Abstract
The Pak4 serine/threonine kinase is highly expressed in many cancer cell lines and human tumors. While several studies have addressed the role for Pak4 in transformation of fibroblasts, most human cancers are epithelial in origin. Epithelial cancers are associated not only with changes in cell growth, but also with changes in the cellular organization within the three dimensional (3D) architecture of the affected tissues. Here we used immortalized mouse mammary epithelial cells (iMMECs) as a model system to study the role for Pak4 in mammary tumorigenesis. iMMECs are an excellent model system for studying breast cancer they can grow in 3D-epithelial cell culture, where they form acinar structures that recapitulate in vivo mammary morphogenesis. While Pak4 is expressed at low levels in wild type iMMECs, it is overexpressed in response to oncogenes, such as oncogenic Ras and Her2/neu. Here we found that overexpression of Pak4 in iMMECs leads to changes in 3D acinar architecture that are consistent with oncogenic transformation. These include decreased central acinar cell death, abrogation of lumen formation, cell polarity alterations, and deregulation of acinar size and cell number. Furthermore, iMMECs overexpressing Pak4 form tumors when implanted into the fat pads of athymic mice. Our results suggest that overexpression of Pak4 triggers events that are important for the transformation of mammary epithelial cells. This is likely to be due to the ability of Pak4 to inhibit apoptosis and promote cell survival, and thus subsequent uncontrolled proliferation, and to its ability to deregulate cell shape and polarity.
Keywords: Pak4, mammary epithelial cells, breast cancer, apoptosis, cell survival
Introduction
Oncogenic transformation can occur in response to improperly controlled cell proliferation, increased levels of cell survival, failure to differentiate, or failure to maintain proper cell shape and polarity. These changes are often associated with improper regulation of intracellular signaling pathways that control cell growth and survival. Protein kinases play key roles in such pathways. The serine/threonine kinase Pak4 has been implicated in signaling pathways leading to malignant transformation (Callow et al., 2002; Gnesutta and Minden, 2003; Li and Minden, 2005; Qu et al., 2001). Pak4 was first identified as an effector protein for the Rho GTPase Cdc42, which leads to changes in cytoskeletal organization (Abo et al., 1998). Later, Pak4 was also found to be activated by Rho GTPase independent stimuli (Paliouras et al., 2009). Pak4 is highly overexpressed in many different types of cancer cell lines and tumors (Callow et al., 2002; Liu, 2008; Chen et al., 2008; Kimmelman et al., 2008). When overexpressed in fibroblasts, activated Pak4 causes cells to grow in an anchorage independent manner (Callow et al., 2002; Kimmelman et al., 2008; Qu et al., 2001). Pak4 transforms these cells as strongly as activated Ras (Qu et al., 2001), and it causes cells to become resistant to apoptosis (Gnesutta and Minden, 2003; Gnesutta et al., 2001; Li and Minden, 2005). When injected into athymic mice, fibroblasts overexpressing Pak4 lead to the formation of large tumors (Liu, 2008).
While a number of studies have shown Pak4 to be highly transforming in fibroblasts, most human cancers are epithelial in origin. Expression studies suggest that Pak4 has a role in epithelial cell tumorigenesis as well (Callow et al., 2002; Liu, 2008; Chen et al., 2008; Kimmelman et al., 2008). The mechanism by which Pak4 transforms epithelial cells, however, is poorly understood. An extensively studied prototype of epithelial tumorigenesis is breast cancer. Pak4 is highly overexpressed in human breast tumors and rat mammary tumors (Liu, 2008), and it was found to be overexpressed in 8 out of 8 breast cancer cell lines that were examined (Callow et al., 2002).
The signaling pathways involved in the progression of breast cancer are complex and incompletely understood. Mammary carcinomas are tumors of the glandular epithelial cells. The normal mammary gland consists of an ordered structure of individual spherically shaped acini. These acini contain hollow lumens, surrounded by polarized epithelial cells. Mammary tumorigenesis is associated with disruption of this well ordered structure (Debnath, 2002; Debnath, 2003), caused in part by increased epithelial cell proliferation and inhibition of apoptosis, resulting in filling of the acinar luminal space with cells (Muthuswamy, 2001). Use of two dimensional (2D) cell cultures can be limiting with respect to studying three dimensional (3D) glandular structures. A more relevant model involves the use of 3D basement membrane cell culture methods that recapitulate glandular epithelium morphogenesis. One such model system involves use of immortalized mouse mammary epithelial cells (iMMECs) generated from wild-type female mice. iMMECs are poorly transforming on their own, and they are an excellent model for studying genes that promote breast cancer (Karantza-Wadsworth, 2008). In 3D cultures, iMMECs behave similarly to the human non-transformed mammary epithelial cell line MCF10A. They follow a temporal sequence of events leading to the formation of spherical acini (Debnath, 2003). First, the cells proliferate and form a cluster of cells, which constitute the beginning of an acinar structure. This is followed by the initial steps of cell polarization. A polarized outer layer of cells forms and is in direct contact with the basement membrane. An inner group of cells is more poorly polarized and does not come into contact with the matrix. These inner, nonpolarized cells begin to die by apoptosis, resulting in hollow lumen formation. At the end of this process, there is a suppression of cell proliferation throughout the acinus. The final structure consists of a single layer of polarized epithelial cells around a hollow lumen and surrounded by basement membrane. Although there are differences between the acini formed by iMMEC cells and normal mammary tissue, in many respects the iMMEC acini mimic the architecture of glandular epithelium. Like normal breast epithelium, the acini formed in this 3D culture system have low levels of proliferation and have a stable cell number (Debnath, 2003). The acinar structures formed by mammary epithelial cells in 3D culture are an excellent model for studying the factors that disrupt the balance between apoptosis and proliferation at a spatial and temporal level.
While changes in cell proliferation and survival are important features of oncogenesis, there are other types of cellular changes that also lead to oncogenic transformation. One example is the disruption of cell polarity. Cells in the glandular epithelium are normally highly polarized. The apical surface of the membrane faces the lumen, and the basolateral surface contacts the underlying basement membrane (Moreno-Bueno, 2008). Disruption of cell polarity is an important hallmark of many epithelial cancers and is sometimes linked to changes in growth and proliferation. Disruption of polarity can contribute to the progression of a premalignant lesion into carcinoma, and it is an important aspect of the epithelial-mesenchymal transition and progression to invasive cancer cells (Tanos, 2008). Acini formed by iMMECs consist of highly polarized cells, and are thus an excellent model for studying factors that can disrupt normal cell polarity.
In this paper we studied the role for Pak4 in controlling growth of mammary epithelial cells. We found that wild type iMMECs have low levels of Pak4. We found that overexpression of Pak4 in iMMECs leads to changes in 3D acinar morphogenesis that are consistent with oncogenic transformation. These include decreased central acinar cell death, abrogation of lumen formation, alterations of cell polarity, and deregulation of acinar size and cell number. Furthermore, iMMECs overexpressing Pak4 form mammary tumors when orthotopically implanted in athymic mice. Our results suggest that overexpression of Pak4 triggers events that are important for the transformation of mammary epithelial cells.
Results
Pak4 is overexpressed in human tumors and cancer cell lines, and in oncogene-expressing iMMECs
To study whether Pak4 is overexpressed in human epithelial cancers, we carried out real-time PCR with human normal and tumor tissue cDNA (see Figure 1A). We found that Pak4 is overexpressed in tumors of the breast, endometrium, esophagus, ovary, prostate, and urinary bladder. Thus Pak4 appears to be broadly expressed in different types of human tumors. In previous studies, Pak4 was shown to be overexpressed in primary tumors from human breast and rat mammary gland, and in a panel of breast cancer cell lines (Liu, 2008). To further study the levels of Pak4 in other epithelial cell lines, we examined the protein levels of Pak4 in human colon and prostate cancer cell lines. We found that Pak4 levels were significantly higher in the tumor cell lines compared with the control cell lines (see Figure 1B). Pak4 was poorly expressed in iMMECs from wild-type mice (see Figures 1C and 2A). Interestingly, however, when the iMMEC cells stably expressed oncogenic Ras or Her2/neu, which are highly transforming in these cells (Karantza-Wadsworth, 2007; Karantza-Wadsworth, 2008), Pak4 levels became highly elevated (see Figure 1C). Pak4 is thus overexpressed in a variety of conditions that are associated with malignant transformation.
Fig. 1.
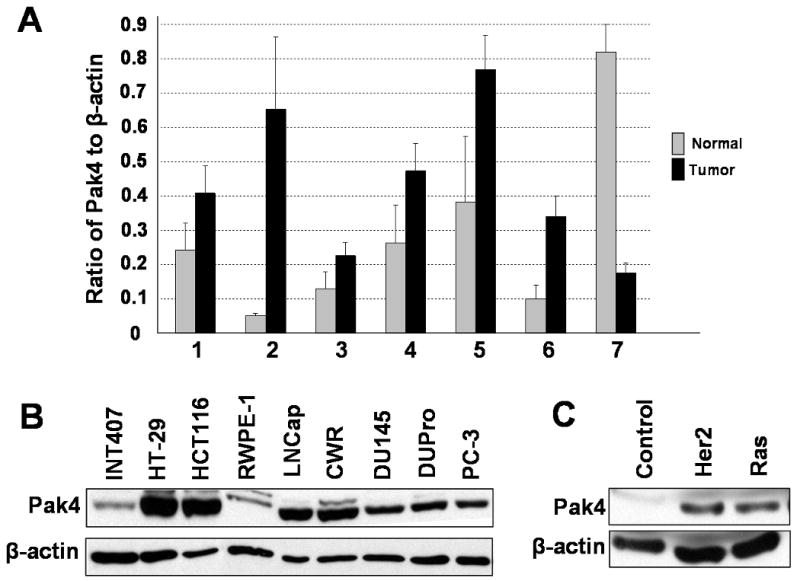
Pak4 is overexpressed in many different types of human tumors and cancer cell lines. A. Quantitative PCR analysis of Pak4 gene expression using cDNA from different types of human tumor tissues and normal tissue. Data are represented as the ratio normalized to β-actin gene expression. 1, Breast. 2, Endometrium. 3, Esophagus. 4, Ovary. 5, Prostate. 6, Urinary bladder. 7, Testis. B. Western blot analysis illustrates high levels of Pak4 expression in human cancer cell lines compared to normal cell lines. The following cell lines were analyzed: Colon cancer cell lines (HT29 and HCT116), prostate cancer cell lines (LNCap, CWR, DU145, DUPro, and PC-3), and normal small intestine and prostate cell lines (INT407 and RWPE-1). C. Pak4 is overexpressed in response to oncogene expression in iMMECs. In B and C, 40 μg of protein extract was used; β-actin served as a loading control.
Fig. 2.
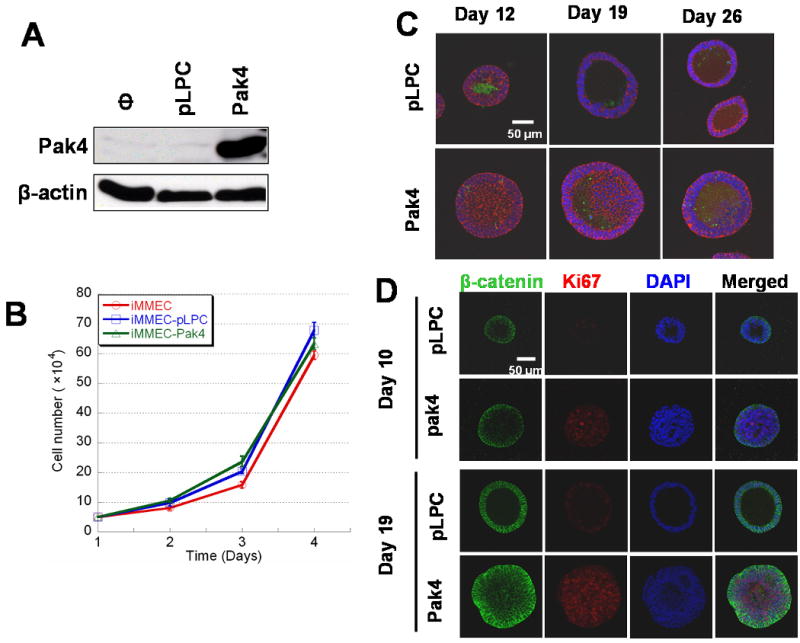
Overexpression of Pak4 inhibits apoptosis and promotes proliferation in iMMECs in 3D culture. A. Generation of stable cell lines in which iMMECs overexpress wild-type Pak4. Stable iMMEC cell lines were generated by retroviral infection of the empty vector (pLPC), or wild-type Pak4 (pLPC-Pak4) vector. Cell extracts were analyzed by for western blot. In each case, 20 μg of protein extract was used. B. Overexpression of Pak4 does not affect the overall growth rate of iMMECs. Control iMMECs (blue), iMMECs infected with empty vector (pLPC, red), and iMMECs infected with Pak4 (yellow) were plated. Cells were counted every day (Y axis is cell number, X axis is days after plating). Each point on the curve is the average of triplicate measurements. C. Inhibition of apoptosis in iMMECs overexpressing Pak4. iMMECs stably expressing either empty vector (top) or Pak4 (bottom) were grown in 3D cultures. Acini were stained with DAPI (blue), beta-catenin (red; to visualize epithelial cells), and cleaved caspase-3 (green). D. Overexpression of Pak4 promotes proliferation in iMMECs. iMMECs expressing empty vector (pLPC), or Pak4, were plated in 3D cultures. Acini are shown at day 10 and day 19 after plating. Acini were stained with beta-catenin (green) to visualize epithelial cells, DAPI (blue) for nuclear counterstaining, and Ki67 (red/purple) to visualize proliferating cells. Proliferating cells could be seen in the lumens of acini generated by Pak4-expressing iMMECs, while this region was empty in the wild-type acini.
Overexpression of Pak4 does not affect the overall growth rate of iMMECs in 2D culture
Western blot analysis showed that Pak4 is poorly expressed in wild-type iMMEC cells (see Figure 2A). This is consistent with the idea that these cells serve as a model for nontransformed mammary epithelial cells. Retroviral infection was used to generate iMMECs that overexpress Pak4. Western blot analysis of a Pak4-overexpressing iMMEC line is shown in Figure 2A. iMMECs expressing Pak4 or empty vector were grown in typical 2D culture conditions, and cells were counted every day. Growth curves were recorded. Growth curves from the first 4 days are shown in Figure 2B. Overexpression of Pak4 did not lead to an overall increase in iMMEC growth rate.
Overexpression of Pak4 abrogates lumen formation and attenuates luminal apoptosis in 3D morphogenesis
The Pak4- and empty vector-transfected-iMMECs were grown on a layer of basement membrane in a 3D culture system, as described (Karantza-Wadsworth, 2008). Acinar structures were then examined by confocal microscopy at different time points. Similar to the vector control cells, Pak4-overexpressing iMMECs formed spherical acini. There were important differences, however, between the two types of acinar structures, as described below.
The structures formed by iMMECs transfected with empty vector are referred to as wild-type acini, and those formed by Pak4-overexpressing iMMECs are referred to as Pak4 acini. Wild-type acini and Pak4 acini were subjected to immunofluorescence and visualized by confocal microscopy at different time points after plating, as shown in Figure 2C. By day 12, a lumen was clearly beginning to form in the wild-type acini. Apoptotic cells could be seen in the centers of these structures, as assessed by staining with anti active caspase-3 antibody. In contrast, in Pak4 acini, hardly any apoptosis could be seen at this time point, and the lumen was mostly filled with cells. By day 19 there was a largely hollow lumen in the wild-type acini, while in the Pak4 acini the lumen still contained many cells. Finally, by day 26, typical acini formed from the wild-type cells. These consisted of a single layer of cells surrounding a completely hollow lumen. The Pak4 acini, however, rarely developed a completely hollow lumen, although at 26 days there was an increased volume of empty space, and some apoptosis could be seen. Furthermore, rather than a single layer of cells surrounding the lumen, there often appeared to be several layers of cells. Pak4 acini also generally grew to a larger size than the wild type acini. The differences between wild-type acini and Pak4 acini with respect to lumen size and overall size of the structures are summarized in Table 1.
Table 1.
Relative sizes (circumferential area) of the different acini (relative to the size of wild-type acini at 10-12 days) are shown. Also shown is the amount of empty lumen in the different acini, shown as a percentage of the total acini. The area of each acini or area of empty lumen was measured using Zeiss LSM software and plotted with the free shape curved drawing mode. These data represents three independent experiments. The mean values with standard error are presented for the empty lumen measurements.
| pLPC | pLPC-Pak4 | |||
|---|---|---|---|---|
| Relative size of the acini (number of acini examined) |
% of empty lumen | Relative size of the acini (number of acini examined) |
% of empty lumen | |
| 10∼12 days | 1 (85) | 25.9%±2.9% | 1.65 (84) | 17.9%±2.2% |
| 19 days | 1.41 (88) | 38.8%±2.5% | 2.43 (88) | 23.3%±2.3% |
| 24∼26 days | 1.31 (66) | 45.8%±2.2% | 2.35 (65) | 27.1%±2.7% |
Overexpression of Pak4 in iMMECs promotes cell proliferation
To determine whether Pak4 expression led to increased cell proliferation, we stained acinar structures with the proliferation marker Ki67. Acini are shown at day 10 and 19 after plating. Compared with wild-type acini, Pak4 acini showed higher levels of cell proliferation. Proliferating cells can be seen in the lumens of the structures that contain Pak4, while this region is hollow in the wild-type structures (see Figure 2D).
Overexpression of Pak4 in iMMECs leads to prolonged activation of the ERK pathway
The results described above indicate that Pak4 overexpression promotes cell survival and proliferation. This prompted us to investigate the role for Pak4 in regulating the ERK MAP Kinase pathway, which plays an important role in both cell growth and proliferation (Xia et al., 1995). We grew cells in 3D cultures and stained acinar structures with total ERK antibody and phospho-ERK antibody, which recognizes the activated form of ERK. Acini are shown at day 10, 19 and 26 after plating (see Figure 3). At the earliest time point examined, both wild-type acini and Pak4-acini had similar high levels of phospho-ERK. After 19 days, however, wild-type acini had a significant decrease in phospho ERK levels, while Pak4 acini continuously maintained the high level of phospho-ERK activation, both in the lumen and in the surrounding cells. The results indicate that Pak4 has a role in promoting sustained activation of ERK in iMMECs.
Fig. 3.
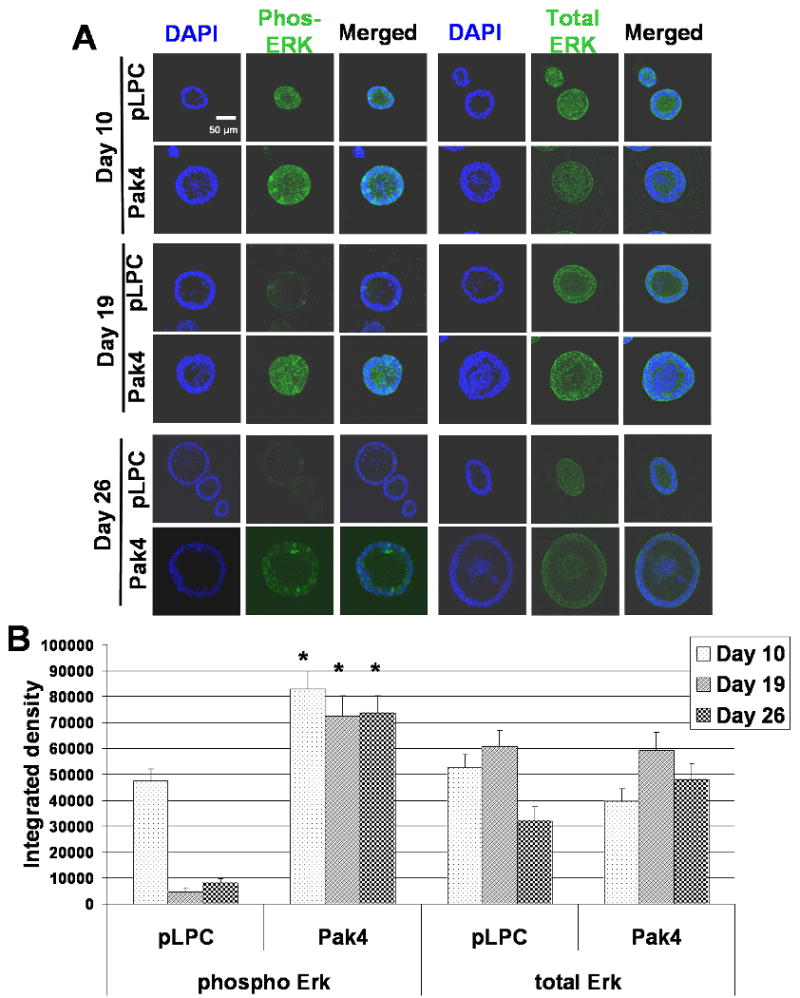
Overexpression of Pak4 promotes ERK activation. A. iMMECs expressing empty vector (pLPC) or Pak4, were plated in 3D cultures. Acini are shown at day 10, day 19 and day 26 after plating. Acini were stained with phospho-ERK or total ERK (green) to visualize ERK activation, and DAPI (blue) to visualize nuclei of all cells. B. Graphs show average integrated density of phosphorylated ERK and total ERK at day 10, day 19, and day 26 in wild-type acini and Pak4 acini. Acini integrated density was measured with Adobe Photoshop CS4 software. For each condition, three typical acini were selected and at least five areas were randomly selected from each acini for integrated density measurement. Error bars indicate s.e.m., * p<0.05 for Pak4 acini verses wild-type acini.
MEK inhibition restores luminal apoptosis and decreases cell proliferation in Pak4 acini
To further establish the role for the MEK–ERK pathway in mediating Pak4 pro-survival effects, we treated cells with the MEK inhibitor, U0126. The Pak4 and empty vector transfected iMMECs were grown in 3D culture. At day 5 after seeding the cells, both Pak4 acini and wild-type acini were treated with U0126 or dimethyl sulfoxide (DMSO, vehicle control), and the treated acini were assessed after 7 days treatment. Consistent with our earlier observations, an increase in ERK phosphorylation was found in Pak4 acini compared with wild-type acini (see Figure 4), In contrast, treatment with U0126 dramatically attenuated ERK phosphorylation, so that the levels of phosphorylated ERK in the Pak4 acini were similar to the levels in the wild-type acini (see Figure 4). These observations confirmed that the MEK-ERK pathway was inhibited in response to U0126 treatment.
Fig. 4.
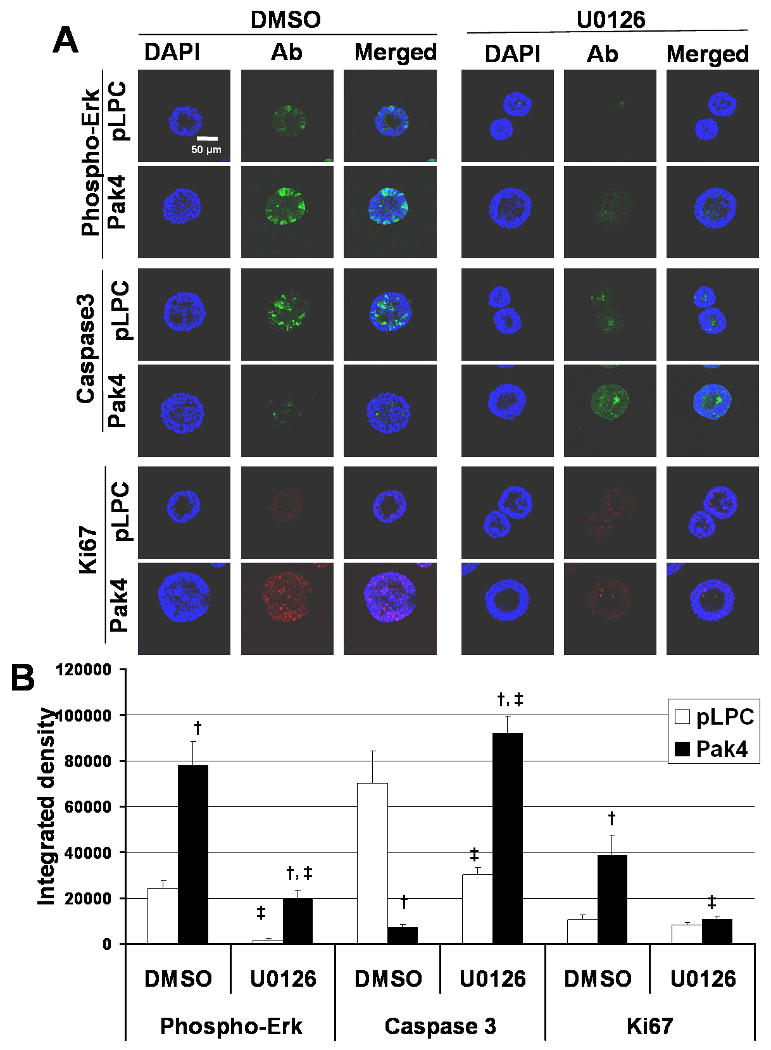
MEK inhibition restores luminal apoptosis and decreases cell proliferation in Pak4 acini. A. Wild-type acini and Pak4 acini are shown at day 12 of 3D culture, after 7 days of treatment with the MEK inhibitor, U0126, or vehicle control, DMSO. Acini were stained with phosphor-ERK (green) to visualize ERK activation, Caspase 3 (green) to visualize apoptotic cells, Ki67 (red) to visualize proliferating cells, and DAPI to visualize nuclei of all cells. B. Graphs show average integrated density of phosphoralated ERK, caspase3 and ki67 staining in wild-type acini and Pak4 acini at day 12 of 3D culture, after 7 days treatment with U0126 or DMSO. Error bars indicate s.e.m., † p<0.05 for Pak4 acini versus wild-type acini at the same treatment condition; ‡ p<0.05 for U0126 treatment versus DMSO treatment in the wild-type acini or Pak4 acini.
We next examined the effects of MEK inhibition on Pak4-mediated survival at day 12 of 3D culture. Results from DMSO-treated acini confirmed our earlier results (Figure 2C) that overexpression of Pak4 significantly attenuated apoptosis compared with wild-type acini, as assessed by anti-caspase-3 staining. Following U0126 treatment, however, Pak4 acini displayed levels of apoptosis similar to those observed in wild-type acini (see Figure 4). Consistent with this, MEK inhibition by U0126 led to a decreased in cell proliferation in Pak4 acini, as assessed by Ki67 staining (see Figure 4). These data suggest that increased cell proliferation and loss of apoptosis in the acini is mediated by prolonged activation of the ERK pathway.
Overexpression of Pak4 leads to changes in iMMEC cell polarity
To determine whether Pak4 affects cell polarity, four types of antibodies were used to stain acini: anti ZO-1 antibody (core tight junction protein; apical membrane staining), phospho-ERM antibody (ezrin/radixin/moesin; stains entire membrane but with strong apical staining), Dlg antibody (basolateral staining), and GM130 antibody (stains the Golgi; apical staining) (Debnath, 2003). Acini were examined 24 days after seeding. At this time point there is considerable variability in the morphologies of the acini, but differences in cell polarity could clearly be seen. While the majority of wild-type acini (62%) showed a normal pattern of GM130 staining (Golgi facing the lumen), only a small percentage of the Pak4 acini (26%) showed the expected staining pattern. In the majority of the Pak4 acini the orientation of the Golgi appeared to be somewhat random, with many Golgi facing away from the lumen. ZO-1 also showed correct apical staining in most (67%) of the wild-type acini, but only 17% of the Pak4 acini showed this pattern. In the rest of the Pak4 acini there was a more disorganized pattern of staining and a significantly lower amount of apical staining. Similar results were also found with phospho-ERM staining which is also a strong apical marker (68% of the wild-type and only of the Pak4 acini showed the expected apical staining pattern). Basolateral staining was examined with the Dlg marker. While Dlg staining in the Pak4 acini was weaker, we did not see big differences in its membrane localization (76% of wild-type acini and 64% of Pak4 acini showed the expected basolateral staining pattern). These results indicate that overexpression of Pak4 alters cell polarity, but that apical polarity is more dramatically affected than basal polarity. Representative acini stained with each of the markers are shown in Figure 5.
Fig. 5.
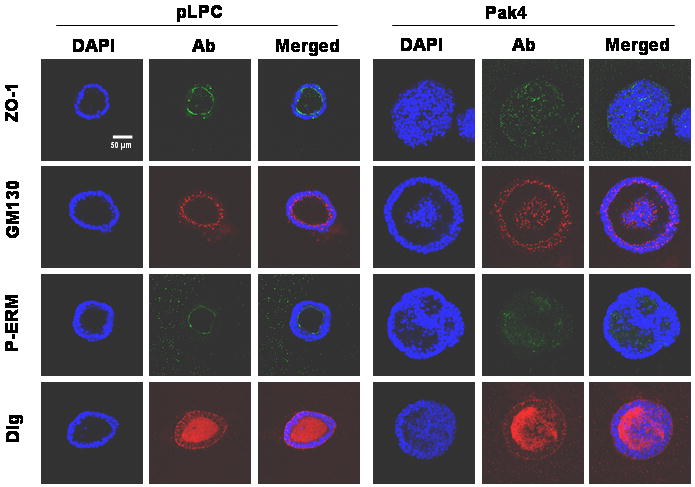
Pak4 overexpression leads to changes in cell polarity. Acini are shown at day 24 after plating. Four types of antibodies were used to examine the cell polarity. ZO-1 (green), a tight junction marker, stains the apical portion of the cells. GM130 (red) stains the apical orientation of the Golgi apparatus toward the hollow lumen of the acini. Phosphor-ERM (ezrin/radixin/moesin; green) stains the entire cell membrane but with the strong apical staining. Dlg (red) stains basolateral membrane. DAPI stains to visualize nuclei of all cells. A total of 50 acini were observed for each condition. Representative acini for each condition are shown.
Overexpression of Pak4 results in tumor formation in athymic mice
The tumorigenicity of iMMECs stably expressing Pak4 was assessed by orthotopic implantation into the mammary fat pads of athymic mice. Five weeks after implantation, iMMECs expressing wild-type Pak4 formed mammary tumors in 6 out of 10 mice, whereas control iMMECs (expressing empty vector) did not generate any tumors. By day 58 after implantation, 10 out of 10 mice implanted with wild-type Pak4-expressing iMMECs had mammary tumors (see Figure 6A). The tumor growth kinetics for iMMECs expressing Pak4 is shown in Figure 6B.
Fig. 6.
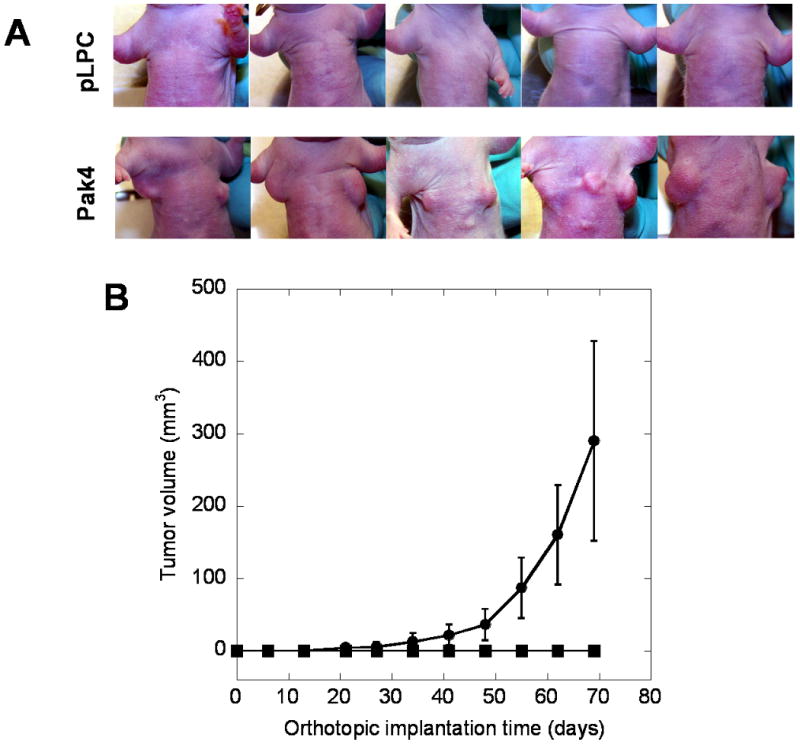
Overexpression of Pak4 results in mammary tumor formation in athymic mice: A. By day 58, iMMECs expressing wild-type Pak4 generated tumors in athymic mice. iMMECs expressing empty vector did not form tumors. B. Tumor volumes from mice implanted with iMMECs expressing empty vector (no tumors) or wild-type Pak4 were assessed.
Discussion
There is increasing evidence that Pak4 is overexpressed in human tumors and cancer cell lines, possibly due to gene amplification (Callow et al., 2002; Liu, 2008; Chen et al., 2008; Kimmelman et al., 2008). Our real time PCR results presented here provide further evidence that Pak4 is overexpressed in a wide array of tumor cell types. While these results have some limitations due to cellular and tissue heterogeneity, taken together with a number of other studies, our results strongly support the idea that Pak4 has a broad and important role in cancer. Several studies have been carried out to investigate the role of Pak4 in oncogenic transformation of fibroblasts (Callow et al., 2002; Liu, 2008; Qu et al., 2001). Since most tumors are of epithelial origin, however, it is important to study Pak4 in epithelial cell models. Here we used immortalized mouse mammary cells (iMMECs) from wild-type mice as a model for studying the role for Pak4 in mammary tumorigenesis. These cells have low levels of endogenous Pak4, and in this respect they resemble an early, non-tumorigenic stage in epithelial transformation. When grown in 3D culture, they form polarized, hollow acini, which recapitulate some aspects of the in vivo structure of the mammary glandular epithelium. Studying iMMECs allows us to go beyond two dimensional cell culture and to study Pak4 in the context of a more complex and physiologically relevant three dimensional system (Karantza-Wadsworth, 2008). In this paper we found that stable overexpression of Pak4 in iMMECs led to the formation of mostly non-hollow acinar structures with a lower level of apoptosis and a thicker layer of cells surrounding the lumen. Most strikingly, iMMECs overexpressing Pak4 resulted in mammary tumors when implanted orthotopically in athymic mice. The fact that overall cell growth rates in 2D cultures were unaffected by Pak4 suggests that changes triggered by Pak4 are influenced by cell interactions in a 3D-context.
The development and maintenance of polarized epithelial cells is critical for the normal function of epithelial tissue, and loss of epithelial polarity is frequently associated with carcinogenesis (Zhan et al., 2008). Here we found evidence that overexpression of Pak4 is associated with disruption of cell polarization, especially apical polarization. Apical polarity loss has been used as a parameter for the characterization of early lesions in certain cancers (Konska et al., 1998). When apical polarity organization is altered, mammary epithelial cells were reported to be pushed into the cell cycle (Chandramouly et al., 2007). The disruption of apicobasal polarity is thought to lead to uncontrolled cell proliferation and survival, resulting in profound effects on glandular structure (Chandramouly et al., 2007; Zhan et al., 2008).
Lumen formation requires apoptosis of the central acinar cells (Debnath, 2002). Pak4 has been implicated in both cell survival and cell proliferation pathways (Li and Minden, 2005; Liu, 2008). We therefore proposed that inhibition of apoptosis and subsequent increased cell survival within the context of the 3D acinar structures play key roles in Pak4 induced tumorigenesis. In the 3D model, we found that a lumen was clearly beginning to form in the wild-type acini by day 12, with apoptotic cells in the centers. In contrast, in Pak4 acini, very little apoptosis could be seen at this time point, and the lumen was mostly filled with cells. By day 19 there was a clear hollow lumen in the wild-type acini, while in the Pak4 acini the lumen still contained many cells. Finally, by day 26, typical acini formed from the wild-type cells. These consisted of a single layer of cells surrounding a completely hollow lumen. The Pak4 acini, however, rarely developed a completely hollow lumen, although at 26 days there was an increased volume of empty space, and some apoptosis could be seen in the lumen. In MCF10A cells, it was previously shown that luminal apoptosis is involved in clearance of cells during lumen formation. Blocking apoptosis during acinar morphogenesis, however, could only delay the clearance of the cells, and the resulting structures eventually exhibited a hollow architecture (Debnath, 2002). Pak4 overexpressing iMMECs rarely formed a completely hollow lumen. One possible explanation is that in addition to inhibiting apoptosis, pak4 may have other roles, such as promoting cell proliferation.
Cell proliferation occurs during early acini development, followed by suppression of proliferation several days after the onset of luminal apoptosis (Debnath, 2002). This is consistent with our findings that wild-type acini showed low levels of proliferation and a hollow lumen by day 19. In contrast, at this time point more proliferating cells could be seen in the lumens of the Pak4 acini. Interestingly, by day 26, a very late stage in acinar formation, Pak4 expressing acini no longer showed higher levels of proliferating cells (data not shown). One possible explanation is that at earlier stages Pak4 promotes both survival and proliferation pathways, while at later stages of acinar formation, Pak4 may function more in cell survival pathways.
We previously showed that Pak4 specifically activates pro-survival pathways, which are associated with activation of pathways such as the NF-κB and ERK MAP Kinase pathways (Li and Minden, 2005). The ERK pathway, which also has key roles in cell proliferation, was examined here in the context of 3D morphogenesis. By day 10 after plating, both wild-type and Pak4 acini had similar levels of activated ERK. By day 19, however, the levels of activated ERK dropped off significantly in the control acini, but remained high in the Pak4 acini. At this stage we also observed increased proliferation as well as decreased apoptosis in the Pak4 acini relative to the control acini (as seen in Figure 2C). On the other hand, inhibition of MEK-ERK signaling with the MEK inhibitor reversed both the increased in proliferation and the attenuation of apoptosis in Pak4 acini at an early stage (as seen in Figure 4). By day 26 after plating, activated ERK staining still can be seen in the Pak4 acini. Interestingly, at this time point, while we continued to observe a decreased level of apoptosis in the Pak4 acini relative to the controls, there was only a low level of proliferation in the Pak4 acini (data not shown). These data suggest that Pak4 may induce cell proliferation and suppress apoptosis by promoting activation of the ERK pathway, although at least at the later time points, the increase in ERK activity is associated more with protection from apoptosis, rather than increased proliferation.
While we have found that Pak4 overexpression affects acinar structure in a way that is consistent with tumorigenesis, the key test for oncogenic transformation is tumorigenicity in animals. To test this, we implanted iMMECs expressing either empty vector or wild-type Pak4 into the mammary fat pads of athymic mice. Remarkably, when Pak4 is overexpressed, iMMECs formed tumors in mice. These results support the idea that Pak4 has an important role in tumorigenesis. A number of other oncogenes have also been studied using the iMMEC model system, and different oncogenes have different effects on the acinar structures (Karantza-Wadsworth, 2007; Karantza-Wadsworth, 2008). When overexpressed and then subject to 3D culture, Bcl2, AKT, Her2/neu, and Ras all lead to the formation of acini with abnormal structures. Among these different oncogenes, however, only Ras and Her2/neu also rendered the iMMECs highly tumorigenic in mice (Karantza-Wadsworth, 2007; Karantza-Wadsworth, 2008). In this sense Pak4 seems to have functions that are similar to Her2/neu and Ras. The effects of these different oncogenes on acinar structures, however, have both similarities and differences. Her2/neu expression, for example, stimulates acinar growth, but does not appear to block apoptosis or inhibit lumen formation. Ha-Ras stimulates acinar growth, but it disrupts acinar structure more severely than we have seen with Pak4 (Karantza-Wadsworth, 2007; Karantza-Wadsworth, 2008). These results raise the intriguing possibility that Pak4 has a role in Her2/neu and Ras signaling in mammary tumorigenesis, but suggest that additional pathways may also be involved in the morphological changes induced by these oncogenes.
This is the first report demonstrating that epithelial cells overexpressing pak4 form tumors in mice. Our studies suggest that the mechanism behind this may involve regulation of cell growth and polarity within the complex 3D structure of the mammary epithelia. Our results, combined with the finding that Pak4 is overexpressed in human breast tumors and breast cancer cell lines, suggest that Pak4 plays an important role in initiation and progression of breast cancer. Our findings may have preventive or therapeutic implications for the rational design of drugs that target proteins that are activated in breast cancer.
Materials and Methods
Real Time PCR
Human normal and tumor quantitative PCR tissue arrays were purchased from OriGene. This array contained 381 tissues covering 22 different cancers. All relevant clinical information can be found on the OriGene web site (http://www.origene.com/geneexpression/disease-panels/products/SCRT102.aspx). Real time PCR quantitative measurements of Pak4 mRNA were made on cDNA samples provided by OriGene. Briefly, primers to human Pak4 were designed using ABI Prism Primer Express software (Applied Biosystems, Foster City, CA). Primers were evaluated with National Center for Biotechnology Information Blast (Bethesda, MD) to confirm product specificity, and products were designed to cross intron/exon borders. The sequences of the forward and reverse primers for Pak4 were 5-GACATCAAGAGCGACTCGATCC -3 and 5-ATCACCATTATCCCCAGCGAC -3 respectively. The sequences of the forward and reverse primers for β-actin were 5-CAGCCATGTACGTTGCTATCCAGG -3 and 5-AGGTCCAGACGCAGGATGGCATG -3, respectively. For all experiments, mRNA expression was measured on an ABI Prism 7900HT Sequence Detection System. Syber Green dye was used for signal detection. Dissociation curves of all analysis were used to ensure specific template amplification. Serial dilutions of a control cDNA (HCT116) were used to determine standard curves, and curves with R2 > 0.97 were then used to determine the mRNA levels in individual samples. Target gene expression level was calculated as a ratio of the mRNA level relative to the mRNA level for β-actin in the same cDNA.
Cell culture, Transfection and Generation of Stable cell lines
iMMECs were cultured as described (Karantza-Wadsworth, 2008). MMECs are isolated from young, C57BL/6 virgin female mice as described (Karantza-Wadsworth, 2008), and immortalized as described (Degenhardt and White, 2006). Inactivation of the Rb and p53 pathways was achieved by stable expression of E1A protein and a dominant negative p53 protein (Degenhardt and White, 2006). Stable iMMEC cell lines were generated by retroviral infection as previously described for fibroblasts (Liu, 2008), and grown on 3 μg/mL puromycin.
The 3D culture of iMMECs were generated as described (Karantza-Wadsworth, 2008). Mammary acini were grown in F12 medium containing 5 μg/ml insulin, 1 μg/ml hydrocortisone, 5 μg/ml EGF, 3 μg/ml puromycin, and 2% Matrigel. The medium was replaced every four days. For MEK inhibition, 0.5 μM MEK Inhibitor U0126 (Sigma) or DMSO were added on day 5 of 3D culture, and the medium was replaced every 2 days thereafter.
Growth Curves
Equal numbers of cells from each cell line were seeded in growth medium in six-well plates. Each day after the seeding one set of cells was trypsinized, collected and counted.
3D Morphogenesis and immunofluorescence (IF)
Mammary acini were fixed and processed for IF as previously described (Karantza-Wadsworth, 2008). Acini were incubated with primary antibodies overnight at 4 °C, washed, and then incubated with fluorescein- or rhodamine-coupled secondary antibodies (from Jackson ImmunoResearch Laboratory) for 2 hr at room temperature. Finally, acini were stained with DAPI (4′, 6′-diamidino-2-phenylindole; Sigma), washed, and mounted with Prolong anti-fade (Molecular Probes). Confocal laser scanning microscope was carried out with a Zeiss LSM510-META confocal microscope system at the W.M. Keck Center for Collaborative Neuroscience, Rutgers University. IF reagents and related antibodies for the analysis of iMMEC-generated acini are shown in Table 2.
Table 2.
Antibodies and fluorescent reagents used for the analysis of acini formed by iMMEC cells.
| Antibody or stain | Purpose | Normal Localization | company | Species |
|---|---|---|---|---|
| Active (cleaved) caspase3 | Apoptosis maker | Apoptotic cells in lumen | Cell Signaling | Rabbit |
| β-catenin | Cell-cell junction | Basolateral | ZYMED Lab | Mouse |
| DAPI | Nuclear counterstain | Nuclei | Boehringer Manheim | N/A |
| Dlg | Basolateral polarity | Strong basal with weaker lateral staining | BD | Mouse |
| GM130 | Apical polarity | Golgi apparatus (apically located) | BD | Mouse |
| Ki67 | Proliferation marker | Nuclei of proliferating cells | Dako Cytomation | Rat |
| P-ERM (Ezrin/Radixin/Moesin) | Plasma membrane, apical | Plasma membrane with strong apical staining | Cell signaling | Rabbit |
| Phospho-Erk | Erk kinase activation | Cytosal and nucleus | Cell Signaling | Rabbit |
| Total Erk | Erk kinase activation control | Cytosol and nucleus | Cell Signaling | Rabbit |
| ZO-1 | Plasma membrane, apical | Apical staining | Invitrogen | Rabbit |
Western blotting
Western blotting was carried out as described (Abo et al., 1998). Polyclonal Pak4 antibody was from Cell signaling. Horseradish peroxidase–conjugated secondary antibodies were from Sigma.
Tumorigenicity assays
iMMECs expressing Pak4 or empty vector were harvested by trypsinization and resuspended in PBS (108 cells/ml). Orthotopic mammary gland implantation of iMMECs was performed according to an Institutional Animal Care and Use Committee approved protocol. NCR nude female mice, 15 weeks old, were anesthetized with 2,2,2-tribromoethanol (avertin, Sigma-Aldrich, St. Louis) at the dose of 175 mg/kg (i.p.). A small incision was made to expose the third pair of mammary fat pads on both sides, and each mammary gland pad was subjected to implantation of 107 cells. The incision was closed with surgical clips that were removed 10 days later. Tumor growth was monitored by weekly measurement of tumor length (L) and width (W). Tumor volume was calculated through πLW2/6.
Acknowledgments
We thank Dr. Chung S. Yang and Dr. Zhihong Yang for providing human colon cancer cell lines. This work was supported by R01 CA076342 to AM and R00 CA133181 to VK.
Footnotes
Conflict of Interest: There are no competing financial interests in relation to this work.
References
- Abo A, Qu J, Cammarano MS, Dan C, Fritsch A, Baud V, et al. PAK4, a novel effector for Cdc42Hs, is implicated in the reorganization of the actin cytoskeleton and in the formation of filopodia. EMBO J. 1998;17:6527–6540. doi: 10.1093/emboj/17.22.6527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callow MG, Clairvoyant F, Zhu S, Schryver B, Whyte DB, Bischoff JR, et al. Requirement for PAK4 in the anchorage-independent growth of human cancer cell lines. J Biol Chem. 2002;277:550–558. doi: 10.1074/jbc.M105732200. [DOI] [PubMed] [Google Scholar]
- Chandramouly G, Abad PC, Knowles DW, Lelievre SA. The control of tissue architecture over nuclear organization is crucial for epithelial cell fate. J Cell Sci. 2007;120:1596–15606. doi: 10.1242/jcs.03439. [DOI] [PubMed] [Google Scholar]
- Chen S, Auletta T, Dovirak O, Hutter C, Kuntz K, El-Ftesi S, et al. Copy number alterations in pancreatic cancer identify recurrent PAK4 amplification. Cancer Biol Ther. 2008;7 doi: 10.4161/cbt.7.11.6840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debnath J, Mills KR, Collins NL, Reginato MJ, Muthuswamy SK, Brugge JS. The role of apoptosis in creating and maintaining luminal space within normal and oncogene-expressing mammary acini. Cell. 2002;111:29–40. doi: 10.1016/s0092-8674(02)01001-2. [DOI] [PubMed] [Google Scholar]
- Debnath J, Muthuswamy SK, Brugge JS. Morphogenesis and oncogeesis of MCF-10A mammary epithelial acini grown in thre-dimensional basement membrane cultures. Methods. 2003;30:256–268. doi: 10.1016/s1046-2023(03)00032-x. [DOI] [PubMed] [Google Scholar]
- Degenhardt K, White E. A mouse model system to genetically dissect the molecular mechanisms regulating tumorigenesis. Clin Cancer Res. 2006;12:5298–304. doi: 10.1158/1078-0432.CCR-06-0439. [DOI] [PubMed] [Google Scholar]
- Gnesutta N, Minden A. Death Receptor Induced Activation of Initiator Caspase-8 is Antagonized by the Serine/Threonine Kinase PAK4. Mol Cell Biol. 2003;23:7838–7848. doi: 10.1128/MCB.23.21.7838-7848.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnesutta N, Qu J, Minden A. The Serine/Threonine Kinase PAK4 prevents caspase activation and protects cells from apoptosis. J Biol Chem. 2001;276:14414–14419. doi: 10.1074/jbc.M011046200. [DOI] [PubMed] [Google Scholar]
- Karantza-Wadsworth V, Patel S, Kravchuk O, Chen G, Mathew R, Jin S, White E. Autophagy mitigates metabolic stress and genome damage in mammary tumorigenesis. Genes and Development. 2007;21:1621–1635. doi: 10.1101/gad.1565707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karantza-Wadsworth V, White E. A mouse mammary epithelial cell model to identify molecular mechanisms regulating breast cancer progression. Meth Enzymol. 2008;446:61–76. doi: 10.1016/S0076-6879(08)01604-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmelman AC, Hezel AF, Aguirre AJ, Zheng H, Paik JH, Ying H, et al. Genomic alterations link Rho family of GTPases to the highly invasive phenotype of pancreas cancer. Proc Natl Acad Sci U S A. 2008;105:19372–7. doi: 10.1073/pnas.0809966105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konska G, Guillot J, De Latour M, Fonck Y. Expression of Tn antigen and N-acetyllactosamine residues in malignant and benign human breast tumors detected by lectins and monoclonal antibody 83D4. Int J Oncol. 1998;12:361–7. doi: 10.3892/ijo.12.2.361. [DOI] [PubMed] [Google Scholar]
- Li X, Minden A. PAK4 functions in tumor necrosis factor (TNF) alpha-induced survival pathways by facilitating TRADD binding to the TNF receptor. J Biol Chem. 2005;280:41192–200. doi: 10.1074/jbc.M506884200. [DOI] [PubMed] [Google Scholar]
- Liu Y, Xiao H, Tian Y, Nekrasova T, Hao X, Lee HJ, Suh N, Yang CS, Minden A. The Pak4 protein kinase plays a key role in cell survival and tumorigenesis in athymic mice. Molecular Cancer Research. 2008;6:1215–1224. doi: 10.1158/1541-7786.MCR-08-0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Bueno G, Portillo F, Cano A. Transcriptional regulation of cell polarity in EMT and cancer. Oncogene. 2008;27:6958–6969. doi: 10.1038/onc.2008.346. [DOI] [PubMed] [Google Scholar]
- Muthuswamy S, Li D, Lelievre S, Bissell M, Brugge JS. ErbB2, but not ErbB1, reinitiates proliferation and induces luminal repopulation in epithelial acini. Nature Cell Biol. 2001;3:785–792. doi: 10.1038/ncb0901-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paliouras GN, Naujokas MA, Park M. Pak4, a novel Gab1 binding partner, modulates cell migration and invasion by the Met receptor. Mol Cell Biol. 2009;29:3018–32. doi: 10.1128/MCB.01286-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu J, Cammarano MS, Shi Q, Ha KC, de Lanerolle P, Minden A. Activated PAK4 Regulates Cell Adhesion and Anchorage-Independent Growth. Mol Cell Biol. 2001;21:3523–3533. doi: 10.1128/MCB.21.10.3523-3533.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanos B, Rodriguez-Boulan E. The epithelial polarity program: machineries involved and their hijacking by cancer. Oncogene. 2008;27:6939–6957. doi: 10.1038/onc.2008.345. [DOI] [PubMed] [Google Scholar]
- Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- Zhan L, Rosenberg A, Bergami KC, Yu M, Xuan Z, Jaffe AB, et al. Deregulation of scribble promotes mammary tumorigenesis and reveals a role for cell polarity in carcinoma. Cell. 2008;135:865–78. doi: 10.1016/j.cell.2008.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]


