success by acquiring multiple mates in these two situations. With respect to the lower half of Fig. 2, analogous arguments can be made for how a female's fecundity presumably varies as a function of her number of mates in species with these various pregnancy modes. Note also, however, that the intercepts are lower and most of the slopes are shallower in the bottom half of the figure than their counterparts in the upper half, due ultimately to the fact that anisogamy limits the maximum fecundity that any female can achieve.
Empirical Data: Incidences of Multiple Mating Under Different Pregnancy Modes.
Our literature review uncovered estimates of mate numbers and rates of multiple mating by the brooding sex in a total of more than 760 sampled broods representing 29 fish species with one or another mode of pregnancy (Table 1). Fig. 3 provides a pictorial overview of these findings across all broods, and Fig. 4 further breaks down these reports for species displaying the three categories of pregnancy that we wish to compare. The papers included approximately equal numbers of species (10, 8, and 11) and comparable numbers of broods (ca 295, 238, and 232) from fishes with internal female pregnancy, internal male pregnancy, and external male pregnancy, respectively. We found no reports for species with “external female pregnancy” (which is a rare phenomenon in fishes).
Table 1.
Multiple mating in microsatellite-based studies of genetic parentage of wild fishes with internal female pregnancy, internal male pregnancy, and external male pregnancy (nest tending)
| Species | No. of clutches examined | Frequency of multiple mating (%) | Mean no. of mates (range) | Ref. |
| Female-pregnant species | ||||
| Swordtail, Xiphophorus multilineatus | 18 | 28 (5/18) | 1.4 (1–3) | (32) |
| Green swordtail, Xiphophorus helleri | 69 | 64 (44/69) | 1.8 (1–4) | (33) |
| Green swordtail, Xiphophorus helleri | 14 | 57 (8/14) | 1.7 (1–2) | (34) |
| Guppy, Poecilia reticulata | 22 | 95 (21/22) | 3.0 (1–6) | (35) |
| Guppy, Poecilia reticulata | 101* | 95 (96/101) | 3.5 (1–9) | (36)† |
| Mosquitofish, Gambusia holbrooki | 50 | 86 (43/50) | 2.2 (1–3) | (37) |
| Least killifish, Heterandria formosa | 36‡ | 47 (17/36) | 1.5 (N/A) | (38)† |
| Black surfperch, Embiotoca jacksoni | 12 | 100 (12/12) | 3.6 (2–6) | (39) |
| Striped surfperch, Embiotoca lateralis | 12 | 100 (12/12) | 3.5 (2–9) | (39) |
| Shiner perch, Cymatogaster aggregata | 27 | 96 (26/27) | 4.6 (1–8) | Table S1 |
| Pacific ocean perch, Sebastes alutus | 66 | 71 (47/66) | 1.9 (1–4) | (40) |
| Black rockfish, Sebastes inermis | 5 | 20 (1/5) | 1.2 (1–2) | (41) |
| Male-pregnant species | ||||
| Gulf pipefish, Syngnathus scovelli | 40 | 3 (1/40) | 1 (1–2) | (14) |
| Dusky pipefish, Syngnathus floridae | 22 | 73 (16/22) | 1.9 (1–3) | (15) |
| Dusky pipefish, Syngnathus floridae | 52§ | 85 (44/52) | 2.3 (1–4) | (42) |
| Broad-nosed pipefish, Syngnathus typhle | 30 | 90 (27/30) | 3.1 (1–6) | (17) |
| Broad-nosed pipefish, Syngnathus typhle | 38¶ | 76 (29/38) | 2.7 (1–5) | (43) |
| Bay pipefish, Syngnathus leptorhynchus | 7 | 86 (6/7) | 2.1 (1–3) | (44) |
| Barred pipefish, Syngnathus auliscus | 7 | 86 (6/7) | 2.1 (1–3) | (44) |
| Straight-nosed pipefish, Nerophis ophidian | 15 | 0 (0/15) | 1 (1) | (45) |
| Western Australian seahorse, Hippocampus angustus | 15 | 0 (0/15) | 1 (1) | (16) |
| Pot-bellied seahorse, Hippocampus abdominalis | 12 | 0 (0/12) | 1 (1) | (46) |
| Male nest-tending species | ||||
| Redbreast sunfish, Lepomis auritus | 25 | 100 (25/25) | 3.6 (2–6) | (47) |
| Spotted sunfish, Lepomis punctatus | 30 | 93 (28/30) | 4.4 (1–6) | (48) |
| Dollar sunfish, Lepomis marginatus | 23 | 83 (19/23) | 2.5 (1–7) | (49) |
| Tessellated darter, Etheostoma olmstedi | 13 | 94 (15/16) | 3.2 (1–4) | (50) |
| Fifteenspine stickleback, Spinachia spinachia | 25 | 68 (17/25) | 2.6 (1–7) | (51) |
| Sand goby, Pomatoschistus minutus | 24 | 100 (24/24) | 3.4 (2–6) | (52) |
| Striped darter, Etheostoma virgatum | 20 | 100 (24/24) | 4.7 (2–7) | (53) |
| Mottled sculpin, Cottus bairdi | 23 | 74 (17/23) | 2.8 (1–6) | (54) |
| Molly Miller, Scartella cristata | 23 | 100 (23/23) | 4.9 (3–8) | (55) |
| Channel catfish, Ictalurus punctatus | 5 | 0 (0/5) | 1 (1) | (56) |
| Two-spotted goby, Gobiusculus flavescens | 21 | 100 (21/21) | 4.3 (2–6) | (57) |
N/A, not available.
*Data from 10 populations combined.
†Data not used in brood analysis.
‡Data from 3 populations combined.
§Data from 2 populations combined.
¶Data from 4 populations combined.
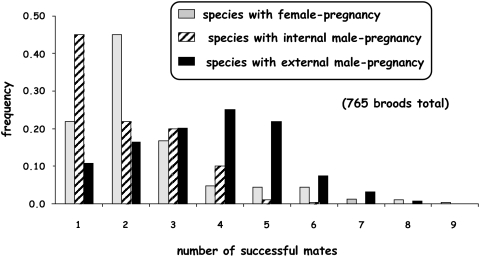
Fig. 3.
Frequency distribution of broods with different number of mates in fish species with female pregnancy, male pregnancy, and male nest tending.
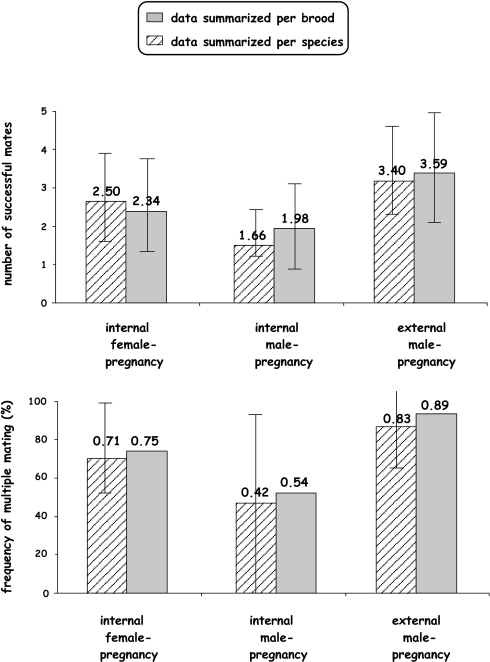
Fig. 4.
Frequencies of multiple mating by the brooding sex and estimated mate numbers (plus their SEs) in 765 genetically monitored “pregnancies” representing 29 fish species that display internal gestation by females, internal gestation by males, or external gestation by nest-tending males.
Both the frequencies of multiple mating by the brooding sex (42–89%) and the mean number of mates per brood (1.7–3.6) were quite high for fish species in all three categories of pregnancy (Figs. 3 and 4; Table 1). Furthermore, the mean numbers of mates per brood were significantly higher in nest-tending fishes than in those with either internal male pregnancy or female pregnancy, and also tended to be higher in fishes with female pregnancy compared with those with internal male pregnancy (Figs. 3 and 4; Table 2). These outcomes were statistically highly significant when the data were analyzed on a per-brood basis (in which case each assayed brood receives equal weighting), and the trends were also in the same direction, albeit sometimes only marginally significant, for data analyzed on a per-species basis (in which case each species receives equal weight regardless of how many broods were genotyped) (Fig. 4; Table 2).
Table 2.
Comparisons of multiple-mating parameters (two-tailed t tests assuming unequal variances, as calculated in Microsoft Excel) for fishes with different forms of pregnancy. Similar statistical outcomes emerged from a one-way ANOVA followed by a post hoc Tukey test (in XLSTAT)
| Comparison | t statistic | Df | P |
| No. of successful mates based on per-brood data | |||
| Internal female pregnancy vs. internal male pregnancy | 3.30 | 531 | 0.00 |
| External male pregnancy vs. internal female pregnancy | 9.67 | 467 | 0.00 |
| External male pregnancy vs. internal male pregnancy | 12.94 | 417 | 0.00 |
| No. of successful mates based on per-species data | |||
| Internal female pregnancy vs. internal male pregnancy | 1.83 | 15 | 0.09 (0.04*) |
| External male pregnancy vs. internal female pregnancy | 1.76 | 19 | 0.09 (0.04*) |
| External male pregnancy vs. internal male pregnancy | 3.96 | 17 | 0.00 |
*These t statistics are significant in a directional one-tailed t test.
Discussion
Although the effect of anisogamy on female fecundity is widely acknowledged to be a key selective factor that ultimately underlies the evolution of different mating behaviors by the two sexes in most animal species, much less attention has been devoted to how the phenomenon of pregnancy might further impact genetic mating systems. By placing a physical constraint on the space available for brooding embryos, pregnancy can dramatically truncate the fecundity potential of a pregnant individual and its mate(s), with sometimes major consequences—such as in the evolution of classical polyandry in some fish species with male pregnancy (58). Furthermore, the fecundity-truncation effect of internal pregnancy goes beyond what anisogamy imposes, and it can apply to members of both sexes depending on which sex does the brooding. Here we have used graphical and verbal arguments to speculate how various forms of pregnancy might impact the evolution of fish mating systems. We have also summarized the empirical genetic-parentage literature on estimated rates of multiple mating by the embryo-brooding parent in fish species with male pregnancy vis-à-vis those with female pregnancy. Although multiple mating by the brooding gender is not the same as multiple paternity or multiple maternity (59), the latter do register the realized or the genetic (as opposed to merely the social) mating system of a species and hence should be especially germane for understanding selection pressures that might have impacted the evolution of mating behaviors.
Our review of genetic parentage in pregnant fish species uncovered the following: (i) broods in all categories of pregnant fishes routinely consist of groups of half-sib progeny, thus evidencing high rates of multiple mating by the brooding parent; (ii) fishes with external male pregnancy (the nest tenders) have significantly higher incidences of multiple maternity than do fish species in which males carry the embryos internally; and (iii) incidences of multiple paternity within broods of female-pregnant fish species are significantly higher than incidences of multiple maternity within the broods of male-pregnant fishes.
Can these empirical observations be reconciled with the theoretical framework outlined earlier (Figs. 1 and 2)? Under that theory, several qualitative predictions can be made with regard to how the fecundity-truncating effects of pregnancy and anisogamy might impact the evolution of mating behaviors and genetic mating systems in fishes. First, because of the high but sex-specific fecundities of males and females in many fish species with pregnancy, and because brooding space provided by the pregnant parent can be a limiting resource in reproduction, members of the nonpregnant sex generally should be under strong selection to seek and welcome mating opportunities with members of the pregnant sex. This expectation is thus consistent with observation (i) in the preceding paragraph.
Second, due to the fact that internal brood space is more limited than brooding space in nests, nest-tending bourgeois males generally should be under stronger selection (as well as have greater physical scope) for successfully rearing broods from multiple females than should males in species with internal male pregnancy. This expectation is thus consistent with observation (ii) above. Third, given the fact (due to anisogamy) that gravid females in female-pregnant species can physically accept many more sperm cells than can males accept eggs in male-pregnant species, opportunities for multiple mating should be higher for pregnant females than for pregnant males, all else being equal. This prediction is thus consistent with observation (iii) above. On the other hand, similarly flat Bateman gradients (compare the lowest lines in the upper and lower panels of Fig. 2) imply that there is otherwise no compelling reason to suspect that male-pregnant fish should experience pronounced differences from female-pregnant fish with respect to selective benefits from having multiple mates. By this reasoning, perhaps it is not surprising that although rates of multiple mating in female-pregnant fishes were significantly higher than those in male-pregnant fishes, the magnitude of this difference was not very large (Figs. 3 and 4).
Overall, all categories of pregnancy in fishes appear to be associated with high rates of successful multiple mating by the brooding sex. Thus, another kind of interpretation is simply that multiple mating by the pregnant gender routinely confers important reproductive payoffs to the participants regardless of whether it is the sire or the dam that gestates the resulting half-sib cohorts. One possibility is that multiple mating may have larger bonus fitness effects than we have heretofore assumed (Fig. 1). In other words, perhaps all pregnant fish parents benefit substantially from having two or more mates for reasons having to do with factors such as better genes for their progeny, higher genetic diversity within their broods, or other such reproductive bonuses that in some cases may derive from improved offspring viability rather than from an enhanced parental fecundity per se. If so, selection pressures could favor the evolution of multiple-mating tendencies by females as well as males in almost any fish species (with or without the phenomenon of pregnancy, and regardless of which sex might provide any gestation services). Currently available genetic-parentage data, which have documented high rates of multiple mating by both sexes in a wide variety of fish species, would not be inconsistent with this possibility.
The current study is only a first step toward understanding how alternative expressions of pregnancy impact the evolution of animal mating behaviors. A more comprehensive theory will have to incorporate many considerations beyond parental fecundity itself, such as how various forms of pregnancy differentially affect survivorship (for example, embryonic mortality might be much higher in nest-tending species than in those that carry their broods internally). Another major challenge will be to formalize how selection pressures related to pregnancy might impact an individual's lifetime genetic fitness (i.e., its future as well as current reproductive success). This task too will require examining tradeoffs: between fecundity and survival, and between natural and sexual selection.
Materials and Methods
Our literature review, conducted in the summer of 2010, sought to identify all substantive papers that have reported rates of multiple paternity or maternity, respectively, within the broods of female-pregnant and male-pregnant fish species, as estimated from parentage analyses using highly polymorphic molecular markers (typically microsatellite loci). For each such paper, we summarized reported information on the proportion of broods with multiple sires or dams as well as mate numbers for the brooding sex. Different authors sometimes used slightly different statistical procedures (60) to estimate the incidence of multiple mating by the brooding sex, but we have accepted the authors’ reported values without further statistical adjustments.
Supplementary Material
Acknowledgments
We thank Giacomo Bernardi, Louis Bernatchez, Rosemary Bryne, Gunilla Rosenqvist, and Andrei Tatarenkov for helpful comments on the manuscript. Work was supported by funds from the University of California at Irvine.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1013786107/-/DCSupplemental.
References
- 1.Parker GA, Baker RR, Smith VGF. The origin and evolution of gamete dimorphism and the male-female phenomenon. J Theor Biol. 1972;36:529–553. doi: 10.1016/0022-5193(72)90007-0. [DOI] [PubMed] [Google Scholar]
- 2.Avise JC. Hermaphroditism: The Biology, Ecology, and Evolution of Dual Sexuality. New York: Columbia Univ Press; 2010. [Google Scholar]
- 3.Bateman AJ. Intra-sexual selection in Drosophila. Heredity. 1948;2:349–368. doi: 10.1038/hdy.1948.21. [DOI] [PubMed] [Google Scholar]
- 4.Arnold SJ, Duvall D. Animal mating systems: A synthesis based on selection theory. Am Nat. 1994;143:317–348. [Google Scholar]
- 5.Emlen ST, Oring LW. Ecology, sexual selection, and the evolution of mating systems. Science. 1977;197:215–223. doi: 10.1126/science.327542. [DOI] [PubMed] [Google Scholar]
- 6.Kvarnemo C, Ahnesjö I. The dynamics of operational sex ratios and competition for mates. Trends Ecol Evol. 1996;11:404–408. doi: 10.1016/0169-5347(96)10056-2. [DOI] [PubMed] [Google Scholar]
- 7.Trivers RL. Parental investment and sexual selection. In: Campbell B, editor. Sexual Selection and the Descent of Man, 1871–1971. Chicago: Aldine; 1972. pp. 136–179. [Google Scholar]
- 8.Parker GA, Simmons LW. Parental investment and the control of sexual selection: Predicting the direction of sexual competition. Proc Biol Sci. 1996;263:315–321. [Google Scholar]
- 9.Clutton-Brock TH, Parker GA. Potential reproductive rates and the operation of sexual selection. Q Rev Biol. 1992;67:437–456. [Google Scholar]
- 10.Clutton-Brock TH, Vincent ACJ. Sexual selection and the potential reproductive rates of males and females. Nature. 1991;351:58–60. doi: 10.1038/351058a0. [DOI] [PubMed] [Google Scholar]
- 11.Payne RB. Sexual selection and intersexual differences in variance of breeding success. Am Nat. 1979;114:447–452. [Google Scholar]
- 12.Wade MJ, Arnold SJ. The intensity of sexual selection in relation to male sexual behaviour, female choice, and sperm precedence. Anim Behav. 1980;28:446–461. [Google Scholar]
- 13.Jones AG, Avise JC. Mating systems and sexual selection in male-pregnant pipefishes and seahorses: Insights from microsatellite-based studies of maternity. J Hered. 2001;92:150–158. doi: 10.1093/jhered/92.2.150. [DOI] [PubMed] [Google Scholar]
- 14.Jones AG, Avise JC. Microsatellite analysis of maternity and the mating system in the Gulf pipefish Syngnathus scovelli, a species with male pregnancy and sex-role reversal. Mol Ecol. 1997;6:203–213. doi: 10.1046/j.1365-294x.1997.00173.x. [DOI] [PubMed] [Google Scholar]
- 15.Jones AG, Avise JC. Polygynandry in the dusky pipefish Syngnathus floridae revealed by microsatellite DNA markers. Evolution. 1997;51:1611–1622. doi: 10.1111/j.1558-5646.1997.tb01484.x. [DOI] [PubMed] [Google Scholar]
- 16.Jones AG, Kvarnemo C, Moore GI, Simmons LW, Avise JC. Microsatellite evidence for monogamy and sex-biased recombination in the Western Australian seahorse Hippocampus angustus. Mol Ecol. 1998;7:1497–1505. doi: 10.1046/j.1365-294x.1998.00481.x. [DOI] [PubMed] [Google Scholar]
- 17.Jones AG, Rosenqvist G, Berglund A, Avise JC. The genetic mating system of a sex-role-reversed pipefish (Syngnathus typhle): A molecular inquiry. Behav Ecol Sociobiol. 1999;46:357–365. [Google Scholar]
- 18.Berglund A, Rosenqvist G, Svensson I. Reversed sex roles and parental energy investment in zygotes of two pipefish (Syngnathidae) species. Mar Ecol Prog Ser. 1986;29:209–215. [Google Scholar]
- 19.Rosenqvist G. Sex role reversal in a pipefish. Mar Behav Physiol. 1993;23:219–230. [Google Scholar]
- 20.Jones AG, Rosenqvist G, Berglund A, Arnold SJ, Avise JC. The Bateman gradient and the cause of sexual selection in a sex-role-reversed pipefish. Proc Biol Sci. 2000;267:677–680. doi: 10.1098/rspb.2000.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taborsky M. Sperm competition in fish: ‘Bourgeois’ males and parasitic spawning. Trends Ecol Evol. 1998;13:222–227. doi: 10.1016/s0169-5347(97)01318-9. [DOI] [PubMed] [Google Scholar]
- 22.Blumer LS. Male parental care in the bony fishes. Q Rev Biol. 1979;54:149–161. [Google Scholar]
- 23.Blumer LS. A bibliography and categorization of bony fishes exhibiting parental care. Zool J Linn Soc. 1982;75:1–22. [Google Scholar]
- 24.Taborsky M. The evolution of bourgeois, parasitic, and cooperative reproductive behaviors in fishes. J Hered. 2001;92:100–110. doi: 10.1093/jhered/92.2.100. [DOI] [PubMed] [Google Scholar]
- 25.Avise JC, Jones AG, Walker D, DeWoody JA. Genetic mating systems and reproductive natural histories of fishes: Lessons for ecology and evolution. Annu Rev Genet. 2002;36:19–45. doi: 10.1146/annurev.genet.36.030602.090831. [DOI] [PubMed] [Google Scholar]
- 26.DeWoody JA, Walker D, Avise JC. Genetic parentage in large half-sib clutches: Theoretical estimates and empirical appraisals. Genetics. 2000;154:1907–1912. doi: 10.1093/genetics/154.4.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neff BD. Genetic paternity analysis and breeding success in bluegill sunfish (Lepomis macrochirus) J Hered. 2001;92:111–119. doi: 10.1093/jhered/92.2.111. [DOI] [PubMed] [Google Scholar]
- 28.Yasui Y. The ‘genetic benefits’ of female multiple mating reconsidered. Trends Ecol Evol. 1998;13:246–250. doi: 10.1016/s0169-5347(98)01383-4. [DOI] [PubMed] [Google Scholar]
- 29.Yasui Y. Female multiple mating as a genetic bet-hedging strategy when mate choice criteria are unreliable. Ecol Res. 2001;16:605–616. [Google Scholar]
- 30.Møller AP, Alatalo RV. Good-genes effects in sexual selection. Proc Biol Sci. 1999;266:85–91. [Google Scholar]
- 31.Byers JA, Waits L. Good genes sexual selection in nature. Proc Natl Acad Sci USA. 2006;103:16343–16345. doi: 10.1073/pnas.0608184103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luo J, Sanetra M, Schartl M, Meyer A. Strong reproductive skew among males in the multiply mated swordtail Xiphophorus multilineatus (Teleostei) J Hered. 2005;96:346–355. doi: 10.1093/jhered/esi042. [DOI] [PubMed] [Google Scholar]
- 33.Tatarenkov A, Healey CIM, Grether GF, Avise JC. Pronounced reproductive skew in a natural population of green swordtails, Xiphophorus helleri. Mol Ecol. 2008;17:4522–4534. doi: 10.1111/j.1365-294X.2008.03936.x. [DOI] [PubMed] [Google Scholar]
- 34.Simmons LW, Beveridge M, Evans JP. Molecular evidence for multiple paternity in a feral population of green swordtails. J Hered. 2008;99:610–615. doi: 10.1093/jhered/esn053. [DOI] [PubMed] [Google Scholar]
- 35.Hain TJA, Neff BD. Multiple paternity and kin recognition mechanisms in a guppy population. Mol Ecol. 2007;16:3938–3946. doi: 10.1111/j.1365-294X.2007.03443.x. [DOI] [PubMed] [Google Scholar]
- 36.Neff BD, Pitcher TE, Ramnarine IW. Inter-population variation in multiple paternity and reproductive skew in the guppy. Mol Ecol. 2008;17:2975–2984. doi: 10.1111/j.1365-294X.2008.03816.x. [DOI] [PubMed] [Google Scholar]
- 37.Zane L, Nelson WS, Jones AG, Avise JC. Microsatellite assessment of multiple paternity in natural populations of a live-bearing fish, Gambusia holbrooki. J Evol Biol. 1999;12:61–69. [Google Scholar]
- 38.Soucy S, Travis J. Multiple paternity and population genetic structure in natural populations of the poeciliid fish, Heterandria formosa. J Evol Biol. 2003;16:1328–1336. doi: 10.1046/j.1420-9101.2003.00608.x. [DOI] [PubMed] [Google Scholar]
- 39.Reisser CMO, Beldade R, Bernardi G. Multiple paternity and competition in sympatric congeneric reef fishes, Embiotoca jacksoni and E. lateralis. Mol Ecol. 2009;18:1504–1510. doi: 10.1111/j.1365-294X.2009.04123.x. [DOI] [PubMed] [Google Scholar]
- 40.Van Doornik DM, Parker SJ, Millard SR, Berntson EA, Moran P. Multiple paternity is prevalent in Pacific ocean perch (Sebastes alutus) off the Oregon coast, and is correlated with female size and age. Environ Biol Fishes. 2008;83:269–275. [Google Scholar]
- 41.Gonzalez EB, et al. Paternity testing of wild black rockfish Sebastes inermis (brownish type) from Seto Inland Sea of Japan. Ichthyol Res. 2009;56:87–91. [Google Scholar]
- 42.Mobley KB, Jones AG. Geographical variation in the mating system of the dusky pipefish (Syngnathus floridae) Mol Ecol. 2007;16:2596–2606. doi: 10.1111/j.1365-294X.2007.03337.x. [DOI] [PubMed] [Google Scholar]
- 43.Rispoli VF, Wilson AB. Sexual size dimorphism predicts the frequency of multiple mating in the sex-role reversed pipefish Syngnathus typhle. J Evol Biol. 2008;21:30–38. doi: 10.1111/j.1420-9101.2007.01470.x. [DOI] [PubMed] [Google Scholar]
- 44.Wilson AB. Interspecies mating in sympatric species of Syngnathus pipefish. Mol Ecol. 2006;15:809–824. doi: 10.1111/j.1365-294X.2006.02831.x. [DOI] [PubMed] [Google Scholar]
- 45.McCoy EE, Jones AG, Avise JC. The genetic mating system and tests for cuckoldry in a pipefish species in which males fertilize eggs and brood offspring externally. Mol Ecol. 2001;10:1793–1800. doi: 10.1046/j.0962-1083.2001.01320.x. [DOI] [PubMed] [Google Scholar]
- 46.Wilson AB, Martin-Smith KM. Genetic monogamy despite social promiscuity in the pot-bellied seahorse (Hippocampus abdominalis) Mol Ecol. 2007;16:2345–2352. doi: 10.1111/j.1365-294X.2007.03243.x. [DOI] [PubMed] [Google Scholar]
- 47.DeWoody JA, Fletcher DE, Wilkins SD, Nelson WS, Avise JC. Molecular genetic dissection of spawning, parentage, and reproductive tactics in a population of redbreast sunfish, Lepomis auritus. Evolution. 1998;52:1802–1810. doi: 10.1111/j.1558-5646.1998.tb02257.x. [DOI] [PubMed] [Google Scholar]
- 48.Dewoody JA, Fletcher DE, Mackiewicz M, Wilkins SD, Avise JC. The genetic mating system of spotted sunfish (Lepomis punctatus): Mate numbers and the influence of male reproductive parasites. Mol Ecol. 2000;9:2119–2128. doi: 10.1046/j.1365-294x.2000.01123.x. [DOI] [PubMed] [Google Scholar]
- 49.Mackiewicz M, Fletcher DE, Wilkins SD, DeWoody JA, Avise JC. A genetic assessment of parentage in a natural population of dollar sunfish (Lepomis marginatus) based on microsatellite markers. Mol Ecol. 2002;11:1877–1883. doi: 10.1046/j.1365-294x.2002.01577.x. [DOI] [PubMed] [Google Scholar]
- 50.DeWoody JA, Fletcher DE, Wilkins SD, Avise JC. Parentage and nest guarding in the tessellated darter (Etheostoma olmstedi) assayed by microsatellite markers (Perciformes: Percidae) Copeia. 2000;3:740–747. [Google Scholar]
- 51.Jones AG, Östlund-Nilsson S, Avise JC. A microsatellite assessment of sneaked fertilizations and egg thievery in the fifteenspine stickleback. Evolution. 1998;52:848–858. doi: 10.1111/j.1558-5646.1998.tb03709.x. [DOI] [PubMed] [Google Scholar]
- 52.Jones AG, Walker D, Kvarnemo C, Lindström K, Avise JC. How cuckoldry can decrease the opportunity for sexual selection: Data and theory from a genetic parentage analysis of the sand goby, Pomatoschistus minutus. Proc Natl Acad Sci USA. 2001;98:9151–9156. doi: 10.1073/pnas.171310198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Porter BA, Fiumera AC, Avise JC. Egg mimicry and allopaternal care: Two mate-attracting tactics by which nesting striped darter (Etheostoma virgatum) males enhance reproductive success. Behav Ecol Sociobiol. 2002;51:350–359. [Google Scholar]
- 54.Fiumera AC, Porter BA, Grossman GD, Avise JC. Intensive genetic assessment of the mating system and reproductive success in a semi-closed population of the mottled sculpin, Cottus bairdi. Mol Ecol. 2002;11:2367–2377. doi: 10.1046/j.1365-294x.2002.01585.x. [DOI] [PubMed] [Google Scholar]
- 55.Mackiewicz M, Porter BA, Dakin EE, Avise JC. Cuckoldry rates in the Molly Miller (Scartella cristata, Blenniidae), a hole-nesting marine fish with alternative reproductive tactics. Mar Biol. 2005;148:213–221. [Google Scholar]
- 56.Tatarenkov A, Barreto F, Winkelman DL, Avise JC. Genetic monogamy in the channel catfish, Ictalurus punctatus, a species with uniparental nest guarding. Copeia. 2006;4:735–741. [Google Scholar]
- 57.Mobley KB, Amundsen T, Forsgren E, Svensson PA, Jones AG. Multiple mating and a low incidence of cuckoldry for nest-holding males in the two-spotted goby, Gobiusculus flavescens. BMC Evol Biol. 2009;9:6. doi: 10.1186/1471-2148-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Andersson M. Evolution of classical polyandry: Three steps to female emancipation. Ethology. 2005;111:1–23. [Google Scholar]
- 59.Uller T, Olsson M. Multiple paternity in reptiles: Patterns and processes. Mol Ecol. 2008;17:2566–2580. doi: 10.1111/j.1365-294X.2008.03772.x. [DOI] [PubMed] [Google Scholar]
- 60.Jones AG, Ardren WR. Methods of parentage analysis in natural populations. Mol Ecol. 2003;12:2511–2523. doi: 10.1046/j.1365-294x.2003.01928.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.