Abstract
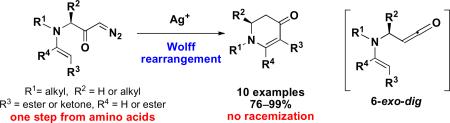
Cyclic enaminones were synthesized in high yields from amino acids in two steps via Wolff rearrangement. The cyclization represents a rare 6-exo-dig cyclization involving a ketene as an electrophile. No racemization was observed during this reaction.
Piperidine, indolizidine and quinolizidine alkaloids (Figure 1) display important biological properties and therefore have been frequent targets for synthetic chemistry efforts.1 It is well–known that monocyclic and bicyclic enaminones (2,3-dihydropyridin-4(1H)-ones) can serve as versatile intermediates for their synthesis.2 The most common synthetic strategies (Scheme 1) to obtain enaminones in optically active form are: 1) the hetero Diels-Alder reaction, and 2) the nucleophilic addition to pyridinium salts utilizing chiral auxiliaries or chiral catalysts.2,3 In 2006 we reported a concise method that relies on the cyclization of amino acid-derived amino ynones to prepare enantioenriched enaminones under very mild conditions.4 In some instances, however, partial racemization of the reaction products was observed during these reactions.4 To address this issue a different disconnection, that retained the use of amino acids, was sought. We hypothesized that enaminones could be derived from diazoketones carrying a pendant enamine moiety that could trap the ketene generated by a Wolff rearrangement (Scheme 2).5
Figure 1.
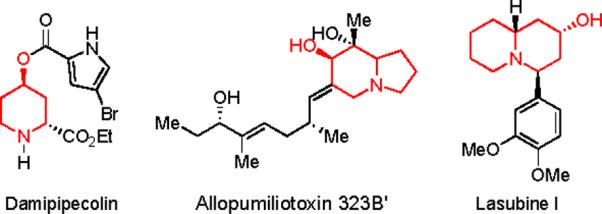
Examples of biologically active molecules containing piperidine, indolizidine and quinolizidine core structures
Scheme 1.
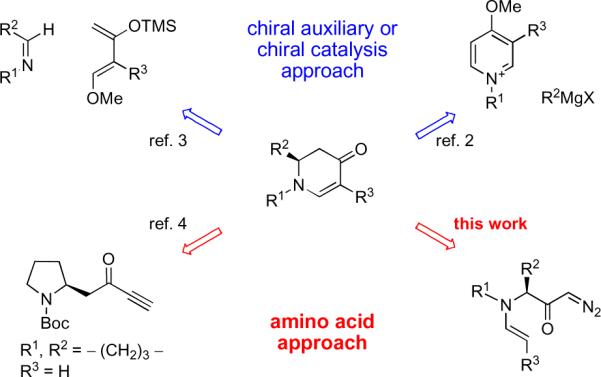
Various approaches for enaminone syntheses
Scheme 2.
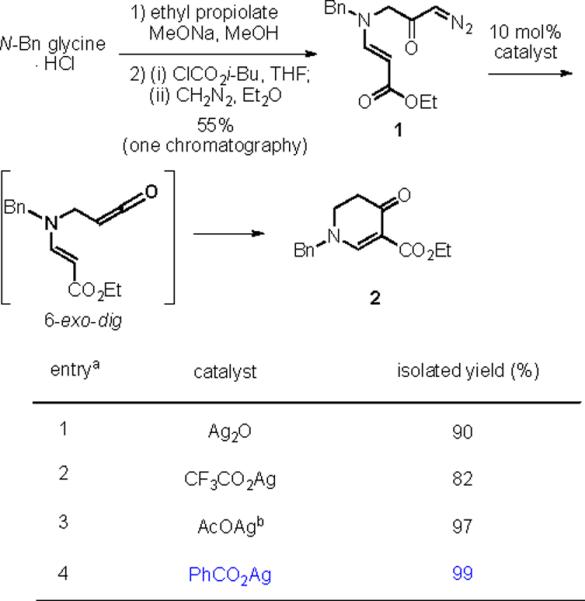
Optimization of reaction conditions
aReaction conditions: diazoketone 1 in CH2Cl2 (0.2M), 24 h, dark
bSonication was used.
The Wolff rearrangement and its ketene intermediate have been studied extensively. Reactions involving the reactive ketene species are classified as either cycloaddition or nucleophilic addition reactions, exemplified by the Staudinger reaction and the Arndt-Eistert homologation.6,7 While a C–C bond is formed in cycloadditions, C–C bond formation in nucleophilic addition reactions of ketenes are less common. Although ketenes have been investigated in reactions with organolithium and magnesium reagents, other type of carbon nucleophiles have been studied less frequently,8 and typically involve intermolecular reactions. Only a few reports have appeared where a carbon nucleophile was employed in an intramolecular fashion.9 Herein, we detail our new synthetic strategy to prepare enaminones from amino acids via Wolff rearrangement using vinylogous amides as carbon nucleophiles. The cyclization represents a rare example of a 6-exo-dig cyclization involving a ketene as the electrophile.10
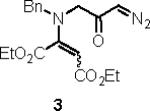
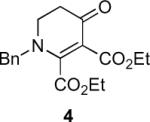
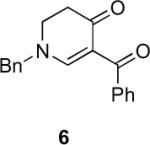
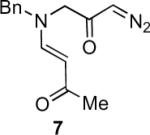
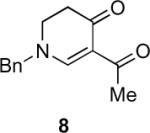
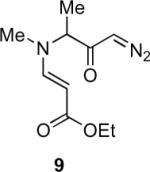
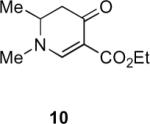
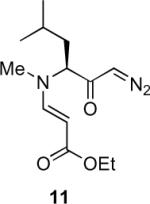
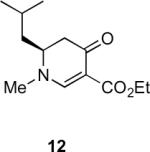
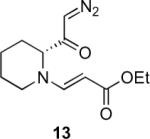
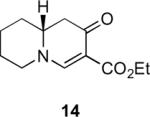
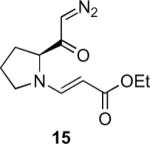
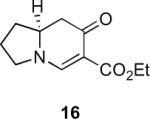
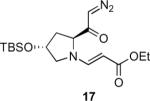
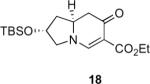
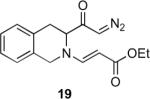
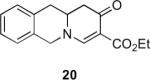
Our efforts started with the synthesis of diazoketone 1 as a starting material for the reaction in order to test the feasibility of our hypothesis (Scheme 2). N-Benzylglycine readily underwent Michael addition to ethyl propiolate, and the acid group of the reaction product was subsequently converted into a diazo moiety. With diazoketone 1 in hand, we embarked on screening reaction conditions. A variety of silver salts, known to induce the Wolff rearrangement, as well as other reaction conditions were tested. We found that the use of halogenated solvents, PhCO2Ag, CF3CO2Ag, and AcOAg readily facilitated the rearrangement to furnish the desired product in excellent yields. The presence of triethylamine, which is often used in Arndt-Eistert homologations as an additive, however diminished the yield. As a result of this study, we selected PhCO2Ag as the catalyst and CH2Cl2 as the solvent for further studies of this method. With optimized reaction conditions in hand, we investigated the scope of the reaction (Table 1). First, diazoketones 3, 5, and 7 (entries 1–3) were synthesized from N-benzylglycine and the corresponding alkynes. Upon treatment with PhCO2Ag, the desired enaminones 4, 6, and 8 were obtained in good yields. Next, α-substituted diazoketone 9 and 11 (entries 4–5) were prepared from N-methylalanine and N-methylleucine respectively, which underwent cyclization to afford β-alkyl enaminones 10 and 12. 19F NMR analysis of the Mosher ester of (S)-12 in comparison to the Mosher esters of (±)-12 revealed that only a single diastereoisomer of the (S)-12 Mosher ester had formed (see SI).11 Diazoketones 13, 15, 17, and 19 (entries 6–9) derived from cyclic amino acids afforded bicyclic enaminones 14, 16, 18, 20 in good yields. These derivatives are known to be excellent intermediates for indolizidine, and quinolizidine syntheses.2 Mosher ester derivatives of 14 and 16 showed single diastereoisomers by HPLC analysis (see SI).11 (2R,8aS)-18 was formed as a single diastereoisomer as determined by 1H NMR.
Table 1.
Substrate scope of the reactiona
Reaction conditions: diazoketone and PhCO2Ag (10 mol%) in CH2Cl2 (0.2 M).
20 mol% of PhCO2Ag was used.
ee was determined by 19F NMR (Mosher ester derivatives); only one isomer was observed.
Ag2O (10 mol%) and C2H4Cl2 (0.2 M) were used.
dr was determined by 1H NMR; only one isomer was observed.
In summary, we have developed a novel enantiospecific synthetic method to obtain monocyclic, bicyclic and tricyclic enaminones from amino acids in two steps. Due to the lack of racemization, a simple nucleophilic 6-exo-dig cyclization is a plausible mechanism, which is a favorable transformation according to Baldwin's rules.10 Further investigation regarding the synthetic utility of this methodology is ongoing.
Supplementary Material
Acknowledgement
This work was supported by National Institutes of Health Grant GM081267 and the University of Minnesota through the Vince and McKnight Endowed Chairs. We thank Dr. Subhashree Rangarajan for mass spectrometry assistance.
Footnotes
Supporting Information Available: Experimental procedures and spectroscopic data for all new compounds (PDF). This material is available free of charge via the Internet at http://pubs.acs.org.
References
- (1).For review: Michael JP. Nat. Prod. Rep. 2008;25:139. doi: 10.1039/b612166g. Chemler SA. Current Bioact. Compd. 2009;5:2. doi: 10.2174/157340709787580928.
- (2).For review: Comins DL, Joseph SP. Adv. Nitrogen Heterocycl. 1996;2:251. Joseph S, Comins DL. Curr. Opin. Drug Discovery Dev. 2002;5:870. Comins DL, O'Connor S, Al-awar RS. In: Comprehensive Heterocyclic Chemistry III. Alan RK, Christopher AR, Eric FVS, Richard JKT, editors. Elsevier; Oxford: 2008. p. 41.
- (3).(a) Danishefsky S, Kitahara T. J. Am. Chem. Soc. 1974;96:7807. [Google Scholar]; (b) Pfrengle W, Kunz H. J. Org. Chem. 1989;54:4261. [Google Scholar]; (c) Ishihara K, Miyata M, Hattori K, Tada T, Yamamoto H. J. Am. Chem. Soc. 1994;116:10520. [Google Scholar]; (d) Kobayashi S, Kusakabe K.-i., Komiyama S, Ishitani H. J. Org. Chem. 1999;64:4220. [Google Scholar]; (e) Yao S, Saaby S, Hazell RG, Jorgensen KA. Chem.--Eur. J. 2000;6:2435. doi: 10.1002/1521-3765(20000703)6:13<2435::aid-chem2435>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]; (f) Josephsohn NS, Snapper ML, Hoveyda AH. J. Am. Chem. Soc. 2003;125:4018. doi: 10.1021/ja030033p. [DOI] [PubMed] [Google Scholar]; (g) Mancheno OG, Arrayas RG, Carretero JC. J. Am. Chem. Soc. 2004;126:456. doi: 10.1021/ja038494y. [DOI] [PubMed] [Google Scholar]; (h) Yamashita Y, Mizuki Y, Kobayashi S. Tetrahedron Lett. 2005;46:1803. [Google Scholar]
- (4).(a) Turunen BJ, Georg GI. J. Am. Chem. Soc. 2006;128:8702. doi: 10.1021/ja0609046. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Niphakis MJ, Turunen BJ, Georg GI. J. Org. Chem. 2010;75:6019. doi: 10.1021/jo101051w. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Niphakis MJ, Georg GI. J. Org. Chem. 2010 doi: 10.1021/jo101051w. ASAP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).For Review: Meier H, Zeller KP. Angew. Chem. 1975;87:52. Kirmse W. Eur. J. Org. Chem. 2002:2193. Tidwell TT. Eur. J. Org. Chem. 2006:563.
- (6).For review: Georg GI, Ravikumar VT. Stereocontrolled Ketene-Imine Cycloaddition Reactions. In: Georg GI, editor. The Organic Chemistry of β-Lactams. Verlag Chemie; New York: 1993. p. 295.
- (7).(a) Bachmann WE, Struve WS. Org. React. 1942:38–62. [Google Scholar]; (b) Seikaly HR, Tidwell TT. Tetrahedron. 1986;42:2587–613. [Google Scholar]
- (8).For ketene reactions with organo lithium/magnesium reagents, see: Haener R, Laube T, Seebach D. J. Am. Chem. Soc. 1985;107:5396. Baigrie LM, Seiklay HR, Tidwell TT. J. Am. Chem. Soc. 1985;107:5391. For reactions with other type of carbon nucleophiles, see: Rathke MW, Sullivan DF. Tetrahedron Lett. 1973:1297. Kita Y, Matsuda S, Kitagaki S, Tsuzuki Y, Akai S. Synlett. 1991:401. Negri G, Kascheres C. J. Heterocycl. Chem. 2001;38:109.
- (9).(a) Hickmott PW, Giasuddin Ahmed M, Ahmed SA, Wood S, Kapon M. J. Chem. Soc., Perkin Trans. 1985:2559. [Google Scholar]; (b) Hickmott PW. S. Afr. J. Chem. 1989;42:17. [Google Scholar]; (c) Byeon C-H, Hart DJ, Lai C-S, Unch J. Synlett. 2000:119. [Google Scholar]
- (10).(a) Baldwin JE. J. Chem. Soc., Chem. Commun. 1976:734. [Google Scholar]; (b) Johnson CD. Acc. Chem. Res. 1993;26:476. [Google Scholar]
- (11).Dale JA, Dull DL, Mosher HS. J. Org. Chem. 1969;34:2543. [Google Scholar]; See SI for experimental details
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.