Abstract
Derivatives of guanidine, such as phenethylbiguanide, are potent inhibitors of mitochondrial respiration in vitro, but the relevance of this inhibition to their in vivo blood sugar-lowering action is not clear. We have studied the metabolism of pyruvate and long chain fatty acids by mitochondria from several tissues of guinea pigs and rats and observed the effects of phenethylbiguanide on these processes. The rate of pyruvate decarboxylation and of β-oxidation of long chain fatty acyl-CoA derivatives by guinea pig heart mitochondria in vitro has been found to exceed the flux of substrate through the citric acid cycle, both in the presence and absence of phosphate acceptor.
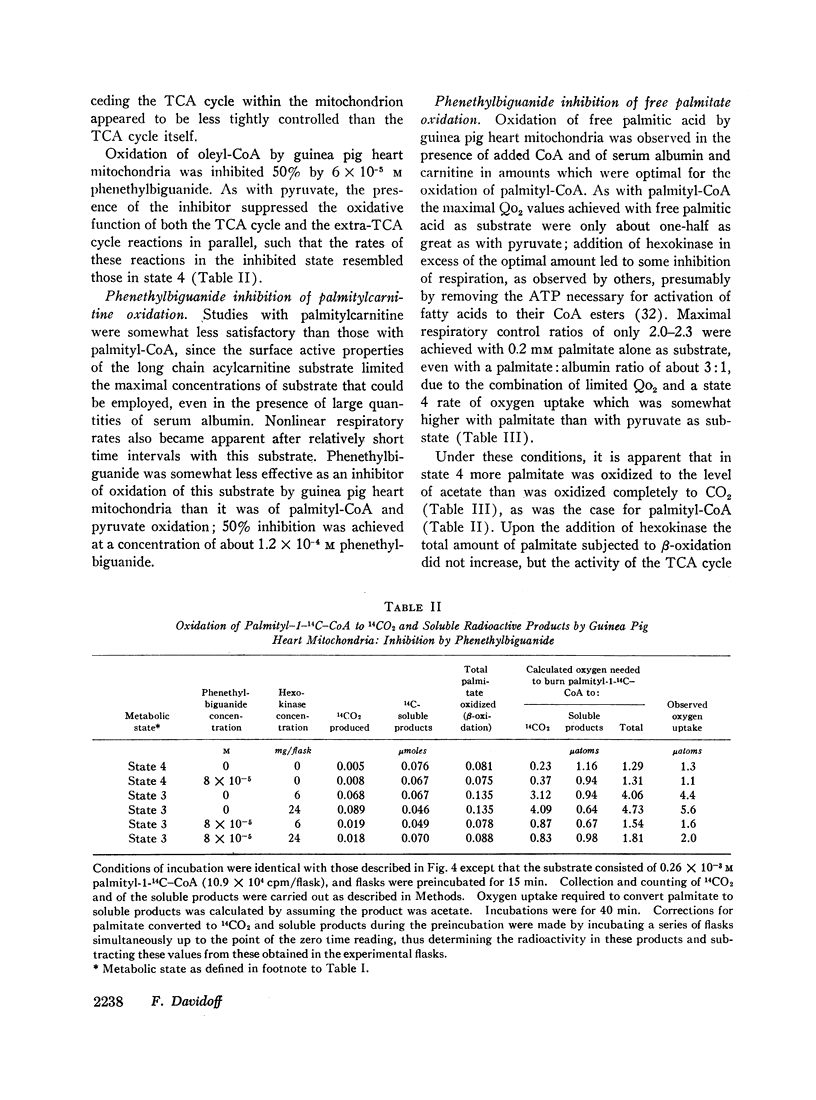
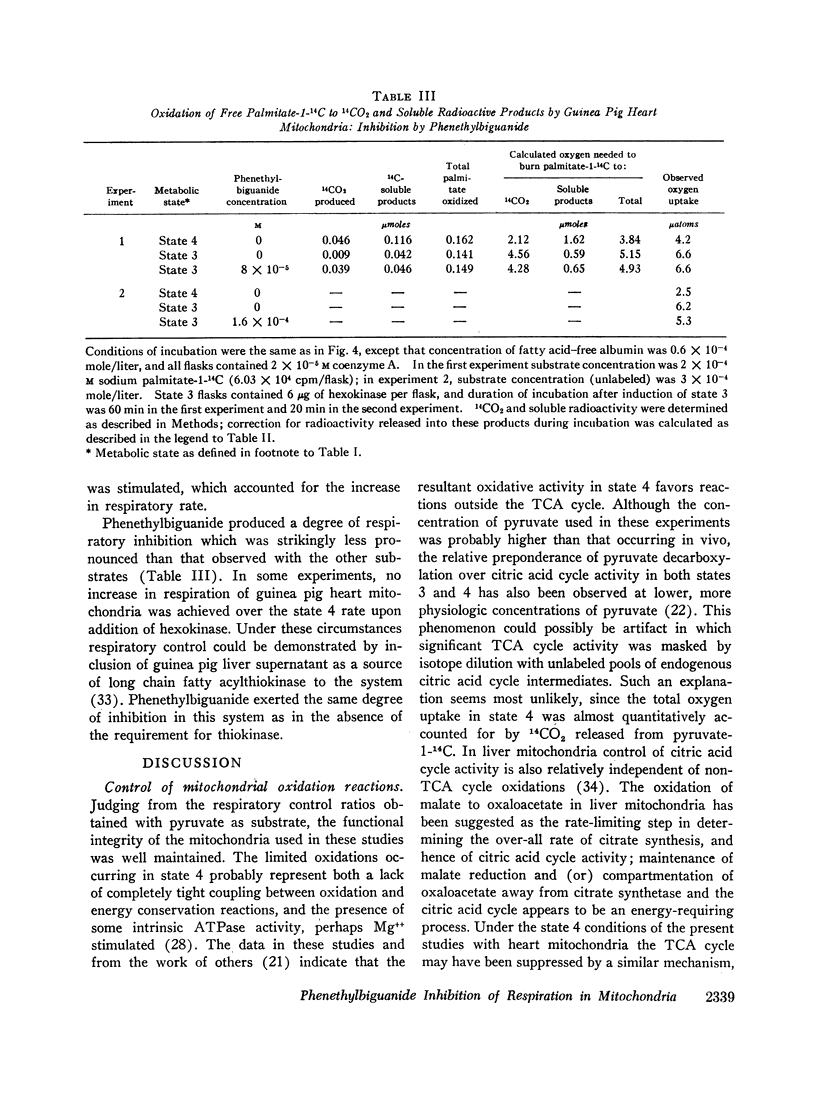
When serum albumin is included in the incubation medium, the respiration of guinea pig heart, skeletal muscle, and liver mitochondria is inhibited by concentrations of phenethylbiguanide which approximate the levels achieved in those tissues in vivo. In the absence of albumin, the mitochondria are several fold less sensitive to phenethylbiguanide inhibition. Mitochondria from rat tissues are less sensitive than those of guinea pig to in vitro inhibition by phenethylbiguanide, but serum albumin alters sensitivity to inhibition in similar fashion in both species. During the breakdown of pyruvate or long chain fatty acyl-CoA, phenethylbiguanide demonstrates no specificity of inhibition toward the oxidative reactions before the citric acid cycle versus those of the cycle itself. However, oxidation of free fatty acids is relatively resistant to inhibition.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allmann D. W., Galzigna L., McCaman R. E., Green D. E. The membrane systems of the mitochondrion. IV. The localization of the fatty acid oxidizing system. Arch Biochem Biophys. 1966 Nov;117(2):413–422. doi: 10.1016/0003-9861(66)90430-9. [DOI] [PubMed] [Google Scholar]
- Altschuld R. A., Kruger F. A. Inhibition of hepatic gluconeogenesis in guinea pig by phenformin. Ann N Y Acad Sci. 1968 Mar 26;148(3):612–622. doi: 10.1111/j.1749-6632.1968.tb27735.x. [DOI] [PubMed] [Google Scholar]
- BODE C., KLINGENBERG M. DIE VERATMUNG VON FETTSAEUREN IN ISOLIERTEN MITOCHONDRIEN. Biochem Z. 1965 Feb 24;341:271–299. [PubMed] [Google Scholar]
- BRESSLER R., KATZ R. I. THE ROLE OF CARNITINE IN ACETOACETATE PRODUCTION AND FATTY ACID SYNTHESIS. J Clin Invest. 1965 May;44:840–848. doi: 10.1172/JCI105196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann R. Uber die Resorption und den biologischen Abbau von 1-(beta-Phenäthyl)-biguanid. Diabetologia. 1967 Aug;3(4):368–376. doi: 10.1007/BF02342630. [DOI] [PubMed] [Google Scholar]
- Björntorp P. The oxidation of fatty acids combined with albumin by isolated rat-liver mitochondria in the presence of fluorocitrate. Biochim Biophys Acta. 1966 Nov 15;128(2):221–227. doi: 10.1016/0926-6593(66)90169-x. [DOI] [PubMed] [Google Scholar]
- Bremer J. Comparison of acylcarnitines and pyruvate as substrates for rat-liver mitochondria. Biochim Biophys Acta. 1966 Feb 1;116(1):1–11. doi: 10.1016/0005-2760(66)90087-7. [DOI] [PubMed] [Google Scholar]
- Brendel K., Bressler R. The resolution of (plus or minus)-carnitine and the synthesis of acylcarnitines. Biochim Biophys Acta. 1967 Feb 14;137(1):98–106. doi: 10.1016/0005-2760(67)90012-4. [DOI] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- CLARKE D. W., FORBATH N. Some studies on the mode of action of DBI. Metabolism. 1959 Jul 2;8(4 Pt 2):553–556. [PubMed] [Google Scholar]
- Childress C. C., Sacktor B., Traynor D. R. Function of carnitine in the fatty acid oxidase-deficient insect flight muscle. J Biol Chem. 1967 Feb 25;242(4):754–760. [PubMed] [Google Scholar]
- DAVIDOFF F., KORN E. D. THE CONVERSION OF LONG CHAIN SATURATED FATTY ACIDS TO THEIR ALPHA, BETA-UNSATURATED, BETA, GAMMA-UNSATURATED, AND BETA-HYDROXY DERIVATIVES BY ENZYMES FROM THE CELLULAR SLIME MOLD, DICTYOSTELIUM DISCOIDEUM. J Biol Chem. 1964 Aug;239:2496–2506. [PubMed] [Google Scholar]
- DUNCAN L. J., BAIRD J. D. Compounds administered orally in the treatment of diabetes mellitus. Pharmacol Rev. 1960 Mar;12:91–158. [PubMed] [Google Scholar]
- Davidoff F. Effects of guanidine derivatives on mitochondrial function. II. Reversal of guanidine-derivative inhibiton by free fatty acids. J Clin Invest. 1968 Oct;47(10):2344–2358. doi: 10.1172/JCI105919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson S. M., Williams G. R. The effect of malate and other dicarboxylic acids on mitochondrial isocitrate metabolism. J Biol Chem. 1966 Aug 25;241(16):3696–3700. [PubMed] [Google Scholar]
- Fritz I. B., Marquis N. R. The role of acylcarnitine esters and carnitine palmityltransferase in the transport of fatty acyl groups across mitochondrial membranes. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1226–1233. doi: 10.1073/pnas.54.4.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDMAN P., VAGELOS P. R. The specificity of triglyceride synthesis from diglycerides in chicken adipose tissue. J Biol Chem. 1961 Oct;236:2620–2623. [PubMed] [Google Scholar]
- GOODMAN D. S. Preparation of human serum albumin free of long-chain fatty acids. Science. 1957 Jun 28;125(3261):1296–1297. doi: 10.1126/science.125.3261.1296. [DOI] [PubMed] [Google Scholar]
- KORNBERG A., PRICER W. E., Jr Enzymatic synthesis of the coenzyme A derivatives of long chain fatty acids. J Biol Chem. 1953 Sep;204(1):329–343. [PubMed] [Google Scholar]
- Krebs H. A. The regulation of the release of ketone bodies by the liver. Adv Enzyme Regul. 1966;4:339–354. doi: 10.1016/0065-2571(66)90027-6. [DOI] [PubMed] [Google Scholar]
- MADISON L. L., UNGER R. H. Effect of phenoformin on peripheral glucose utilization in human diabetic and nondiabetic subjects. Diabetes. 1960 May-Jun;9:202–206. doi: 10.2337/diab.9.3.202. [DOI] [PubMed] [Google Scholar]
- Newsholme E. A., Randle P. J. Regulation of glucose uptake by muscle. 7. Effects of fatty acids, ketone bodies and pyruvate, and of alloxan-diabetes, starvation, hypophysectomy and adrenalectomy, on the concentrations of hexose phosphates, nucleotides and inorganic phosphate in perfused rat heart. Biochem J. 1964 Dec;93(3):641–651. doi: 10.1042/bj0930641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norum K. R., Farstad M., Bremer J. The submitochondrial distribution of acid:CoA ligase (AMP) and palmityl-CoA:carnitine palmityltransferase in rat liver mitochondria. Biochem Biophys Res Commun. 1966 Sep 8;24(5):797–804. doi: 10.1016/0006-291x(66)90397-4. [DOI] [PubMed] [Google Scholar]
- Patrick S. J. Effects of phenfromin and hypoglycin on gluconeogenesis of rat tissues. Can J Biochem. 1966 Jan;44(1):27–33. doi: 10.1139/o66-004. [DOI] [PubMed] [Google Scholar]
- Pogson C. I., Randle P. J. The control of rat-heart phosphofructokinase by citrate and other regulators. Biochem J. 1966 Sep;100(3):683–693. doi: 10.1042/bj1000683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi C. R., Galzigna L., Alexandre A., Gibson D. M. Oxidation of long chain fatty acids by rat liver mitochondria. J Biol Chem. 1967 May 10;242(9):2102–2110. [PubMed] [Google Scholar]
- SOELING H. D., WERCHAU H., CREUTZFELDT W. [Studies on the metabolic effects of hypoglycemic biguanides in various animal species]. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1963;244:290–310. [PubMed] [Google Scholar]
- Searle G. L., Schilling S., Porte D., Barbaccia J., De Grazia J., Cavalieri R. R. Body glucose kinetics in nondiabetic human subjects after phenethylbiguanide. Diabetes. 1966 Mar;15(3):173–178. doi: 10.2337/diab.15.3.173. [DOI] [PubMed] [Google Scholar]
- Söling H. D., Moshagen D., Skutella E., Kneer P., Creutzfeldt W. Die Wirkung von N1, n-Butylbiguanid auf den Stoffwechsel der isolierten perfundierten Leber normaler und alloxandiabetischer ketostischer Ratten. Diabetologia. 1967 Jun;3(3):318–330. doi: 10.1007/BF00429864. [DOI] [PubMed] [Google Scholar]
- TRANQUADA R. E., KLEEMAN C., BROWN J. Some effects of phenethylbiguanide on human hepatic metabolism as measured by hepatic vein catheterization. Diabetes. 1960 May-Jun;9:207–214. doi: 10.2337/diab.9.3.207. [DOI] [PubMed] [Google Scholar]
- UNGAR G., PSYCHOYOS S., HALL H. A. Action of phenethylbiguanide, a hypoglycemic agent, on tricarboxylic acid cycle. Metabolism. 1960 Jan;9:36–51. [PubMed] [Google Scholar]
- VONKORFF R. W. METABOLIC CHARACTERISTICS OF ISOLATED RABBIT HEART MITOCHONDRIA. J Biol Chem. 1965 Mar;240:1351–1358. [PubMed] [Google Scholar]
- WILLIAMSON J. R. GLYCOLYTIC CONTROL MECHANISMS. I. INHIBITION OF GLYCOLYSIS BY ACETATE AND PYRUVATE IN THE ISOLATED, PERFUSED RAT HEART. J Biol Chem. 1965 Jun;240:2308–2321. [PubMed] [Google Scholar]
- WILLIAMSON J. R., WALKER R. S., RENOLD A. E. METABOLIC EFFECTS OF PHENETHYLBIGUANIDE (DBI) ON THE ISOLATED PERFUSED RAT HEART. Metabolism. 1963 Dec;12:1141–1152. [PubMed] [Google Scholar]
- WILLIAMS R. H., TYBERGHEIN J. M., HYDE P. M., NIELSEN R. L. Studies related to the hypoglycemic action of phenethyldiguanide. Metabolism. 1957 Jul;6(4):311–319. [PubMed] [Google Scholar]
- Weinbach E. C., Garbus J. Restoration by albumin of oxidative phosphorylation and related reactions. J Biol Chem. 1966 Jan 10;241(1):169–175. [PubMed] [Google Scholar]
- Whereat A. F., Hull F. E., Orishimo M. W. The role of succinate in the regulation of fatty acid synthesis by heart mitochondria. J Biol Chem. 1967 Sep 25;242(18):4013–4022. [PubMed] [Google Scholar]
- von Jagow G., Westermann B., Wieland O. Suppression of pyruvate oxidation in liver mitochondria in the presence of long-chain fatty acid. Eur J Biochem. 1968 Feb;3(4):512–518. doi: 10.1111/j.1432-1033.1967.tb19561.x. [DOI] [PubMed] [Google Scholar]



