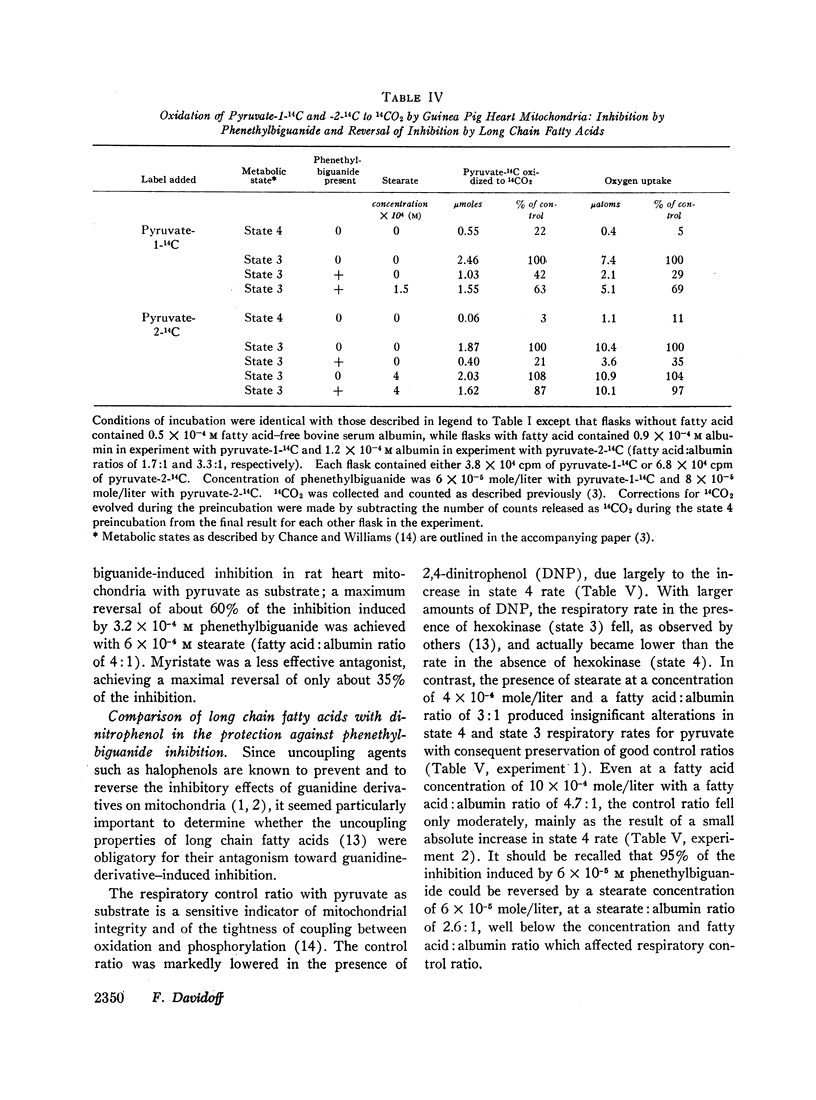
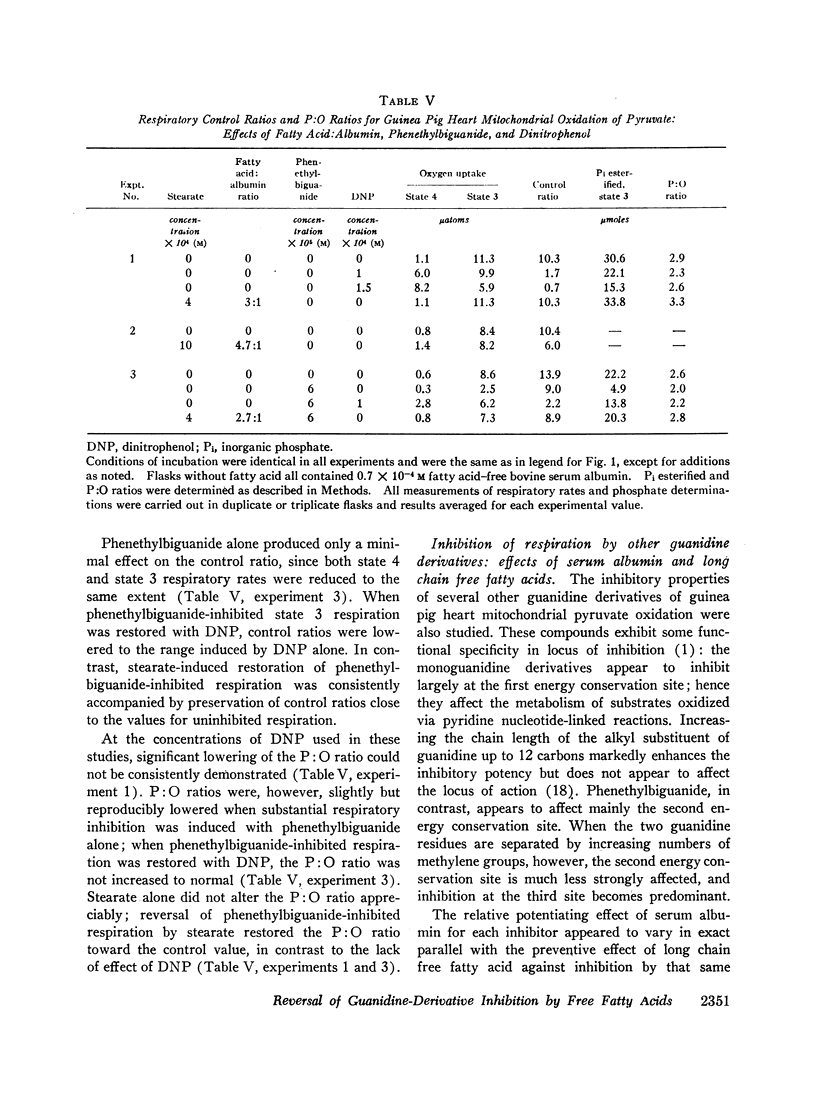
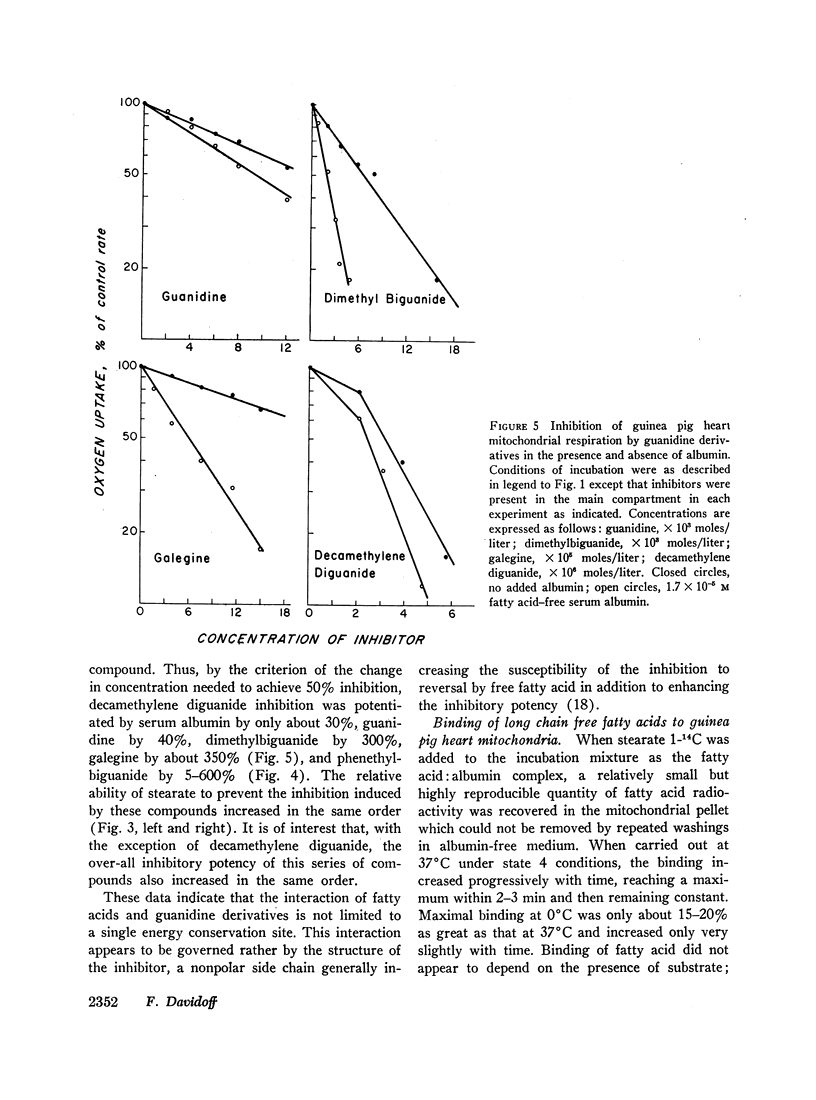
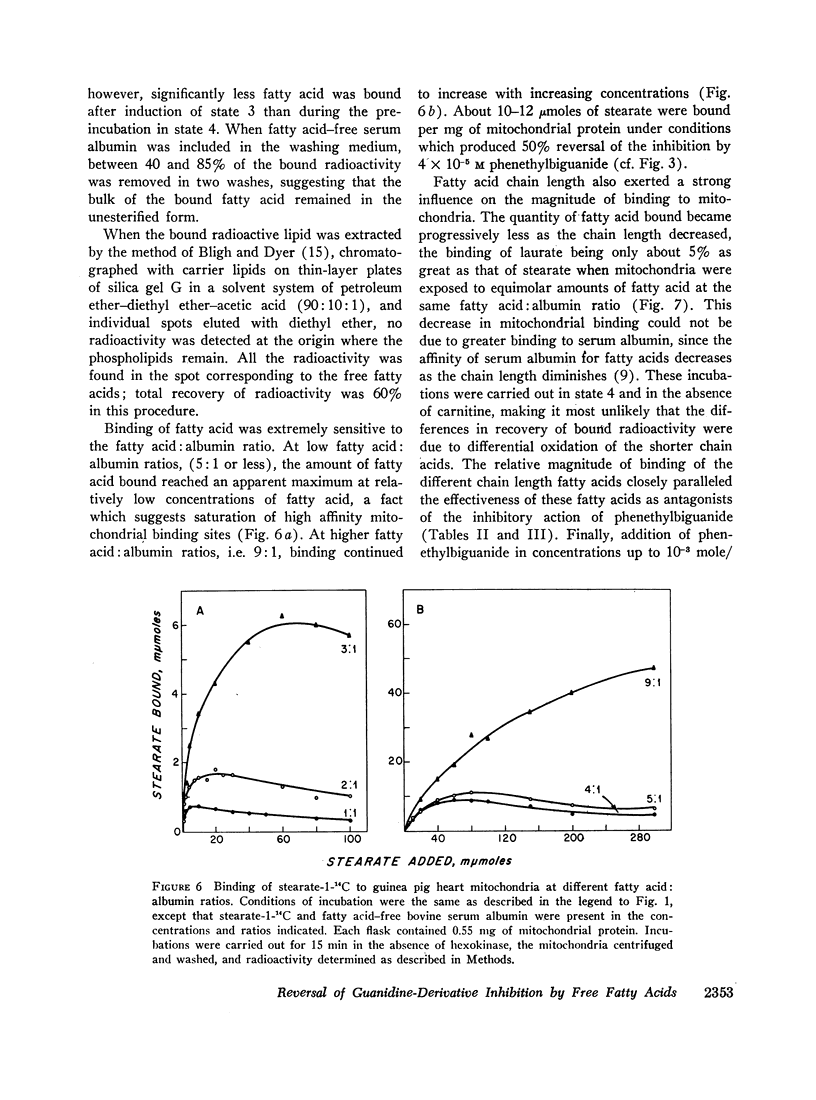
Abstract
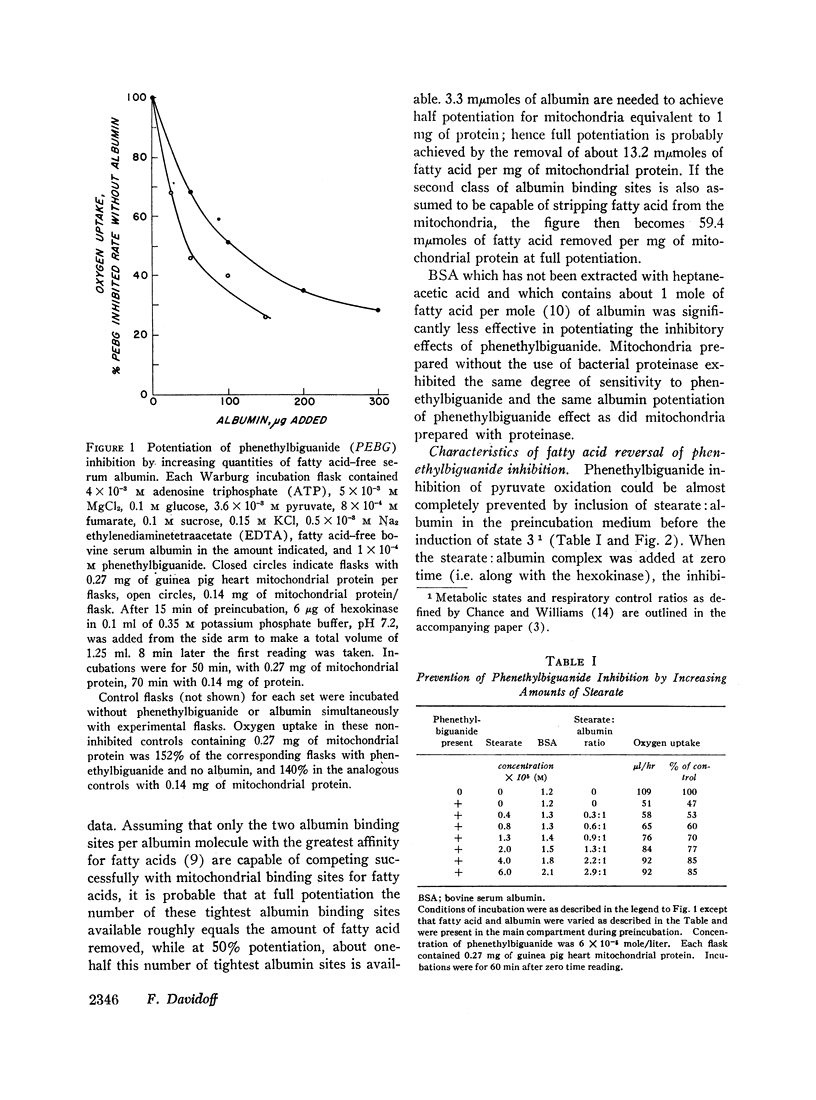
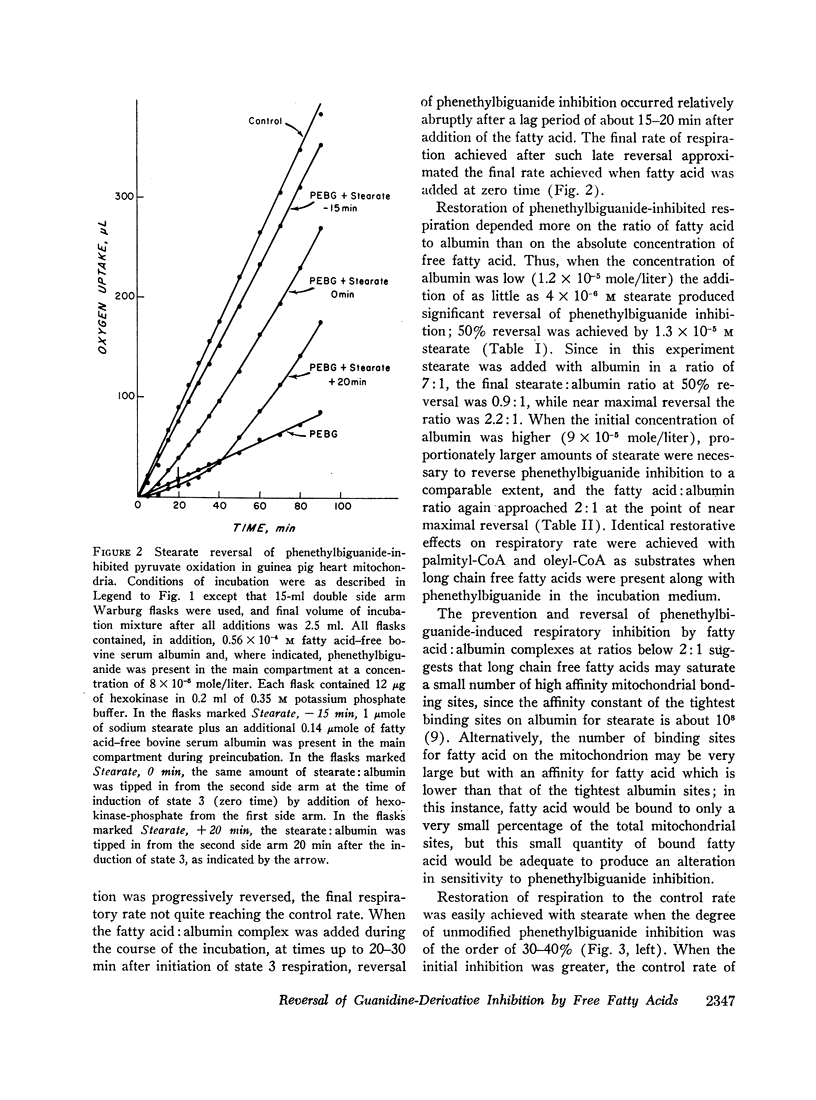
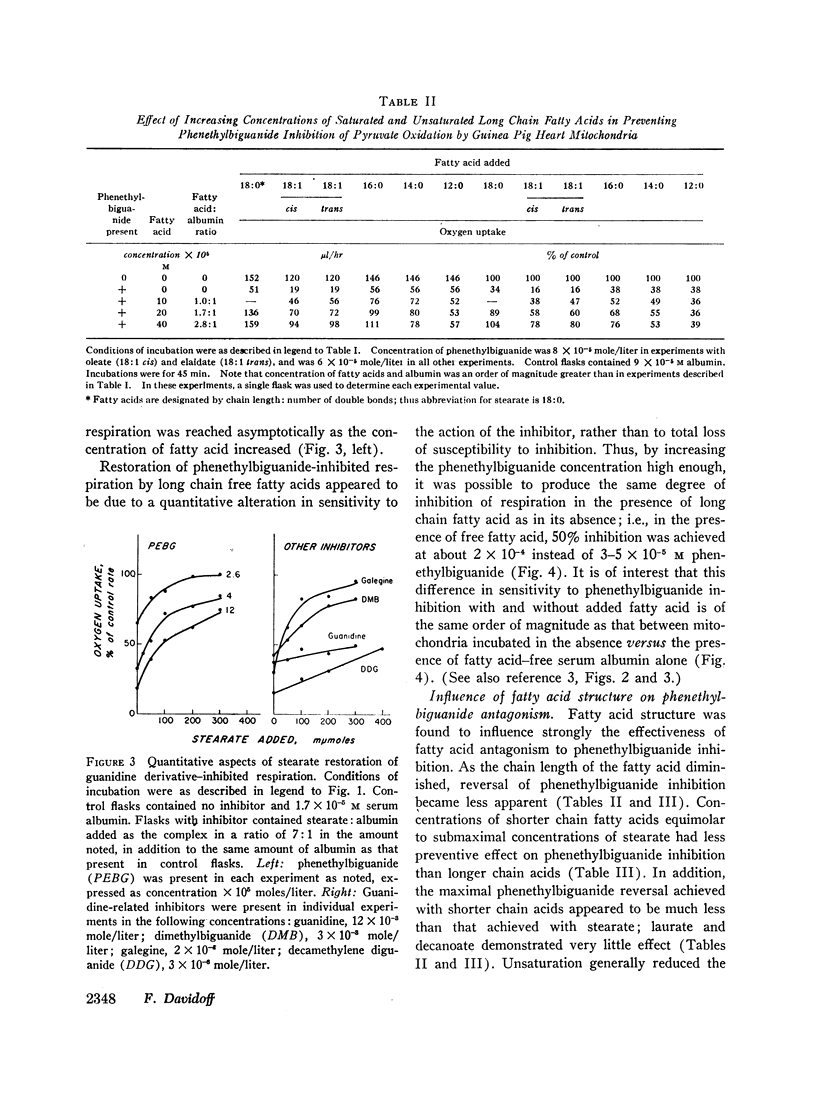
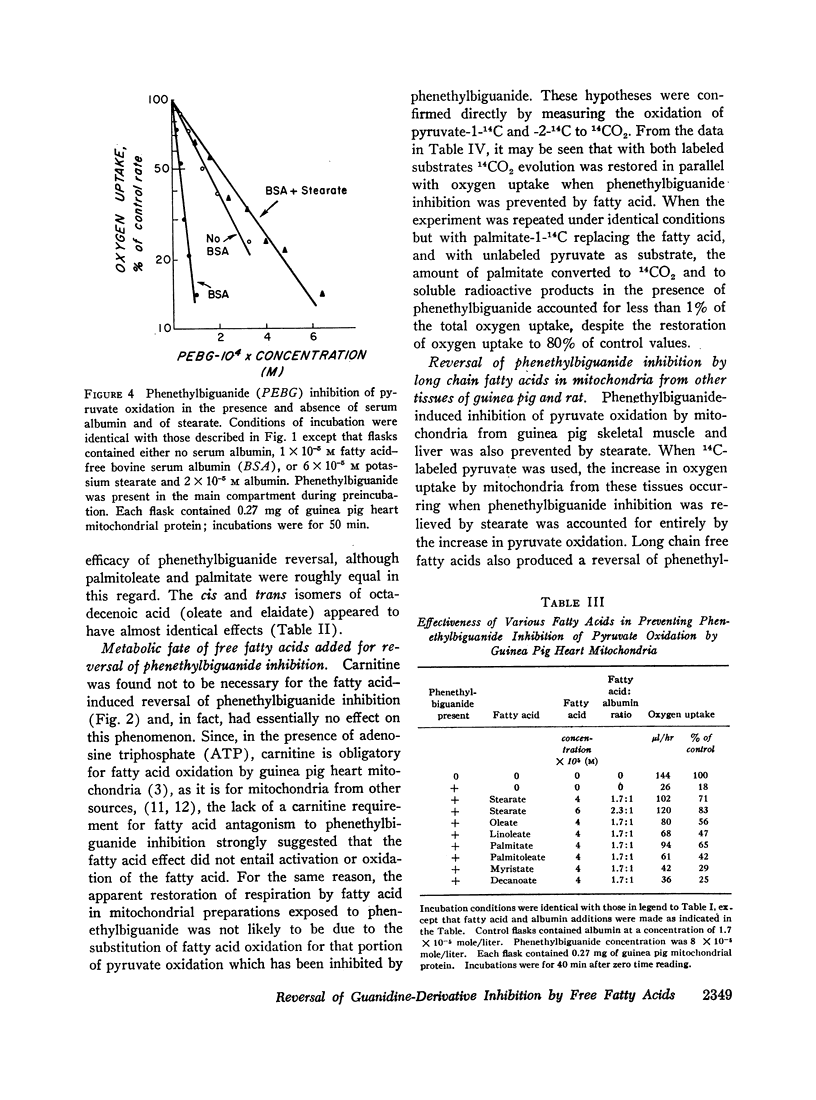
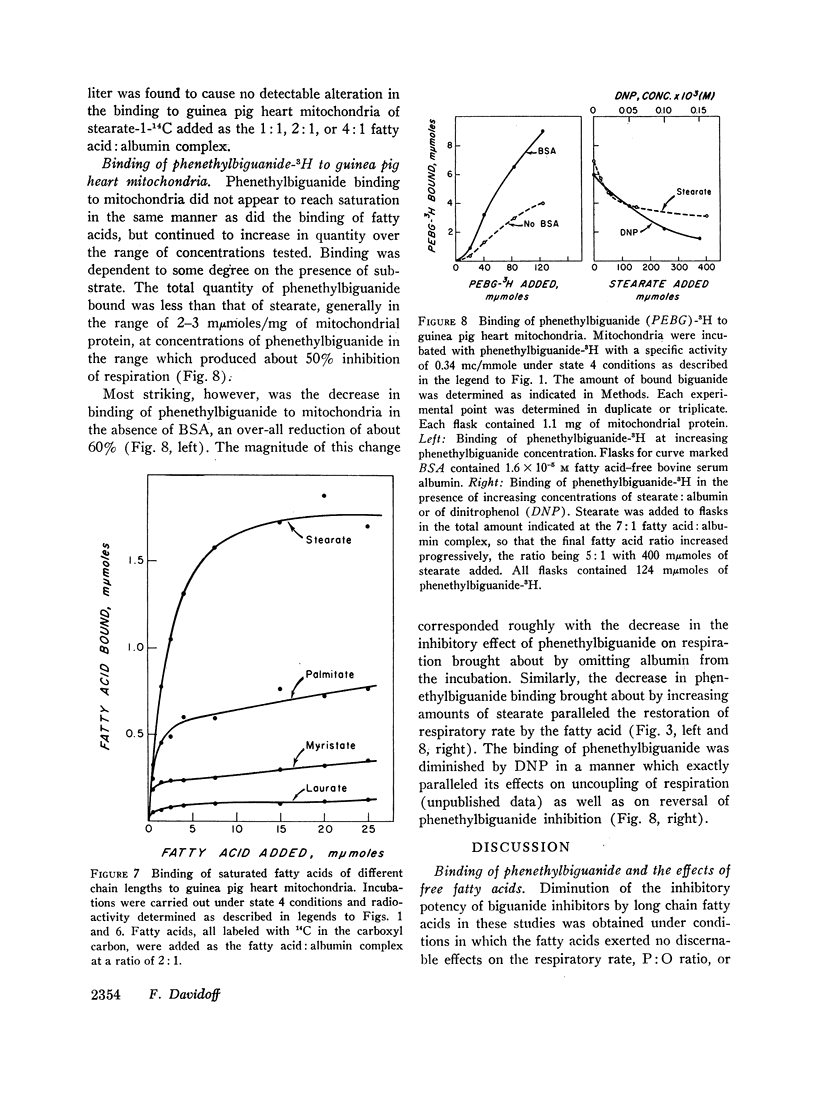
Long chain free fatty acids interfere with the inhibitory action of phenethylbiguanide and related compounds on mitochondrial respiration in vitro. This interference depends on binding of fatty acids to mitochondria and diminishes with decreasing chain length. Reversal of guanidine-derivative inhibition by fatty acids differs from that caused by dinitrophenol in that the effect of fatty acid is achieved without alteration in coupling or respiratory control. The binding of phenethylbiguanide to mitochondria is inhibited by both fatty acid and dinitrophenol. Serum albumin potentiates the inhibitory potency of guanidine derivatives, probably by removing endogenous mitochondrial free fatty acids.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- BODE C., KLINGENBERG M. DIE VERATMUNG VON FETTSAEUREN IN ISOLIERTEN MITOCHONDRIEN. Biochem Z. 1965 Feb 24;341:271–299. [PubMed] [Google Scholar]
- Ballard F. J., Hanson R. W. The citrate cleavage pathway and lipogenesis in rat adipose tissue: replenishment of oxaloacetate. J Lipid Res. 1967 Mar;8(2):73–79. [PubMed] [Google Scholar]
- Bauer C., Von Korff R. W. Variation in endogenous substrates and pyruvate metabolism in isolated heart mitochondria of several species. Biochim Biophys Acta. 1967 Mar 8;131(2):280–287. doi: 10.1016/0005-2728(67)90141-7. [DOI] [PubMed] [Google Scholar]
- Beckmann R. Uber die Resorption und den biologischen Abbau von 1-(beta-Phenäthyl)-biguanid. Diabetologia. 1967 Aug;3(4):368–376. doi: 10.1007/BF02342630. [DOI] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- Davidoff F. Effects of guanidine derivatives on mitochondrial function. I. Phenethylbiguanide inhibition of respiration in mitochondria from guinea pig and rat tissues. J Clin Invest. 1968 Oct;47(10):2331–2343. doi: 10.1172/JCI105918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EISENHARDT R. H., ROSENTHAL O. 2,4-DINITROPHENOL: LACK OF INTERACTION WITH HIGH-ENERGY INTERMEDIATES OF OXIDATIVE PHOSPHORYLATION. Science. 1964 Jan 31;143(3605):476–477. doi: 10.1126/science.143.3605.476. [DOI] [PubMed] [Google Scholar]
- Entman M., Bressler R. The mechanism of action of hypoglycin on long-chain fatty acid oxidation. Mol Pharmacol. 1967 Jul;3(4):333–340. [PubMed] [Google Scholar]
- Falcone A. B., Mao R. L. Catalysis of an oxygen exchange reaction between long-chain fatty acids and water by rat-liver mitochondria. Biochim Biophys Acta. 1965 Aug 24;105(2):246–252. doi: 10.1016/s0926-6593(65)80149-7. [DOI] [PubMed] [Google Scholar]
- Falcone A. B., Mao R. L. The effect of long-chain fatty acids on orthophosphate-adenosine 5'-triphosphate exchange activity associated with oxidative phosphorylation. Biochim Biophys Acta. 1965 Aug 24;105(2):233–245. doi: 10.1016/s0926-6593(65)80148-5. [DOI] [PubMed] [Google Scholar]
- Ferguson S. M., Williams G. R. The effect of malate and other dicarboxylic acids on mitochondrial isocitrate metabolism. J Biol Chem. 1966 Aug 25;241(16):3696–3700. [PubMed] [Google Scholar]
- Fritz I. B., Marquis N. R. The role of acylcarnitine esters and carnitine palmityltransferase in the transport of fatty acyl groups across mitochondrial membranes. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1226–1233. doi: 10.1073/pnas.54.4.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODMAN D. S. Preparation of human serum albumin free of long-chain fatty acids. Science. 1957 Jun 28;125(3261):1296–1297. doi: 10.1126/science.125.3261.1296. [DOI] [PubMed] [Google Scholar]
- Johnson C. L., Safer B., Schwartz A. The effects of histones and other polycations on cellular energetics. IV. Further studies on the stimulation of mitochondrial oxygen consumption. J Biol Chem. 1966 Oct 10;241(19):4513–4521. [PubMed] [Google Scholar]
- Lardy H. A., Paetkau V., Walter P. Paths of carbon in gluconeogenesis and lipogenesis: the role of mitochondria in supplying precursors of phosphoenolpyruvate. Proc Natl Acad Sci U S A. 1965 Jun;53(6):1410–1415. doi: 10.1073/pnas.53.6.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogson C. I., Randle P. J. The control of rat-heart phosphofructokinase by citrate and other regulators. Biochem J. 1966 Sep;100(3):683–693. doi: 10.1042/bj1000683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi C. R., Galzigna L., Alexandre A., Gibson D. M. Oxidation of long chain fatty acids by rat liver mitochondria. J Biol Chem. 1967 May 10;242(9):2102–2110. [PubMed] [Google Scholar]
- Schatz G., Racker E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. VII. Oxidative phosphorylation in the diphosphopyridine nucleotide-cytochrome b segment of the respiratory chain: assay and properties in submitochondrial particles. J Biol Chem. 1966 Mar 25;241(6):1429–1438. [PubMed] [Google Scholar]
- Spencer A., Corman L., Lowenstein J. M. Citrate and the conversion of carbohydrate into fat. A comparison of citrate and acetate incorporation into fatty acids. Biochem J. 1964 Nov;93(2):378–388. doi: 10.1042/bj0930378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söling H. D., Moshagen D., Skutella E., Kneer P., Creutzfeldt W. Die Wirkung von N1, n-Butylbiguanid auf den Stoffwechsel der isolierten perfundierten Leber normaler und alloxandiabetischer ketostischer Ratten. Diabetologia. 1967 Jun;3(3):318–330. doi: 10.1007/BF00429864. [DOI] [PubMed] [Google Scholar]
- Vázquez-Colón L., Ziegler F. D., Elliott W. B. On the mechanism of fatty acid inhibition of mitochondrial metabolism. Biochemistry. 1966 Apr;5(4):1134–1139. doi: 10.1021/bi00868a004. [DOI] [PubMed] [Google Scholar]
- Williamson J. R., Kreisberg R. A., Felts P. W. Mechanism for the stimulation of gluconeogenesis by fatty acids in perfused rat liver. Proc Natl Acad Sci U S A. 1966 Jul;56(1):247–254. doi: 10.1073/pnas.56.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates D. W., Shepherd D., Garland P. B. Organization of fatty-acid activation in rat liver mitochondria. Nature. 1966 Mar 19;209(5029):1213–1215. doi: 10.1038/2091213a0. [DOI] [PubMed] [Google Scholar]


