Abstract
The rates of catabolism of human γG-immunoglobulins of subclasses γG1, γG2, γG3, and γG4 were studied by determining the rates of elimination from the circulation of pairs of 131I-and 125I-labeled γG-myeloma proteins in 57 patients suffering from cancer other than multiple myeloma. On the average, γG1-, γG2-, and γG4-myeloma proteins were catabolized at a rate similar to that of normal γG-immunoglobulin, whereas γG3-myeloma proteins were catabolized more rapidly than normal γG-immunoglobulin. The average half-lives for the myeloma proteins were 12.3 days for normal γG, 11.6 days for γG1, 12.4 days for γG2, 8.2 days for γG3, and 11.3 days for γG4. However, significant differences in catabolic rates were observed when individual myeloma proteins of a single subclass were compared. These individual variations were present within all four heavy chain subclasses. The extent of differences ranged from 10 to 47%. The catabolic rate of normal γG was in an intermediate range when compared with myeloma proteins of relatively long and short half-lives. The rate of catabolism of an individual myeloma protein did not correlate with its light chain type, Gm factor, carbohydrate content, or electrophoretic mobility. These findings indicate that the structure(s) related to the catabolism of γG-immunoglobulins are complex and differ from one immunoglobulin molecule to another.
Full text
PDF

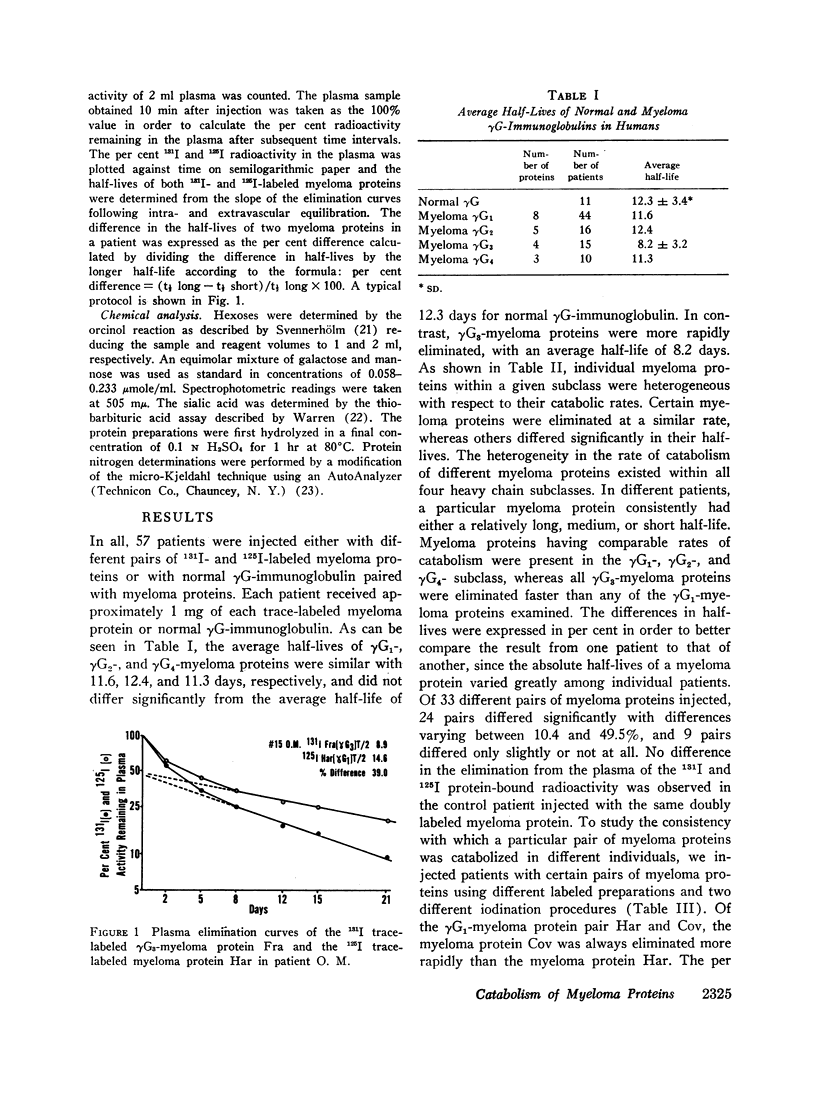
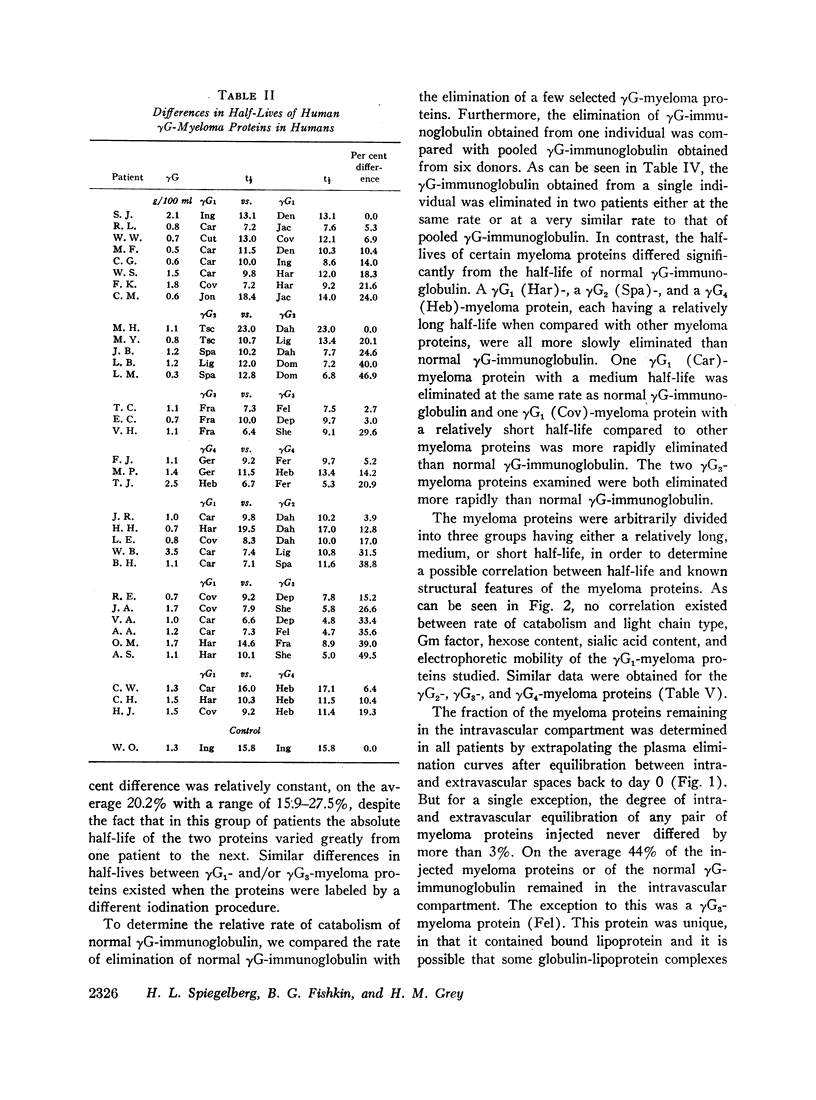
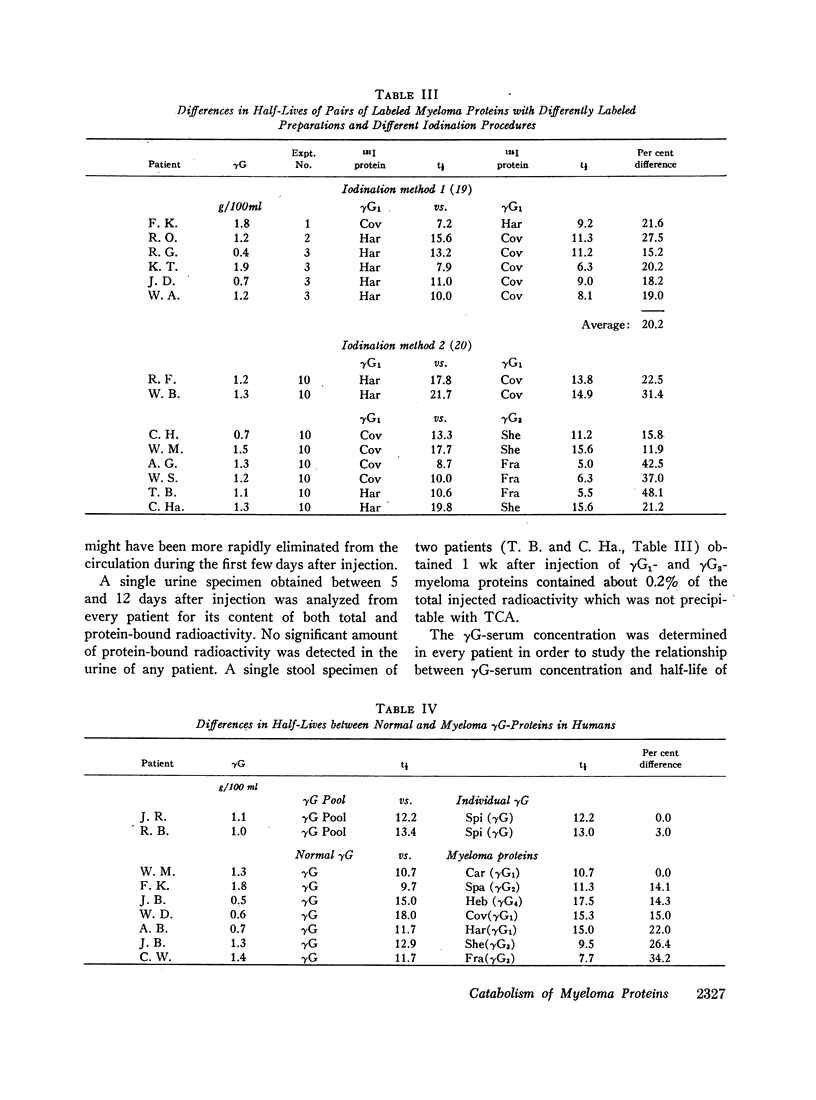
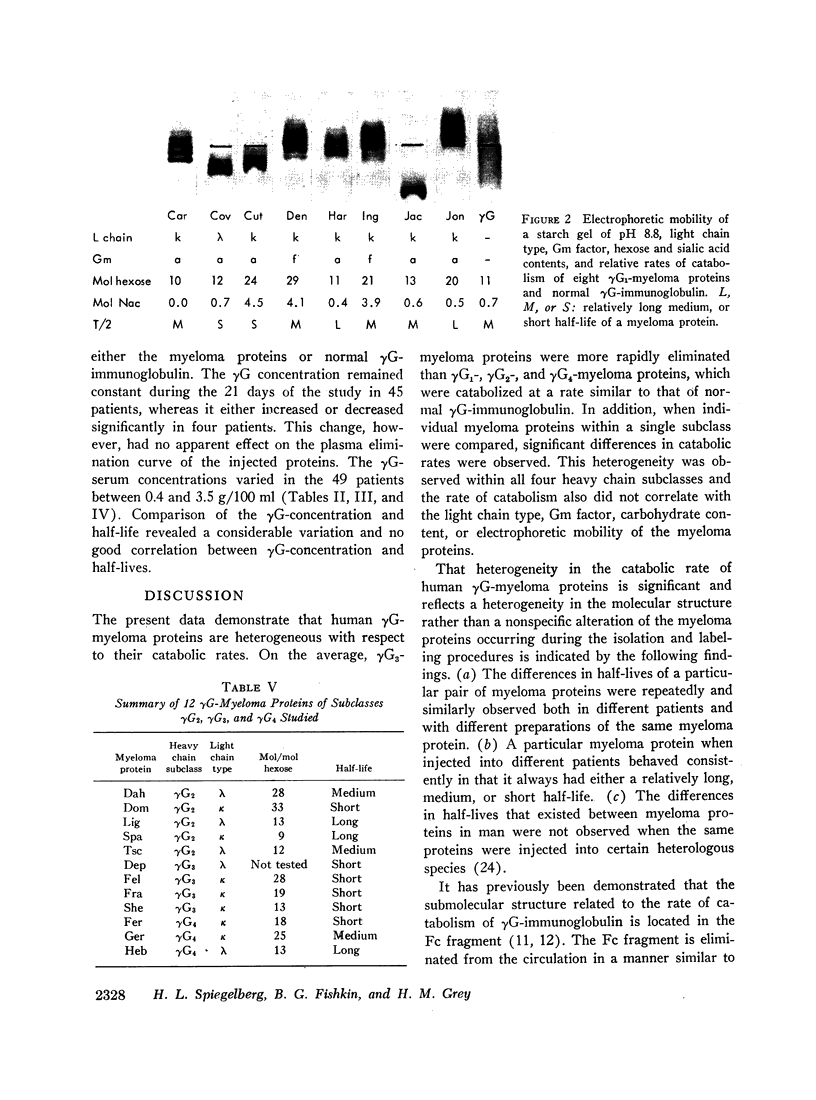


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTH W. F., WOCHNER R. D., WALDMANN T. A., FAHEY J. L. METABOLISM OF HUMAN GAMMA MACROGLOBULINS. J Clin Invest. 1964 Jun;43:1036–1048. doi: 10.1172/JCI104987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambell F. W. The transmission of immunity from mother to young and the catabolism of immunoglobulins. Lancet. 1966 Nov 19;2(7473):1087–1093. doi: 10.1016/s0140-6736(66)92190-8. [DOI] [PubMed] [Google Scholar]
- COHEN S., FREEMAN T. Metabolic heterogeneity of human gamma-globulin. Biochem J. 1960 Sep;76:475–487. doi: 10.1042/bj0760475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAHEY J. L., MCLAUGHLIN C. PREPARATION OF ANTISERA SPECIFIC FOR 6.6 S GAMMA-GLOBULINS, BETA 2A-GLOBULINS, GAMMA-1.-MACROGLOBULINS, AND FOR TYPE I AND II COMMON GAMMA-GLOBULIN DETERMINANTS. J Immunol. 1963 Oct;91:484–497. [PubMed] [Google Scholar]
- FAHEY J. L., ROBINSON A. G. FACTORS CONTROLLING SERUM GAMMA-GLOBULIN CONCENTRATION. J Exp Med. 1963 Nov 1;118:845–868. doi: 10.1084/jem.118.5.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAHEY J. L., SELL S. THE IMMUNOGLOBULINS OF MICE. V. THE METABOLIC (CATABOLIC) PROPERTIES OF FIVE IMMUNOGLOBULIN CLASSES. J Exp Med. 1965 Jul 1;122:41–58. doi: 10.1084/jem.122.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERRARI A. Nitrogen determination by a continuous digestion and analysis system. Ann N Y Acad Sci. 1960 Jul 22;87:792–800. doi: 10.1111/j.1749-6632.1960.tb23236.x. [DOI] [PubMed] [Google Scholar]
- Fahey J. L. HETEROGENEITY OF MYELOMA PROTEINS. J Clin Invest. 1963 Jan;42(1):111–123. doi: 10.1172/JCI104688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangione B., Franklin E. C., Fudenberg H. H., Koshland M. E. Structural studies of human gamma-G-myeloma proteins of different antigenic subgroups and genetic specificities. J Exp Med. 1966 Oct 1;124(4):715–732. doi: 10.1084/jem.124.4.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREY H. M., KUNKEL H. G. H CHAIN SUBGROUPS OF MYELOMA PROTEINS AND NORMAL 7S GAMMA-GLOBULIN. J Exp Med. 1964 Aug 1;120:253–266. doi: 10.1084/jem.120.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grey H. M., Kunkel H. G. Heavy-chain subclasses of human gamma-G-globulin. Peptide and immunochemical relationships. Biochemistry. 1967 Aug;6(8):2326–2334. doi: 10.1021/bi00860a007. [DOI] [PubMed] [Google Scholar]
- Ishizaka T., Ishizaka K., Salmon S., Fudenberg H. Biologic activities of aggregated gamma-globulin. 8. Aggregated immunoglobulins of different classes. J Immunol. 1967 Jul;99(1):82–91. [PubMed] [Google Scholar]
- MULLER-EBERHARD H. J. A new supporting medium for preparative electrophoresis. Scand J Clin Lab Invest. 1960;12:33–37. [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- McConahey P. J., Dixon F. J. A method of trace iodination of proteins for immunologic studies. Int Arch Allergy Appl Immunol. 1966;29(2):185–189. doi: 10.1159/000229699. [DOI] [PubMed] [Google Scholar]
- Rogentine G. N., Jr, Rowe D. S., Bradley J., Waldmann T. A., Fahey J. L. Metabolism of human immunoglobulin D (IgD). J Clin Invest. 1966 Sep;45(9):1467–1478. doi: 10.1172/JCI105454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPIEGELBERG H. L., WEIGLE W. O. THE CATABOLISM OF HOMOLOGOUS AND HETEROLOGOUS 7S GAMMA GLOBULIN FRAGMENTS. J Exp Med. 1965 Mar 1;121:323–338. doi: 10.1084/jem.121.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEINBERG A. G., WILSON J. A., LANSET S. A new human gamma globulin factor determined by an allele at the Inv locus. Vox Sang. 1962;7:151–156. doi: 10.1111/j.1423-0410.1962.tb03239.x. [DOI] [PubMed] [Google Scholar]
- SVENNERHOLM L. The quantitative estimation of cerebrosides in nervous tissue. J Neurochem. 1956 May;1(1):42–53. doi: 10.1111/j.1471-4159.1956.tb12053.x. [DOI] [PubMed] [Google Scholar]
- Spiegelberg H. L., Weigle W. O. Studies on the catabolism of gamma- G subunits and chains. J Immunol. 1965 Dec;95(6):1034–1040. [PubMed] [Google Scholar]
- TAKATSUKI K., OSSERMAN E. F. STRUCTURAL DIFFERENCES BETWEEN 2 TYPES OF "HEAVY CHAIN" DISEASE PROTEINS AND MYELOMA GLOBULINS OF CORRESPONDING TYPES. Science. 1964 Jul 31;145(3631):499–500. doi: 10.1126/science.145.3631.499. [DOI] [PubMed] [Google Scholar]
- TERRY W. D., FAHEY J. L. SUBCLASSES OF HUMAN GAMMA-2-GLOBULIN BASED ON DIFFERENCES IN THE HEAVY POLYPEPTIDE CHAINS. Science. 1964 Oct 16;146(3642):400–401. doi: 10.1126/science.146.3642.400. [DOI] [PubMed] [Google Scholar]
- Terry W. D. Skin-sensitizing activity related to gamma- polypeptide chain characteristics of human IgG. J Immunol. 1965 Dec;95(6):1041–1047. [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- WEIGLE W. O., DIXON F. J. The antibody response of lymph node cells transferred to tolerant recipients. J Immunol. 1959 Jun;82(6):516–519. [PubMed] [Google Scholar]




