Abstract
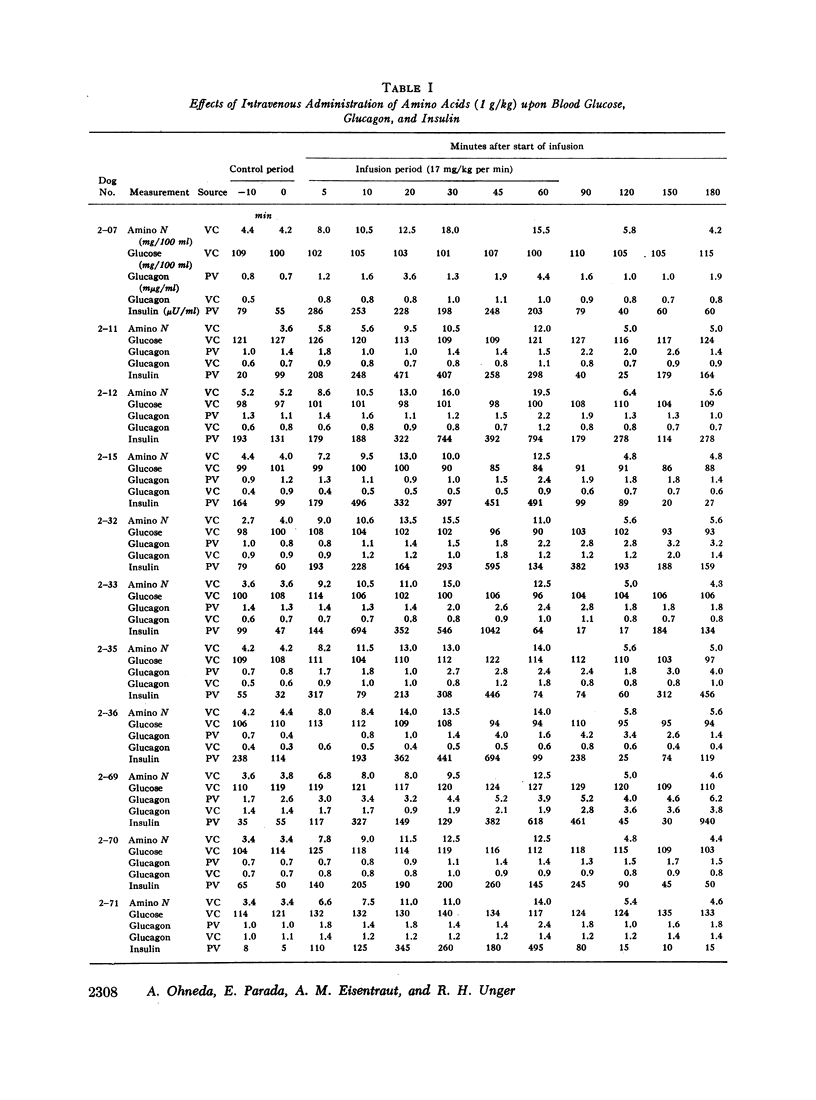
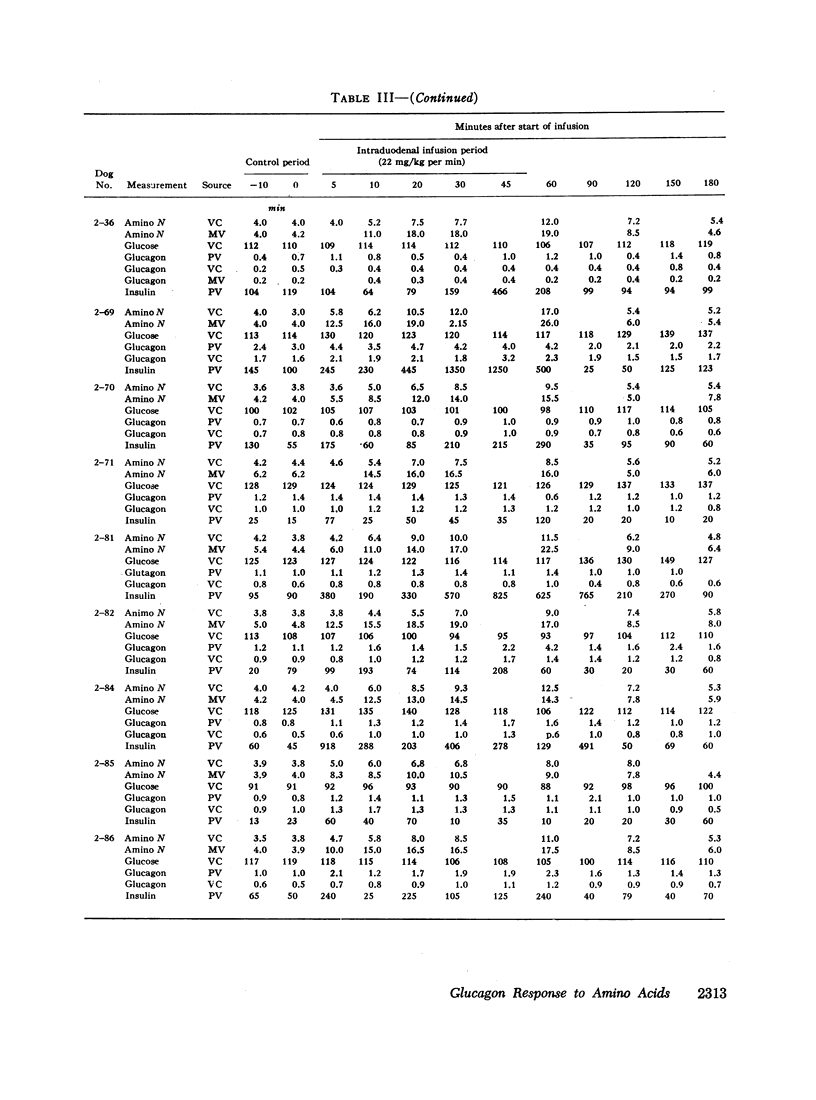
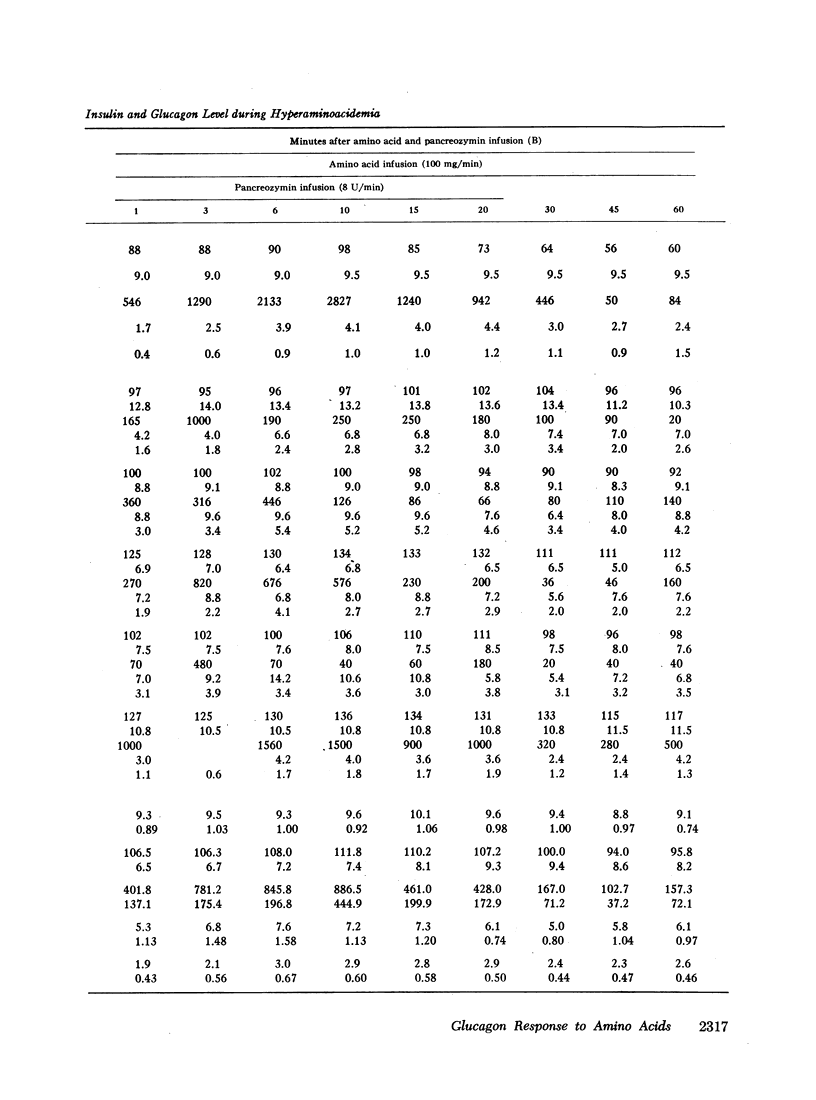
Studies were carried out to determine if hyperaminoacidemia stimulates the secretion of pancreatic glucagon, and, if so, to evaluate the effect of endogenous and exogenous pancreozymin and of hyperglycemia upon this response. The intravenous administration to 16 dogs of 1 g/kg of a 10 amino acid mixture over a 60 min period raised amino nitrogen to a mean level of 13.5 mg/100 ml; mean pancreaticoduodenal vein insulin rose from 84 to 459 μU/ml and glucagon from 1.1 to 2.7 mμg/ml. Further augmentation of both insulin and glucagon secretion was achieved during hyperaminoacidemia by infusing pancreozymin.
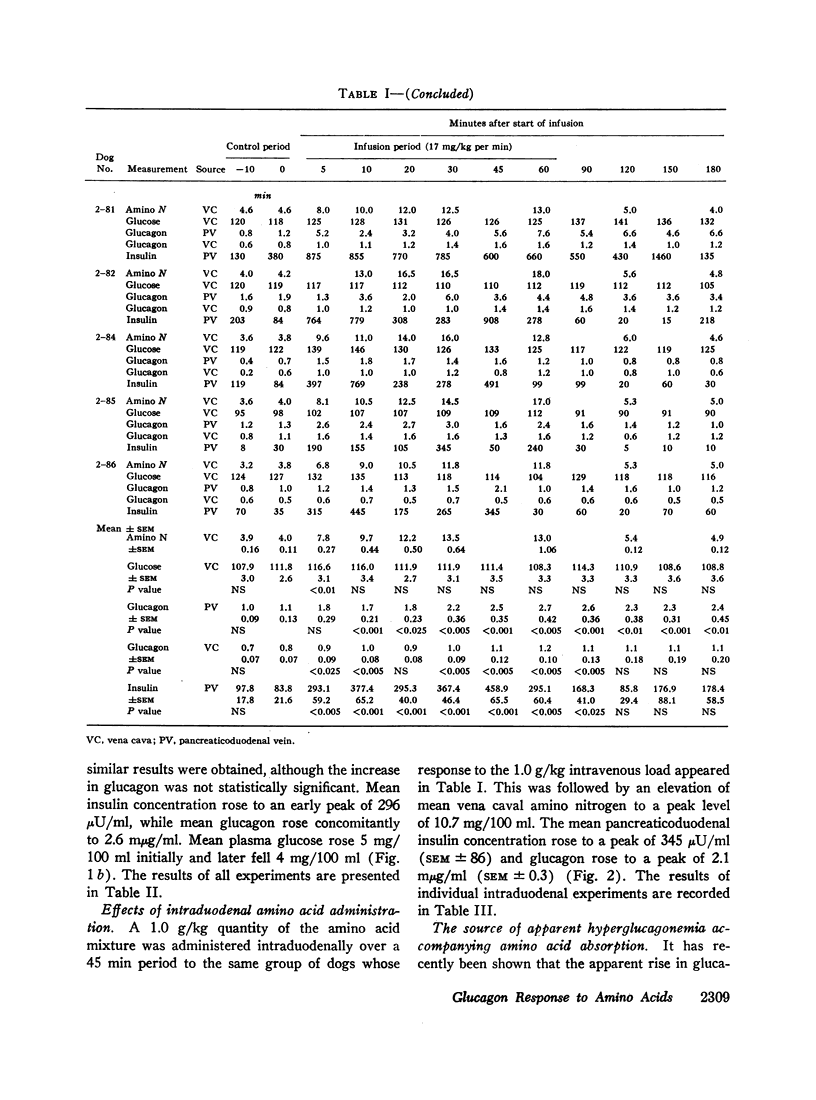
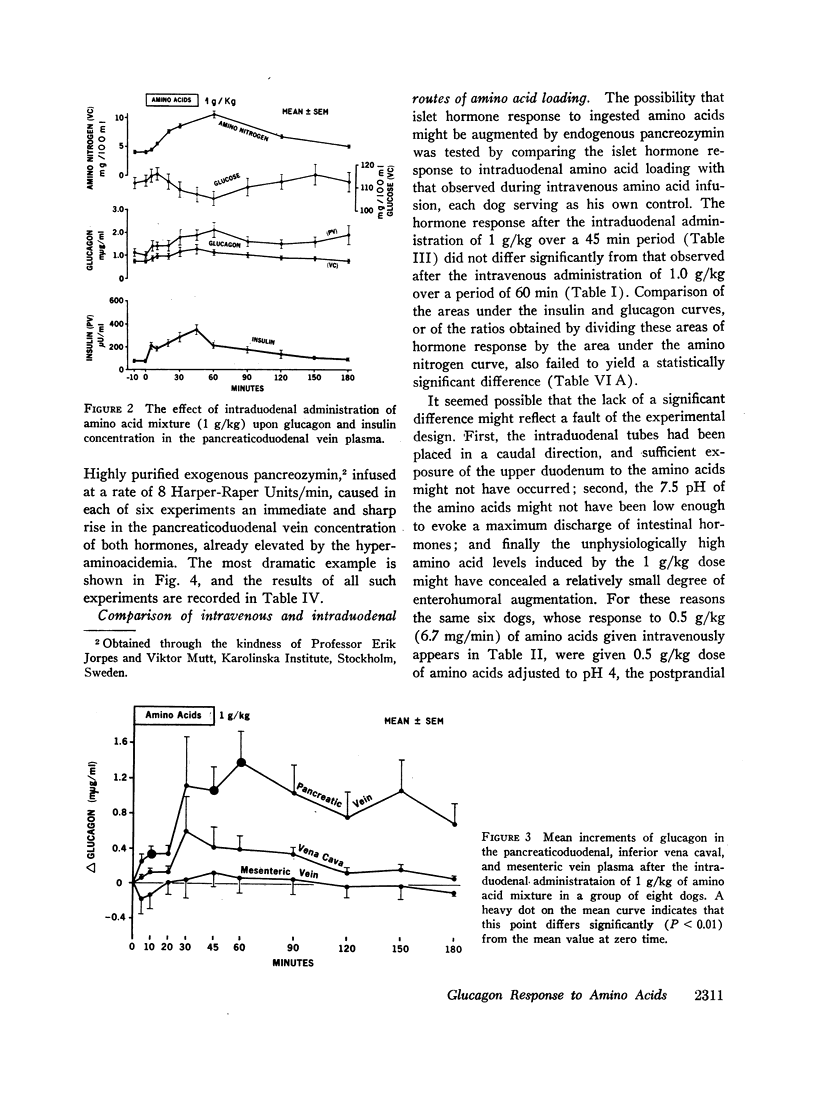
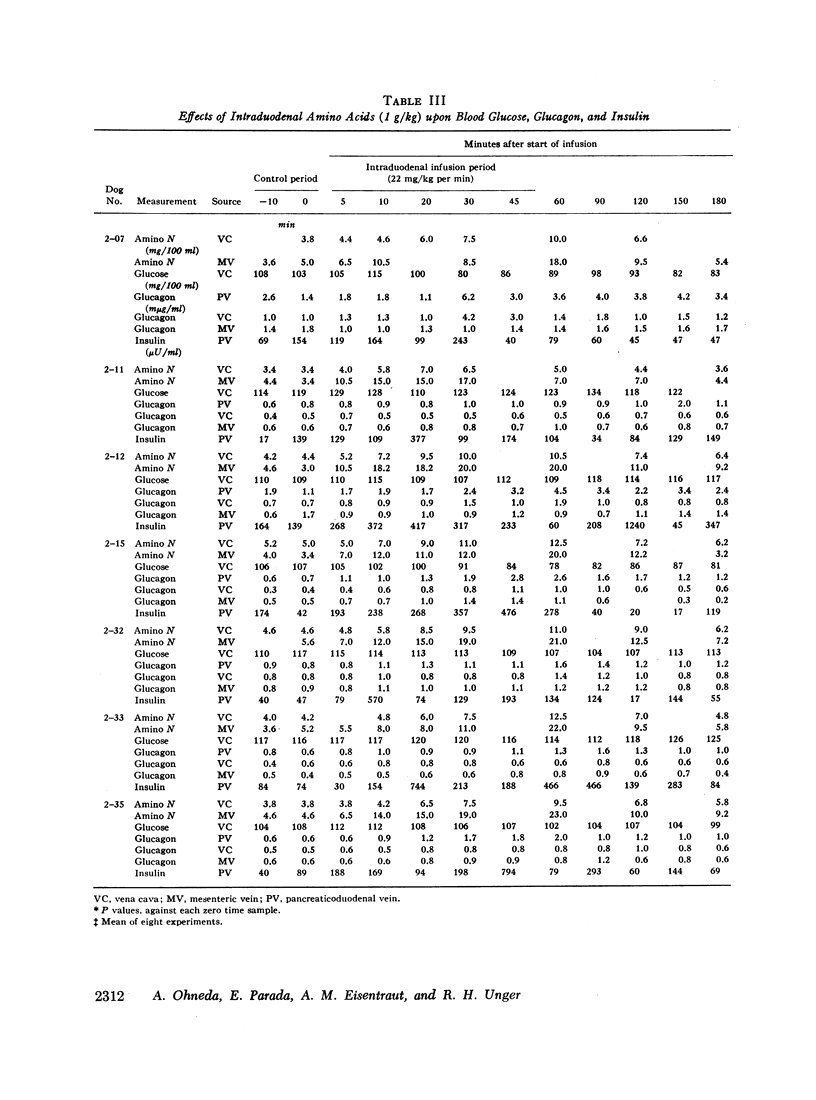
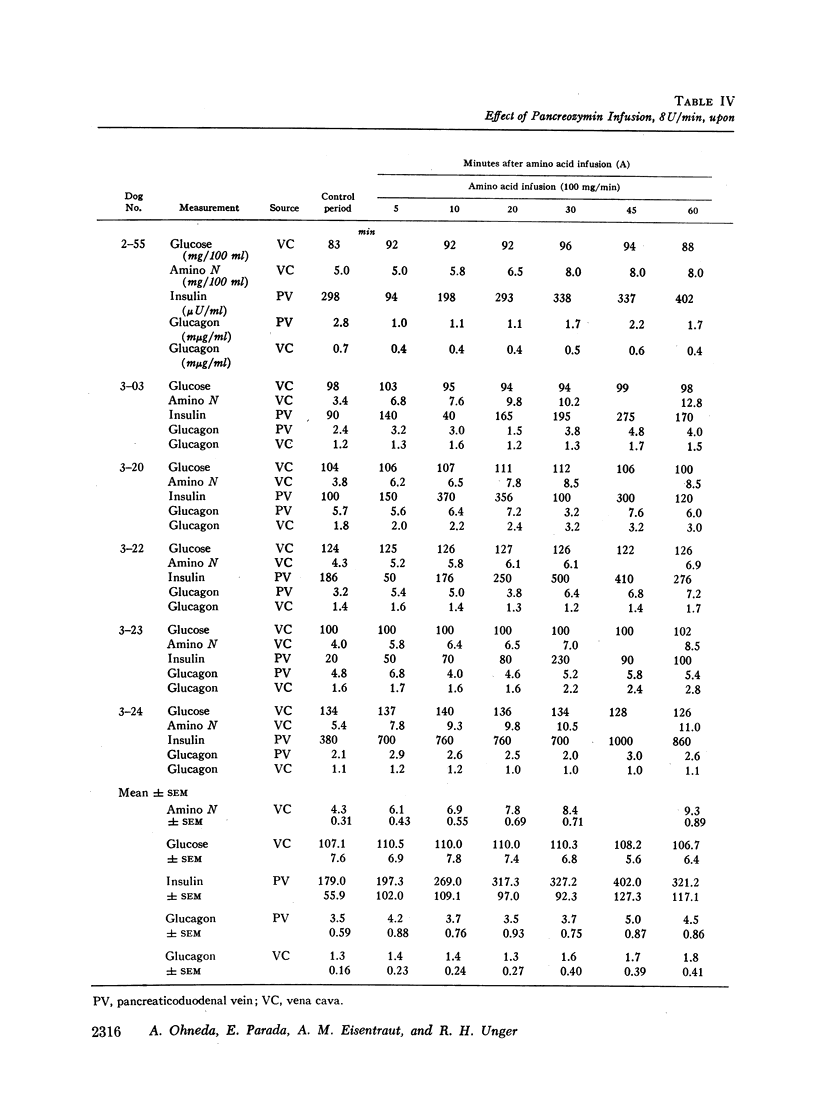
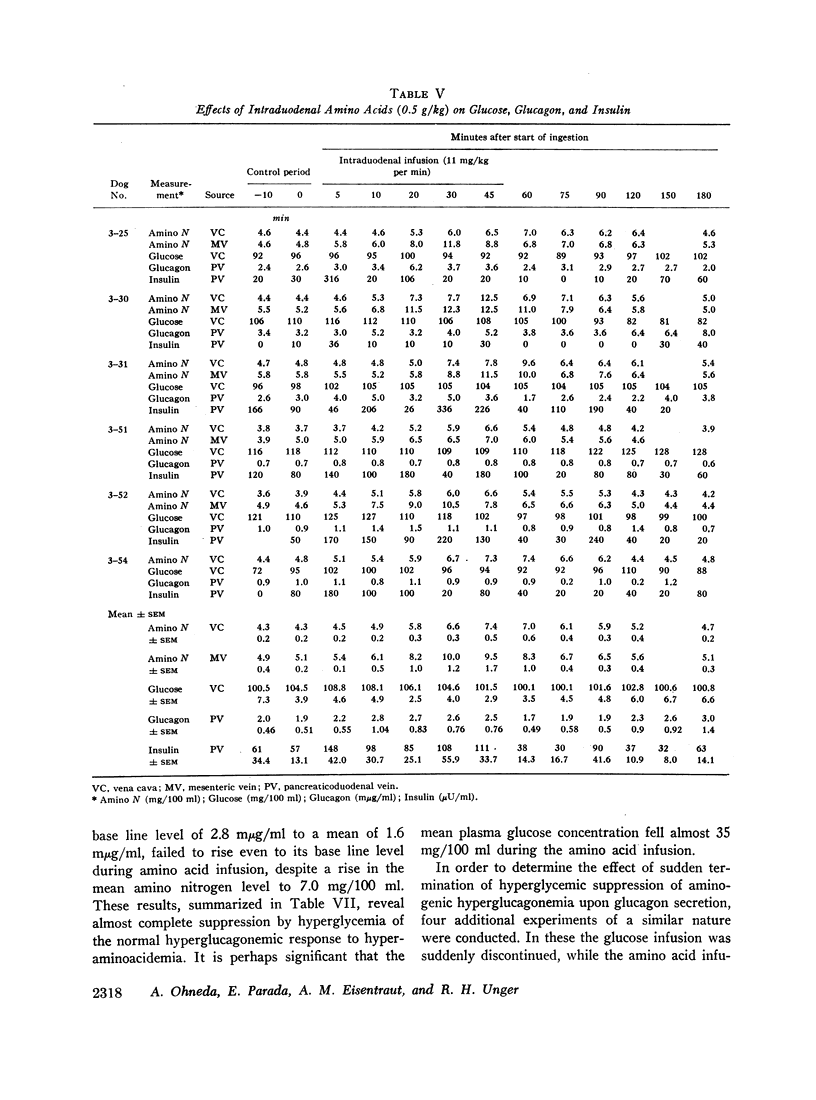
Since endogenous pancreozymin is known to be stimulated by amino acids in the gut, it seemed possible that intraduodenal loading of amino acids would elicit a greater insulin and glucagon response than could be explained by the accompanying hyperaminoacidemia. The intraduodenal administration of 1 g/kg of the amino acid mixture was followed by substantial hyperinsulinemia and hyperglucagonemia, which frequently anticipated the hyperaminoacidemia, and in many of the dogs the ratio of hormone rise to amino nitrogen rise was greater after intraduodenal than after the intravenous route of amino acid administration in the same animal. Intraduodenal administration of amino acids did not cause measurable release of intestinal glucagon-like immunoreactivity into the mesenteric vein plasma.
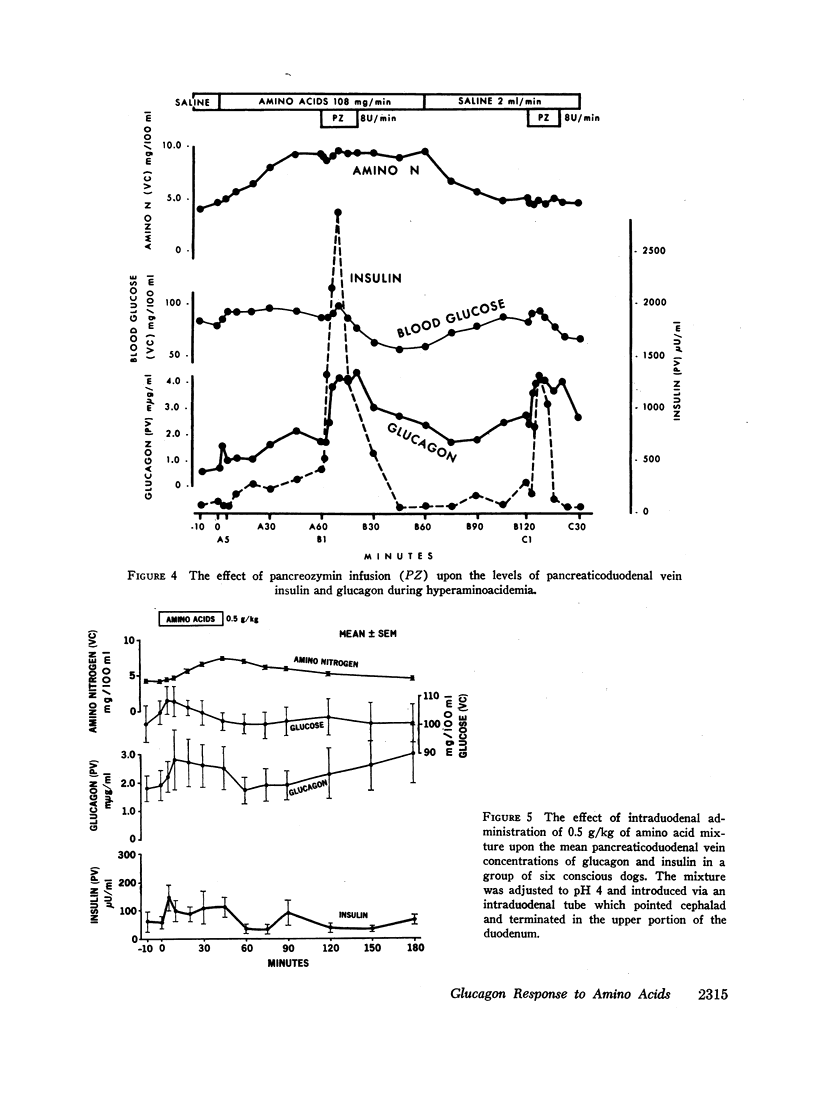
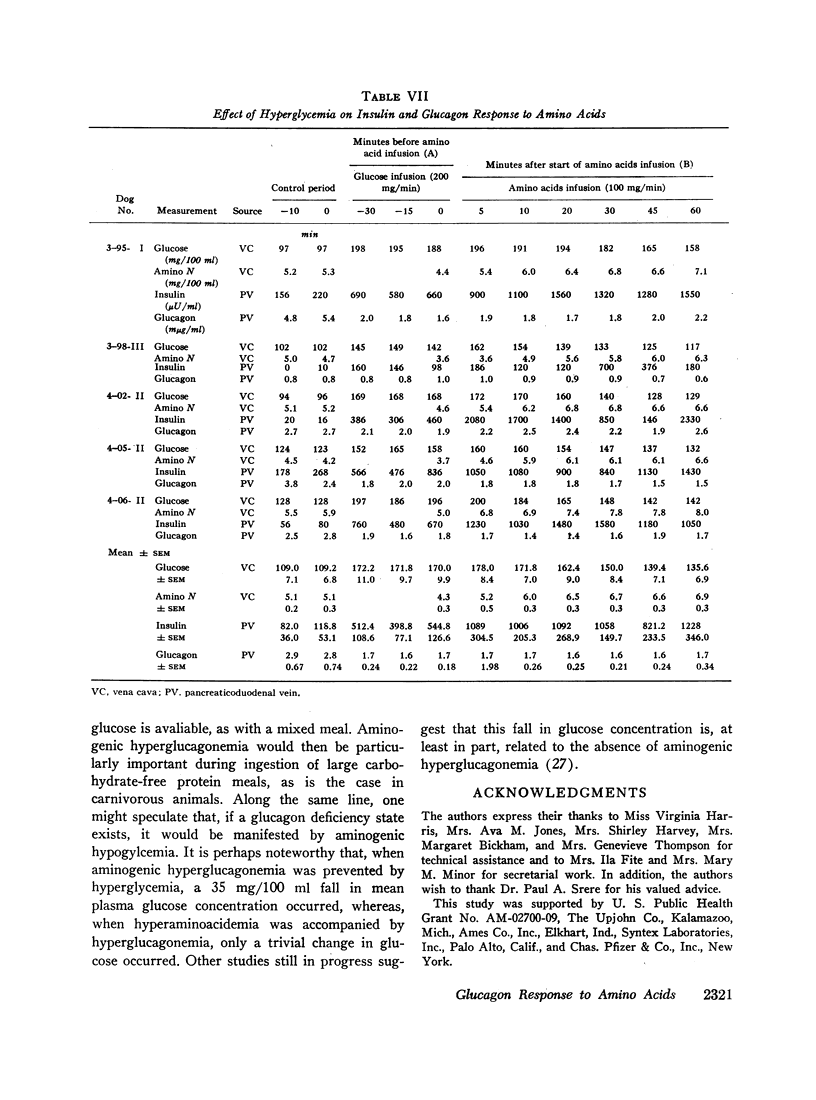
Hyperglycemia induced by constant glucose infusion prevented aminogenic hyperglucagonemia and even suppressed the augmenting action of pancreozymin; sudden termination of the infusion with continued amino acid infusion was associated with a striking rise in glucagon.
It is concluded (a) that hyperaminoacidemia stimulates pancreatic glucagon secretion, (b) that aminogenic hyperglucagonemia is augmented by the infusion of pancreozymin, (c) that intraduodenal administration of amino acids stimulates pancreatic glucagon secretion without measurable release of glucagon-like immunoreactivity into the mesenteric vein, and (d) that hyperglycemia prevents aminogenic hyperglucagonemia even during augmentation with pancreozymin. This conclusion suggests that the prevention of hypoglycemia during amino acid-induced insulin secretion may be an important function of glucagon.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assan R., Rosselin G., Dolais J. Effets sur la glucagonémie des perfusions et ingestions d'acides aminés. Journ Annu Diabetol Hotel Dieu. 1967;7:25–41. [PubMed] [Google Scholar]
- CURRY D. M., BEATON G. H. Glucagon administration in pregnant rats. Endocrinology. 1958 Aug;63(2):252–254. doi: 10.1210/endo-63-2-252. [DOI] [PubMed] [Google Scholar]
- Exton J. H., Park C. R. The role of cyclic AMP in the control of liver metabolism. Adv Enzyme Regul. 1968;6:391–407. doi: 10.1016/0065-2571(68)90024-1. [DOI] [PubMed] [Google Scholar]
- Fajans S. S., Floyd J. C., Jr, Knopf R. F., Conn F. W. Effect of amino acids and proteins on insulin secretion in man. Recent Prog Horm Res. 1967;23:617–662. doi: 10.1016/b978-1-4831-9826-2.50017-9. [DOI] [PubMed] [Google Scholar]
- Floyd J. C., Jr, Fajans S. S., Conn J. W., Knopf R. F., Rull J. Stimulation of insulin secretion by amino acids. J Clin Invest. 1966 Sep;45(9):1487–1502. doi: 10.1172/JCI105456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KALANT N. The effect of glucagon on metabolism of glycine-1-C14. Arch Biochem Biophys. 1956 Dec;65(2):469–474. doi: 10.1016/0003-9861(56)90206-5. [DOI] [PubMed] [Google Scholar]
- Ketterer H., Eisentraut A. M., Unger R. H. Effect upon insulin secretion of physiologic doses of glucagon administered via the portal vein. Diabetes. 1967 May;16(5):283–288. doi: 10.2337/diab.16.5.283. [DOI] [PubMed] [Google Scholar]
- Lawrence A. M. Radioimmunoassayable glucagon levels in man: effects of starvation, hypoglycemia, and glucose administration. Proc Natl Acad Sci U S A. 1966 Feb;55(2):316–320. doi: 10.1073/pnas.55.2.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitz D., Merimee T. J., Burgess J. A., Riggs L. Growth hormone and insulin release after arginine: indifference to hyperglycemia and epinephrine. J Clin Endocrinol Metab. 1966 Oct;26(10):1170–1172. doi: 10.1210/jcem-26-10-1170. [DOI] [PubMed] [Google Scholar]
- SHOEMAKER W. C., VAN ITALLIE T. B. The hepatic response to glucagon in the unanesthetized dog. Endocrinology. 1960 Feb;66:260–268. doi: 10.1210/endo-66-2-260. [DOI] [PubMed] [Google Scholar]
- Samols E., Tyler J., Marri G., Marks V. Stimulation of glucagon secretion by oral glucose. Lancet. 1965 Dec 18;2(7425):1257–1259. doi: 10.1016/s0140-6736(65)92278-6. [DOI] [PubMed] [Google Scholar]
- Samols E., Tyler J., Megyesi C., Marks V. Immunochemical glucagon in human pancreas, gut, and plasma. Lancet. 1966 Oct 1;2(7466):727–729. doi: 10.1016/s0140-6736(66)92982-5. [DOI] [PubMed] [Google Scholar]
- UNGER R. H., EISENTRAUT A. M., McCALL M. S., MADISON L. L. Glucagon antibodies and an immunoassay for glucagon. J Clin Invest. 1961 Jul;40:1280–1289. doi: 10.1172/JCI104357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNGER R. H., EISENTRAUT A. M., McCALL M. S., MADISON L. L. Measurements of endogenous glucagon in plasma and the influence of blood glucose concentration upon its secretion. J Clin Invest. 1962 Apr;41:682–689. doi: 10.1172/JCI104525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger R. H., Ketterer H., Dupré J., Eisentraut A. M. The effects of secretin, pancreozymin, and gastrin on insulin and glucagon secretion in anesthetized dogs. J Clin Invest. 1967 Apr;46(4):630–645. doi: 10.1172/JCI105565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger R. H., Ketterer H., Eisentraut A. M. Distribution of immunoassayable glucagon in gastrointestinal tissues. Metabolism. 1966 Oct;15(10):865–867. doi: 10.1016/0026-0495(66)90156-9. [DOI] [PubMed] [Google Scholar]
- Unger R. H., Ohneda A., Valverde I., Eisentraut A. M., Exton J. Characterization of the responses of circulating glucagon-like immunoreactivity to intraduodenal and intravenous administration of glucose. J Clin Invest. 1968 Jan;47(1):48–65. doi: 10.1172/JCI105714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG C. C., GROSSMAN M. I. Physiological determination of release of secretin and pancreozymin from intestine of dogs with transplanted pancreas. Am J Physiol. 1951 Feb;164(2):527–545. doi: 10.1152/ajplegacy.1951.164.2.527. [DOI] [PubMed] [Google Scholar]
- WEINGES K. F. [The effect of glucagon on the total amino acids in the serum]. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1959;237:17–21. [PubMed] [Google Scholar]
- YALOW R. S., BERSON S. A. Immunoassay of endogenous plasma insulin in man. J Clin Invest. 1960 Jul;39:1157–1175. doi: 10.1172/JCI104130. [DOI] [PMC free article] [PubMed] [Google Scholar]


