Abstract
We describe here a method, based on iterative colony filter screening, for the rapid isolation of binding specificities from a large synthetic repertoire of human antibody fragments in single-chain Fv configuration. Escherichia coli cells, expressing the library of antibody fragments, are grown on a porous master filter, in contact with a second filter coated with the antigen, onto which antibodies secreted by the bacteria are able to diffuse. Detection of antigen binding on the second filter allows the recovery of a number of E.coli cells, including those expressing the binding specificity of interest, which can be submitted to a second round of screening for the isolation of specific monoclonal antibodies. We tested the methodology using as antigen the ED-B domain of fibronectin, a marker of angiogenesis. From an antibody library of 7 × 108 clones, we recovered a number of specifically-binding antibodies of different aminoacid sequence. The antibody clone showing the strongest enzyme-linked immunosorbent assay signal (ME4C) was further characterised. Its epitope on the ED-B domain was mapped using the SPOT synthesis method, which uses a set of decapeptides spanning the antigen sequence synthesised and anchored on cellulose. ME4C binds to the ED-B domain with a dissociation constant Kd = 1 × 10–7 M and specifically stains tumour blood vessels, as shown by immunohistochemical analysis on tumour sections of human and murine origin.
INTRODUCTION
With the advent of hybridoma technology (1), monoclonal antibodies have established themselves as invaluable reagents in biological and clinical research. Furthermore, 18 antibody-based biopharmaceuticals have been approved for clinical use by the Food and Drug Administration, while more than 700 antibodies by more than 200 companies are currently in clinical development (2).
The demonstration that functional antibody fragments can be produced in Escherichia coli (3,4) and displayed on phages (5) opened new avenues for antibody engineering and for the isolation of useful binding specificities. The display of antibody repertoires on the surface of bacteriophages, together with stringent selection protocols, allow to by-pass hybridoma technology and to isolate specific monoclonal antibody fragments against virtually any antigen, both self and foreign (6). It is generally recognised that good quality antibody phage libraries are reliable sources of useful antibodies when panned against purified antigen (6–10) and even complex antigen mixtures (11). However, it has been noticed that multiple rounds of biopanning may give rise to immunodominance, with the loss of diverse binding specificities and of valuable antibody clones (12). Furthermore, a complex patent situation has limited the availability of commercial antibody phage libraries for academic laboratories (13).
Filter screening techniques could in principle represent a valuable alternative to phage display. Clones (typically <106) are simultaneously assayed for their ability to generate the binding specificities of interest, thereby preventing the loss of binders that may occur during biopanning. Gherardi et al. (14) described a filter-screening methodology for the identification of the few clones secreting an antibody of given antigen specificity, out of several thousand hybridoma clones. This screening methodology was improved by Skerra and colleagues (15,16), who developed a two-membrane system for detection of antigen binding by antibody Fab fragments secreted by bacterial colonies. In brief, antibody-expressing bacterial colonies were grown on a porous master filter. Secreted Fab fragments were captured on a second membrane, which was later probed for antigen binding. Clones expressing binding antibodies could be identified on the master filter and regrown. However, this interesting screening methodology was only tested in model screening experiments with two antibody clones, and it was not clear whether it could work with libraries containing billions of antibody clones.
In this article we investigated whether the two-membrane screening methodology allows the isolation of good quality monoclonal antibodies from a large (>7 × 108 clones) synthetic antibody repertoire expressed in E.coli, bypassing phage display and yielding clones that express antibody fragments in soluble form.
We used the ETH-2 library as antibody source (7). This library consists of antibody fragments in single-chain Fv (scFv) configuration, containing the DP47 germline variable heavy (VH) gene (17), linked either to a VK domain based on the DPK-22 germline gene (18) or to a Vλ domain based on the DPL-16 germline gene (19). Judiciously chosen residues in the complementarity determining region 3 (CDR3) of VH and variable light (VL) had been combinatorially mutated by means of partially-degenerated oligonucleotide primers (7).
As antigen for our screening experiments, we chose the ED-B domain of fibronectin, a marker of angiogenesis (9,20–22). The ED-B domain, a 91 amino acid protein domain whose solution structure has been solved by NMR spectroscopy (23) and which can be inserted or omitted in the fibronectin molecule by a mechanism of alternative splicing at the level of the mRNA (24) primary transcript, is present in neoplastic tissues, mainly around proliferating new blood vessels, but it is undetectable in mature blood vessels and in the vast majority of normal tissues. To date, the production of monoclonal antibodies directly recognising the ED-B domain in fibronectin has not been possible using hybridoma technology, because of tolerance. This problem, however, has been overcome using antibody phage technology (6) with large synthetic antibody repertoires (9,21). Several antibody fragments specific for the ED-B domain of fibronectin have recently been generated, which stain vascular structures in tumour sections and selectively target tumour neovasculature, as shown in tumour-bearing mice using infrared fluorescence and radioactive techniques (25–28).
In this article, we show that a number of potentially useful monoclonal human antibody fragments can be isolated from the ETH-2 library by our iterative screening methodology. One of them (ME4C) was analysed in detail, in terms of the epitope recognised on the ED-B molecule, and of its binding affinity and kinetic binding constant to the antigen. ME4C strongly and selectively stains new blood vessels in sections of glioblastoma multiforme, a highly angiogenetic aggressive solid brain tumour, and in sections of human SKMEL-28 melanoma, grafted in nude mice.
MATERIALS AND METHODS
The ETH-2 antibody library
The description of the ETH-2 library, its construction and use, have been described previously (7). In brief, the ETH-2 is a phage display library of recombinant antibody fragments in the scFv format (7), containing >7 × 108 individual clones. Sequence diversity is inserted in the antibody molecule at the level of the CDR3 of both VH and VL chains. The genes coding for VH and VL are cloned in a phagemid vector, which allows either the display of antibodies on phage or their expression in soluble form. Moreover, the vector appends a FLAG tag at the C-terminus of the scFvs (7,9) enabling their immunodetection by means of the M2 monoclonal antibody (Sigma, St Louis, MO).
Colony growth and expression of scFvs
The ETH-2 library was stored as bacteria (E.coli TG1 strain) harbouring phagemid DNA. Bacteria were grown in liquid medium as described (7) and, once in the exponential phase of growth, 108 bacteria were spread on a 20 cm diameter PVDF filter membrane Durapore (type GVWP; Millipore, Bedford, MA). This master filter was placed on a 20 cm diameter Petri dish containing TYE agar (1 l TYE agar contains 15 g agar, 10 g bactotryptone, 5 g yeast extract), 100 µg/ml ampicillin, 1% glucose and incubated at 37°C for 8 h. After incubation, a lawn of completely confluent bacterial colonies was visible on the filter.
A second (capture) membrane was prepared as follows. A 20 cm diameter PVDF membrane (Immobilon-P; Millipore) was pre-wet and coated with the capture antigen, the recombinant protein 7B89 (29) and a fibronectin fragment containing the ED-B domain. Preliminary screening experiments had shown that the small recombinant ED-B domain of fibronectin alone did not bind to the membrane. The filter coating was achieved by incubation of the membrane for 6 h at 37°C in phosphate-buffered saline (PBS: 50 mM phosphate, pH 7.4, 100 mM NaCl) containing 150 µg/ml 7B89. The filter was then blocked in 5% milk/PBS (MPBS) for 2 h at 37°C, washed four times in PBS 0.2% (v/v) Tween-20 (PBST) and soaked in 2× TY medium containing 100 µg/ml ampicillin and 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG).
The capture membrane was then placed onto a TYE agar plate containing 100 µg/ml ampicillin and 1 mM IPTG and covered in turn with the Durapore membrane, with the bacterial colonies on top. The resulting ‘sandwich’ was incubated at room temperature for 16 h. The presence of IPTG in the medium and the absence of glucose allow for the Lac-promoter regulated expression of the scFvs. These antibody fragments can diffuse; those specifically binding to the 7B89 protein are ‘captured’ by the second membrane.
Detection of antigen binding
The capture membrane was washed four times with PBST and then blocked in MPBS for 6 h at 37°C. To detect bound scFvs, the filter was incubated with M2 anti-flag murine antibody (F-3165; Sigma) diluted 1:3000 in MBST [MPBS + 0.2% (v/v) Tween-20] for 1 h at 37°C, washed four times in PBST and incubated with horseradish peroxidase conjugated rabbit anti-mouse IgG (172–1011; Bio-Rad) diluted 1:3000 in MBST for 1 h at 37°C. The filter was then washed extensively with PBST and incubated in 4-chloro-1-naphthol until dark spots were visible. The enzymatic colourimetric reaction was stopped by washing the filter with water and then allowing it to dry.
Alignment marks, made when superimposing the two filters, allowed us to go back from colour spots on the capture filter to the areas containing, among other colonies, the anti ED-B producing antibody clones on the first filter.
Amplification of positive clones
After the first filter screening, positive signals on the capture filter corresponded to areas of confluent bacteria colonies on the first filter, which were rescued with a cell scraper and grown in 2× TY, 100 µg/ml ampicillin, 1% (w/v) glucose until an OD600 nm of 0.7 was reached.
At this stage, 106 bacteria were spread on a new Durapore membrane and the screening procedure was repeated. At the end of this second round, positive signals in the capture filter corresponded to single colonies, which were picked, grown and used for a further screening, in which 103 bacteria were plated on a Durapore filter. After the third round of screening, positive signals on the capture membrane corresponded to single positive colonies, each representing a potential source of monoclonal antibody directed against the 7B89 antigen.
Characterisation of the selected antibodies
Individual clones positive at the third round of filter screening were grown in liquid medium as described (7) and allowed to produce soluble scFv fragments. The scFv binding specificity was then determined by a soluble enzyme-linked immunosorbent assay (ELISA) as described (7), performed on ED-B, 7B89 or irrelevant antigens (MPBS, bovine serum albumin, ovalbumin, lysozyme) (7). Clones positive for both ED-B and 7B89, but negative for all other control antigens, were selected for further characterisation.
Kinetic association and dissociation rates of antibody fragments were determined by surface plasmon resonance on a BIAcore 1000 instrument (Pharmacia) as described (25,30), using monomeric fraction of the scFv (27) and sensor chips coated with both 7B89 and ED-B (25).
DNA sequencing of selected clones was performed with a 377 DNA sequencer ABI Prism apparatus (Perkin-Elmer, Norwalk, CT).
The epitopes on ED-B recognised by one of the selected scFvs (ME4C) were identified using the SPOT synthesis method (31), as described for other anti-ED-B antibody fragments (23).
Immunostaining of sections of glioblastoma multiforme and SK-MEL human melanoma was performed as described (9,20,21).
RESULTS
Isolation of anti ED-B antibody fragments by colony filter screening
We used the naive ETH-2 antibody library (7), in the form of a pool of antibody-secreting bacteria, to investigate whether monoclonal antibodies specific for the ED-B domain of fibronectin could be isolated, using a modified screening methodology based on the two membrane colony filter screening of Skerra et al. (15).
The iterative screening procedure that we used is schematically depicted in Figure 1. One hundred million bacteria from the ETH-2 library, each potentially harbouring a different scFv coding sequence, were grown on a porous master filter until a lawn of small confluent colonies was visible. These bacteria represent only a sub-set of the ETH-2 library, which had been shown to routinely yield specific binders against a variety of different antigens in phage selection experiments (9). The master filter was then laid on a capture filter embedded with the antigen (an ED-B containing fibronectin fragment), and bacteria on the master filter were induced to express the recombinant antibodies by placing the filters on solid medium containing IPTG. Recombinant antibodies can diffuse from the master to the capture membrane, where the ones specific for the antigen are trapped onto the capture filter. A FLAG-tag at the C-terminal extremity of the scFv antibody fragments allowed detection of their binding to the antigen-coated capture membrane by an enzymatic colourimetric reaction. Due to the huge number of bacteria on the master filter, the first round of screening did not allow the identification of single positive colonies, but only of positive areas of confluent colonies that, by superposition of the two filters, could be rescued from the master membrane and grown to perform a new round of screening. We performed three rounds of screening, plating 106 bacteria on a master filter for the second round and 103 for the third round (the diversity of the bacterial pool decreases at each round of screening, since the pool becomes enriched of specifically binding clones).
Figure 1.
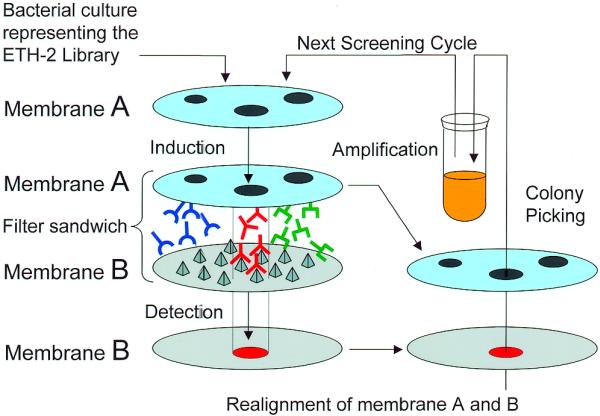
Schematic representation of the iterative colony screening method. Bacteria expressing a potentially different antibody fragment were spread on a Durapore filter membrane (A). On the filter, placed on a solid medium that represses expression of the antibody fragments, colonies were visible after 8 h incubation at 37°C (here three different colonies are depicted schematically). A second filter membrane (B) was coated with the antigen of interest (represented by pyramids), laid on a solid medium capable of inducing the expression of scFvs, and in contact with membrane A. Antibody fragments that diffused from membrane A and that bound to the antigen (in red) were captured on membrane B, and could be detected by an enzymatic colourimetric reaction. The corresponding colonies could be identified on membrane A, regrown and the procedure could be repeated until single positive colonies producing monoclonal antibodies were identified.
Figure 2A shows the master membrane, and Figure 2B shows the capture membrane after immunometric detection, in the third round of colony screening for binding to the ED-B antigen. At this stage, colonies on the master filter were no longer confluent. Approximately 30% of the plated colonies (filter A) produced scFvs that bound to filter B, therefore representing potential anti-ED-B monoclonal antibody fragments.
Figure 2.
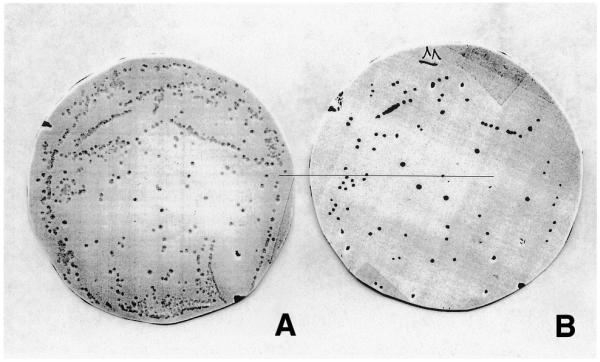
Anti ED-B antibodies identified after three rounds of colony filter screening. (A) The Durapore filter membrane, where 103 bacterial clones were plated and which were rescued from the second round of iterative filter screening. (B) The capture filter membrane, coated with the recombinant protein 7B89, onto which antibodies secreted by the colonies grown on filter (A) could diffuse. The clones secreting antibodies binding to 7B89 were detected by enzymatic colorimetric reaction. Around 30% of the colonies in (A) result positive in (B).
The positive clones at the third round of filter screening were regrown and the binding specificity of the corresponding antibodies was analysed by ELISA (data not shown). Sixteen percent produced antibodies that bound strongly both to the ED-B domain of fibronectin and to the ED-B containing 7B89 fibronectin fragment (21), but not to a palette of irrelevant antigens. In total nine different clones were sequenced. Among them the clone showing the strongest ELISA signal [named scFv(ME4C)], was further characterised.
Characterisation of the anti-ED-B antibody ME4C
Table 1 shows the amino acid sequence of scFv(ME4C). The portions of the CDR3 regions that had been combinatorially mutated in the ETH-2 library are shown in bold. The sequence of scFv(ME4C) is different from the sequence of all the anti-ED-B antibodies selected in our laboratory (9,25; data not shown).
Table 1. Sequence of the variable immunoglobulin domains of scFv(ME4C).
| E1 | V | Q | L | L | E | S | G | G | G10 | L |
| V | Q | P | G | G | S | L | R | L20 | S | C |
| A | A | S | G | F | T | F | S30 | S | F | S |
| M | S | W | V | R | Q | A40 | P | G | K | G |
| L | E | W | V | S | S50 | I | S | G | S | S |
| G | T | T | Y | Y60 | A | D | S | V | K | G |
| R | F | T | I70 | S | R | D | N | S | K | N |
| T | L | Y80 | L | Q | M | N | S | L | R | A |
| E | D90 | T | A | V | Y | Y | C | A | K | Q99 |
| K | S | A | P103 | F | D | Y | W | G | Q | G110 |
| T | L | V | T | V | S | S | G | D | G120 | S |
| S | G | G | S | G | G | A | S | S130 | S | E |
| L | T | Q | D | P | A | V | S140 | V | A | L |
| G | Q | T | V | R | I | T150 | C | Q | G | D |
| S | L | R | S | Y | Y160 | A | S | W | Y | Q |
| Q | K | P | G | Q170 | A | P | V | L | V | I |
| Y | G | K | N180 | N | R | P | S | G | I | P |
| D | R | F190 | S | G | S | S | S | G | N | T |
| A | S200 | L | T | I | T | G | A | Q | A | E |
| D210 | E | A | D | Y | Y | C | N | S | S | A220 |
| P | V | S | N | R225 | V | V | F | G | G230 | G |
| T | K | L | T | V | L | G238 |
Primary sequence of the variable immunoglobulin domains of scFv(ME4C) (DDBJ/EMBL/GenBank accession no. AJ297960). Single amino acid codes are used according to standard IUPAC nomenclature. Amino acid positions in the CDR3, which are combinatorially mutated in the ETH-2 antibody library, are indicated in bold. A flexible linker connecting VH and VL domains is located between residues number 118 and 129.
The affinity of scFv(ME4C) towards the ED-B domain of fibronectin was measured by real-time interaction analysis using surface plasmon resonance detection on a BIAcore instrument. Monomeric fractions of scFv(ME4C) were allowed to bind to biotinylated ED-B, immobilised at low surface density on a streptavidin-coated microsensor chip, yielding a Kd = 1 × 10–7 M–1, with a kinetic dissociation constant (koff) of 6 × 10–3 s–1 and a kinetic association constant (kon) of 6 × 104 s–1 M–1.
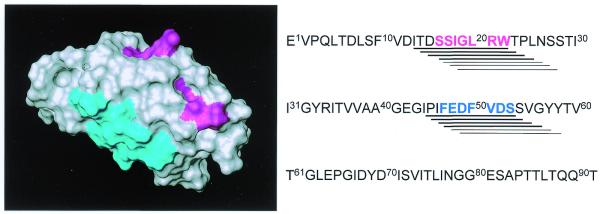
In order to further characterise the binding of scFv(ME4C) to ED-B, we identified the epitope on the target antigen using the SPOT method (31). A series of 82 decapeptides, spanning the 91 amino acid sequence of the ED-B domain, was synthesised on a cellulose support and was assayed for binding to scFv(ME4C). Figure 3 (right) shows the sequence of the synthetic decapeptides that are recognised by scFv(ME4C). In Figure 3 (left), these peptides are mapped onto the solution structure of the ED-B domain determined by NMR spectroscopy, outlining a discontinuous epitope, which overlaps to a large extent with the epitopes recognised by the CGS-1, CGS-2 and L19 antibodies (23).
Figure 3.
Epitope mapping of the recombinant antibody ME4C using the SPOT method. The binding of the antibody ME4C to synthetic decapeptides spanning the ED-B sequence is reported as a horizontal line (right). The thickness of the line is proportional to the observed binding signal. Two minimal sequences recognised by the antibodies in the array of synthetic ED-B peptides are coloured in cyan and magenta. The same colours were used to map the two epitopes on the three-dimensional structure of ED-B (left).
In order to investigate whether scFv(ME4C) recognises the ED-B domain in the intact oncofoetal fibronectin molecule and whether it could be a useful reagent for the detection of angiogenesis, we performed an immunohistochemical analysis on frozen tumour sections.
Figure 4A shows the immunohistochemical findings obtained with frozen sections of glioblastoma multiforme and Figure 4B of the SKMEL-28 human melanoma grafted in nude mice. In both cases, ME4C strongly stains the abundant neo-vascular structures of the tumour, but does not stain normal tissues (data not shown). The staining pattern of scFv(ME4C) is indistinguishable from that of previously described anti-ED-B antibodies obtained by phage display (21,27).
Figure 4.
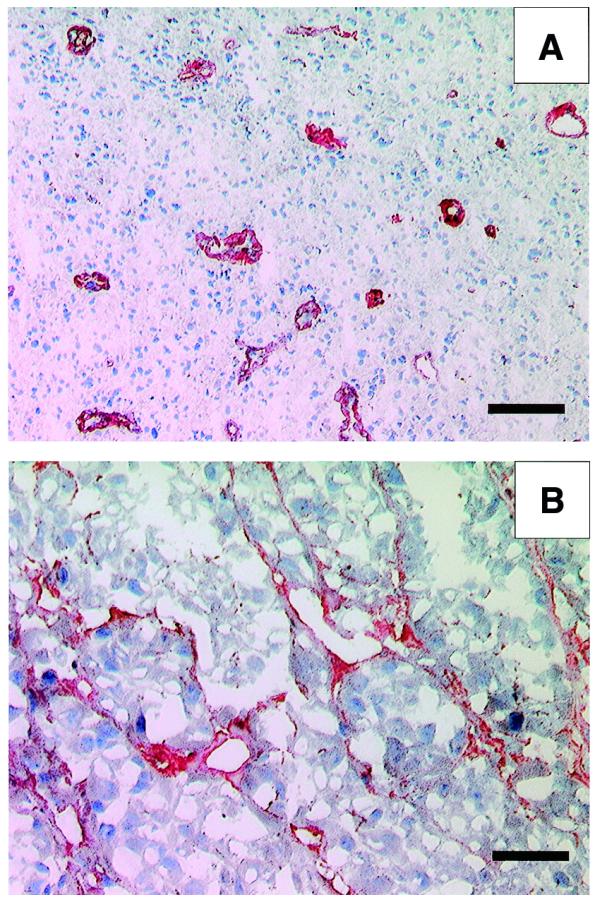
Immunohistochemical experiments with the antibody ME4C. (A) A section of glioblastoma multiforme specimen. The typical glomerulus-like vascular structures are stained in red by the scFv(ME4C). Scale bar, 100 µm. (B) A section of SKMEL-28 human melanoma, grafted in a nude mouse, stained with scFv(ME4C). The antibody localises around vascular structures and proliferating cells. Scale bar, 50 µm
DISCUSSION
In this article, we have shown that a colony filter screening method can be used in an iterative fashion, for the isolation of useful binding specificities from a lawn of confluent bacteria, expressing a synthetic library of human antibody fragments. In particular, we have obtained a number of antibodies specific for the ED-B domain of fibronectin, a marker of angiogenesis, without immunisation and bypassing both hybridoma and phage technology. Angiogenesis, i.e. the proliferation of new blood vessels from pre-existing ones, is a characteristic feature of aggressive solid tumours and of relevant disorders, such as age-related macular degeneration, diabetic retinopathy and rheumatoid arthritis (32). Molecules capable of selective targeting and destruction of new blood vessels are promising agents for the treatment of angiogenesis-related diseases (28,33).
Colony filter screening techniques have been used in the past for the identification of streptavidin-binding peptides from a plasmid-encoded library of random peptides (106 different clones), which were displayed at the C-terminus of an anti-lysozyme antibody Fv fragment (34). To our knowledge, however, the isolation of monoclonal antibodies from naive antibody libraries by filter screening had not been reported previously. This is probably due to the fact that naive antibody libraries must be sufficiently large (typically >100 000 000 clones) (6,8,35), in order to contain binders of the desired specificity. In this article, we have screened 100 000 000 bacterial clones, representing a subset of the ETH-2 antibody library (size 7 × 108 clones). The resulting anti-ED-B antibodies (including clone ME4C) have a typical expression yield of ∼5–6 mg scFv per l of bacterial culture.
The colony filter screening method of Skerra et al. (15) has recently been used by de Wildt et al. (12), who have applied the high-throughput robotic colony filter screening of antibody libraries after one or two rounds of phage biopanning. The approach is certainly of great interest, particularly as it allowed the authors to screen up to 18 342 antibody clones at a time and to directly recover binding specificities of interest. However, direct screening of a large antibody library by robotic methods may be limited by the number of clones that can be simultaneously picked, grown and assayed. In this respect, the iterative colony screening method described in this article may represent a valuable alternative, particularly as it bypasses phage display selection. An advantage of performing expression in situ is that it avoids the unfolding and denaturation of isolated antibodies that can occur when they come in contact with chemically treated surfaces. In order to use a lower amount of antigen for coating membranes without reducing coating efficiency, the procedure may have to be optimised (i.e. membranes of different materials, fusion of the antigen to carrier proteins). The anti-ED-B antibody ME4C, isolated by iterative colony filter screening, binds to native ED-B-containing fibronectin, as shown by immunohistochemical analysis. The affinity of the ME4C antibody (Ka = 107 M–1) is sufficient for its use as immunochemical reagent (36). For in vivo tumour-targeting applications, its affinity would have to be matured (27). Antibodies from the ETH-2 library have been successfully affinity matured by combinatorially mutating judiciously selected residues located in the complementary determining regions 1 and 2 of the heavy and light chains (9,37). The same approach, followed by an extensive screening for kinetic association and dissociation constants could in principle be used for scFv ME4C. Interestingly, the epitope on the ED-B domain recognised by the ME4C antibody is to a large extent overlapping with the epitopes recognised by all the other anti-ED-B antibodies isolated in our group from two different naive synthetic antibody libraries (7,9,21,23,36). These results indicate that an epitopic surface on the ED-B molecule is immunodominant, irrespective of the library used and of the method chosen for antibody isolation.
Acknowledgments
ACKNOWLEDGEMENTS
We thank Paola Battestin for her help in screening. D.N. acknowledges financial support from the Swiss National Science Foundation (project, ‘Isolation of novel enzymes’).
DDBJ/EMBL/GenBank accession no. AJ297960
References
- 1.Kohler G. and Milstein,C. (1975) Continuous cultures of fused cells secreting antibody of predefined specificity. Nature, 256, 495–497. [DOI] [PubMed] [Google Scholar]
- 2.Walsh G. (2000) Biopharmaceutical benchmark. Nat. Biotechnol., 18, 831–833. [DOI] [PubMed] [Google Scholar]
- 3.Better M., Chang,C.P., Robinson,R.R. and Horwitz,A.H. (1988) Escherichia coli secretion of an active chimeric antibody fragment. Science, 240, 1041–1043. [DOI] [PubMed] [Google Scholar]
- 4.Skerra A. and Plueckthun,A. (1988) Assembly of a functional immunoglobulin Fv fragment in Escherichia coli. Science, 240, 1038–1041. [DOI] [PubMed] [Google Scholar]
- 5.McCafferty J., Griffiths,A.D., Winter,G. and Chiswell,D.J. (1990) Phage antibodies: filamentous phage displaying antibody variable domains. Nature, 348, 552–554. [DOI] [PubMed] [Google Scholar]
- 6.Winter G., Griffiths,A.D., Hawkins, RE. and Hoogenboom,H.R. (1994) Making antibodies by phage display technology. Annu. Rev. Immunol., 12, 433–455. [DOI] [PubMed] [Google Scholar]
- 7.Viti F., Nilsson,F., Demartis,S., Huber,A. and Neri,D. (2000) Design and use of phage display libraries for the selection of antibodies and enzymes. Methods Enzymol., 326, 480–505. [DOI] [PubMed] [Google Scholar]
- 8.Vaughan T.J., Williams,A.J., Pritchard,K, Osbourn,J.K., Pope,A.R., Earnshaw,J.C., McCafferty,J., Hodits,R.A., Wilton,J. and Johnson,K.S. (1996) Human antibodies with sub-nanomolar affinities isolated from a large non-immunized phage display library. Nat. Biotechnol., 14, 309–314. [DOI] [PubMed] [Google Scholar]
- 9.Pini, A., Viti,F., Santucci,A., Carnemolla,B., Zardi,L., Neri,P. and Neri,D. (1998) Design and use of a phage display library. Human antibodies with subnanomolar affinity against a marker of angiogenesis eluted from a two-dimensional gel. J. Biol. Chem., 273, 21769–21776. [DOI] [PubMed] [Google Scholar]
- 10.Neri, D., Pini,A. and Nissim,A. (1998) Antibodies from phage display libraries as immunochemical reagents. Methods Mol. Biol., 80, 475–500. [DOI] [PubMed] [Google Scholar]
- 11.Marks, J.D., Ouwehand,W.H., Bye,J.M., Finnern,R., Gorick,B.D., Voak,D., Thorpe,S.J., Hughes-Jones,N.C. and Winter,G. (1993) Human antibody fragments specific for human blood group antigens from a phage display library. Biotechnology, 11, 1145–1149. [DOI] [PubMed] [Google Scholar]
- 12.de Wildt, R.M., Mundy,C.R., Gorick,B.D. and Tomlinson,I.M. (2000) Antibody arrays for high-throughput screening of antibody-antigen interactions. Nat. Biotechnol., 18, 989–994. [DOI] [PubMed] [Google Scholar]
- 13.Brower, V. (1999) Tumor angiogenesis-new drugs on the block. Nat. Biotechnol., 17, 963–968. [DOI] [PubMed] [Google Scholar]
- 14.Gherardi E., Pannell,R. and Milstein,C. (1990) A single-step procedure for cloning and selection of antibody-secreting hybridomas. J. Immunol. Methods, 126, 61–68. [DOI] [PubMed] [Google Scholar]
- 15.Skerra A., Dreher,M.L. and Winter,G. (1991) Filter screening of antibody Fab Fragments secreted from individual bacterial clones: specific detection of antigen binding with a two-membrane system. Anal. Biochem., 196, 151–155. [DOI] [PubMed] [Google Scholar]
- 16.Dreher, M.L., Gherardi,E., Skerra,A. and Milstein,C. (1991) Colony assays for antibody fragments expressed in bacteria. J. Immunol. Methods, 139, 197–205. [DOI] [PubMed] [Google Scholar]
- 17.Tomlinson I.M., Walter,G., Marks,J.D., Lewelyn,M.B. and Winter,G. (1992) The repertoire of human germline VH sequences reveals about fifty groups of VH segments with different hypervariable loops. J. Mol. Biol., 227, 776–798. [DOI] [PubMed] [Google Scholar]
- 18.Cox J.P., Tomlinson,I.M. and Winter,G. (1994) A directory of human germ-line V kappa segments reveals a strong bias in their usage. Eur. J. Immunol., 24, 827–836. [DOI] [PubMed] [Google Scholar]
- 19.Williams S.C., Frippiat,J.P., Tomlinson,I.M., Ignatovich,O., Lefranc,M.P. and Winter,G. (1996) Sequence and evolution of the human germline V lambda repertoire. J. Mol. Biol., 264, 220–232. [DOI] [PubMed] [Google Scholar]
- 20.Castellani P., Viale,G., Dorcaratto,A., Nicolo,G., Kazmarek,J., Querze,G. and Zardi,L. (1994) The fibronectin isoform containing the ED-B oncofetal domain: a marker of angiogenesis. Int. J. Cancer, 59, 612–618. [DOI] [PubMed] [Google Scholar]
- 21.Carnemolla B., Neri,D., Castellani,P., Leprini,A., Neri,G., Pini,A., Winter,G. and Zardi,L. (1996) Phage antibodies with pan-species recognition of the oncofoetal angiogenesis marker fibronectin ED-B domain. Int. J. Cancer, 68, 397–405. [DOI] [PubMed] [Google Scholar]
- 22.Zardi, L., Carnemolla,B., Siri,A., Petersen,T.E., Paolella,G., Sebastio,G. and Baralle,F.E. (1987) Transformed human cells produce a new fibronectin isoform by preferential alternative splicing of a previously unobserved exon. EMBO J., 6, 2337–2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fattorusso R., Pellecchia,M., Viti,F., Neri,P., Neri,D. and Wuthrich,K. (1999) NMR structure of the human oncofoetal fibronectin ED-B domain, a specific marker for angiogenesis. Structure Fold Des., 7, 381–390. [DOI] [PubMed] [Google Scholar]
- 24.Carnemolla B., Balza,E., Siri,A., Zardi,L., Nicotra,M.R., Bigotti,A. and Natali,P.G. (1989) A tumor-associated fibronectin isoform generated by alternative splicing of messenger RNA precursors. J. Cell Biol., 108, 1139–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neri D., Carnemolla,B., Nissim,A., Balza,E., Leprini,A., Querze,G., Pini,A., Tarli,L., Halin,C., Neri,P., Zardi,L. and Winter,G. (1997) Targeting by affinity-matured recombinant antibody fragments of an angiogenesis associated fibronectin isoform. Nat. Biotechnol., 15, 1271–1275. [DOI] [PubMed] [Google Scholar]
- 26.Tarli L., Balza,E., Viti,F., Borsi,L., Castellani,P., Berndorff,D., Dinkelborg,L., Neri,D. and Zardi,L. (1999) A high-affinity human antibody that targets tumoral blood vessels. Blood, 94, 192–198. [PubMed] [Google Scholar]
- 27.Viti F., Tarli,L., Giovannoni,L., Zardi,L. and Neri,D. (1999) Increased binding affinity and valence of recombinant antibody fragments lead to improved targeting of tumoral angiogenesis. Cancer Res., 59, 347–352. [PubMed] [Google Scholar]
- 28.Birchler M., Viti,F., Zardi,L., Spiess,B. and Neri,D. (1999) Selective targeting and photocoagulation of ocular angiogenesis mediated by a phage-derived human antibody fragment. Nat. Biotechnol., 17, 984–988. [DOI] [PubMed] [Google Scholar]
- 29.Carnemolla B., Leprini,A., Allemanni,G., Saginati,M. and Zardi,L. (1992) The inclusion of the Type III repeat ED-B in the Fibronectin molecule generates conformational modifications that unmask a cryptic sequence. J. Biol. Chem., 267, 24689–24692. [PubMed] [Google Scholar]
- 30.Jönsson U., Fägerstam,L., Ivarsson,B., Johnsson,B., Karlsson,R., Lundh,K., Löfås,S., Persson,B., Roos,H., Rönnberg,I., Sjölander,S., Stenberg,E., Ståhlberg, Urbaniczky,C., Östlin,H. and Malmqvist,M. (1991) Real-time biospecific interaction analysis using surface plasmon resonance and a sensor chip technology. Biotechniques, 11, 620–627. [PubMed] [Google Scholar]
- 31.Frank R. and Overwin,H. (1996) SPOT synthesis. Epitope analysis with arrays of synthetic peptides prepared on cellulose membranes. Methods Mol. Biol., 66, 149–169. [DOI] [PubMed] [Google Scholar]
- 32.Folkman J. (1995) Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat. Med., 1, 27–31. [DOI] [PubMed] [Google Scholar]
- 33.Nilsson F., Kosmehl,H., Zardi,L. and Neri,D. (2001) Targeted delivery of tissue factor to the ED-B domain of fibronectin, a marker of angiogenesis, mediates the infarction of solid tumours in mice. Cancer Res., in press. [PubMed] [Google Scholar]
- 34.Schmidt T.G. and Skerra,A. (1993) The random peptide library-assisted engineering of a C-terminal affinity peptide, useful for the detection and purification of a functional Ig Fv fragment. Protein Eng., 1, 109–122. [DOI] [PubMed] [Google Scholar]
- 35.Griffiths A.D., Williams,S.C., Hartley,O., Tomlinson,I.M., Waterhouse,P., Crosby,W.L., Kontermann,R.E., Jones,P.T., Low,N.M., Allison,T.J., Prospero,T.D., Hoogenboom,H.R., Nissim,A., Co,J.P.L., Harrison,J.L., Zaccolo,M., Gherardi,E. and Winter,G. (1994) Isolation of high affinity human antibodies directly from large synthetic repertoires. EMBO J., 13, 3245–3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nissim A., Hoogenboom,H.R., Tomlinson,I.M., Flynn,G., Midgley,C., Lane,D. and Winter,G. (1994) Antibody fragments from a ‘single pot’ phage display library as immunochemical reagents. EMBO J., 3, 692–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang W.P., Green,K., Pinz-Sweeney,S., Briones,A.T., Burton,D.R. and Barbas,C.F.,III (1995) CDR walking mutagenesis for the affinity maturation of a potent human anti-HIV-1 antibody into the picomolar range. J. Mol. Biol., 3, 392–403. [DOI] [PubMed] [Google Scholar]