Abstract
Neurotransmitter release is achieved through the fusion of synaptic vesicles with the neuronal plasma membrane (exocytosis). Vesicles are then retrieved from the plasma membrane (endocytosis). It was hypothesized more than 3 decades ago that endosomes participate in vesicle recycling, constituting a slow endocytosis pathway required especially after prolonged stimulation. This recycling model predicts that newly endocytosed vesicles fuse with an endosome, which sorts (organizes) the molecules and buds exocytosis-competent vesicles. We analyzed here the endosome function using hippocampal neurons, isolated nerve terminals (synaptosomes), and PC12 cells by stimulated emission depletion microscopy, photooxidation EM, and several conventional microscopy assays. Surprisingly, we found that endosomal sorting is a rapid pathway, which appeared to be involved in the recycling of the initial vesicles to be released on stimulation, the readily releasable pool. In agreement with the endosomal model, the vesicle composition changed after endocytosis, with the newly formed vesicles being enriched in plasma membrane proteins. Vesicle proteins were organized in clusters both in the plasma membrane (on exocytosis) and in the endosome. In the latter compartment, they segregated from plasma membrane components in a process that is likely important for sorting/budding of newly developed vesicles from the endosome.
Keywords: endosome, photooxidation, recycling, readily releasable pool, stimulated emission depletion microscopy
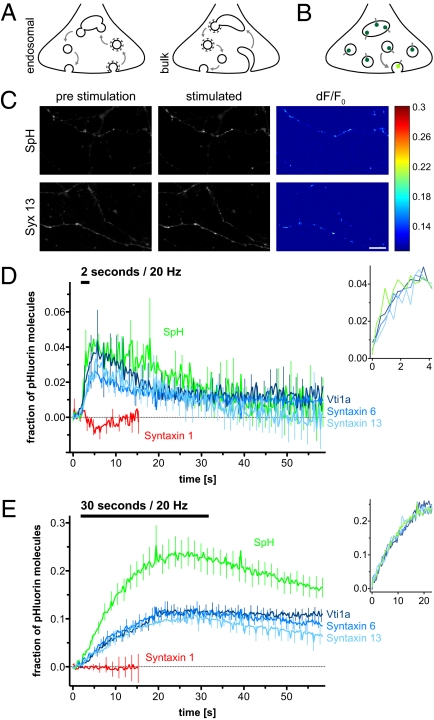
Synaptic vesicles collapse into the plasma membrane to release neurotransmitter, followed by retrieval via clathrin-coated vesicles. These may simply uncoat to generate exocytosis-competent synaptic vesicles, or they may first need to fuse with what is termed a “sorting” (or early) endosome, from which exocytosis-competent synaptic vesicles would subsequently bud (1). Coated vesicles connected to endosome-like structures were noted in EM more than 3 decades ago (2). However, they were soon afterward interpreted as evidence for endocytosis of plasma membrane infoldings (bulk endocytosis), followed by vesicle budding from the infoldings (e.g., 3, 4; reviewed in 5) (Fig. 1A).
Fig. 1.
Endosomal pHluorins reveal participation of endosomal proteins in synaptic vesicle recycling. (A) “Endosomal recycling” and “bulk endocytosis” models. Clathrin-coated vesicles retrieve membrane in both models. They uncoat and fuse to the endosome in the first model. In the second model, they bud from (rather than fuse to) plasma membrane infoldings. (B) Function of pHluorin. The pH-sensitive GFP moiety is quenched at the low pH of the acidic endosomes/synaptic vesicles (dark green). GFP fluorescence increases on fusion (change from acidic to neutral pH). (C) Synaptic boutons expressing synaptopHluorin (Upper) or syntaxin 13-pHluorin (Lower) respond to stimulation; resting preparations (Left) and preparations stimulated for 17 s (Center) are indicated. (Right) The fractional change in fluorescence (dF) is indicated, normalized to the prestimulation fluorescence (F0), in the form of dF/F0. (Scale bar: 15 μm.) (D) Change in pHluorin signal on RRP-releasing stimuli (20 Hz/2 s). Means ± SEM from 9–38 experiments are shown. For clarity, only every fifth error bar and only a short stretch of the syntaxin 1-pHluorin signal are shown. (Inset) Signal rise on stimulation is indicated, with all traces normalized to the synaptopHluorin maximum. (E) Change in pHluorin signal on stimuli releasing the entire recycling pool (20 Hz/30 s). Means ± SEM from 5–27 experiments are shown. Significantly more synaptopHluorin than endosomal pHluorin is exocytosed (P < 0.0001, Kolmogorov–Smirnov test). (Inset) Signal rise on stimulation is indicated normalizing all traces to the synaptopHluorin maximum.
Several lines of evidence currently support the existence of endosomes in the synapse (although none are free of controversy). First, synaptic vesicles contain sorting (early) endosome markers, such as endosomal soluble N-ethylmaleimide-sensitive factor attachment receptor (SNARE) fusion proteins and the membrane organizer Rab5 (6–9). However, they also contain a plethora of other SNAREs and Rabs (9). Second, an organelle containing the lipid phosphatidyl-inositol-3-phosphate (PI3P, a known component of sorting endosomes) has been observed in the Drosophila neuromuscular junction; importantly, it recycles along with synaptic vesicles (10). However, this compartment is unknown in mammalian neurons (11), and organelles purified from mammalian nerve terminals fuse at very low levels with bona fide sorting endosomes in vitro (8). Finally, perturbation of endosomal protein activity has resulted in (generally mild) changes in synaptic vesicle recycling, as observed for Rab5 mutants (10, 12), for the adaptor protein AP3 (13), and for the PI3-kinase (14). In agreement with these findings, it has been recently observed that deletion of the adaptor protein σ1B-adaptin (thought to participate in sorting endosome dynamics) perturbs synaptic vesicle recycling, inducing the appearance of large organelles with attached clathrin-coated vesicles (15), which may represent bulk endocytosis intermediates that may later fuse to a (presumably small-sized) endosome (16).
The quantitative role played by the endosome and the recycling step at which the molecular identity of the vesicle changes (thus requiring the endosomal sorting step) remain enigmatic (16). To address these issues, we studied synaptic vesicle recycling by a combination of microscopy approaches. Surprisingly, we found that endosomal sorting is a rapid pathway, functioning for the initial vesicles to be released on stimulation, the readily releasable pool (RRP).
Results
Endosomal Proteins Recycle with Rapid Kinetics.
We have demonstrated in the past that the endosomal SNARE fusion proteins syntaxin 13, syntaxin 6, and vti1a are strongly enriched on synaptic vesicles and that they participate in the fusion between purified recently endocytosed synaptic organelles and bona fide endosomes in vitro (8). To test the involvement of endosomal proteins in vesicle recycling directly, we generated three unique fluorescent reporters by linking a pH-sensitive GFP (pHluorin) (17) to the intravesicular domains of these three SNAREs. The GFP fluorescence is quenched by the low pH inside the synaptic vesicle and increases on exocytosis (Fig. 1B).
As pHluorin controls, we used synaptobrevin, the major SNARE of the synaptic vesicle [using the well-characterized synaptopHluorin construct (17)], and the plasma membrane SNARE syntaxin 1 (18). All endosomal pHluorin constructs recycled during stimulation (Fig. 1C) in hippocampal cultured neurons; they did not affect vesicle recycling, and were properly expressed on intracellular organelles (Fig. S1).
Both synaptopHluorin and the endosomal pHluorins indicated sizeable release on a 20-Hz/2-s stimulation train, which recycles the RRP of synaptic vesicles [∼10–20% of all vesicles (19)] (Fig. 1D). Confirming the validity of the assay, no increase in syntaxin 1-pHluorin signal could be detected on stimulation, in agreement with the fact that it is mainly expressed on the plasma membrane (Fig. S1). Scaling the pHluorin signals to the responses to NH4Cl application (which neutralizes the low pH of the vesicles, and thus reveals the entire pool of pHluorin molecules, not shown in the figure) indicated that the fraction of endosomal SNAREs participating in RRP exocytosis is similar to that of synaptopHluorin (with similar kinetics) (Fig. 1D Inset). In contrast, prolonged stimulation (20 Hz/30 s) caused the release of a substantially smaller proportion of endosomal SNAREs vs. synaptopHluorin (with similar, although slightly slower, kinetics: half-times of 12.2, 11.7, and 18.2 s for syntaxin 13, syntaxin 6, and vti1a, respectively, compared with 10.5 s for synaptopHluorin) (Fig. 1E).
We conclude that endosomal proteins can recycle with rapid kinetics during vesicle recycling, and that they seem to be mainly present on the RRP rather than on slower releasing vesicles.
Morphological Evidence for Endosomal Fusion of RRP Vesicles.
We next addressed directly the question of whether RRP vesicles participate in endosomal fusion. We first investigated the morphology of the endocytosed organelles, by resorting to labeling with the styryl dye FM 1-43, followed by diaminobenzidine photooxidation [a technique allowing for identification of the fluorescently labeled organelles in EM (20)].
The RRP-releasing stimulus (20 Hz/2 s) was delivered in presence of FM 1-43 dye, followed by fixation at 4, 10, or 30 s after stimulation (Fig. S2 A and B). Small labeled vesicles were visible at 4 s after stimulation. They increased in size, although decreasing in number, at 10 s after stimulation. The 30 s distribution was again dominated by small vesicles (Fig. S2 C and D). The total amount of label did not appear to change throughout (Fig. S2E). The result is in agreement with a model in which the recently endocytosed vesicles fuse with endosomes (and/or to each other, homotypic fusion), followed by endosomal budding (Fig. 1A Left and Fig. S2A Upper); in contrast, the formation of large organelles through bulk endocytosis at 10 s would have caused an increase in both the number of labeled organelles and the total amount of label.
To verify whether synaptic vesicles fuse with endosomes (and/or to each other) by a different assay, we marked vesicle proteins by differently colored Quantum Dots (Qdots; Invitrogen) and monitored their endocytosis. The synaptotagmin molecules on the surface membrane (corresponding to fused vesicle proteins) were labeled using a biotinylated antibody (21), which was, in turn, detected by streptavidin-coated Qdots (22). We applied the same RRP-releasing stimulus as above (20 Hz/2 s) and monitored the distance between the Qdots at 4, 10, and 30 s after stimulation (Fig. S2 F and G). At 10 s after stimulation, a substantial proportion of Qdots appeared tightly pressed against each other (within less than ∼25 nm from each other; note that the radius of the Qdot, plus the synaptotagmin antibody, is at least ∼12 nm); they separated again at 30 s, in agreement with the EM data (Fig. S2 H and I; details presented in SI Materials and Methods).
Transport of Vesicle Protein to Bona Fide Endosomes.
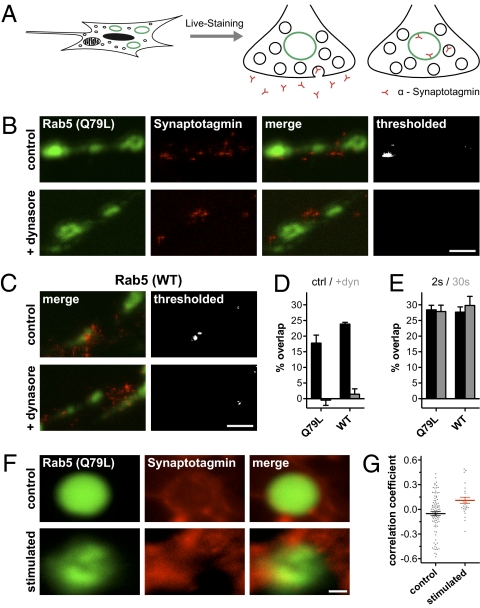
The RRP vesicles should also recycle at normal physiological culture activity (5). If they indeed use endosomal recycling, they would do so during culture activity as well and not only after a stimulus. However, the assays we used above rely on temporally defined changes taking place after a stimulus and cannot be used to test this issue. We therefore developed a different approach, in which we labeled the endosomal compartment by expressing GFP-tagged variants of the marker Rab5 [both WT and a constitutively active GTP-bound mutant, Rab5 Q79L (23)]; expression of these proteins does not seem to perturb endosomal trafficking in the neuroendocrine cell line PC12 (Fig. S3). We then tested whether synaptotagmin antibodies added to the cultures could reach the endosomes (Fig. 2A). We investigated this by superresolution stimulated emission depletion (STED) microscopy in ultrathin sections, with a 3D resolution of ∼70–80 nm. The antibodies reached the endosome equally well for constitutively active Rab5 and WT Rab5 (Fig. 2 B–D); the reaction is specific and depends on dynamin-dependent endocytosis of the vesicles (Fig. 2 B–D and Fig. S4). Confirming the results obtained above, the RRP-releasing stimulus (20 Hz/2 s) also induced a substantial colocalization of the synaptotagmin antibodies with the endosomal signal (Fig. 2E). To test whether strong stimulation (releasing more than just the RRP) would further increase the fraction of molecules recycling via endosomes, we repeated the experiments using a 20-Hz/30-s stimulus. Importantly, the amount of colocalization after the longer stimulus was comparable to that obtained on the RRP-releasing stimulus (Fig. 2E), in agreement with Fig. 1.
Fig. 2.
Fusion of recently endocytosed vesicles with bona fide endosomes. (A) We labeled the endosomes by expressing GFP-Rab5 (green) and the recycling vesicles by incubation with a fluorescently labeled synaptotagmin antibody (red) for 5 min at 37 °C. The colocalization of the two signals would indicate fusion of the recently endocytosed vesicle to the endosome. High-resolution imaging of preparations expressing a dominant active GFP-Rab5 (Q79L) (B) or WT GFP-Rab5 (C) is shown. The vesicle marker is imaged by STED microscopy, with a lateral resolution of ∼70–80 nm. The preparations were cut in ultrathin (∼80 nm) sections, and Rab5 is imaged with confocal lateral resolution. Recently endocytosed vesicles (red) reach the endosomes (green) in controls (Upper) but not in preparations treated with the dynamin inhibitor Dynasore (ChemBridge Corporation) (Lower). (Right) Area of overlap is shown (thresholded). (Scale bar: 1 μm.) Dynasore effects on styryl dye uptake and release are presented in Fig. S4. (D) Quantification of overlap for dominant active GFP-Rab5 (Q79L) and WT GFP-Rab5. The fraction of the endosome area colocalizing with synaptotagmin spots was determined and normalized to a maximum colocalization control [obtained from synaptotagmin double-immunostaining (20)]. Dynasore treatment abolishes the overlap (P < 0.01, t test). Data represent the mean ± SEM of three independent experiments. Overlap values were corrected by subtracting the overlap between the green signal and a mirror image of the red signal (which accounts for the negative values). (E) Overlap of the synaptic vesicle marker with endosomes on electrical stimulation. Experiments were performed as in A–D, after stimulating the samples using RRP-releasing protocols (20 Hz/2 s) or recycling pool-releasing protocols (20 Hz/30 s). The samples were then fixed and processed after 10 s (as in Fig. S2). Data represent the mean ± SEM from four to six experiments. (F) Live STED imaging. Neurons expressing dominant active Rab5 (Q79L; green) were labeled with antibodies recognizing the luminal domain of synaptotagmin (red) (24). Summed movie (Movie S1) frames are shown, indicating the general colocalization of the endosome and the vesicles before (Upper) or during stimulation (20 Hz/30 s; Lower). (Scale bar: 500 nm.) (G) Correlation between Rab5 (Q79L) and synaptotagmin. The correlation between endosome and synaptotagmin signals increases slightly on stimulation (P < 0.05, t test). Data represent the mean ± SEM from 92 (nonstimulated) and 27 (stimulated) endosomal regions (from 63 and 18 movies, respectively). The individual values are shown by the scatter plots.
To verify this observation, we investigated the interaction of the synaptotagmin molecules with the endosome by live STED imaging (details presented in 24). We monitored synaptic vesicle motion both at rest and during strong stimulation (20 Hz/30 s) (Fig. 2F and Movie S1). The fusion of vesicles to the endosomes was quantified by the correlation between the Rab5-GFP and synaptotagmin signals. As in the fixed samples (Fig. 2 B–E), prolonged stimulation only caused a small increase in correlation when compared with normal culture activity (Fig. 2G). This is in agreement with the hypothesis that endosomal sorting predominantly targets the RRP.
Inhibition of Endosomal Sorting Reduces the RRP.
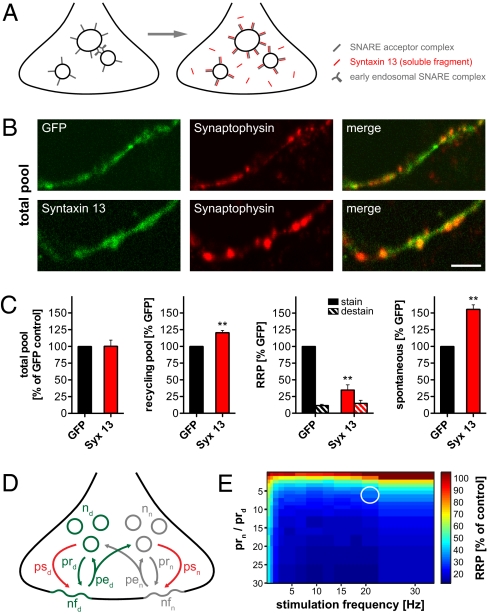
To verify whether RRP recycling strictly requires endosomal activity, we sought to inhibit endosomal function by expressing soluble fragments of syntaxin 13. These fragments are designed specifically to perturb the fusion of the vesicles to the endosomes (Fig. 3A), without interfering with general endosome activity (the recycling of several endosome-targeted markers did not appear to have severe effects in PC12 cells) (Fig. S5).
Fig. 3.
RRP is strongly reduced by inhibiting endosomal recycling. (A) Blocking endosomal fusion. Soluble syntaxin 13 fragments are expressed (red); they engage the endosomal SNAREs on the membranes of endosomes and synaptic vesicles, thus blocking the formation of fusogenic SNARE complexes (8). GFP was expressed together with the syntaxin 13 fragments to identify transfected neurons; GFP alone was expressed as a control. (B) Total vesicle pool of hippocampal cultures visualized by immunostaining for synaptophysin (red). (Scale bar: 2.5 μm.) (Upper) Axons expressing GFP alone. (Lower) Axons expressing both GFP and the syntaxin 13 fragment. Labeling examples of other vesicle pools are presented in Fig. S6 A–C. (C) Quantification of vesicle pools. Note that all values are presented as percentages of the GFP-only control. (Left) Total pool of vesicles was measured from the synaptophysin immunostaining. Different vesicle pools were labeled in independent experiments with the styryl dye FM 4-64FX (10 μM): the recycling pool by 20-Hz/30-s stimulation, the RRP by 20-Hz/2-s stimulation, and the spontaneous pool by incubation with FM 4-64FX for 15 min in presence of 1 μM tetrodotoxin. Note that the FM-loaded RRP vesicles could be re-released by a 20-Hz/30-s stimulus in both conditions (“destain,” hashed bars). Data are presented as the mean ± SEM from three independent experiments. Asterisks indicate significant changes (**P < 0.01, t test). (D) Monte Carlo model of RRP recycling under endosomal inhibition. Normal (n) vesicles were placed in the synapse; endocytosis occasionally retrieved improperly sorted vesicles (containing plasma membrane components; also illustrated in Fig. 4), which we termed “dirty” for the sake of simplicity in the context of this model. Under normal circumstances, these vesicles would be sorted by the endosome. The vesicle numbers (n), the numbers of fused vesicles (nf), their probabilities to release on stimulation (pr) or spontaneously (ps), their probabilities to be endocytosed (pe), and the probability that a normal vesicle would be retrieved as a dirty one (and vice versa) were all modeled according to our own data and values obtained from the literature (details presented in SI Materials and Methods). The RRP size on a 20-action potential (AP) train (at different frequencies) is monitored first in the absence of any dirty vesicles. We then block endosomal sorting and allow the accumulation of dirty vesicles during a prolonged activity period (1,000-AP train, 1 Hz). We finally induce a second 20-AP train and measure the different RRP size as a percentage of the size on the initial train. (E) RRP release on the second 20-AP train, expressed as a percentage of the first train (control), in relation to the stimulation frequency (x axis) and the probability of release of dirty vesicles relative to normal ones (y axis). The experimental results from C are best fit by a model in which the dirty vesicles have a 6-fold lower release probability than the normal ones (circle).
We expressed the syntaxin 13 fragments using an internal ribosomal entry site system, in which simultaneously expressed GFP (not linked to syntaxin 13) serves to identify the modified neurons. We expressed GFP alone as a control. We first analyzed the total vesicle pool of the transfected neurons. We fixed the cultures and immunostained them against the synaptic vesicle marker synaptophysin (Fig. 3B). As indicated in Fig. 3CLeft, neurons transfected with syntaxin 13 fragments contained the same amount of synaptophysin (i.e., the same amount of vesicles).
Second, we incubated the neurons with the fixable styryl dye FM 4-64FX (10 μM) and stimulated them for different periods of time to determine the size of the different functional synaptic vesicle pools. The pool loaded by a 20-Hz/30-s stimulus (recycling pool) increased significantly, by ∼20%; in contrast, the RRP (loaded by a 20-Hz/2-s stimulus as above) decreased by more than 50% (Fig. 3C). The simplest interpretation is that the inhibition of endosomal recycling decreased the ability of the RRP vesicles to release rapidly, and thus integrated them into the slower releasing recycling pool. Additionally, spontaneous release (taking place during a 15-min incubation in the presence of tetrodotoxin) increased significantly (Fig. 3C) (typical images are presented in Fig. S6 A–C). Finally, a syntaxin 13 soluble fragment lacking the SNARE domain had no effects on any of these parameters (Fig. S6D).
A 60% reduction of the RRP is not necessarily equivalent to a 60% reduction in the release probability for all RRP vesicles. This issue is relatively complex, because some RRP vesicles may be sorted correctly at the plasma membrane during the endocytosis step even when endosomal sorting is inhibited. Therefore, some correctly sorted RRP vesicles may be present even during endosomal inhibition and provide much of the RRP release. To obtain an approximation of how efficient the release of an RRP vesicle would be after failing to sort correctly, we modeled the vesicle recycling system using parameters from the literature and parameters we found in this study (Fig. 3D; description presented in SI Materials and Methods). The model suggests that a 60% drop in RRP size corresponds to a 6-fold reduction in the ability to release for a vesicle that failed to sort correctly (Fig. 3E, circle).
Vesicular Molecular Identity Changes on Endocytosis but Not on Exocytosis.
We next investigated the recycling step at which the vesicle identity changes and induces a requirement for endosomal sorting. Does the vesicle lose its identity in the plasma membrane on exocytosis? Conversely, does endocytosis retrieve not just the vesicle but contaminating molecules from the plasma membrane?
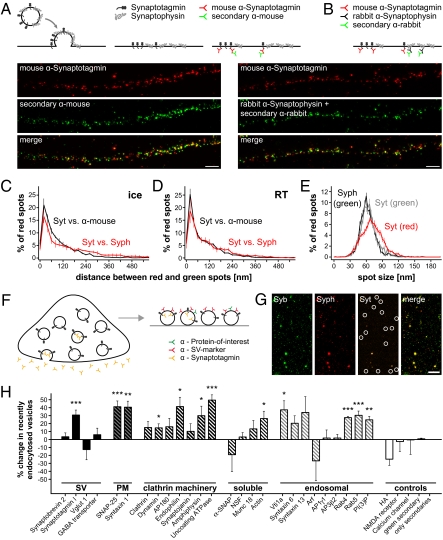
The issue of whether the vesicle molecules diffuse into the plasma membrane on exocytosis is highly contested in the current synaptic literature. Synaptotagmin forms clusters in the plasma membrane (21), and the clusters formed by different vesicles do not appear to interchange molecules (20); however, an interchange of molecules between different fused vesicles was suggested by pHluorin-dependent techniques (discussed in 25). To differentiate between these scenarios, we investigated simultaneously the exocytosis of two synaptic vesicle markers, synaptotagmin and synaptophysin. We blocked synaptotagmin epitopes present on the plasma membrane and then allowed for exocytosis (while inhibiting endocytosis, either at a low temperature or in the absence of divalent ions) (Fig. S7 A and B). The recently exocytosed vesicles were monitored by two-color STED microscopy (Fig. 4 A and B); confocal microscopy cannot be used here because it barely detects the synapses (Fig. S7 C and D). The colocalization of the two proteins was very similar to the positive control (synaptotagmin antibodies detected by anti-mouse antibodies), suggesting that the two vesicle markers remain clustered on exocytosis (Fig. 4 C–E). Thus, the vesicle appears to maintain its identity on fusion, at least at a broad level.
Fig. 4.
Maintenance of the molecular identity of the vesicle during recycling. (A–E) Synaptotagmin and synaptophysin remain largely coclustered on exocytosis. (A) Colocalization positive control. Only recently exocytosed vesicles are investigated, with the experiments performed after blocking the surface synaptotagmin epitopes with nonfluorescent antibodies (not shown in the cartoon; Fig. S7A). Neurons are then incubated with Atto647N-coupled mouse anti-synaptotagmin antibodies (Atto-tec; red). Endocytosis is blocked either by keeping the neurons on ice or by incubating them at room temperature in absence of divalents (Fig. S7B) to prevent the internalization of the label. After fixation, the samples are immunostained with secondary anti-mouse antibodies coupled to Atto590 (Atto-tec; green). Note the high colocalization in two-color STED microscopy. (Scale bar: 1 μm.) (B) Synaptotagmin/synaptophysin colocalization. Neurons are incubated with Atto647N-coupled mouse anti-synaptotagmin antibodies (red) and rabbit antibodies that recognize the luminal domain of synaptophysin. After fixation, the samples are immunostained with secondary anti-rabbit antibodies (Atto590; green) to visualize the synaptophysin staining. (Scale bar: 1 μm.) Spot-to-spot distance analysis is performed for experiments in which endocytosis was blocked by low temperature on ice (C) or at room temperature in the absence of divalents (D). Note that the two sets of distributions are similar [although Kolmogorov Smirnov tests detect a significant difference for C (P = 0.0057)]. (E) Spot size. Both synaptotagmin and synaptophysin form well-defined clusters of about 60–70 nm, within the resolution of the microscope. (C–E) All data represent the mean ± SEM from 3 to 6 independent experiments. (F–H) Change of synaptic vesicle composition on endocytosis. (F) Synaptosomes were purified and stimulated by depolarization (50 mM KCl) in presence of fluorescently coupled antibodies against the luminal domain of synaptotagmin. The vesicles were isolated, adsorbed onto glass coverslips, and immunostained for a synaptic vesicle marker (red) and a protein of interest (green). (G) Typical images of vesicles immunostained for synaptobrevin (Syb) as protein of interest and synaptophysin (Syph) as a synaptic vesicle marker. Several recently endocytosed vesicles are indicated in the (dim) synaptotagmin (Syt) channel. (Scale bar: 7.5 μm.) (H) Summary of the change in protein composition for the recently endocytosed vesicles vs. general pool vesicles. Several plasma membrane, clathrin-mediated endocytosis machinery, and early endosomal proteins are enriched in the recently endocytosed vesicle pool. Asterisks indicate significant changes (*P < 0.05; **P < 0.01; ***P < 0.001; t tests). Data are presented as the mean ± SEM of 3–39 independent experiments (typically 3–6 experiments; data for HA show the mean ± range of values for 2 independent experiments, data for “only secondaries” represent 1 experiment). A complete description and discussion are presented in Fig. S8.
Does endocytosis subsequently retrieve plasma membrane components along with the vesicle molecules? Stimulation of cultured neurons resulted in an increase of plasma membrane proteins in the vesicle cluster, although this can be explained by both bulk endocytosis and endosomal recycling (Fig. S8 A–C). To test the issue directly, we purified synaptosomes from rat brain and depolarized them (50 mM KCl) in the presence of a fluorescently coupled synaptotagmin antibody (8) (Fig. 4F). Endocytosis on depolarization resulted in a relatively homogeneous fraction of small vesicles (i.e., bulk endocytosis events must have been relatively rare) (Fig. S8 D and E). After disrupting the synaptosomes by hypoosmotic shock, the fluorescently labeled vesicles were immunostained for different markers (Fig. 4G). The recently endocytosed vesicles contained the same levels of general vesicle constituents as all other vesicles but presented much higher levels of the plasma membrane marker SNAREs syntaxin 1 and SNAP-25 (Fig. 4H). This is in agreement with the slight but significant endocytosis of syntaxin 1-pHluorin immediately after the RRP-releasing stimulus (Fig. 1D; P < 0.05, Kolmogorov–Smirnov test). We conclude that endocytosis tends to retrieve plasma membrane components in synaptosomes, although one should note that not every newly endocytosed vesicle is necessarily enriched in such components. These vesicles were also enriched in endosomal molecules, confirming the suggestion that they use endosomal sorting (Fig. 4H).
Synaptic Vesicle and Plasma Membrane Components Segregate in Cholesterol-Dependent Domains on Endosomes.
How is the segregation between vesicular and plasma membrane components achieved in the endosome? We first sought to verify whether these components do indeed separate in the endosome. We investigated single endosomes [bona fide early endosomes purified from neuroendocrine cells (8) or synaptosomal organelles] by two-color STED microscopy. Synaptic vesicle markers and plasma membrane proteins appeared to avoid each other (Fig. S9A Upper). Interestingly, this avoidance was eliminated by removing cholesterol from the membranes by incubation with methyl-β-cyclodextrin (26): The two types of proteins now appeared to intermix (Fig. S9A Lower). In contrast, the colocalization of two vesicle markers (synaptophysin and synaptobrevin) decreased slightly on cholesterol removal or remained similar (Fig. S9 B and C). These results suggest that the vesicular and plasma membrane components prefer different lipid phases in the endosome, which may contribute to their segregation and, ultimately, to the sorting process.
Discussion
We suggest here that endosomal sorting is not a slow recycling pathway. It is about as fast as clathrin-mediated endocytosis would normally be (up to ∼30 s needed for the cycle) (Fig. S2), which puts it on almost the same time scale required for the rerelease of a vesicle using the fastest vesicle recycling model ever proposed, kiss-and-run (27).
Importantly, the identity of the molecules budding synaptic vesicles from the endosome is still unknown (although some factors have been suggested in studies on microvesicle formation in PC12 cells; e.g., ref. 28). However, it is likely that few of the large array of molecules forming a synaptic vesicle (9) would be able to interact directly with the sorting machinery, because many seem unable to interact even with the components of the much more strictly required clathrin pathway (29). Our observations favor a view in which the synaptic vesicle components are held together throughout the vesicle cycle, perhaps forming multicomponent domains reminiscent of membrane rafts (30). The clathrin machinery would then be able to retrieve quantal amounts of vesicle material from the plasma membrane by binding to a limited number of vesicle components, most notably synaptotagmin (29). Furthermore, the presence of domains would serve sorting in the endosome, as suggested in Fig. S9. The fact that several important vesicle components remain together after detergent solubilization (31) correlates with this observation, as does the finding that synaptic protein domains on endosomes are quite resistant to changes in their membrane environment (26). It is unclear why such protein clusters would be segregated from plasma membrane components in the endosome but not directly at the plasma membrane (during clathrin-dependent endocytosis); Rab5 effectors or PI3P-binding proteins, which would be selectively available at the endosome membrane, may play a part.
Although not many vesicles strictly require the endosome, those that do are the most important components of the synapse—the vesicles that normally recycle on physiological activity, the RRP. Importantly, endosomal recycling would thus be one of the few molecular mechanisms differentiating the RRP from other vesicles. Why would RRP vesicles preferentially sort their membranes via endosomes? It is possible that the endosome's role in removing contaminating components (e.g., plasma membrane proteins) is only important for these frequently recycling vesicles; other vesicles may not even fuse too often in vivo, because nonphysiological stimulation is required for their recycling in vitro.
Materials and Methods
Materials, reagents, and previously published methods are described in detail in SI Materials and Methods. PHluorin imaging (Fig. 1) was performed largely as described (18). Photooxidation and EM (Fig. S2) were performed as described (20). Thin section imaging (Fig. 2) and sample processing were performed as described (20). Live STED imaging (Fig. 2) was performed as described (24). Cell culturing, transfection, and conventional confocal imaging (Fig. 3) were performed according to standard procedures. Surface staining of hippocampal cultured neurons was performed largely as described (21). Two-color STED microscopy was performed exactly as described (20). Synaptosomes were prepared and live-stained using a fluorescently labeled antibody against the luminal (exposed) domain of synaptotagmin I exactly as described (8). Synaptosome-derived vesicles were adsorbed onto coverslips as described (8); their immunostaining, imaging, and data analysis details are presented in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank R. Jahn (Max Planck Institute for Biophysical Chemistry) for helpful discussions and ideas as well as the kind gift of antisynaptic vesicle protein antibodies. We thank C. Schäfer and K. Kröhnert for technical assistance and H. Magliarelli and J. Seefeldt for help with experiments. S.O.R. thanks A. Bock for excellent assistance. P.H. acknowledges support from the Boehringer Ingelheim Fonds (PhD Fellowship) and the MSc/PhD program “Molecular Biology.” S.O.R. acknowledges the support of a Starting Grant from the European Research Council, Program FP7, and support from the Deutsche Forschungsgemeinschaft (Grant RI 1967/1-1, Superresolution investigation of synaptic vesicle recycling).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1007037107/-/DCSupplemental.
References
- 1.Südhof TC. The synaptic vesicle cycle. Annu Rev Neurosci. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- 2.Heuser JE, Reese TS. Evidence for recycling of synaptic vesicle membrane during transmitter release at the frog neuromuscular junction. J Cell Biol. 1973;57:315–344. doi: 10.1083/jcb.57.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gennaro JF, Jr, Nastuk WL, Rutherford DT. Reversible depletion of synaptic vesicles induced by application of high external potassium to the frog neuromuscular junction. J Physiol. 1978;280:237–247. doi: 10.1113/jphysiol.1978.sp012382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heuser J. The role of coated vesicles in recycling of synaptic vesicle membrane. Cell Biol Int Rep. 1989;13:1063–1076. doi: 10.1016/0309-1651(89)90020-9. [DOI] [PubMed] [Google Scholar]
- 5.Rizzoli SO, Betz WJ. Synaptic vesicle pools. Nat Rev Neurosci. 2005;6:57–69. doi: 10.1038/nrn1583. [DOI] [PubMed] [Google Scholar]
- 6.Antonin W, Riedel D, von Mollard GF. The SNARE Vti1a-beta is localized to small synaptic vesicles and participates in a novel SNARE complex. J Neurosci. 2000;20:5724–5732. doi: 10.1523/JNEUROSCI.20-15-05724.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fischer von Mollard G, Stahl B, Li C, Südhof TC, Jahn R. Rab proteins in regulated exocytosis. Trends Biochem Sci. 1994;19:164–168. doi: 10.1016/0968-0004(94)90278-x. [DOI] [PubMed] [Google Scholar]
- 8.Rizzoli SO, et al. Evidence for early endosome-like fusion of recently endocytosed synaptic vesicles. Traffic. 2006;7:1163–1176. doi: 10.1111/j.1600-0854.2006.00466.x. [DOI] [PubMed] [Google Scholar]
- 9.Takamori S, et al. Molecular anatomy of a trafficking organelle. Cell. 2006;127:831–846. doi: 10.1016/j.cell.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 10.Wucherpfennig T, Wilsch-Bräuninger M, González-Gaitán M. Role of Drosophila Rab5 during endosomal trafficking at the synapse and evoked neurotransmitter release. J Cell Biol. 2003;161:609–624. doi: 10.1083/jcb.200211087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murthy VN, Stevens CF. Synaptic vesicles retain their identity through the endocytic cycle. Nature. 1998;392:497–501. doi: 10.1038/33152. [DOI] [PubMed] [Google Scholar]
- 12.de Hoop MJ, et al. The involvement of the small GTP-binding protein Rab5a in neuronal endocytosis. Neuron. 1994;13:11–22. doi: 10.1016/0896-6273(94)90456-1. [DOI] [PubMed] [Google Scholar]
- 13.Voglmaier SM, et al. Distinct endocytic pathways control the rate and extent of synaptic vesicle protein recycling. Neuron. 2006;51:71–84. doi: 10.1016/j.neuron.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 14.Rizzoli SO, Betz WJ. Effects of 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one on synaptic vesicle cycling at the frog neuromuscular junction. J Neurosci. 2002;22:10680–10689. doi: 10.1523/JNEUROSCI.22-24-10680.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glyvuk N, et al. AP-1/sigma1B-adaptin mediates endosomal synaptic vesicle recycling, learning and memory. EMBO J. 2010;29:1318–1330. doi: 10.1038/emboj.2010.15. and correction (2010) 29:1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krauss M, Haucke V. Adaptin’ endosomes for synaptic vesicle recycling, learning and memory. EMBO J. 2010;29:1313–1315. doi: 10.1038/emboj.2010.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miesenböck G, De Angelis DA, Rothman JE. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature. 1998;394:192–195. doi: 10.1038/28190. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell SJ, Ryan TA. Syntaxin-1A is excluded from recycling synaptic vesicles at nerve terminals. J Neurosci. 2004;24:4884–4888. doi: 10.1523/JNEUROSCI.0174-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schikorski T, Stevens CF. Morphological correlates of functionally defined synaptic vesicle populations. Nat Neurosci. 2001;4:391–395. doi: 10.1038/86042. [DOI] [PubMed] [Google Scholar]
- 20.Opazo F, et al. Limited intermixing of synaptic vesicle components upon vesicle recycling. Traffic. 2010;11:800–812. doi: 10.1111/j.1600-0854.2010.01058.x. [DOI] [PubMed] [Google Scholar]
- 21.Willig KI, Rizzoli SO, Westphal V, Jahn R, Hell SW. STED microscopy reveals that synaptotagmin remains clustered after synaptic vesicle exocytosis. Nature. 2006;440:935–939. doi: 10.1038/nature04592. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Q, Li Y, Tsien RW. The dynamic control of kiss-and-run and vesicular reuse probed with single nanoparticles. Science. 2009;323:1448–1453. doi: 10.1126/science.1167373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stenmark H, et al. Inhibition of rab5 GTPase activity stimulates membrane fusion in endocytosis. EMBO J. 1994;13:1287–1296. doi: 10.1002/j.1460-2075.1994.tb06381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Westphal V, et al. Video-rate far-field optical nanoscopy dissects synaptic vesicle movement. Science. 2008;320:246–249. doi: 10.1126/science.1154228. [DOI] [PubMed] [Google Scholar]
- 25.Fernández-Alfonso T, Ryan TA. The efficiency of the synaptic vesicle cycle at central nervous system synapses. Trends Cell Biol. 2006;16:413–420. doi: 10.1016/j.tcb.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 26.Geumann U, Schafer C, Riedel D, Jahn R, Rizzoli SO. Synaptic membrane proteins form stable microdomains in early endosomes. Microsc Res Tech. 2010;73:606–617. doi: 10.1002/jemt.20800. [DOI] [PubMed] [Google Scholar]
- 27.Aravanis AM, Pyle JL, Tsien RW. Single synaptic vesicles fusing transiently and successively without loss of identity. Nature. 2003;423:643–647. doi: 10.1038/nature01686. [DOI] [PubMed] [Google Scholar]
- 28.Lichtenstein Y, Desnos C, Faúndez V, Kelly RB, Clift-O'Grady L. Vesiculation and sorting from PC12-derived endosomes in vitro. Proc Natl Acad Sci USA. 1998;95:11223–11228. doi: 10.1073/pnas.95.19.11223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jung N, Haucke V. Clathrin-mediated endocytosis at synapses. Traffic. 2007;8:1129–1136. doi: 10.1111/j.1600-0854.2007.00595.x. [DOI] [PubMed] [Google Scholar]
- 30.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 31.Bennett MK, Calakos N, Kreiner T, Scheller RH. Synaptic vesicle membrane proteins interact to form a multimeric complex. J Cell Biol. 1992;116:761–775. doi: 10.1083/jcb.116.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grabenbauer M, et al. Correlative microscopy and electron tomography of GFP through photooxidation. Nat Methods. 2005;2:857–862. doi: 10.1038/nmeth806. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.