Abstract
IL-17 and its receptor are founding members of a novel inflammatory cytokine family. To date, only one IL-17 receptor subunit has been identified, termed IL-17RA. All known cytokine receptors consist of a complex of multiple subunits. Although IL-17-family cytokines exist as homodimers, the configuration and stoichiometry of the IL-17R complex remain unknown. We used fluorescence resonance energy transfer (FRET) to determine whether IL-17RA subunits multimerize, and, if so, whether they are preassembled in the plasma membrane. HEK293 cells coexpressing IL-17RA fused to cyan or yellow fluorescent proteins (CFP or YFP) were used to evaluate FRET before and after IL-17A or IL-17F treatment. In the absence of ligand, IL-17RA molecules exhibited significant specific FRET efficiency, demonstrating that they exist in a multimeric, preformed receptor complex. Strikingly, treatment with IL-17A or IL-17F markedly reduced FRET efficiency, suggesting that IL-17RA subunits within the IL-17R complex undergo a conformational change upon ligand binding.
Interleukin 17A (IL-17 or CTLA8) and its receptor (IL-17R or IL-17RA) are the founding members of a novel family of proinflammatory cytokines (1). Most cells are potential targets of IL-17A, since IL-17RA is ubiquitously expressed, but the molecular biology of this receptor family is still poorly understood (2). Produced almost exclusively by effector memory T cells (3, 4), IL-17A elicits production of numerous cytokines and chemokines involved in the inflammatory response (5) and is a particularly potent activator of neutrophils (1, 6). Consequently, IL-17RA-deficient mice exhibit enhanced susceptibility to multiple infectious agents, including Klebsiella pneumoniae, Toxoplasma gondii, and Candida albicans (7–9). In contrast to its protective role in infection, IL-17A plays a pathogenic role in autoimmune disease, especially rheumatoid arthritis (RA)3 (5, 10). Blocking IL-17A in animal models of RA ameliorates symptoms (11), and other mouse models in which IL-17A production is impaired show reduced susceptibility to arthritis (12, 13). Indeed, IL-17A was recently described as the hallmark cytokine produced by a subset of “pathogenic” T cells involved in autoimmune inflammation (14). Thus, like many inflammatory cytokines, IL-17A can be protective or destructive depending on context.
All known cytokine receptors are multimeric and oligomerization of receptor subunits is essential for function. Some, like the growth hormone (GH) and erythropoietin (EPO) receptors, are homodimers, while others are heterodimers or higher order multimers. It is generally considered that cytokines trigger signal transduction by inducing association of receptor subunits, thereby bringing into proximity crucial intracellular signaling intermediates. However, there is convincing evidence that some cytokine receptors are preassembled before binding ligand. For example, crystallographic and genetic studies showed that the EPO receptor (EPOR) exists as preformed homodimer without EPO. Binding of EPO triggers structural alterations that bring together EPOR-associated JAK2 kinases, facilitating their phosphorylation and initiating signaling (15–19). Similarly, studies of the IFN-γ, Fas, and TNF receptors showed that their constituent subunits preassociate even in the absence of ligand (20–22). However, not all cytokine receptors exist as preformed multimers; for example, most evidence to date indicates that binding of GH triggers receptor dimerization (18).
Fluorescence resonance energy transfer (FRET) is a powerful tool to measure protein-protein interactions. In FRET, nonradiated energy is transferred from a donor to an acceptor fluorophore when the donor emission spectrum significantly overlaps the acceptor excitation spectrum and the fluorophores are closely approximated. FRET has been used to detect interactions between proteins that are tagged with a FRET donor/acceptor pair, such as cyan or yellow fluorescent proteins (CFP or YFP). These are generally considered to be the fluorophores of choice because they have excitation and emission wavelengths favorable for FRET, appropriate extinction coefficients and quantum yields, and CFP has high photostability (23).
Surprisingly little is currently known about the configuration of the IL-17R-binding complex. In mice, only one IL-17A-binding subunit has been identified, termed IL-17RA, which also binds weakly to IL-17F (2, 24). Since all known cytokine receptors contain multiple peptides, we hypothesized that IL-17RA also signals as part of a multisubunit complex. In this study, we provide evidence that the IL-17R-binding complex contains multiple IL-17RA subunits, which are preassembled in the plasma membrane before ligand binding.
Materials and Methods
Constructs, cell culture and cytokine stimulations
Murine IL-17RA constructs were generated by RT-PCR and fused to FLAG and enhanced YFP and CFP (BD Clontech) (see Fig. 1). The 24p3 promoter-luciferase construct was previously described (25). TNFRΔp60/YFP (hemagglutinin tagged) was a gift from F. Chan (University of Massachusetts Medical School, Worcester, MA). HEK293, ST2, and IL-17R-deficient fibroblasts (derived from mice provided by Amgen) were maintained in α-MEM (Sigma-Aldrich) with 10% FBS (Gemini Bioproducts). Cells were transfected with calcium phosphate or Fugene 6 (Roche). IL-17A, IL-17F, and TNF-α were obtained from R&D Systems.
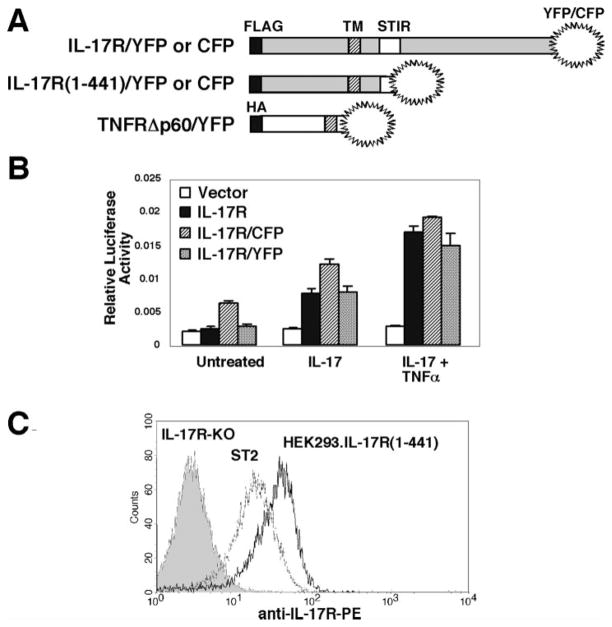
FIGURE 1.
IL-17RA constructs used in this study. A, Schematic diagram of IL-17RA and TNFR FRET constructs. Full-length and truncated murine IL-17RA cD-NAs were fused in-frame to YFP or CFP. A truncated TNFR p60 fused to YFP was used as a control. IL-17R constructs have N-terminal FLAG tags, and the TNFR construct has an N-terminal hemagglutinin tag. TM, transmembrane domain, STIR, similar expression of fibroblast growth factor receptor/Toll/IL-1 receptor domain. B, IL-17R/CFP and IL-17R/YFP deliver normal IL-17-dependent signals. IL-17R-deficient murine fibroblasts were transiently transfected with a reporter construct containing the murine 24p3 proximal promoter upstream of luciferase (25) and an internal Renilla-luciferase plasmid along with a vector control (□), wild-type IL-17RA (■), or IL-17R/CFP and IL-17R/YFP constructs (▨ and  , respectively). Cells were stimulated with IL-17 (100 ng/ml) and/or TNF-α (2 ng/ml) for 6 h and luciferase assays were performed in triplicate. SDs are shown. C, Normal cell surface expression of IL-17R (1-441). HEK293.IL-17R(1-441)/CFP and/YFP cells (black line), ST2 cells (gray line), or IL-17RA-deficient fibroblasts (solid histogram) were stained with anti-mouse IL-17RA-PE.
, respectively). Cells were stimulated with IL-17 (100 ng/ml) and/or TNF-α (2 ng/ml) for 6 h and luciferase assays were performed in triplicate. SDs are shown. C, Normal cell surface expression of IL-17R (1-441). HEK293.IL-17R(1-441)/CFP and/YFP cells (black line), ST2 cells (gray line), or IL-17RA-deficient fibroblasts (solid histogram) were stained with anti-mouse IL-17RA-PE.
Luciferase assays
IL-17R-deficient MF cells were transfected with indicated constructs and normalized to an internal Renilla-luciferase control. Six hours after stimulation, standard luciferase assays were performed in triplicate as described previously (26).
Flow cytometry and FRET
For FACS, 0.25–1.5 × 106 cells were incubated on ice with 3.4 μg mAb M750 (rat anti-mouse IL-17R, IgG2a, provided by Amgen) followed by goat anti-rat-PE (BD Pharmingen). Data were analyzed on a FACSCalibur with CellQuest software (BD Biosciences). FRET data were obtained from three channel images using the macro of LSM FRET tool software (Zeiss AIM software) as previously described (27). FRET efficiencies were calculated by the FRETN and N-FRET methods, with similar results (28, 29).
Results
To assess IL-17RA multimerization in live, single cells by FRET, murine IL-17RA with an N-terminal FLAG tag was fused to CFP or YFP (Fig. 1A). The IL-17RA has an unusually long cytoplasmic tail that could potentially increase nonspecific receptor-receptor interactions in FRET (15, 23). Therefore, for these studies, we examined FRET in both full-length and truncated forms of IL-17RA. It was previously shown that the N-terminal FLAG tag does not interfere with ligand binding or signal transduction (30), and we confirmed that the full-length IL-17R/CFP and IL-17R/YFP constructs activated signaling equivalently to wild-type IL-17RA. Specifically, IL-17R/CFP and/YFP directed transcriptional activation of the 24p3 target gene promoter linked to a luciferase reporter (Fig. 1B). However, no signals were activated by the IL-17R (1-441) truncation, because this receptor lacks key cytoplasmic signaling domains (data not shown and Ref. 31). Although the truncated receptor does not signal, it can be readily detected at the cell surface with an IL-17R-specific mAb, indicating that it appears to be conformationally intact (Fig. 1C).
To assess receptor interactions by FRET, HEK293 cells were transfected with these IL-17RA constructs. All receptors were expressed efficiently at the plasma membrane, although there was also significant expression within the cytoplasm, which is typical of ectopically expressed cytokine receptors (Figs. 1C and 2 and data not shown). As a negative control, we paired a truncated TNF receptor (TNFRΔp60/YFP) (21) with IL-17R/CFP constructs (Fig. 1A and Ref 21). The sensitized emission method was used to analyze FRET efficiency. Using the multi-track and line-scanning mode of a laser-scanning confocal microscope, cells were simultaneously recorded in the CFP, YFP, or FRET channels. We first obtained bleed-through (cross-talk) coefficients by analyzing images from cells expressing individual YFP- or CFP-tagged constructs and then obtained FRET efficiency and FRET images from cells expressing both constructs. FRET efficiencies were calculated for each pixel using methods to correct for donor and acceptor concentrations.
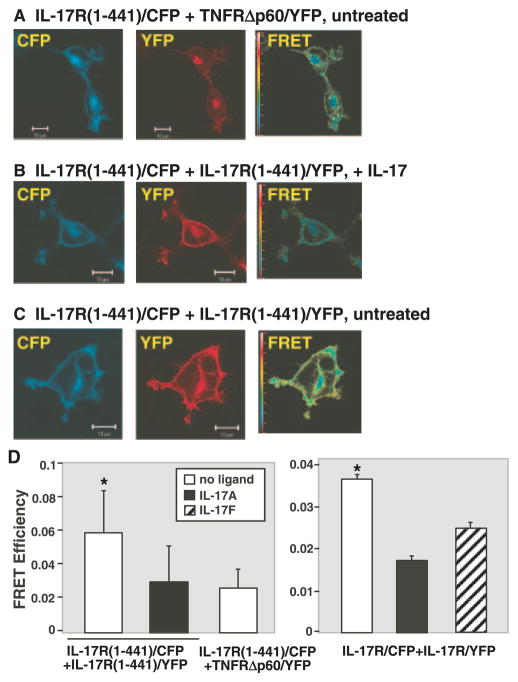
A major advantage of using confocal microscopy to evaluate FRET is that individual regions of interest (ROI) within a cell can be selectively examined for FRET fluorescence. In contrast, flow cytometric approaches can only assess total cellular FRET, which often includes high concentrations of fluorophores in intracellular compartments. Therefore, since we were interested in IL-17RA interactions at the cell surface, only the plasma membrane region of the cell was used as a ROI. Although the IL-17RA and TNF receptors both localize to the cell membrane, they do not associate as a receptor complex, and thus were used to determine baseline FRET efficiencies. Strikingly, cells coexpressing either full-length or truncated IL-17R/CFP and IL-17R/YFP constructs showed a marked enhancement of FRET (images for the truncated receptor are shown here, Fig. 2, C and D). Surprisingly, however, FRET between IL-17RA subunits disappeared following treatment with IL-17A (Fig. 2, B–D, and data not shown). Treatment of cells with IL-17F, another ligand for IL-17RA (24), also reduced FRET efficiency, although to a lesser extent. This result is likely due to the 10- to 30-fold reduced binding affinity of IL-17F to the IL-17RA complex (S. Levin, personal communication). Quantitative analyses of FRET efficiencies indicated that there is a statistically significant increase in FRET in cells coexpressing full-length or truncated IL-17R/CFP and IL-17R/YFP constructs in the absence of ligand compared with cells treated with IL-17A, IL-17F, or to the negative control (Fig. 2D). Together, these data show that IL-17RA subunits multimerize at the plasma membrane in the absence of ligand. Moreover, binding to IL-17RA by either ligand induces a conformational change in the receptor complex, causing a loss of FRET signal.
FIGURE 2.
IL-17R subunits exhibit FRET in the absence of ligand. HEK293 cells were transfected with IL-17R(1-441)/CFP and TNFRΔp60/YFP (A) or IL-17R(1-441)/YFP (B and C). Image of the CFP channel (excitation, 458 nm and emission, 475–525 nm) is shown in the left panels and images of the YFP channel (excitation, 514 nm and emission, 530 nm) is shown in the middle panels. N-FRET image is shown in the right panels and the color bar shows FRET efficiency of the N-FRET image. B, Cells were pretreated with 200 ng/ml IL-17A for 10 min before imaging. Representative images from each condition are shown. Similar data were obtained for full-length IL-17R/CFP and/YFP (data not shown). D, Quantitative analysis of FRET efficiency. HEK293 cells transfected with full-length or IL-17R(1-441)/CFP and/YFP were analyzed for FRET efficiency in plasma membrane ROI from multiple cells (n > 50). Cells were stimulated with 100 ng/ml IL-17A or IL-17F for 10 min before imaging. Data for N-FRET are shown with SEs. Statistical significance was assessed using a t test assuming equal variance. *, Significantly enhanced compared to negative controls, p < 0.01.
Discussion
Neutralization of inflammatory mediators to reduce progression of RA has been used successfully for several cytokines, particularly TNF-α (32). IL-17A is also an important mediator of RA pathology, as blockade of IL-17A in rodent arthritis models reduces joint inflammation and bone erosion (10, 11, 33). Therefore, understanding the positioning of IL-17RA subunits relative to one another, both in the presence and absence of ligand, is likely to aid in the design of agents to inhibit inflammatory IL-17 signaling, since knowing the distance between subunits may allow for accurate prediction of the structural properties of putative receptor antagonists.
The data presented here show that IL-17RA subunits preassemble as multimers in the plasma membrane. In some cases, FRET has been used to calculate the distance between the donor and acceptor and thereby infer the distance between the molecules to which they are linked. It is difficult to make meaningful measurements between IL-17RA subunits, since no structural information about the cytoplasmic tail of IL-17RA is available, and therefore the relative orientations of CFP and YFP are unknown. Although a free rotation of CFP or YFP is assumed in calculating FRET efficiencies, this is probably not actually the case when fluorophores are tethered to larger proteins. However, FRET between CFP and YFP is detectable only in the range of 10–100 Å, and FRET efficiency decreases exponentially as the distance between fluorophores increases (23). Thus, these data indicate that CFP and YFP are held in extremely close proximity through their association with IL-17RA. Upon ligand stimulation, we observed a decrease in FRET efficiency similar to that of the negative control. This unexpected result suggests that ligand binding induces a conformational change in the IL-17R complex, causing the cytoplasmic tails to move further than 100 Å apart.
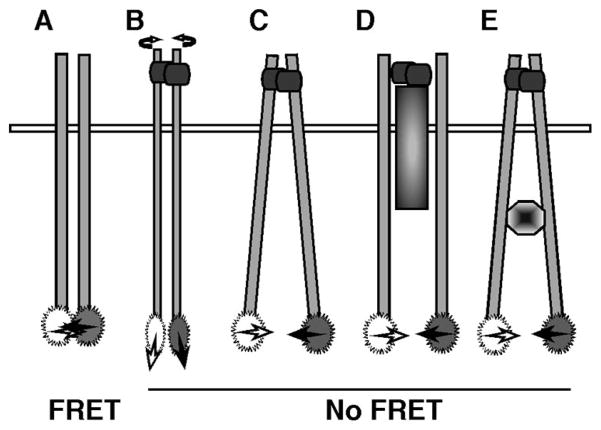
The loss of FRET following interaction with IL-17A or IL-17F suggests several alternative mechanisms of IL-17R signaling. Although it is formally possible that the IL-17RA subunits completely dissociate, it is more likely that the receptor complex is preserved but the C-terminal ends of the receptor tails become repositioned. For example, the receptor may be conformationally rigid, such that rotation of the receptor mediated by ligand causes changes in the relative orientations of CFP and YFP, thus reducing energy transfer (Fig. 3, A and B). It is also possible that the juxtamembrane regions of the IL-17R are pushed apart (Fig. 3C). It is known in the EPOR that alterations in the orientation or spacing of the extracellular domain can be transmitted to the cytoplasmic tails and their associated signaling molecules (17, 34–36). Alternatively, the recruitment of an intracellular signaling molecule or additional receptor subunit might physically separate IL-17RA tails (Fig. 3, D and E). Results from this study do not preclude the possibility of an additional subunit in the IL-17R complex, which has been postulated based on a discrepancy between IL-17R affinity and concentrations of IL-17A needed for biological responsiveness (2). The loss of FRET could also be due to receptor internalization and degradation, although we only see minimal loss of receptor upon short-term treatment with IL-17 (our unpublished data). Finally, these models are not mutually exclusive, and some or all may come into play upon interaction of IL-17A or IL-17F with the receptor.
FIGURE 3.
Alternative models of IL-17RA multimerization. In the absence of ligand, IL-17RA subunits exhibit FRET, indicating they are in close proximity (A). Upon binding of ligand (either IL-17A or IL-17F), FRET is reduced or lost, indicating some sort of conformational change between subunits. The receptors may rotate with respect to each other (B), the receptor transmembrane and cytoplasmic domains may move apart (C) or additional receptor subunits or intracellular signaling intermediates may physically separate the receptor subunits (D and E).
These data predict the existence of a pre-ligand assembly domain in IL-17RA, analogous to a similar domain in the TNFR superfamily (21). This domain not only dictates receptor assembly, but has been shown to be a possibly useful therapeutic for inflammatory arthritis (37). Although there is no obvious homology between the extracellular domains of the TNFR and IL-17RA receptors, the pre-ligand assembly domains are quite degenerate (F. Chan, personal communication). However, these experiments set the stage for the identification of such a domain within IL-17RA.
Because IL-17A and IL-17F exist as homodimers, it has long been presumed that their cognate receptors would also be homodimeric. Consistent with this, human IL-17RD (or similar expression of fibroblast growth factor receptor, SEF) was recently shown to form a homodimer in an overexpression system (38). Although the composition and stoichiometry of the IL-17A-binding complex remains to be fully elucidated, this study demonstrates that it contains at least two identical IL-17RA subunits that are preassembled in the plasma membrane, and a conformational change in the receptor appears to occur following ligand binding.
Supplementary Material
Acknowledgments
We thank Dr. W. Sigurdson (University at Buffalo Confocal and Imaging Facility) and Dr. S. Pierce (National Institute of Allergy and Infectious Diseases) for helpful suggestions. We are grateful to Dr. F. K. M. Chan (University of Massachusetts) for TNFRp60/YFP and Drs. J. Tocker and J. Peschon (Amgen, Seattle, WA) for IL-17R-deficient mice, anti-IL-17R mAbs, and valuable comments. We also thank Dr. X. Xu, Dr. J. Fang, and Dr. C. Mullin and M. Gaffen for valuable contributions.
Footnotes
This study was supported by National Institutes of Health Grants to J.M.K. (DE014831) and S.L.G. (AR050458), the Arthritis Foundation (to S.L.G.), and National Institute of Allergy and Infectious Diseases intramural funding (to T.J., L.Y., and X.J.).
Abbreviations used in this paper: RA, rheumatoid arthritis; FRET, fluorescence resonance energy transfer; CFP, cyan fluorescent protein; YFP, yellow fluorescent protein, EPO, erythropoietin, EPOR, erythropoietin receptor; GH, growth hormone; ROI, region of interest.
Disclosures
G. Gaffen owns stock in Amgen Incorporated.
References
- 1.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 2.Yao Z, Fanslow WC, Seldin MF, Rousseau AM, Painter SL, Comeau MR, Cohen JI, Spriggs MK. Herpesvirus Saimiri encodes a new cytokine, IL-17, which binds to a novel cytokine receptor. Immunity. 1995;3:811–821. doi: 10.1016/1074-7613(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 3.Fossiez F, Djossou O, Chomarat P, Flores-Romo L, Ait-Yahia S, Maat C, Pin JJ, Garrone P, Garcia E, Saeland S, et al. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med. 1996;183:2593–2603. doi: 10.1084/jem.183.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu X, Clements J, Gaffen S. Signaling through the murine T cell receptor induces IL-17 production in the absence of costimulation, IL-23 or dendritic cells. Mol Cells. 2005 In press. [PubMed] [Google Scholar]
- 5.Gaffen SL. Interleukin-17: a unique inflammatory cytokine with roles in bone biology and arthritis. Arthritis Res Ther. 2004;6:240–247. doi: 10.1186/ar1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwarzenberger P, La Russa V, Miller A, Ye P, Huang W, Zieske A, Nelson S, Bagby GJ, Stoltz D, Mynatt RL, Spriggs M, Kolls JK. IL-17 stimulates granulopoiesis in mice: use of an alternate, novel gene therapy-derived method for in vivo evaluation of cytokines. J Immunol. 1998;161:6383–6389. [PubMed] [Google Scholar]
- 7.Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, Oliver P, Huang W, Zhang P, Zhang J, et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang W, Na L, Fidel PL, Schwarzenberger P. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J Infect Dis. 2004;190:624–631. doi: 10.1086/422329. [DOI] [PubMed] [Google Scholar]
- 9.Kelly MN, Kolls JK, Happel K, Schwartzman JD, Schwarzenberger P, Combe C, Moretto M, Khan IA. Interleukin-17/interleukin-17 receptor-mediated signaling is important for generation of an optimal polymorphonuclear response against Toxoplasma gondii infection. Infect Immun. 2005;73:617–621. doi: 10.1128/IAI.73.1.617-621.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miossec P. Interleukin-17 in rheumatoid arthritis: if T cells were to contribute to inflammation and destruction through synergy. Arthritis Rheum. 2003;48:594–601. doi: 10.1002/art.10816. [DOI] [PubMed] [Google Scholar]
- 11.Lubberts E, Koenders MI, Oppers-Walgreen B, van den Bersselaar L, Coenen-de Roo CJ, Joosten LA, van den Berg WB. Treatment with a neutralizing anti-murine interleukin-17 antibody after the onset of collagen-induced arthritis reduces joint inflammation, cartilage destruction, and bone erosion. Arthritis Rheum. 2004;50:650–659. doi: 10.1002/art.20001. [DOI] [PubMed] [Google Scholar]
- 12.Nurieva RI, Treuting P, Duong J, Flavell RA, Dong C. Inducible costimulator is essential for collagen-induced arthritis. J Clin Invest. 2003;111:701–706. doi: 10.1172/JCI17321. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol. 2003;171:6173–6177. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- 14.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Remy I, I, Wilson A, Michnick SW. Erythropoietin receptor activation by a ligand-induced conformation change. Science. 1999;283:990–993. doi: 10.1126/science.283.5404.990. [DOI] [PubMed] [Google Scholar]
- 16.Livnah O, Stura EA, Johnson DL, Middleton SA, Mulcahy LS, Wrighton NC, Dower WJ, Jolliffe LK, Wilson IA. Functional mimicry of a protein hormone by a peptide agonist: The EPO receptor complex at 2.8Å. Science. 1996;273:464–471. doi: 10.1126/science.273.5274.464. [DOI] [PubMed] [Google Scholar]
- 17.Livnah O, Stura EA, Middleton SA, Johnson DL, Jolliffe LK, Wilson IA. Crystallographic evidence for preformed dimers of erythropoietin receptor before ligand activation. Science. 1999;283:987–990. doi: 10.1126/science.283.5404.987. [DOI] [PubMed] [Google Scholar]
- 18.Frank SJ. Receptor dimerization in GH and erythropoietin action–it takes two to tango, but how? Endocrinology. 2002;143:2–10. doi: 10.1210/endo.143.1.8607. [DOI] [PubMed] [Google Scholar]
- 19.Watowich SS. Activation of erythropoietin signaling by receptor dimerization. Int J Biochem Cell Biol. 1999;31:1075–1088. doi: 10.1016/s1357-2725(99)00075-8. [DOI] [PubMed] [Google Scholar]
- 20.Krause CD, Mei E, Xie J, Jia Y, Bopp MA, Hochstrasser RM, Pestka S. Seeing the light: preassembly and ligand-induced changes of the interferon γ receptor complex in cells. Mol Cell Proteomics. 2002;1:805–815. doi: 10.1074/mcp.m200065-mcp200. [DOI] [PubMed] [Google Scholar]
- 21.Chan FK, Chun HJ, Zheng L, Siegel RM, Bui KL, Lenardo MJ. A domain in TNF receptors that mediates ligand-independent receptor assembly and signaling. Science. 2000;288:2351–2354. doi: 10.1126/science.288.5475.2351. [DOI] [PubMed] [Google Scholar]
- 22.Siegel RM, Frederiksen JK, Zacharias DA, Chan FK, Johnson M, Lynch D, Tsien RY, Lenardo MJ. Fas preassociation required for apoptosis signaling and dominant inhibition by pathogenic mutations. Science. 2000;288:2354–2357. doi: 10.1126/science.288.5475.2354. [DOI] [PubMed] [Google Scholar]
- 23.Siegel RM, Chan F, Zacharias D, Swofford R, Holmes K, Tsien R, Lenardo M. Measurement of molecular interactions in living cells by fluorescence resonance energy transfer between variants of the green fluorescent protein. Science’s STKE. 2000;38:PL1. doi: 10.1126/stke.2000.38.pl1. [DOI] [PubMed] [Google Scholar]
- 24.Hymowitz SG, Filvaroff EH, Yin JP, Lee J, Cai L, Risser P, Maruoka M, Mao W, Foster J, Kelley RF, Pan G, et al. IL-17s adopt a cystine knot fold: structure and activity of a novel cytokine, IL-17F, and implications for receptor binding. EMBO J. 2001;20:5332–5341. doi: 10.1093/emboj/20.19.5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen F, Ruddy MJ, Plamondon P, Gaffen SL. Cytokines link osteoblasts and inflammation: micorarray analysis of interleukin-17- and TNFα-induced genes in bone cells. J Leukocyte Biol. 2005;77:388–399. doi: 10.1189/jlb.0904490. [DOI] [PubMed] [Google Scholar]
- 26.Liu X, Lin X, Gaffen SL. Crucial role for nuclear factor of activated T cells (NFAT) in T cell receptor-mediated regulation of the human interleukin-17 gene. J Biol Chem. 2004;279:52762–52771. doi: 10.1074/jbc.M405764200. [DOI] [PubMed] [Google Scholar]
- 27.Xu X, Meier-Schellersheim M, Jiao X, Nelson L, Jin T. Quantitative imaging of single live cells reveals spatiotemporal dynamics of multistep signaling events of chemoattractant gradient sensing in dictyostelium. Mol Biol Cell. 2005;16:676–688. doi: 10.1091/mbc.E04-07-0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gordon GW, Berry G, Liang XH, Levine B, Herman B. Quantitative fluorescence resonance energy transfer measurements using fluorescence microscopy. Biophys J. 1998;74:2702–2713. doi: 10.1016/S0006-3495(98)77976-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xia Z, Liu Y. Reliable and global measurement of fluorescence resonance energy transfer using fluorescence microscopes. Biophys J. 2001;81:2395–2402. doi: 10.1016/S0006-3495(01)75886-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwandner R, Yamaguchi K, Cao Z. Requirement of tumor necrosis factor-associated factor (TRAF)6 in interleukin 17 signal transduction. J Exp Med. 2000;191:1233–1240. doi: 10.1084/jem.191.7.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Novatchkova M, Leibbrandt A, Werzowa J, Neubuser A, Eisenhaber F. The STIR-domain superfamily in signal transduction, development and immunity. Trends Biochem Sci. 2003;28:226–229. doi: 10.1016/S0968-0004(03)00067-7. [DOI] [PubMed] [Google Scholar]
- 32.O’Dell J. Anticytokine therapy –a new era in the treatment of rheumatoid arthritis? N Engl J Med. 1999;340:310–312. doi: 10.1056/NEJM199901283400411. [DOI] [PubMed] [Google Scholar]
- 33.Lubberts E, Joosten LA, Oppers B, van den Bersselaar L, Coenen-de Roo CJ, Kolls JK, Schwarzenberger P, van de Loo FA, van den Berg WB. IL-1-independent role of IL-17 in synovial inflammation and joint destruction during collagen-induced arthritis. J Immunol. 2001;167:1004–1013. doi: 10.4049/jimmunol.167.2.1004. [DOI] [PubMed] [Google Scholar]
- 34.Constantinescu SN, Huang LJ, Nam H, Lodish HF. The erythropoietin receptor cytosolic juxtamembrane domain contains an essential, precisely oriented, hydrophobic motif. Mol Cell. 2001;7:377–385. doi: 10.1016/s1097-2765(01)00185-x. [DOI] [PubMed] [Google Scholar]
- 35.Livnah O, Johnson DL, Stura EA, Farrell FX, Barbone FP, You Y, Liu KD, Goldsmith MA, He W, Krause CD, Pestka S, Jolliffe LK, Wilson IA. An antagonist peptide-EPO receptor complex suggests that receptor dimerization is not sufficient for activation. Nat Struct Biol. 1998;5:993–1004. doi: 10.1038/2965. [DOI] [PubMed] [Google Scholar]
- 36.Syed RS, Reid SW, Li C, Cheetham JC, Aoki KH, Liu B, Zhan H, Osslund TD, Chirino AJ, Zhang J, et al. Efficiency of signalling through cytokine receptors depends critically on receptor orientation. Nature. 1998;395:511–516. doi: 10.1038/26773. [DOI] [PubMed] [Google Scholar]
- 37.Deng GM, Zheng L, Chan FKM, Lenardo M. Amelioration of inflammatory arthritis by targeting the pre-ligand assembly domain of tumor necrosis factor receptors. Nat Med. 2005;11:1066–1072. doi: 10.1038/nm1304. [DOI] [PubMed] [Google Scholar]
- 38.Yang RB, Ng CK, Wasserman SM, Komuves LG, Gerritsen ME, Topper JN. A novel interleukin-17 receptor-like protein identified in human umbilical vein endothelial cells antagonizes basic fibroblast growth factor-induced signaling. J Biol Chem. 2003;278:33232. doi: 10.1074/jbc.M305022200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.