Abstract
Ultraviolet (UV) radiation is the primary environmental risk factor in the development of nonmelanoma skin cancer, and UVB in particular promotes tumor growth through various signaling pathways. Kaempferol, a flavonoid with anti-inflammatory and anti-oxidative properties, has been studied as a chemopreventive agent; however, little is known regarding its effects on UVB-induced photo-carcinogenesis. Here, we examined the effect of kaempferol on UVB-induced skin inflammation. We found that kaempferol suppressed UVB-induced cyclooxygenase-2 (COX-2) protein expression in mouse skin epidermal JB6 P+ cells and attenuated the UVB-induced transcriptional activities of cox-2 and activator protein-1 (AP-1). Kaempferol attenuated the UVB-induced phosphorylation of several mitogen-activated protein kinases (MAPKs), including ERKs, p38, and JNKs, but had no effect on the phosphorylation of the upstream MAPK regulator Src. However, in vitro and ex vivo kinase assays demonstrated that kaempferol suppressed Src kinase activity. Furthermore, in vivo data from mouse skin support the idea that kaempferol suppresses UVB-induced COX-2 expression by blocking Src kinase activity. A pull-down assay revealed that kaempferol competes with ATP for direct binding to Src. Docking data suggest that kaempferol docks easily into the ATP-binding site of Src, which is located between the N and C lobes of the kinase domain. Taken together, these results suggest that kaempferol is a potent chemopreventive agent against skin cancer through its inhibitory interaction with Src.
Keywords: flavonoid, photo-carcinogenesis, skin cancer
1. Introduction
Exposure to ultraviolet (UV) irradiation causes DNA damage and other cellular responses that contribute to the development of inflammation and skin cancer. Skin cancer includes the common but highly curable condition of nonmelanoma skin cancer (i.e., squamous and basal cell carcinomas) and the less common but potentially lethal cutaneous melanomas [1,2]. More than one million new nonmelanoma skin cancer cases are diagnosed annually in the United States, which is roughly equivalent to the incidence of all other cancers combined [3]. UVB is of particular concern for the induction of skin cancer because it can induce mutations in critical target genes, as well as modulate cellular signal transduction pathways [4]. Despite major advances in our understanding of skin biology, the incidence of skin cancer is reaching epidemic proportions, indicating that more effective preventive strategies are required, as are innovative new treatments.
Cyclooxygenase (COX) proteins catalyze the rate-limiting steps in the synthesis of prostaglandins from arachidonic acid. Two COX isoforms, COX-1 and COX-2, are known. Whereas COX-1 is constitutively expressed in most tissues, COX-2 is typically absent from many tissue types, including the epidermis. However, COX-2 expression is highly inducible in response to various stimuli, including UV exposure [5]. In previous studies, transgenic mice overexpressing COX-2 showed increased skin tumor development [6], whereas the inhibition of COX-2 by celecoxib, a COX-2 inhibitor, significantly suppressed UV-induced skin carcinogenesis [7, 8]. These observations suggest a critical role for COX-2 in skin tumor development.
The Src tyrosine kinase is part of a superfamily of membrane-associated non-receptor tyrosine kinases, which were originally identified as proto-oncogene products. Src is involved in multiple signal transduction pathways and cellular processes including as growth, differentiation, adhesion, motility, and survival [9, 10]. Src has been widely studied in tumorigenesis. Recent reports show that Src family kinases are associated with the activation of mitogen-activated protein kinases (MAPKs) in response to genotoxic and oxidative stress. Notably, both Src expression and activity are elevated in tumor tissues relative to normal tissues [11].
Flavonoids have attracted substantial attention in relation to their wide range of activities in reducing cancer risk. Previous studies indicated that some flavonoids including myricetin and quercetin can directly bind to some important kinases such as MEK, Raf, and PI3-K [12-14]. Kaempferol (3,5,7,4′-tetrahydroxy flavone, Fig. 1A), which is found in tea, propolis, and grapefruit, is one of the most common dietary flavonoids [15, 16]. Kaempferol is believed to be an antioxidant and anti-inflammatory agent [17-19]. Previous studies demonstrated that kaempferol reduces lipopolysaccharide-induced COX-2 levels in RAW 264.7 cells [19] and inhibits reactive oxygen species production through the inhibition of iNOS and TNF-α protein expression in aged gingival tissues [20].
Fig. 1.
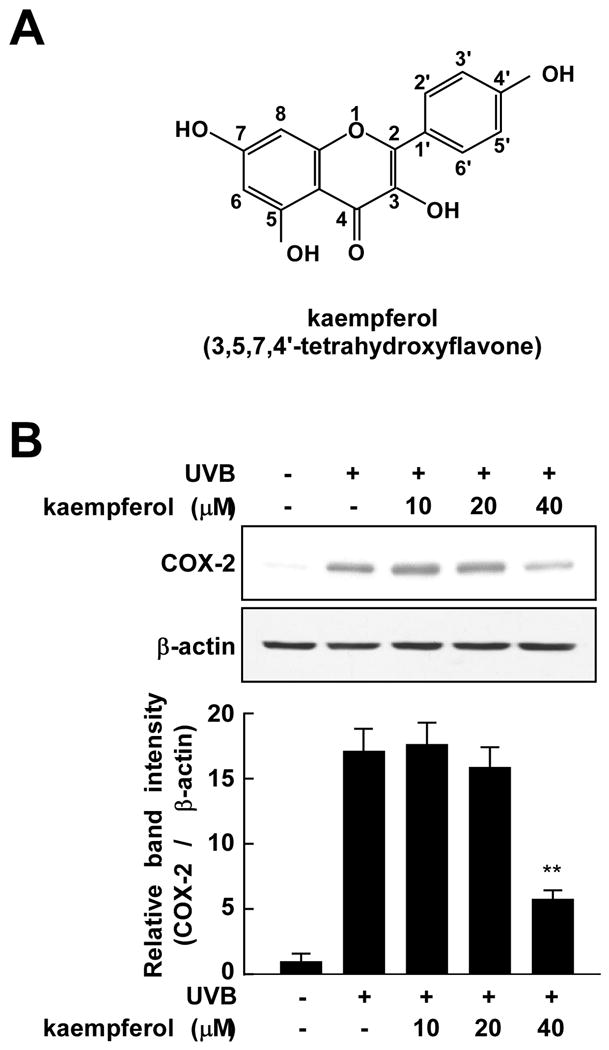
Kaempferol attenuates UVB-induced COX-2 protein expression. (A) Chemical structure of kaempferol with its numbering scheme. (B) Kaempferol inhibits UVB-induced COX-2 protein expression in JB6 P+ cells. Cells were treated with various doses of kaempferol (0, 10, 20, or 40 μM) for 30 min, then stimulated with UVB (0.05 J/cm2) and harvested 4 h later. COX-2 and β-actin protein expression were determined by Western blotting using specific antibodies. Three separate experiments were performed with similar results and representative blots are shown. The asterisks (**) indicate a significant decrease in COX2 activity in groups treated with UVB and kaempferol compared with the group treated with UVB alone (p < 0.01).
Here, we examined the effects of kaempferol on UVB-induced COX-2 protein expression both in cells (ex vivo) and in mouse skin (in vivo). Topical kaempferol treatment exerted strong protective effects against UVB-induced photo-inflammation in mouse skin in vivo, which is proposed to occur through the inhibition of Src activity. These results suggest that kaempferol is a potent antitumor-promoting agent.
2. Materials and Methods
2.1 Materials
Kaempferol was purchased from Indofine Chemical Co. (Somerville, NJ). Eagle's minimum essential medium (MEM), gentamicin, and L-glutamine were obtained from Gibco-BRL (Carlsbad, CA). Fetal bovine serum (FBS) was purchased from Sigma-Aldrich (St. Louis, MO). COX-2 antibodies were purchased from Cayman (Ann Arbor, MI) and anti-β-actin was obtained from Sigma-Aldrich (St. Louis, MO). Antibodies to detect phosphorylated p38 (Tyr180/Tyr182), total p38, phosphorylated c-Jun N-terminal kinases (JNKs, Thr183/Tyr185), total JNKs, and phosphorylated Src (Y416) were purchased from Cell Signaling Biotechnology (Beverly, MA). The antibody against total Src was obtained from Upstate Biotechnology (Lake Placid, NY). CNBr-Sepharose 4B, glutathione-Sepharose 4B, [γ-32P]ATP, and the chemiluminescence detection kit were purchased from Amersham Pharmacia Biotech (Piscataway, NJ). The protein assay kit was obtained from Bio-Rad Laboratories (Hercules, CA).
2.2 Cell culture
JB6 P+ mouse epidermal cells were cultured in monolayers at 37°C in a 5% CO2 incubator in 5% FBS-MEM, 2 mM L-glutamine, and 25 μg/ml gentamicin. The cells were stably transfected with a luciferase reporter plasmid bearing either cox-2 or activator protein-1 (AP-1) and then maintained in MEM supplemented with 5% FBS containing 200 μg/ml G418 to exclude non-transfected cells.
2.3. Animals
Female ICR mice (five weeks of age; mean body weight of 25 g) were purchased from the Institute of Laboratory Animal Resources at Seoul National University (Seoul, Korea). The animals were acclimated for one week prior to the study and had access to food and water ad libitum. The animals were housed in climate-controlled quarters (24°C and 50% humidity) with a 12-h light/12-h dark cycle.
2.4. UVB irradiation
A UVB irradiation system was used to stimulate the cells in serum-free media. The spectral peak of the UVB source (Bio-Link Crosslinker; Vilber Lourmat, Cedex 1, France) was 312 nm. The cells were exposed to UVB (0.5 kJ/m2) and then cultured for either 15 min or 4 h. ICR mice were exposed to UVB at a dose of 5 kJ/m2 and then proteins were isolated from their skin 2 or 6 h later.
2.5. Western blot assay
For Western blotting, cells (1.5 × 106) were cultured in a 10-cm dish for 48 h and then starved in 0.1% FBS-MEM for 24 h to eliminate the FBS-induced activation of MAPKs [extracellular signal-related kinases (ERKs), JNKs, and p38]. The cells were then treated with kaempferol (0, 10, 20, or 40 μM) for 30 min and irradiated with UVB (0.05 J/cm2). The harvested cells were disrupted with lysis buffer [10 mM Tris (pH 7.5), 150 mM NaCl, 5 mM EDTA, 1% Triton X-100, 1 mM DTT, 0.1 mM PMSF, 10% glycerol, and a protease inhibitor cocktail tablet] and the supernatant fractions were boiled for 5 min. The protein concentration was determined using a dye-binding protein assay kit (Bio-Rad Laboratories) as described in the manufacturer's manual. Lysate proteins (40 μg) were subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene difluoride (PVDF) membrane (Amersham Pharmacia Biotech). After blotting, the membrane was incubated overnight with specific primary antibodies at 4°C. The bands were visualized using a chemiluminescence detection kit (Amersham Pharmacia Biotechnologies) after hybridization with a horseradish peroxidase-conjugated secondary antibody. In the in vivo assays, three groups of four ICR mice each received a topical application of kaempferol (0, 40, or 200 nmol) in 200 μl acetone on their shaved backs 1 h before UVB irradiation. To isolate and quantify COX-2 from the skin of the mice, the mice were sacrificed 6 h after UVB treatment and the dorsal skin of each mouse was excised and placed on ice. Any fat was removed, the skin then snap-frozen in liquid nitrogen and immediately pulverized with a mortar and pestle. The pulverized skin was blended on ice with a homogenizer (IKAT10 basic; IKA Laboratory Equipment, Staufen, Germany) and the skin lysates were centrifuged at 9,000 g for 20 min. After the protein content was determined using a Bio-Rad protein assay kit, 100 μg of the extract was subjected to 10% SDS-PAGE.
2.6. Luciferase assay of COX-2 and AP-1 transactivation
Confluent monolayers of JB6 P+ cells that were stably transfected with the cox-2 or AP-1 luciferase plasmid were trypsinized, and 8×103 viable cells suspended in 100 μl of 5% FBS MEM were added to each well of a 96-well plate. The plates were incubated at 37°C in a humidified atmosphere of 5% CO2. When the cultures reached 80–90% confluence, they were starved in 0.1% FBS MEM for an additional 24 h. The cells were treated for 30 min with kaempferol (0, 10, 20, or 40 μM) and irradiated with UVB (0.05 J/cm2) and harvested 4 h later. Thereafter, the cells were disrupted with 100 μl of lysis buffer (0.1 M potassium phosphate [pH 7.8], 1% Triton X-100, 1 mM dithiothreitol [DTT], 2 mM EDTA), and luciferase activity was measured using a luminometer (Luminoskan Ascent; Labsystems, MD).
2.7. Src kinase assay
Src kinase activity was determined directly according to the instructions provided by Upstate Biotechnology. Briefly, each reaction contained 6.25 μl of assay buffer [200 mM Tris-HCl (pH 7.5), 0.4 mM ethyleneglycol-bis(aminoethylether)-tetraacetic acid, and 0.4 mM sodium orthovanadate (Na3VO4)] and a magnesium acetate-ATP cocktail buffer [2.5 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (pH 7.4), 50 mM magnesium acetate, and 0.5 mM ATP]. The Src substrate peptide (250 μM) was also included with 10 ng of active Src. Next, 10 μl of diluted [γ-32P]ATP solution was incubated at 30°C for 10 min with the above assay buffer and substrate peptide and 15-μl aliquots were transferred to p81 paper and washed three times with 0.75% phosphoric acid for 5 min and once with acetone for 5 min. Radioactive incorporation was measured using a scintillation counter. The effects of kaempferol (0-40 μM) were evaluated with the reaction mixtures at 30°C for 10 min.
2.8. Src immunoprecipitation and kinase assays in JB6 P+ cells
JB6 P+ cells were cultured to 80% confluence and then incubated with 0.1% FBS-MEM for 24 h at 37°C to reduce background. Cells were treated with various concentrations of kaempferol (0-40 μM) for 30 min before UVB irradiation (0.5 kJ/m2). The cells were then harvested after 15 min, disrupted with lysis buffer [20 mM Tris-HCl (pH 7.4), 1 mM EDTA, 150 mM NaCl, 1 mM ethyleneglycol-bis(aminoethylether)-tetraacetic acid, 1% Triton X-100, 1 mM β-glycerophosphate, 1 mg/ml leupeptin, 1 mM Na3VO4, and 1 mM PMSF], and centrifuged at 10,000 g for 15 min in a microcentrifuge. Lysates containing 500 μg of protein were immunoprecipitated using anti-Src and then incubated overnight at 4°C with protein A/G Sepharose beads. The beads were washed three times with kinase buffer [200 mM Tris-HCl (pH 7.5), 0.4 mM ethyleneglycol-bis(aminoethylether)-tetraacetic acid, and 0.4 mM Na3VO4] and then re-suspended in 6.25 μl of kinase buffer supplemented with 250 μM Src substrate peptide and 10 μl of diluted [γ-32P]ATP solution. The beads were incubated for 30 min at 10°C. A 15-μl aliquot was transferred to p81 paper and washed three times with 0.75% phosphoric acid for 5 min and once with acetone for 5 min. Radioactive incorporation was measured using a scintillation counter.
2.9. In vivo Src immunoprecipitation and kinase assay
Mice were treated topically with kaempferol (0, 40, or 200 nmol) in 200 μl of acetone and at 2 h after UVB (5 kJ/m2) exposure, they were euthanized and their dorsal skin was excised and prepared for analysis. Proteins were extracted as described above and centrifuged at 10,000 g for 15 min. The mouse skin extract (700 μg) was mixed with protein A/G Sepharose (20 μl) for 1 h at 4°C and centrifuged at 9,000 g for 1 min at 4°C. Anti-Src (20 μl) was added to the supernatant fraction and samples were rocked gently overnight at 4°C. The tubes were then centrifuged and pellets were washed twice. Next, the pellets were suspended in 6.5 μl of kinase buffer supplemented with 10 μl of diluted [γ-32P]ATP solution and 2.5 μl of Src substrate peptide (250 μM) and incubated for 30 min at 30°C. A 15-μl aliquot was then transferred to p81 paper and washed three times with 0.75% phosphoric acid for 5 min per wash and one time with acetone for 5 min. Radioactive incorporation was determined using a scintillation counter. The data are shown as means ± standard deviation (S.D.) of the results from three mice in each treatment group.
2.10. In vitro and ex vivo pull-down assays
Recombinant Src (2 μg) or the supernatant fraction of JB6 P+ cells irradiated with UVB (0.05 J/cm2, 500 μg protein) was incubated with kaempferol-Sepharose 4B or Sepharose 4B (control) beads (100 μl, 50% slurry) in reaction buffer [50 mM Tris-HCl (pH 7.5), 5 mM EDTA, 150 mM NaCl, 1 mM DTT, 0.01% Nonidet P-40, 2 μg/ml bovine serum albumin, 0.02 mM PMSF, and 1 × protease inhibitor mixture]. After incubation with gentle rocking overnight at 4°C, the beads were washed five times with buffer [50 mM Tris-HCl (pH 7.5), 5 mM EDTA, 150 mM NaCl, 1 mM DTT, 0.01% Nonidet P-40, and 0.02 mM PMSF], and the proteins bound to the beads were analyzed by immunoblotting. Three separate experiments were performed.
2.11. ATP and kaempferol competition assay
Briefly, active Src (0.2 μg) was incubated with 100 μl of kaempferol-Sepharose 4B or 100 μl of Sepharose 4B beads in reaction buffer (see in vitro pull-down assay) for 12 h at 4°C, and ATP was added at 10 or 100 μM to a final volume of 500 μl. After 30 h, the samples were washed and the proteins analyzed by Western blotting. Three separate experiments were performed with similar results and representative blots are shown.
2.12. Molecular modeling
Insight II (Accelrys Inc., San Diego, CA) was used for the docking study and structural analysis using the crystal coordinates of Src (accession code 1YOJ) from the Protein Data Bank (PDB; http://www.rcsb.org/pdb/).
2.13. Statistical analyses
When necessary, the data are expressed as means ± S.D. and the student's t-test was used for single statistical comparisons. A p-value of < 0.05 was used as the criterion for statistical significance.
3. Results
3.1. Kaempferol inhibits UVB-induced COX-2 expression
One well-recognized molecular target for chemoprevention is COX-2, which is abnormally up-regulated in many premalignant and malignant tissues [21]. The JB6 P+ mouse epidermal cell line represents a well-developed cell culture system that has been utilized extensively as a model to study the molecular events associated with tumor promotion [22]. To evaluate the inhibitory effects of kaempferol on pro-inflammatory gene expression, cells were treated with various doses of kaempferol for 30 min and harvested and kaempferol at 40 μM effectively inhibited UVB-induced COX-2 protein expression (Fig. 1B).
3.2. Kaempferol attenuates UVB-induced COX-2 and AP-1 activation in JB6 P+ cells
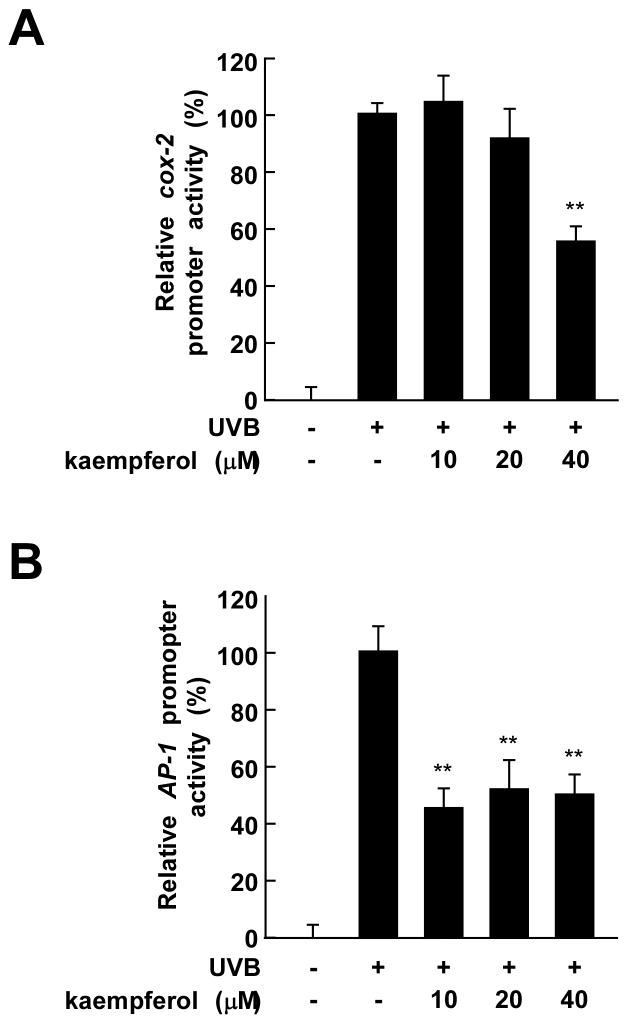
To confirm that kaempferol regulates COX-2 expression, we examined the effect of kaempferol on cox-2 transcription using a reporter gene assay. UVB-induced cox-2 promoter activity decreased significantly in a dose-dependent manner after kaempferol exposure (Fig. 2A). AP-1, a downstream molecule regulated by MAPKs, is a transcription factor involved in cox-2 gene expression [23]. UVB-induced AP-1 transcription has also been previously documented [24]. To determine whether the suppression of cox-2 gene expression by kaempferol involves the inhibition of AP-1 activity, we measured AP-1 transactivation using JB6 P+ cells stably transfected with a luciferase reporter plasmid bearing AP-1. Treatment with kaempferol significantly reduced the UVB-induced transactivation of AP-1 (Fig. 2B). These results indicate that kaempferol suppresses cox-2 promoter activity and AP-1 transactivation in JB6 P+ cells, which might contribute to the antitumor-promoting activity of kaempferol.
Fig. 2.
Kaempferol inhibits UVB-induced cox-2 and AP-1 promoter activity in JB6 P+ cells. (A and B) Kaempferol suppresses UVB-induced cox-2 and AP-1 promoter activity. For the luciferase assay, JB6 P+ cells stably transfected with a luciferase reporter plasmid bearing either cox-2 or AP-1 were cultured as described in Materials and Methods. Cells were then starved in 0.1% FBS-MEM and treated with various doses of kaempferol (0, 10, 20, or 40 μM) for 30 min. The cells were then subjected to UVB irradiation (0.05 J/cm2) and harvested 4 h later. Luciferase activity was assayed and cox-2 and AP-1 activities are expressed relative to control cells without UVB treatment. The values indicate means ± S.D. of cox-2- or AP-1-associated luciferase activity calculated from triplicate samples. The asterisk (**) indicates a significant decrease in luciferase activity in kaempferol treated cells compared to untreated control (p < 0.01).
3.3. Kaempferol inhibits the UVB-induced phosphorylation of ERKs, p38, and JNKs, but has no effect on Src phosphorylation in JB6 P+ cells
Previous studies have shown that UV irradiation activates the MAPKs cascade, and further work has demonstrated that Src family kinases are associated with MAPKs activation [25, 26]. Thus, we investigated the influence of kaempferol on the UVB-induced activation (or phosphorylation) of Src, ERKs, p38, and JNKs. Treatment with kaempferol (20 or 40 μM) inhibited the UVB-induced phosphorylation of ERKs, p38, and JNKs, but had no effect on Src phosphorylation (Fig. 3). These results indicate the kaempferol suppressed phosphorylation and activation of ERKs, p38, and JNKs, which could lead to attenuated UVB-induced COX-2 expression.
Fig. 3.
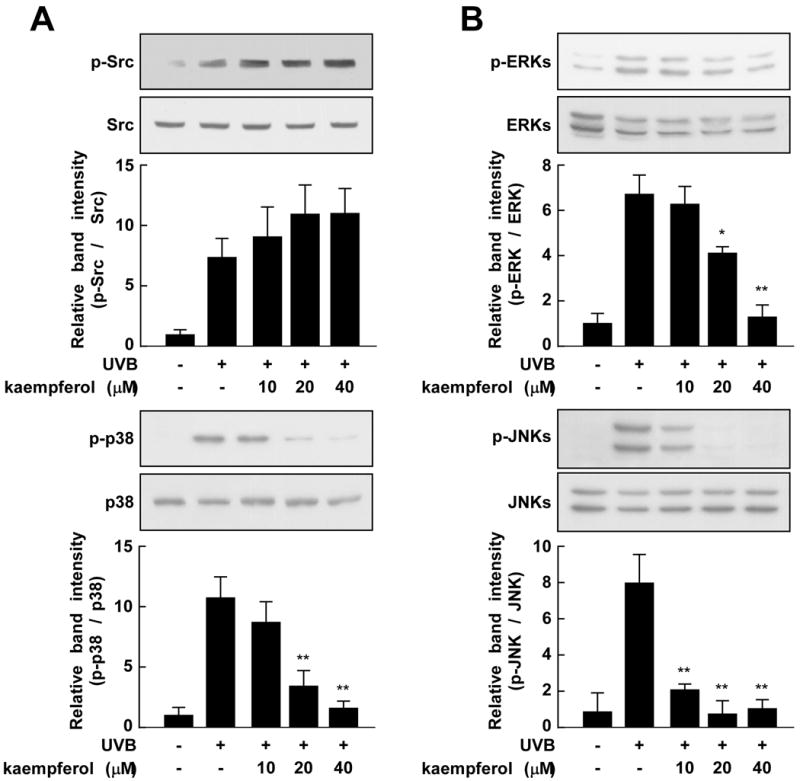
Kaempferol has differential effects on UVB-induced phosphorylation of Src, ERKs, p38, and JNKs in JB6 P+ cells. Cells were starved in 0.1% FBS-MEM and then treated with various doses of kaempferol (0, 10, 20, or 40 μM) for 30 min. The cells were then stimulated with UVB (0.05 J/cm2) and harvested 15 min later. Lysates were prepared and Western blotting was carried out to assess the phosphorylated and total protein levels of Src, ERKs, p38, and JNKs. Three separate experiments were performed with similar results and representative blots are shown. The asterisks (**) indicate a significant difference between groups treated with UVB and kaempferol and the group treated with UVB alone (p < 0.01).
3.4. Kaempferol suppresses UVB-induced COX-2 expression through the inhibition of Src activity
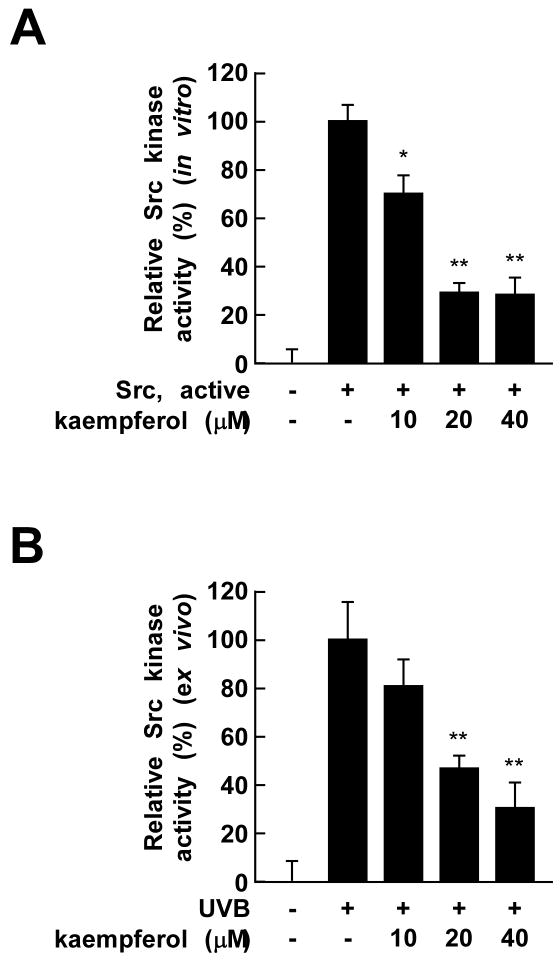
Kaempferol did not block the UVB-induced phosphorylation of Src. However, because kaempferol strongly blocked UVB-induced MAPKs phosphorylation, which is regulated by Src, we hypothesized that kaempferol might directly inhibit Src kinase activity. Thus, we next investigated the effects of kaempferol on Src kinase activity using a specific substrate peptide. The results of an in vitro kinase assay showed that kaempferol strongly inhibited Src kinase activity (Fig. 4A). In JB6 P+ cells, kaempferol also attenuated UVB-induced Src kinase activity (Fig. 4B), suggesting that kaempferol exerts its COX-2-suppressing effects in JB6 P+ cells by inhibiting Src activity, and subsequent downstream signaling.
Fig. 4.
Kaempferol suppresses UVB-induced Src kinase and binding activities. (A) In vitro and (B) ex vivo Src kinase assays were performed as described in Materials and Methods. Active Src was incubated with kaempferol (A) or UVB-irradiated cells were treated with kaempferol (B) and Src kinase activity assessed. The mean 32P count was determined from triplicate samples and the data are expressed as means ± S.D. The asterisks ** indicate a significant decrease in samples treated with kaempferol compared to untreated control samples (p < 0.01).
3.5. Kaempferol attenuates UVB-induced COX-2 expression and Src activity in vivo in mouse skin
We further evaluated the effect of kaempferol on UVB-induced COX-2 expression and Src kinase activity in vivo using mouse skin. Topical pre-treatment with kaempferol blocked both UVB-induced COX-2 protein expression (Fig. 5A) and Src kinase activity (Fig. 5B) in mouse dorsal skin, supporting the idea that the inhibitory effect of kaempferol on COX-2 expression is mediated through the modulation of Src kinase activity in mouse skin.
Fig. 5.
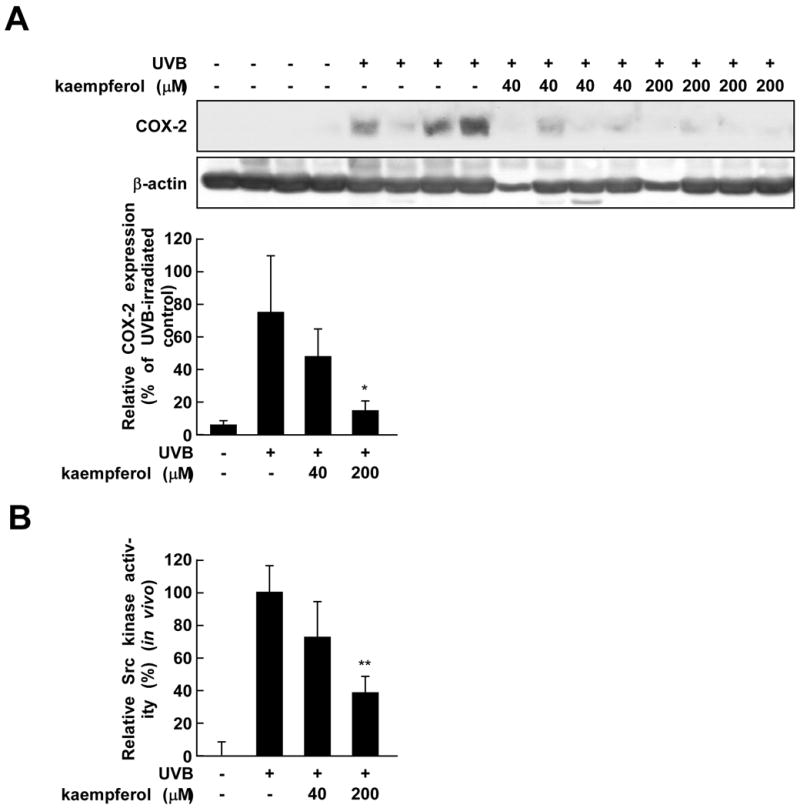
Kaempferol inhibits UVB-induced COX-2 protein expression and Src kinase activity in mouse dorsal skin. (A) In a COX-2 expression assay, COX-2 and β-actin protein expression were analyzed by Western blotting using specific antibodies. Each band was quantified by densitometry. The data are expressed as means ± S.D. (n = 4). The asterisk (*) indicates a significant difference (p < 0.05) between the groups treated with kaempferol and UVB irradiation and the group exposed to UVB alone. (B) In the Src kinase activity assay, dorsal skin protein lysates were prepared from the epidermis and the assays were carried out as described in Materials and Methods. Each band was quantified by densitometry. The data are expressed as means ± S.D. (n = 3). The asterisks (**) indicate a significant difference at p < 0.01 between the groups treated with kaempferol and UVB irradiation and the group exposed to UVB alone.
3.6. Kaempferol competes with ATP for Src binding
To further elucidate the inhibitory effect of kaempferol on Src activity, we performed a kaempferol pull-down assay. Subsequent immunoblotting revealed that Src bound with kaempferol-Sepharose 4B beads, but not with Sepharose 4B beads (Fig. 6A). Src was loaded as a control. Next, we used ex vivo pull-down assays to determine whether kaempferol binds with Src derived from JB6 P+ cell lysates. Cells were treated with UVB and cell lysates were collected 15 min later and subjected to SDS-PAGE. Src was observed in association with kaempferol-Sepharose 4B beads, but not with Sepharose 4B beads (Fig. 6B). These data indicate that kaempferol binds Src directly and subsequently inhibits the activity of Src and the activation of downstream signals. The ability of kaempferol to bind Src was decreased in the presence of ATP in a concentration-dependent manner (Fig. 6C), suggesting that kaempferol is an ATP-competitive inhibitor.
Fig. 6.
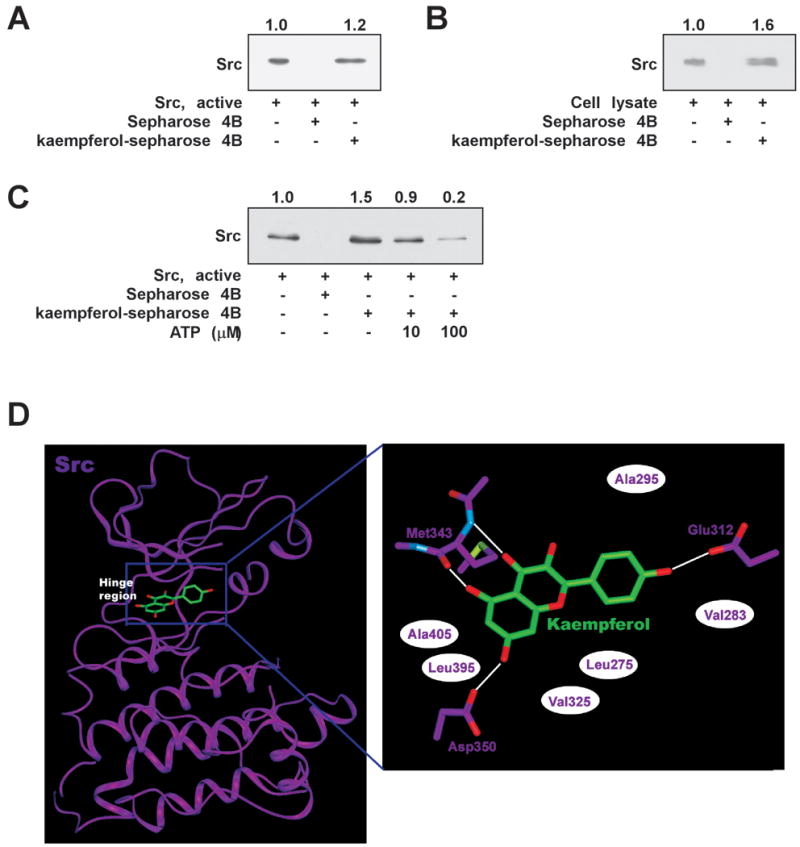
Kaempferol directly binds with Src. (A) Src-kaempferol binding was confirmed by immunoblotting with anti-Src: lane 1 (input control), Src protein standard; lane 2 (control), Sepharose 4B beads; lane 3, kaempferol-Sepharose 4B beads. (B) Kaempferol binds specifically to UVB-activated Src. Src-kaempferol binding in UVB-exposed JB6 P+ cells was confirmed by immunoblotting using anti-Src: lane 1 (input control), whole-cell lysates from JB6 P+ cells; lane 2 (control), lysates from JB6 P+ cells precipitated with Sepharose 4B beads; lane 3, whole-cell lysates from JB6 P+ cells precipitated with kaempferol-Sepharose 4B beads. (C) Kaempferol binds with Src in an ATP-competitive manner. Active Src (2 μg) was incubated with ATP (10 or 100 μM) and kaempferol-Sepharose 4B beads (50 μl) or Sepharose 4B beads (50 μl; as a negative control) in reaction buffer at a final volume of 500 μl. The mixtures were incubated at 4°C overnight with shaking. After washing, the pulled-down proteins were detected by Western blotting: lane 2, negative control, Src kinase cannot bind Sepharose 4B; lane 3, positive control, Src kinase binding with kaempferol-Sepharose 4B; lanes 4 and 5, increasing amounts of ATP decreased kaempferol binding with Src kinase. (D) Hypothetical computational models of Src in complex with kaempferol. In the close-up view, hydrogen bonds are depicted as white lines and hydrophobic contacts as white ellipses.
4. Discussion
Kaempferol is commonly found in fruits, vegetables and tea, and has been reported to exert various biological effects, including anti-oxidative and anti-carcinogenic effects [27]. Compared to other daily dietary flavonols, kaempferol is reported to be associated with a decreased risk of various cancers [28-30]. In the present study, we demonstrated that kaempferol directly targets the Src protein kinase to effectively inhibit UVB-induced COX-2 expression both in vitro and in vivo.
UVB plays a major role in the development of human skin cancer [31, 32] and has been shown to act as a tumor initiator and promoter in animals [33]. The observed inflammatory response following acute and chronic UVB exposure contributes to skin carcinogenesis through oxidative stress mechanisms. Numerous studies have shown that UVB irradiation significantly increases cox-2 gene expression [34, 35] and suggest that COX-2 may function as an early marker for skin tumorigenesis [36]. Given the causal relationship between inflammation and cancer, COX-2 is a promising target for preventing photo-inflammation and skin cancer. In our study, kaempferol inhibited UVB-induced cox-2 expression at the transcriptional level by suppressing cox-2 promoter activity, which resulted from the inhibition of AP-1 transactivation. The effect was not dose-dependent. Previous studies demonstrated that C/EBP β, CREB, and signal transducer and activator of transcription 1 (STAT1) play crucial roles in the shear stress-induced cox-2 gene expression [37, 38]. Therefore, kaempferol could affect other promoter activities. Topical pre-treatment with kaempferol blocked UVB-induced COX-2 protein abundance in mouse dorsal skin. These data support the hypothesis that the anti-inflammatory activity of kaempferol might be due in part to its ability to block COX-2 production.
UVB-induced COX-2 protein expression corresponds with its effect on signal pathway activation. UVB irradiation promotes tumor development by activating various intracellular signaling cascades with major roles in cell growth, differentiation, and proliferation, leading to the clonal expansion of UVB-initiated cells into skin tumors [39]. MAPKs belong to a highly conserved family of serine/threonine protein kinases that includes ERKs, p38, and JNKs, and these proteins have been demonstrated to play a role in tumorigenesis. In this study, we found that kaempferol suppresses UVB-induced MAPKs phosphorylation-activation, which might represent a mechanistic link between kaempferol and the observed attenuation of UVB-stimulated COX-2 expression.
Src is an oncogenic kinase whose activity has been implicated in the progression of many types of cancer [40]. Elevated Src expression and activity have been reported in hyperproliferative epidermal disorders and pre-malignant lesions [41, 42]. Recent studies showed that treatment with AZD0530, a selective Src inhibitor, suppressed keratinocyte proliferation and reduced the number of papillomas formed [43]. We showed that kaempferol inhibited MAPKs activation, suggesting that kaempferol targets an upstream activator. Therefore, we focused on the role of Src in UVB-induced COX-2 protein expression. Our results show that kaempferol significantly inhibited Src activity through its direct binding with Src.
To investigate the molecular mechanism of kaempferol-induced Src inhibition, we conducted a computational modeling study using the crystal structure of the Src kinase domain [44]. The catalytic kinase domain of Src consists of an N lobe and a C lobe, which are linked through a loop called the “hinge region.” The ATP-binding site is flanked by these two lobes and the backbone atoms in this hinge region interact with the adenine moiety of ATP through hydrogen bonding. Considering our experimental results indicating that kaempferol is an ATP-competitive inhibitor of Src, we docked the compound onto the ATP-binding site of the kinase (Fig. 6D). Interestingly, our model suggests that kaempferol interacts with the backbone atoms in the hinge region of Src, as do other protein kinase inhibitors. The hydroxyl group at the 5-position and the carbonyl group at the 4-position in the compound can form two hydrogen bonds with the backbone atoms at Met343 in the hinge region of Src. The hydroxyl groups at the 4′- and 7-positions of kaempferol might form additional hydrogen bonds with Glu312 and Asp350, respectively. In addition, the inhibitor might be sandwiched by the side chains of the hydrophobic residues in the ATP-binding site, including Ala295, Ala405, Leu395, Val325, Leu275, and Val283. Additional studies using x-ray crystallography to determine the inhibitor-complex structure should elucidate the exact binding mode of kaempferol to Src.
Overall, our results reveal a novel molecular mechanism in which kaempferol blocks UVB-induced COX-2 expression by binding directly to Src. Based on our findings, kaempferol shows great potential as a novel chemopreventive agent and might be useful in the treatment of UVB-associated tumorigenesis.
Acknowledgments
This study was supported by the World Class University Program (R31-2008-00-10056-0) and Priority Research Centers Program (2009-0093824), the National Research Foundation of Korea and National Cancer Institute (N01-CN-53301 WA#14).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fears TR, Scotto J. Estimating increases in skin cancer morbidity due to increases in ultraviolet radiation exposure. Cancer Invest. 1983;1:119–26. doi: 10.3109/07357908309042414. [DOI] [PubMed] [Google Scholar]
- 2.Strickland PT, Vitasa BC, West SK, Rosenthal FS, Emmett EA, Taylor HR. Quantitative carcinogenesis in man: solar ultraviolet B dose dependence of skin cancer in Maryland watermen. J Natl Cancer Inst. 1989;81:1910–3. doi: 10.1093/jnci/81.24.1910. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, et al. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 4.Sarasin A. The molecular pathways of ultraviolet-induced carcinogenesis. Mutat Res. 1999;428:5–10. doi: 10.1016/s1383-5742(99)00025-3. [DOI] [PubMed] [Google Scholar]
- 5.Fischer SM. Is cyclooxygenase-2 important in skin carcinogenesis? J Environ Pathol Toxicol Oncol. 2002;21:183–91. [PubMed] [Google Scholar]
- 6.Muller-Decker K, Neufang G, Berger I, Neumann M, Marks F, Furstenberger G. Transgenic cyclooxygenase-2 overexpression sensitizes mouse skin for carcinogenesis. Proc Natl Acad Sci U S A. 2002;99:12483–8. doi: 10.1073/pnas.192323799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fischer SM, Lo HH, Gordon GB, Seibert K, Kelloff G, Lubet RA, et al. Chemopreventive activity of celecoxib, a specific cyclooxygenase-2 inhibitor, and indomethacin against ultraviolet light-induced skin carcinogenesis. Mol Carcinog. 1999;25:231–40. [PubMed] [Google Scholar]
- 8.Fischer SM, Conti CJ, Viner J, Aldaz CM, Lubet RA. Celecoxib and difluoromethylornithine in combination have strong therapeutic activity against UV-induced skin tumors in mice. Carcinogenesis. 2003;24:945–52. doi: 10.1093/carcin/bgg046. [DOI] [PubMed] [Google Scholar]
- 9.Schwartzberg PL. The many faces of Src: multiple functions of a prototypical tyrosine kinase. Oncogene. 1998;17:1463–8. doi: 10.1038/sj.onc.1202176. [DOI] [PubMed] [Google Scholar]
- 10.Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annual review of cell and developmental biology. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- 11.Masaki T, Igarashi K, Tokuda M, Yukimasa S, Han F, Jin YJ, et al. pp60c-src activation in lung adenocarcinoma. Eur J Cancer. 2003;39:1447–55. doi: 10.1016/s0959-8049(03)00276-4. [DOI] [PubMed] [Google Scholar]
- 12.Lee KW, Kang NJ, Rogozin EA, Kim HG, Cho YY, Bode AM, et al. Myricetin is a novel natural inhibitor of neoplastic cell transformation and MEK1. Carcinogenesis. 2007;28:1918–27. doi: 10.1093/carcin/bgm110. [DOI] [PubMed] [Google Scholar]
- 13.Lee KM, Hwang MK, Lee DE, Lee KW, Lee HJ. Protective effect of quercetin against arsenite-induced COX-2 expression by targeting PI3K in rat liver epithelial cells. Journal of agricultural and food chemistry. 58:5815–20. doi: 10.1021/jf903698s. [DOI] [PubMed] [Google Scholar]
- 14.Jung SK, Lee KW, Kim HY, Oh MH, Byun S, Lim SH, et al. Myricetin suppresses UVB-induced wrinkle formation and MMP-9 expression by inhibiting Raf. Biochemical pharmacology. 79:1455–61. doi: 10.1016/j.bcp.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olszewska M. Separation of quercetin, sexangularetin, kaempferol and isorhamnetin for simultaneous HPLC determination of flavonoid aglycones in inflorescences, leaves and fruits of three Sorbus species. Journal of pharmaceutical and biomedical analysis. 2008;48:629–35. doi: 10.1016/j.jpba.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Scheller S, Wilczok T, Imielski S, Krol W, Gabrys J, Shani J. Free radical scavenging by ethanol extract of propolis. International journal of radiation biology. 1990;57:461–5. doi: 10.1080/09553009014552601. [DOI] [PubMed] [Google Scholar]
- 17.Martini ND, Katerere DR, Eloff JN. Biological activity of five antibacterial flavonoids from Combretum erythrophyllum (Combretaceae) Journal of ethnopharmacology. 2004;93:207–12. doi: 10.1016/j.jep.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 18.Comalada M, Ballester I, Bailon E, Sierra S, Xaus J, Galvez J, et al. Inhibition of pro-inflammatory markers in primary bone marrow-derived mouse macrophages by naturally occurring flavonoids: analysis of the structure-activity relationship. Biochemical pharmacology. 2006;72:1010–21. doi: 10.1016/j.bcp.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 19.Kim SK, Kim HJ, Choi SE, Park KH, Choi HK, Lee MW. Anti-oxidative and inhibitory activities on nitric oxide (NO) and prostaglandin E2 (COX-2) production of flavonoids from seeds of Prunus tomentosa Thunberg. Archives of pharmacal research. 2008;31:424–8. doi: 10.1007/s12272-001-1174-9. [DOI] [PubMed] [Google Scholar]
- 20.Kim HK, Park HR, Lee JS, Chung TS, Chung HY, Chung J. Down-regulation of iNOS and TNF-alpha expression by kaempferol via NF-kappaB inactivation in aged rat gingival tissues. Biogerontology. 2007;8:399–408. doi: 10.1007/s10522-007-9083-9. [DOI] [PubMed] [Google Scholar]
- 21.Kim SO, Kundu JK, Shin YK, Park JH, Cho MH, Kim TY, et al. [6]-Gingerol inhibits COX-2 expression by blocking the activation of p38 MAP kinase and NF-kappaB in phorbol ester-stimulated mouse skin. Oncogene. 2005;24:2558–67. doi: 10.1038/sj.onc.1208446. [DOI] [PubMed] [Google Scholar]
- 22.Colburn NH, Wendel EJ, Abruzzo G. Dissociation of mitogenesis and late-stage promotion of tumor cell phenotype by phorbol esters: mitogen-resistant variants are sensitive to promotion. Proc Natl Acad Sci U S A. 1981;78:6912–6. doi: 10.1073/pnas.78.11.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bachelor MA, Cooper SJ, Sikorski ET, Bowden GT. Inhibition of p38 mitogen-activated protein kinase and phosphatidylinositol 3-kinase decreases UVB-induced activator protein-1 and cyclooxygenase-2 in a SKH-1 hairless mouse model. Mol Cancer Res. 2005;3:90–9. doi: 10.1158/1541-7786.MCR-04-0065. [DOI] [PubMed] [Google Scholar]
- 24.Bowden GT. Prevention of non-melanoma skin cancer by targeting ultraviolet-B-light signalling. Nat Rev Cancer. 2004;4:23–35. doi: 10.1038/nrc1253. [DOI] [PubMed] [Google Scholar]
- 25.Summy JM, Trevino JG, Baker CH, Gallick GE. c-Src regulates constitutive and EGF-mediated VEGF expression in pancreatic tumor cells through activation of phosphatidyl inositol-3 kinase and p38 MAPK. Pancreas. 2005;31:263–74. doi: 10.1097/01.mpa.0000178280.50534.0c. [DOI] [PubMed] [Google Scholar]
- 26.Ishizawar R, Parsons SJ. c-Src and cooperating partners in human cancer. Cancer Cell. 2004;6:209–14. doi: 10.1016/j.ccr.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 27.Hertog MG, Hollman PC, Katan MB, Kromhout D. Intake of potentially anticarcinogenic flavonoids and their determinants in adults in The Netherlands. Nutrition and cancer. 1993;20:21–9. doi: 10.1080/01635589309514267. [DOI] [PubMed] [Google Scholar]
- 28.Gates MA, Tworoger SS, Hecht JL, De Vivo I, Rosner B, Hankinson SE. A prospective study of dietary flavonoid intake and incidence of epithelial ovarian cancer. International journal of cancer. 2007;121:2225–32. doi: 10.1002/ijc.22790. [DOI] [PubMed] [Google Scholar]
- 29.Nothlings U, Murphy SP, Wilkens LR, Henderson BE, Kolonel LN. Flavonols and pancreatic cancer risk: the multiethnic cohort study. American journal of epidemiology. 2007;166:924–31. doi: 10.1093/aje/kwm172. [DOI] [PubMed] [Google Scholar]
- 30.Garcia-Closas R, Gonzalez CA, Agudo A, Riboli E. Intake of specific carotenoids and flavonoids and the risk of gastric cancer in Spain. Cancer Causes Control. 1999;10:71–5. doi: 10.1023/a:1008867108960. [DOI] [PubMed] [Google Scholar]
- 31.Kraemer KH, Lee MM, Andrews AD, Lambert WC. The role of sunlight and DNA repair in melanoma and nonmelanoma skin cancer. The xeroderma pigmentosum paradigm. Archives of dermatology. 1994;130:1018–21. [PubMed] [Google Scholar]
- 32.Madronich S, de Gruijl FR. Skin cancer and UV radiation. Nature. 1993;366:23. doi: 10.1038/366023a0. [DOI] [PubMed] [Google Scholar]
- 33.Ananthaswamy HN, Pierceall WE. Molecular mechanisms of ultraviolet radiation carcinogenesis. Photochemistry and photobiology. 1990;52:1119–36. doi: 10.1111/j.1751-1097.1990.tb08452.x. [DOI] [PubMed] [Google Scholar]
- 34.Buckman SY, Gresham A, Hale P, Hruza G, Anast J, Masferrer J, et al. COX-2 expression is induced by UVB exposure in human skin: implications for the development of skin cancer. Carcinogenesis. 1998;19:723–9. doi: 10.1093/carcin/19.5.723. [DOI] [PubMed] [Google Scholar]
- 35.Pentland AP, Schoggins JW, Scott GA, Khan KN, Han R. Reduction of UV-induced skin tumors in hairless mice by selective COX-2 inhibition. Carcinogenesis. 1999;20:1939–44. doi: 10.1093/carcin/20.10.1939. [DOI] [PubMed] [Google Scholar]
- 36.Athar M, An KP, Morel KD, Kim AL, Aszterbaum M, Longley J, et al. Ultraviolet B(UVB)-induced cox-2 expression in murine skin: an immunohistochemical study. Biochemical and biophysical research communications. 2001;280:1042–7. doi: 10.1006/bbrc.2000.4201. [DOI] [PubMed] [Google Scholar]
- 37.Ogasawara A, Arakawa T, Kaneda T, Takuma T, Sato T, Kaneko H, et al. Fluid shear stress-induced cyclooxygenase-2 expression is mediated by C/EBP beta, cAMP-response element-binding protein, and AP-1 in osteoblastic MC3T3-E1 cells. The Journal of biological chemistry. 2001;276:7048–54. doi: 10.1074/jbc.M008070200. [DOI] [PubMed] [Google Scholar]
- 38.Zhang X, Zhang J, Yang X, Han X. Several transcription factors regulate COX-2 gene expression in pancreatic beta-cells. Molecular biology reports. 2007;34:199–206. doi: 10.1007/s11033-007-9085-3. [DOI] [PubMed] [Google Scholar]
- 39.Gupta A, Rosenberger SF, Bowden GT. Increased ROS levels contribute to elevated transcription factor and MAP kinase activities in malignantly progressed mouse keratinocyte cell lines. Carcinogenesis. 1999;20:2063–73. doi: 10.1093/carcin/20.11.2063. [DOI] [PubMed] [Google Scholar]
- 40.Irby RB, Yeatman TJ. Role of Src expression and activation in human cancer. Oncogene. 2000;19:5636–42. doi: 10.1038/sj.onc.1203912. [DOI] [PubMed] [Google Scholar]
- 41.Ayli EE, Li W, Brown TT, Witkiewicz A, Elenitsas R, Seykora JT. Activation of Src-family tyrosine kinases in hyperproliferative epidermal disorders. Journal of cutaneous pathology. 2008;35:273–7. doi: 10.1111/j.1600-0560.2007.00807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Oijen MG, Rijksen G, ten Broek FW, Slootweg PJ. Overexpression of c-Src in areas of hyperproliferation in head and neck cancer, premalignant lesions and benign mucosal disorders. J Oral Pathol Med. 1998;27:147–52. doi: 10.1111/j.1600-0714.1998.tb01931.x. [DOI] [PubMed] [Google Scholar]
- 43.Serrels B, Serrels A, Mason SM, Baldeschi C, Ashton GH, Canel M, et al. A novel Src kinase inhibitor reduces tumour formation in a skin carcinogenesis model. Carcinogenesis. 2009;30:249–57. doi: 10.1093/carcin/bgn278. [DOI] [PubMed] [Google Scholar]
- 44.Breitenlechner CB, Kairies NA, Honold K, Scheiblich S, Koll H, Greiter E, et al. Crystal structures of active SRC kinase domain complexes. Journal of molecular biology. 2005;353:222–31. doi: 10.1016/j.jmb.2005.08.023. [DOI] [PubMed] [Google Scholar]