Abstract
Pancreatic ductal adenocarcinoma (PDAC) is one of the most lethal cancers with dismal survival rates. Its intransigence to conventional therapy renders PDAC an aggressive disease with early metastatic potential. Thus, novel targets for PDAC therapy are urgently needed. Multiple signal transduction pathways are implicated in progression of PDAC. These pathways stimulate production of intracellular messengers in their target cells to modify their behavior, including the lipid-derived diacylglycerol (DAG). One of the prominent intracellular targets of DAG is the protein kinase C (PKC) family. However, the mechanisms by which PKC-mediated signals are decoded by the cell remain incompletely understood. Protein kinase D1 (PKD or PKD1, initially called atypical PKCµ), is the founding member of a novel protein kinase family that includes two additional protein kinases that share extensive overall homology with PKD, termed PKD2, and PKD3. The PKD family occupies a unique position in the signal transduction pathways initiated by DAG and PKC. PKD lies downstream of PKCs in a novel signal transduction pathway implicated in the regulation of multiple fundamental biological processes. We and others have shown that PKD-mediated signaling pathways promote mitogenesis and angiogenesis in PDAC. Our recent observations demonstrate that PKD also potentiates chemoresistance and invasive potential of PDAC cells. This review will briefly highlight diverse biological roles of PKD family in multiple neoplasias including PDAC. Further, this review will underscore our latest advancement with the development of a potent PKD family inhibitor and its effect both in vitro and in vivo in PDAC.
Keywords: Protein kinase D, Protein kinase C, Pancreatic cancer, Signal transduction
1. Introduction
Pancreatic ductal adenocarcinoma (PDAC), which comprises 90% of all human pancreatic cancers, is a devastating disease, with overall 5-year survival rate of only 3–5%. This dismal rate of survival is due to several factors, including late presentation with locally advanced, unresectable tumors, early metastatic disease, and rapidly arising chemoresistance. Even patients that undergo “curative” surgery have a 5-year survival rate of only ~20%. The incidence of this disease in the US has increased recently to more than 42,000 new cases each year and is now the fourth leading cause of cancer mortality in both men and women [1]. As the current therapies offer very limited survival benefits, novel molecular therapeutic targets and strategies are urgently needed to treat this aggressive disease.
It is recognized that PDAC arises from the stepwise progression of precursor lesions, including pancreatic intraepithelial neoplasias [2, 3]. Progression from these non-invasive duct lesions to invasive cancer is associated with the accumulation of genetic alterations [4, 5], including activating mutations in the KRAS oncogene which appears in ~90% of PDACs and inactivating mutations in the tumor suppressors p53, the deleted in pancreatic cancer 4 (DPC4) and p16ink4a genes [5–7]. It is generally accepted that the progressive accumulation of pro-oncogenic mutations during the promotional phase of pancreatic tumorigenesis requires activation of signaling pathways leading to sustained cell proliferation.
2. Protein kinase C (PKC) isoforms and pancreatic ductal adenocarcinoma (PDAC)
Numerous growth and developmental factors, oncogenes, G protein-coupled receptors (GPCRs) and their signal transduction pathways have been implicated in the progression of PDAC. Many of these signals initiate their characteristic effect on target cells by stimulating the synthesis or decreasing the degradation of lipid-derived second messengers with subsequent activation of serine/threonine-specific kinases involved in signal transduction pathways related to growth control and cell cycle progression [8]. A key reaction in this process is the stimulation of the isoforms of the phospholipase C (PLC) family, identified as one of the “core” signaling pathways that undergo somatic alterations in nearly all pancreatic cancers [9]. PLCs, including β, γ, δ and ε, which are activated by multiple stimuli, catalyze the hydrolysis of phosphatidylinositol 4,5-biphosphate to produce two second messengers: Ins (1,4,5)P3 and diacylglycerol (DAG). Ins (1,4,5)P3 triggers the release of Ca2+ from internal stores [10] whereas DAG directly activates a variety of effectors, the most prominent of which is protein kinase C (PKC), a phospholipid-dependent protein kinase family [11] that induces rapid phosphorylation of cytosolic and membrane-bound proteins. PKC isoforms can be classified in three subclasses according to their regulatory properties, which are conferred by specific domains located in the NH2-terminal portion of these proteins. All members of the PKC family, i.e., conventional PKCs (α, βI, βII, γ), novel PKCs (δ, ε, η, θ) and atypical PKCs (ζ, ι), are characterized by a highly conserved catalytic domain and by an autoinhibitory domain that maintains these enzymes in an inactive state in the absence of activating second messengers. Earlier studies demonstrated that PDAC cell lines express multiple PKCs, including α, β, ε and η [12–14]. Furthermore, a number of reports indicate an important role of PKCs in promoting proliferation and in preventing apoptosis of pancreatic cancer cells [12, 14–16] though a different view was also expressed [17]. A recent study demonstrates that atypical PKCι is required for the transformed growth of PDAC cells in vitro and their tumorigenesis in vivo [18]. However, the downstream signaling targets stimulated by PKCs in PDAC cells, as in most other human cancer cells, remain poorly characterized and a major gap in understanding the dysfunctional regulation of mitogenic signaling in cancer cells, including PDAC.
3. Protein kinase D: regulation through PKC
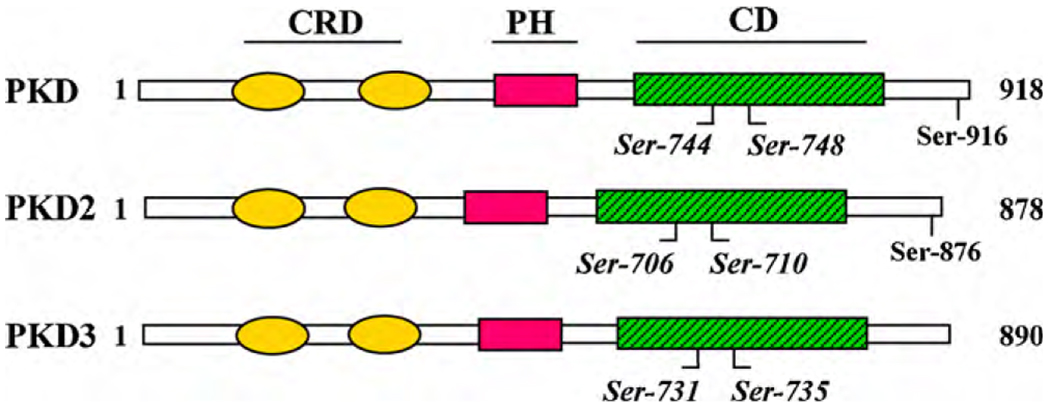
Protein kinase D (PKD), the founding member of a new family of serine/threonine protein kinases and the subject of this mini-review, occupies a unique position in the signal transduction pathways initiated by DAG and PKC in normal and cancer cells. PKD not only is a direct DAG target but it also lies downstream of PKCs in a novel signal transduction pathway implicated in the regulation of multiple fundamental biological processes [19–21]. PKD (also called initially PKCµ) is a serine/threonine protein kinase with structural, enzymological, and regulatory properties different from the PKC family members [19, 22, 23]. The most distinct characteristics of PKD (shown in Fig. 1) are the presence of a catalytic domain distantly related to Ca2+-regulated kinases and a pleckstrin homology (PH) domain that regulates enzyme activity [24–27]. The N-terminal region of PKD also contains a cysteine-rich domain (CRD) comprised by a tandem repeat of cysteine-rich, zinc finger-like motifs, cys1 and cys2, which confer high affinity binding of phorbol esters, and play a role in the regulation of catalytic kinase activity [28, 29]. The identification of PKD2 [30] and PKD3 [31, 32], similar in overall structure and primary amino acid sequence to PKD, confirmed the notion that PKD (henceforth designated as PKD1) is the founding member of a new family of serine/threonine protein kinases [33], now classified in the kinome within the Ca2+/calmodulin-dependent protein kinase (CaMK) group, separate from the PKC family.
Fig. 1. Schematic representation of PKD family.
Serine residues within the activation loop of PKDs that become phosphorylated via novel PKCs are indicated in italics. CRD, cysteine-rich domain; PH, pleckstrin homology domain; and CD, catalytic domain.
PKD1 isolated from multiple cell types, including PDAC cells [13], exhibits very low catalytic activity that can be stimulated by phosphatidylserine micelles and either DAG or phorbol esters [23, 34, 35]. These early studies implied that PKD1 represents a novel component of the signal transduction initiated by DAG production in their target cells [25]. Subsequent studies, aimed to define PKD1 regulation within intact cells, elucidated a mechanism of PKD1 activation distinct from the direct stimulation of enzyme activity by DAG/phorbol ester plus phospholipids obtained in vitro. Specifically, treatment of intact cells with phorbol esters or cell-permeable DAGs induced a dramatic conversion of PKD1 from an inactive to an active form, as shown by in vitro kinase assays performed in the absence of lipid co-activators [21, 35, 36]. In all these cases, PKD1 activation was selectively and potently blocked by cell treatment with PKC inhibitors that did not directly inhibit PKD1 catalytic activity [21, 35], suggesting that PKD1 activation in intact cells is mediated, directly or indirectly, through PKCs. In line with this conclusion, cotransfection of PKD1 with active mutant forms of “novel” PKCs (PKCs δ, ε, η, θ), resulted in robust PKD1 activation in the absence of cell stimulation [21, 27, 37, 38]. Thus, PKD1 occupies a unique position in signal transduction since it is a point of convergence and integration of multiple stimuli that induce DAG accumulation and lies downstream of PKCs in a novel signal transduction cascade. Since PKDs phosphorylate consensus sites (LXRXXS/TV/L/M) different from PKCs, PKD1 also represents a major point of signal dissemination in the network.
Studies with multiple agonists, cell types and PKD1 mutants has led to a model that envisages that PKC-dependent PKD1 phosphorylation at the activation loop serves as a direct “on/off” switch for catalytic activity [see Ref. [33] for details]. Further studies showed that multiple stimuli induce a striking and transient PKD1 translocation from the cytosol to the plasma membrane followed by a rapid, PKC-dependent reverse translocation of PKD1 from the plasma membrane to the cytosol and subsequent accumulation in the nucleus [32, 39–41]. This implies that PKD can phosphorylate targets in a variety of sub-cellular locations and consequently regulate multiple cellular activities, as it will be discussed in the next Section.
4. Biological role of PKD family in neoplasia
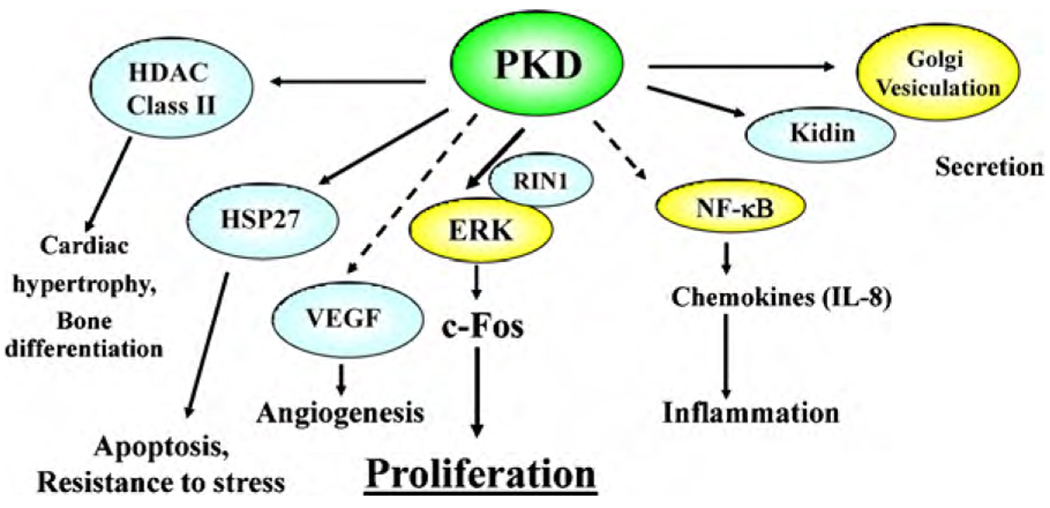
The members of the PKD family are increasingly implicated in the regulation of a remarkable array of fundamental biological processes (summarized in Fig. 2). These include cell proliferation, epithelial cell polarity, function of heat shock proteins implicated in chemoresistance, inflammation, oxidative stress and angiogenesis, which are key characteristics in the stepwise pathogenesis of neoplasia.
Fig. 2. Schematic representation of regulation of multiple biological processes by PKD.
The solid lines indicate direct and the dashed lines represent either direct or indirect regulation of these processes by PKD. HDAC, histone deacetylase complex; HSP27, heat shock protein 27; VEGF, vascular endothelial growth factor; RIN1, Ras and Rab interactor 1; and ERK, extracellular signal-regulated kinase.
4.1. Role of PKD in cell proliferation
GPCRs and their cognate agonists are increasingly implicated as autocrine/paracrine growth factors for multiple solid tumors, including PDAC [8, 42, 43]. Pancreatic cancer cell lines express multiple functional GPCRs, as revealed using a Ca2+ mobilization assay as indicator of productive ligand-receptor interactions [44]. A variety of GPCR agonists, including neurotensin (NT), angiotensin II (ANG II) and bradykinin, stimulated DNA synthesis in PDAC cell lines, including PANC-1 and MIA PaCa-2 [13, 44–47]. Furthermore, a broad-spectrum GPCR antagonist inhibited the growth of pancreatic cancer cells either in vitro or xenografted into nu/nu mice [48]. Other studies demonstrated increased expression of GPCRs for ANG II and NT in pancreatic cancer tissues [49–52]. More recently, crosstalk between insulin/IGFI receptors and GPCR signaling systems in PDAC cells, leading to enhancement of GPCR-induced early signaling has been identified [47, 53]. Consequently, understanding of the signal transduction pathways that mediate GPCR-induced proliferation in PDAC cells provides a different avenue to identify novel targets for therapeutic intervention. Our contention, as discussed below, is that PKD1 is a major downstream element in GPCR mitogenic signaling.
PKD1 can be activated by multiple growth-promoting GPCR agonists acting through Gq, Gi and G12 in a variety of cells types, suggesting that PKD1 functions in mediating mitogenic signaling [see Ref. [33] for review of earlier literature]. Indeed, overexpression of either PKD1 or PKD2 strikingly potentiated the stimulation of DNA synthesis and cell proliferation induced by Gq-coupled receptor agonists in Swiss 3T3 cells [54–57]. In contrast, overexpression of PKD1 mutants lacking catalytic activity, failed to promote any enhancement of GPCR-induced mitogenesis [55]. These results indicate that PKD1 activation plays a critical role in GPCR mitogenic signaling.
A key pathway involved in mitogenic signaling induced by GPCRs is the extracellular-regulated protein kinase (ERK) cascade [58–60]. The duration and intensity of ERK pathway activation are of critical importance for determining specific biological outcomes, including proliferation, differentiation, death and transformation [61, 62]. ERK signal duration is sensed by the cells through the protein products of immediate early genes, including c-Fos [63, 64]. When ERK activation is transient its activity declines before the c-Fos protein accumulates, and c-Fos is degraded rapidly. However, when ERK signaling is sustained, c-Fos is phosphorylated by ERK and RSK and its stability is dramatically increased thereby leading to its accumulation [55, 63, 64]. Consequently, the stimulatory effect of PKD1 on GPCR-induced cell proliferation [54] has been linked to its ability to increase the duration of the MEK/ERK/RSK pathway leading to accumulation of immediate gene products, including c-Fos, that stimulate cell cycle progression [55]. Sustained (rather than transient) ERK signaling has been linked to stimulation of cell proliferation in pancreatic cancer cells [46].
In contrast to the stimulating effect of PKD1 on the duration of ERK pathway activation, we recently found that induced expression of PKD1 suppressed NT-induced c-Jun Ser63 phosphorylation and the upshift of c-Jun protein. In contrast, K618N PKD1 (kinase-dead) failed to suppress c-Jun Ser63 phosphorylation in PANC-1 clones [65]. This strongly indicates that the catalytic activity of PKD1 is required for suppression of GPCR agonist-induced c-Jun Ser63 phosphorylation in PDAC cells. Induced expression of PKD1 markedly attenuated NT mediated JNK/c-Jun activation at or upstream of MKK4. Previous studies implicated PKD1 in the attenuation of EGF-induced JNK signaling [66–68]. Although biological outcomes depend on stimulus and cell type, transient JNK activation was shown to promote cell survival while prolonged JNK activation mediates apoptosis [69]. Consequently, it is plausible that the attenuation of sustained JNK/c-Jun activation mediated by PKD1 facilitates survival and proliferation of pancreatic cancer cells. Taken together, these studies underscore one of the major emerging roles of PKD1, namely concomitant upregulation of GPCR-mediated mitogenic ERK signaling and downregulation of sustained pro-apoptotic JNK signaling pathway.
Although the immediate downstream target(s) required for the transmission of PKD mitogenic signal has not been fully identified, putative substrates are beginning to emerge. Recently a number of scaffolding proteins and endogenous inhibitors have been implicated in the regulation of the intensity and duration of the ERK pathway [70]. Modeling of the ERK pathway indicates that scaffolds regulate the speed and intensity of pathway activation whereas inhibitors modulate its duration in response to stimuli [71]. The activity and sub-cellular localization of these proteins are also regulated by phosphorylation thereby offering potential new mechanisms for controlling the Raf/MEK/ERK pathway. In this regard, it is very interesting that PKD1 has been shown to phosphorylate RIN1 [72], a multidomain protein that binds with high affinity to Ras (in its GTP form) and interferes with the interaction between Ras and Raf. Therefore, RIN1 inhibits ERK activation in its unphosphorylated form [72]. The phosphorylation of RIN1 at Ser351 by PKD1 induces binding of 14-3-3 proteins that confine RIN1 to the cytosol thereby preventing it from inhibiting the stimulatory interaction between Ras and Raf-1 [72]. PKD1-mediated phosphorylation of RIN1 may be one of the molecular mechanisms by which PKD1 modulates the duration of ERK pathway activation.
4.2. PKD and regulation of cell trafficking, motility, and secretion
A function of PKD1 and PKD2 demonstrated in several cell types is to regulate the budding of secretory vesicles from the trans-Golgi network [73, 74]. Specifically, inactivation of PKD1 (e.g. by expression of kinase-deficient mutants of PKD1) blocks fission of trans-Golgi network (TGN) transport carriers, inducing the appearance of long tubules filled with cargo. At the TGN, active PKD1 and PKD2 phosphorylate phosphatidylinositol 4-kinase IIIb (PI4KIIIb), a key player required for fission of TGN-to-plasma membrane carriers [75]. PI4KIIIb is recruited to the TGN membrane by the small GTPase ARF, and subsequently activated by PKD-mediated phosphorylation to generate PI(4)P. This lipid then recruits the machinery that is required for carrier fission [76].
This process has been implicated in fibroblast locomotion and localized Rac1-dependent leading edge activity [77]. In agreement with an important role in cell trafficking and motility, PKD also promotes integrin recruitment to newly formed focal adhesions [78] and invasiveness of cancer cells [79, 80]. In contrast, two proteins implicated in actin dynamics, cofilin [81] and cortactin [82] have been shown to be phosphorylated by PKD, leading to reduced cell motility. Although PKD contributes to cell motility and actin dynamics, its role appears complex and might depend on cell context and exposure to specific stimuli.
Several studies indicate an important role of PKD in secretion in a number of endocrine cell types. PKD has been shown to stimulate the secretion of the gastrointestinal peptide neurotensin (NT) in the human endocrine cell line BON [83]. Further studies determined that the PKD protein substrate Kidins220, [kinase D-interacting substrate of 220 kDa [84, 85]] mediates NT secretion [86]. Interestingly, the PKD/Kidins220 pathway appears to function downstream of PKD-induced fission of TGN carriers, suggesting that PKD regulates different steps of cell secretion. In addition to PKD, PKD2 has been shown to regulate chromogranin release in BON cells [87]. Other studies indicate that PKDs play a critical role in regulating angiotensin II-mediated cortisol and aldosterone secretion from H295R cells, a human adrenocortical cell line [88, 89]. Recent studies using mice deficient in p38δ reveal a novel p38δ-PKD pathway that regulates insulin secretion and survival of pancreatic β cells, suggesting a critical role for PKD in the development of diabetes mellitus [90]. This function of PKD could be relevant to PDAC given the growth-promoting effect of increased insulin secretion from β cells on epithelial acinar and ductal pancreatic cells [91].
4.3. PKD and epithelial cell polarity
Establishing and maintaining cellular polarity is of fundamental importance for the functions of a variety of cell types, including neuronal and epithelial cells. Early neurons develop initial polarity by mechanisms analogous to those used by migrating cells. In line with this notion, PKDs has been shown to play a role in neuronal protein trafficking. In these cells PKD1 and PKD2 regulate TGN-derived sorting of dendritic proteins and axon formation and hence have a role in establishing neuronal polarity [92, 93]. In polarized epithelial cells, PKD1 and PKD2, but not PKD3, specifically regulate the production of TGN carriers destined to the basolateral membrane rather than to the apical membrane and consequently, PKD and PKD2 may play an important role in the generation of epithelial polarity [74].
Another major mechanism involved in establishing cell polarity is mediated by the evolutionary conserved PAR (partitioning-defective) genes [94]. The Par-3/Par6/aPKC complex is located at tight junctions whereas Par-1, a protein kinase, is found in lateral membranes. There is an antagonistic interaction between the Par-3/Par6/aPKC complex and Par-1 mediated by phosphorylation of specific residues that form binding sites for 14-3-3 proteins. Par-1 kinase, activated by mammalian Par-4/LKB1 by phosphorylation of its activation loop, phosphorylates Par-3 thereby destabilizing the complex and removing it from lateral membranes whereas Par-3/Par6/aPKC phosphorylates Par-1 (on Thr595) to dissociate it from apical plasma membranes [94]. Treatment of cells with phorbol-12-myristate-13-acetate (PMA) induced PKD1-mediated phosphorylation of Par-1 on a residue (Ser400) that promotes Par-1 binding to 14-3-3, thereby promoting its dissociation from the plasma membrane and inhibiting its activity [95]. Although these results suggest the attractive hypothesis that PKD1 plays a role in regulating epithelial cell polarity via phosphorylation of Par-1, additional experiments using physiological stimuli rather than PMA and ductal pancreatic cells are necessary to substantiate this important hypothesis and its relevance in the pathogenesis of PDAC.
4.4. PKD and heat shock proteins
The small heat shock proteins (Hsps), including human Hsp27 and mouse Hsp25 play an important role in the regulation of many cellular functions in response to stress, cytokines, growth factors and GPCR agonists. The level of Hsp27 is markedly increased in many cancer cells and its expression contributes to the malignant properties of these cells, including chemoresistance [see Ref. [96] for references]. Many of the functions attributed to Hsp27 require its phosphorylation, especially at Ser-82, a consensus site for PKD1-mediated phosphorylation. Although it is widely recognized that Hsp27 is a substrate of the p38 MAPK/MK2 cascade [97, 98], other studies demonstrated that phorbol esters also stimulate the phosphorylation of Hsp27 via a PKC-dependent but p38/MK2-independent pathway [99]. However, it has remained unclear whether PKCs directly phosphorylate Hsp27. PKD1 has been implicated in the phosphorylation of Hsp27 on Ser82 in HeLa cells exposed to oxidative stress [100], a condition previously shown to activate PKD1 [38, 101, 102] but also the p38 MAPK/MK2 cascade. The relative contribution to Hsp27 phosphorylation of these parallel pathways was not evaluated.
Human pancreatic cancer PANC-1 cells express high levels of Hsp27. Knockdown of both PKD1 and PKD2, virtually abolished NT-induced Hsp27Ser82 phosphorylation in PANC-1 cells treated with SB 202190, to eliminate the p38MAPK/MK-2 pathway [96]. These results demonstrate that NT induces Hsp27 phosphorylation on Ser82 via simultaneous operation of at least two separate pathways in PANC-1 cells and members of the PKD family play a critical role in mediating one of the pathways. PKD1 and PKD3 are also required to regulate Hsp27 phosphorylation in DT40 B-cells [103]. This phosphorylation of Hsp27 is also necessary for PKD repression of androgen receptor transcriptional activity and androgen-dependent proliferation of prostate cancer cells [104]. Thus, PKDs function as upstream kinases for Hsp27 in a variety of cell types, in some cases functioning in conjunction with the p38 MAP kinase pathway.
4.5. Role of PKD in VEGF-induced endothelial angiogenesis
Recent studies implicated PKD1 signaling in ERK activation and DNA synthesis in endothelial cells stimulated by vascular endothelial growth factor (VEGF), which is essential for many angiogenic processes both in normal and abnormal conditions [105]. In addition to stimulate activation loop Ser744 and Ser748 phosphorylation, VEGF, acting via the KDR receptor, also induces PKD1 phosphorylation on Tyr463 [106].
Regulation of chromatin accessibility by acetylation/deacetylation of nucleosomal histones is a key mechanism used to modulate gene expression. Class II histone deacetylases (HDACs), including HADCS 5 and 7, regulate chromatin structure by interacting with various transcription factors to repress their transcriptional activity. PKD1-mediated phosphorylation of specific residues in class II HDACS leads to association with 14-3-3 chaperone proteins thereby regulating their intracellular distribution in a variety of cell types. Sequestration of HDACs in the cytoplasm presumably relieves target genes from HDAC repressive actions, thereby facilitating gene expression. HDAC7 has been implicated in the regulation of endothelial cells morphology, migration, and capacity to form capillary tube-like structures in vitro [107]. Treatment of endothelial cells with PMA or VEGF resulted in the exit of HDAC7 from the nucleus through a PKC/PKD pathway [107, 108]. Further studies indicate that VEGF also stimulates PKD-dependent phosphorylation of HDAC5 at Ser259/498 residues, which leads to HDAC5 nuclear exclusion and transcriptional activation [109]. It is conceivable that the complex program of gene expression and migration triggered by VEGF in endothelial cells leading to angiogenesis is orchestrated by PKD-mediated phosphorylation of both HADC5 and HDAC7, leading to their nuclear extrusion in these cells. Indeed, it has been recently proposed that PKD1 is one of the most attractive targets for anti-angiogenic therapies [110].
4.6. PKDs, inflammation, and oxidative stress
NF-κB is a key transcription factor that is activated by multiple receptors and regulates the expression of a wide variety of proteins that control innate and adaptive immunity. A number of studies indicate that PKD1 is a mediator of NF-κB induction in a variety of cells, including PDAC cells, exposed to GPCR agonists or oxidative stress [38, 111–115]. In view of the increasing recognition of the interplay between inflammation and cancer development, a possible role of PKD1 in linking these processes is of importance. However, the precise molecular mechanisms remain incompletely understood.
Stimulation of human colonic epithelial NCM460 cells with the GPCR agonist and bioactive lipid lysophosphatidic acid (LPA) led to a rapid and striking activation of PKD2, the major isoform of the PKD family expressed by these cells [114]. LPA induced a striking increase in the production of interleukin 8 (IL-8), a potent pro-inflammatory and pro-angiogenic chemokine, and stimulated NF-κB activation. PKD2 gene silencing utilizing small interfering RNAs dramatically reduced LPA-stimulated NF-κB promoter activity and IL-8 production. These results imply that PKD2 mediates LPA-stimulated IL-8 secretion in NCM460 cells through a NF-κB-dependent pathway. PKD2 has also been implicated in mediating NF-κB activation by Bcr-Abl in myeloid leukemia cells [112].
NF-κB also plays a critical role in inflammatory and cell death responses during acute pancreatitis. Previous studies demonstrated that the PKC isoforms PKCδ and ε are key regulators of NF-κB activation induced by cholecystokinin-8 (CCK-8), an agonist that induces pancreatitis when administered to rodents at supramaximal doses. PKD has been shown to function as a key downstream target of PKCδ and PKCδ in pancreatic acinar cells stimulated by CCK-8 or the cholinergic agonist carbachol. Furthermore, PKD was necessary for NF-κB activation induced by these GPCR agonists [116]. The kinetics of PKD1 and NF-κB activation during rat pancreatitis showed that both PKD1 and NF-κB activation were early events during acute pancreatitis and that their time courses of response in vivo were similar [116]. These results identify PKD1 as a novel early point of convergence in the signaling pathways mediating NF-κB activation in pancreatitis, a condition that in its chronic form predisposes to pancreatic cancer.
Since the original finding that oxidative stress induces PKD1 activation, partly via PKC-mediated activation loop phosphorylation, and partly through Src-mediated PKD1 tyrosine phosphorylation [101], a number of reports confirmed that PKD1 is a sensor of oxidative stress [38, 90, 102, 111, 113, 115, 117, 118]. Recently, Tyr95 in PKD1 has been identified as a phosphorylation site that is regulated by oxidative stress and generates a binding motif for PKCδ. Oxidative stress-mediated PKCδ/PKD1 interaction results in PKD1 activation loop phosphorylation on Ser744 and Ser748 leading to catalytic activation [118]. A number of studies have shown that PKD1 opposes the apoptotic effects of oxidative stress in a variety of cells [90, 115, 117, 119–121].
A recent study using pancreatic β cells, demonstrated that stress signals markedly induced TNFAIP3/A20, a zinc finger-containing, immediate early-response gene with potent antiapoptotic and anti-inflammatory functions [122]. In fact, A20 is an early NF-κB-responsive gene that encodes a ubiquitin-editing protein that is involved in the negative feedback regulation of NF-κB signaling [123]. Interestingly, other studies demonstrated that PKD1 induces A20 promoter activity [124]. It is plausible that PKD1 initiates not only an inflammatory response via NF-κB but also stimulates expression of the antiapoptotic and anti-inflammatory A20, as a feedback mechanism that protect cells subject to stress signals, including oxidative stress.
5. Role of the PKD family in PDAC
Given the unmet need for defining molecularly targeted therapies for PDAC, this section is focused more specifically in summarizing recent advances in identifying PKD family members as potential therapeutic targets in PDAC. A previous study reported moderate to strong overexpression of PKD1 in PDAC while only mild to moderate staining in normal pancreatic tissue implicate the significant role of PKD1 in this cancer [125]. However, the results of this study did not distinguish whether the increase in PKD immunoreactivity represented active or inactive PKD. In a recent study, autophosphorylated PKD1/2 (on the C-terminal tail, indicative of catalytic activation) was shown to be significantly up-regulated in PDAC, as compared to normal pancreatic ducts [[126] and unpublished results].
Previously, we demonstrated that the PDAC cell lines PANC-1, MiaPaca-2 and HPAF-II endogenously express PKD1/2 [13]. We also reported that the GPCR agonist neurotensin induces PKD1 activation [13] and translocation to the plasma membrane [32] and subsequently acts as potent growth factor for PDAC cell lines, including PANC-1 [44, 46, 127]. As indicated above, downstream targets of PKD1 include Hsp27 [Heat shock protein 27, [96]] which contributes to gemcitabine resistance in PDAC cells [128]. Interestingly, PKD1 has been reported to be up-regulated in PDAC cell lines highly resistant to chemotherapeutic drugs [125].
As mentioned above, recent results show that induced overexpression of PKD1 in PANC-1 cells led to reciprocal regulation of neurotensin-induced MAPK pathways in these cells. Specifically, PKD1 suppressed pro-apoptotic JNK signaling pathway and concomitantly prolonged mitogenic ERK1/2 signaling [65]. Accordingly, recent results show that PKD1 overexpression in PANC-1 cells stimulated DNA synthesis, anchorage-dependent and anchorage-independent proliferation and markedly enhanced neurotensin-induced DNA synthesis in these cells [[65] and unpublished results]. Thus, PKD1 emerges as a potential novel target for developing therapeutic strategies to restrict the unregulated proliferation of pancreatic cancer cells.
6. PKD family as a therapeutic agent: development of novel PKD inhibitors
As discussed in previous sections, PKD signaling is increasingly implicated in the regulation of multiple cellular activities and in the mechanism of action of multiple stimuli and in the unrestrained proliferation of PDAC cells [13,32,44,46,65,96,125,126,128]. Although several compounds are known to inhibit the catalytic activity PKD1, including Gö-6976 [129] and K252a [57], these agents are not PKD specific. The identification of selective PKD inhibitors would be extremely useful in helping to define the physiological substrates and functions of the members of the PKD family and may open up new avenues for the development of novel therapeutic approaches in a variety of conditions, including PDAC.
In order to test further the hypothesis that the PKD family plays a critical role in PDAC cell proliferation and develop novel potential anti-cancer agent(s), a diverse compound library was screened against purified PKD1 to identify novel inhibitors against this protein kinase family. High throughput screening identified a new family of pyrazine benzamide compounds that are pharmacologically active, cell-permeable, PKD family inhibitors [126]. A lead compound, CRT0066101, was used in vitro to quantitate its effects on the catalytic activity of PKD, as determined by inhibition of peptide substrate phosphorylation. The IC50 values were 1, 2.5 and 2 nM for PKD1, PKD2, and PKD3 respectively. The specificity of CRT0066101 for PKD family members was also confirmed by in vitro kinase assays comprising a panel of >90 protein kinases (including PKCα, PKBα, MEK1, c-Raf, MAPKAP-2, PAK2, CHK1, GSK3β, CAMK-I, CAMK-IV, Aurora kinase, c-Src, EGFR, and PDGFR) that have a role in cancer promotion or progression. As described above, PKD directly phosphorylate Hsp 27 on Ser82 in intact PANC-1 cells stimulated with NT [96]. Our recent results demonstrate that treatment of PANC-1 cells with CRT0066101 (e.g. 1 µM) inhibited PKD1 activation, and prevented Hsp27 phosphorylation on Ser82 [126]. Importantly, CRT0066101 did not interfere with NT-induced phosphorylation of MARCKS on Ser152/156, a well-established target of PKC. These results corroborated the specificity of CRT0066101 within intact PDAC cells. In the context of this article, it is important that CRT0066101 inhibited DNA synthesis (BrdU incorporation) in proliferating PANC-1 cells with an IC50 = 1 µM [126]. Additional results demonstrate that PKD inhibition also abrogates anchorage-independent growth of PDAC cells in semisolid medium, a hallmark of malignant cells.
Plasma concentrations of CRT0066101 were evaluated following oral administration (by gavage) of a dose of 80 mg/kg in CD-1 mice. Optimal therapeutic concentrations (~8 µM) of CRT0066101 were detectable 6 h after the oral administration of this drug. Crucially, administration of CRT0066101 markedly reduced the growth of heterotopic (subcutaneous) or orthotopic (intra-pancreatic) xenografts models of PDAC [126]. These results provide strong evidence indicating that PKD signaling plays a critical role in the growth of human pancreatic cancer cells.
Interestingly, recent results obtained by another laboratory using endothelial cells showed that vascular endothelial growth factor (VEGF)-induced phosphorylation of the PKD1 substrates histone deacetylase (HDAC) 5, CREB and Hsp27 phosphorylation on Ser82, was inhibited by CRT5, another pyrazine benzamide PKD family inhibitor [130]. These results corroborate that pyrazine benzamides are selective PKD antagonists and raise the interesting hypothesis that they inhibit PDAC growth by acting at two different levels: directly on PDAC cell proliferation and indirectly, preventing angiogenesis necessary to support tumor cell growth.
It is noteworthy that benzoxoloazepinolone, a previously identified PKD inhibitor termed CID755673 [131], appears to induce biological effects, including stimulation of cell cycle progression, independently of PKD1 [132]. It appears that CID755673 has other cellular target(s) in addition to PKD1 and therefore, experiments using this compound to elucidate the role of the PKD1 family in cell regulation should be interpreted with great caution. Recently, several analogs with equal or greater potencies as CID755673 were identified [133]. Modifications to the aromatic core structure of this inhibitor significantly increased potency while retaining high specificity for PKD1. In line with the notion that PKD1 plays a role in stimulating the proliferation of cancer cells, the new PKD1 inhibitors identified in Ref. [133] arrested proliferation when applied to prostate cancer cells. Cell migration and invasion were also inhibited by these analogs with varying potencies that correlated to their cellular activity[133]. These compounds are being tested as pharmacological tools for dissecting PKD1 function and as potential anti-cancer agents in the treatment of prostate cancer.
Using high throughput screening and medicinal chemistry, another group identified a series of selective small molecule inhibitors of PKD family members [134]. One of these compounds, referred as bipyridyl PKD inhibitor (BPKDi), inhibited the three members of the PKD family, PKD1, PKD2 and PKD3, with IC50 values of 1, 9 and 1 nM, respectively [134]. BPKDi inhibited PKD-mediated phosphorylation of class IIa HDAC kinases in cardiac myocytes but did not significantly inhibit other putative class IIa HDAC kinases. In cultured cardiac myocytes, BPKDi blocked agonist-dependent PKD activation and phosphorylation-dependent nuclear export of class IIa HDACs -4 and -5. Pharmacological inhibition of PKD activity was associated with attenuation of myocyte hypertrophy [134]. It will be important to determine whether these compounds inhibit cancer cell growth.
As different inhibitors of PKD catalytic activity are emerging, it will be of interest to determine if they inhibit PKD through similar or different mechanisms. If different mechanisms are defined, it might be possible to use combinations of PKD inhibitors at lower concentrations that may increase target specificity and anti-proliferative activity and reduce undesirable off-target effects.
7. Conclusions and implications
A great deal of progress has been made in understanding the regulatory mechanisms of activation and sub-cellular localization of PKD1 and the role of novel PKCs in mediating rapid phosphorylation at the activation loop. As in other phosphorylation cascades, inducible activation loop phosphorylation provides a mechanism of signal integration and amplification. Interestingly, new results uncovered that the regulation of the activation loop phosphorylation of PKD1 is more complex than previously thought, with the participation of different mechanism at different times, especially in cells stimulated by Gq-coupled receptor agonists [57, 135].
Accumulating evidence demonstrate that PKD plays an important role in an array of cellular processes and activities, including signal transduction [55, 56, 72, 136], chromatin organization [137], Golgi function [73, 77], gene expression [111, 112, 114], prostaglandin synthesis via COX-2 induction [138], immune regulation [137] and cell survival, adhesion, motility, differentiation, DNA synthesis and proliferation [reviewed in Ref. [33]]. The involvement of PKDs in mediating such a diverse array of normal and abnormal biological activities in different sub-cellular compartments is likely to depend on the dynamic changes in their spatial and temporal localization, combined with its distinct substrate specificity. As originally predicted [24], it seems that a variety of biological responses attributed originally to PKCs are in fact executed by PKDs. Animal models using PKD transgenics or tissue specific knockout are emerging and will serve to further clarify the function(s) of PKD isoforms in vivo. In this context, it is important to point out that global knockout of PRKD1 in mice induces embryonic lethality with incomplete penetrance [139].
In conclusion, it is increasingly apparent that the members of the PKD family are key players in the regulation of fundamental cellular activities and processes in normal and cancer cells. Pancreatic ductal adenocarcinoma is one of the most lethal human diseases and novel molecularly targeted therapies are urgently needed. In this article we posit that PKD1 plays a role in promoting the development of human pancreatic cancer at least via three different mechanisms: (1) PKD plays a critical role in PDAC cell proliferation by reciprocal regulation of ERK and JNK cascades; (2) PKD is increasingly implicated in the mechanisms by which VEGF induces angiogenesis and (3) PKD plays an important role in stimulating the secretion of insulin and GPCR agonists that activate mitogenic signaling in pancreatic ductal adenocarcinoma cells. The role of PKD in multiple aspects of pancreatic cancer development may explain the success of orally active PKD inhibitors in reducing the growth of orthotopic xenografts of PDAC. PKD is emerging as a valuable target for development of novel therapeutic approaches in important and still intractable diseases, including human pancreatic cancer.
Acknowledgements
Work in the laboratory of SG is supported by MD Anderson Cancer Center Physician Scientist Program Award, McNair Foundation Scholar Award, Institutional Research Grant, NIH grants 5P30CA16672, and R21CA135218. Work in the laboratory of ER is supported by NIH Grants R21CA137292, R01DK56930, R01DK55003, and P30DK41301. ER holds the Hirshberg Chair of Pancreatic Cancer Research.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer Statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–450. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- 3.Maitra A, Fukushima N, Takaori K, Hruban RH. Precursors to invasive pancreatic cancer. Adv Anat Pathol. 2005;12:81–91. doi: 10.1097/01.pap.0000155055.14238.25. [DOI] [PubMed] [Google Scholar]
- 4.Brand RE, Tempero MA. Pancreatic cancer. Curr Opin Oncol. 1998;10:362–366. doi: 10.1097/00001622-199807000-00014. [DOI] [PubMed] [Google Scholar]
- 5.Hezel AF, Kimmelman AC, Stanger BZ, Bardeesy N, Depinho RA. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 2006;20:1218–1249. doi: 10.1101/gad.1415606. [DOI] [PubMed] [Google Scholar]
- 6.Kern S, Hruban R, Hollingsworth MA, Brand R, Adrian TE, Jaffee E, et al. A white paper: the product of a pancreas cancer think tank. Cancer Res. 2001;61:4923–4932. [PubMed] [Google Scholar]
- 7.Hruban RH, Goggins M, Parsons J, Kern SE. Progression model for pancreatic cancer. Clin Cancer Res. 2000;6:2969–2972. [PubMed] [Google Scholar]
- 8.Rozengurt E. Mitogenic signaling pathways induced by G protein-coupled receptors. J Cell Physiol. 2007;213:589–602. doi: 10.1002/jcp.21246. [DOI] [PubMed] [Google Scholar]
- 9.Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–1806. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mikoshiba K. The InsP3 receptor and intracellular Ca2+ signaling. Curr Opin Neurobiol. 1997;7:339–345. doi: 10.1016/s0959-4388(97)80061-x. [DOI] [PubMed] [Google Scholar]
- 11.Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992;258:607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- 12.Denham DW, Franz MG, Denham W, Zervos EE, Gower WR, Jr, Rosemurgy AS, et al. Directed antisense therapy confirms the role of protein kinase C-alpha in the tumorigenicity of pancreatic cancer. Surgery. 1998;124:218–222. [PubMed] [Google Scholar]
- 13.Guha S, Rey O, Rozengurt E. Neurotensin induces protein kinase C-dependent protein kinase D activation and DNA synthesis in human pancreatic carcinoma cell line PANC-1. Cancer Res. 2002;62:1632–1640. [PubMed] [Google Scholar]
- 14.Ishino K, Fukazawa H, Shikano M, Ohba M, Kuroki T, Uehara Y. Enhancement of anchorage-independent growth of human pancreatic carcinoma MIA PaCa-2 cells by signaling from protein kinase C to mitogen-activated protein kinase. Mol Carcinog. 2002;34:180–186. doi: 10.1002/mc.10063. [DOI] [PubMed] [Google Scholar]
- 15.Trauzold A, Wermann H, Arlt A, Schuetze S, Schaefer H, Oestern S, et al. CD95 and TRAIL receptor-mediated activation of protein kinase C and NF-kappaB contributes to apoptosis resistance in ductal pancreatic adenocarcinoma cells. Oncogene. 2001;20:4258–4269. doi: 10.1038/sj.onc.1204559. [DOI] [PubMed] [Google Scholar]
- 16.Way D, Smith S, Sivendran S, Chie L, Kanovsky M, Brandt-Rauf PW, et al. A protein kinase C inhibitor induces phenotypic reversion of ras-transformed pancreatic cancer cells and cooperatively blocks tumor cell proliferation with an anti-ras peptide. Cancer Chemother Pharmacol. 2002;49:429–437. doi: 10.1007/s00280-002-0432-8. [DOI] [PubMed] [Google Scholar]
- 17.Detjen KM, Brembeck FH, Welzel M, Kaiser A, Haller H, Wiedenmann B, et al. Activation of protein kinase C alpha inhibits growth of pancreatic cancer cells via p21(cip)-mediated G(1) arrest. J Cell Sci. 2000;113:3025–3035. doi: 10.1242/jcs.113.17.3025. [DOI] [PubMed] [Google Scholar]
- 18.Scotti ML, Bamlet WR, Smyrk TC, Fields AP, Murray NR. Protein Kinase C{iota} is required for pancreatic cancer cell transformed growth and tumorigenesis. Cancer Res. 2010;70:2064–2074. doi: 10.1158/0008-5472.CAN-09-2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valverde AM, Sinnett-Smith J, Van Lint J, Rozengurt E. Molecular cloning and characterization of protein kinase D: a target for diacylglycerol and phorbol esters with a distinctive catalytic domain. Proc Natl Acad Sci USA. 1994;91:8572–8576. doi: 10.1073/pnas.91.18.8572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zugaza JL, Waldron RT, Sinnett-Smith J, Rozengurt E. Bombesin, vasopressin, endothelin, bradykinin, and platelet-derived growth factor rapidly activate protein kinase D through a protein kinase C-dependent signal transduction pathway. J Biol Chem. 1997;272:23952–23960. doi: 10.1074/jbc.272.38.23952. [DOI] [PubMed] [Google Scholar]
- 21.Zugaza JL, Sinnett-Smith J, Van Lint J, Rozengurt E. Protein kinase D (PKD) activation in intact cells through a protein kinase C-dependent signal transduction pathway. EMBO J. 1996;15:6220–6230. [PMC free article] [PubMed] [Google Scholar]
- 22.Johannes FJ, Prestle J, Eis S, Oberhagemann P, Pfizenmaier K. PKCu is a novel, atypical member of the protein kinase C family. J Biol Chem. 1994;269:6140–6148. [PubMed] [Google Scholar]
- 23.Van Lint JV, Sinnett-Smith J, Rozengurt E. Expression and characterization of PKD, a phorbol ester and diacylglycerol-stimulated serine protein kinase. J Biol Chem. 1995;270:1455–1461. doi: 10.1074/jbc.270.3.1455. [DOI] [PubMed] [Google Scholar]
- 24.Rozengurt E, Sinnett-Smith J, Van Lint J, Valverde AM. Protein kinase D (PKD): a novel target for diacylglycerol and phorbol esters. Mutat Res. 1995;333:153–160. doi: 10.1016/0027-5107(95)00141-7. [DOI] [PubMed] [Google Scholar]
- 25.Rozengurt E, Sinnett-Smith J, Zugaza JL. Protein kinase D: a novel target for diacylglycerol and phorbol esters. Bochem Soc Trans. 1997;25:565–571. doi: 10.1042/bst0250565. [DOI] [PubMed] [Google Scholar]
- 26.Iglesias T, Rozengurt E. Protein kinase D activation by mutations within its pleckstrin homology domain. J Biol Chem. 1998;273:410–416. doi: 10.1074/jbc.273.1.410. [DOI] [PubMed] [Google Scholar]
- 27.Waldron RT, Iglesias T, Rozengurt E. The pleckstrin homology domain of protein kinase D interacts preferentially with the eta isoform of protein kinase C. J Biol Chem. 1999;274:9224–9230. doi: 10.1074/jbc.274.14.9224. [DOI] [PubMed] [Google Scholar]
- 28.Iglesias T, Matthews S, Rozengurt E. Dissimilar phorbol ester binding properties of the individual cysteine-rich motifs of protein kinase D. FEBS Lett. 1998;437:19–23. doi: 10.1016/s0014-5793(98)01189-2. [DOI] [PubMed] [Google Scholar]
- 29.Iglesias T, Rozengurt E. Protein kinase D activation by deletion of its cysteine-rich motifs. FEBS Lett. 1999;454:53–56. doi: 10.1016/s0014-5793(99)00772-3. [DOI] [PubMed] [Google Scholar]
- 30.Sturany S, Van Lint J, Mueller F, Wilda M, Hameister H, Hoecker M, et al. Molecular cloning and characterization of the human protein kinase D2: A novel member of the protein kinase D family of serine threonine kinases. J Biol Chem. 2001;276:3310–3318. doi: 10.1074/jbc.M008719200. [DOI] [PubMed] [Google Scholar]
- 31.Hayashi A, Seki N, Hattori A, Kozuma S, Saito T. PKCnu, a new member of the protein kinase C family, composes a fourth subfamily with PKCmu. Biochim Biophys Acta. 1999;1450:99–106. doi: 10.1016/s0167-4889(99)00040-3. [DOI] [PubMed] [Google Scholar]
- 32.Rey O, Yuan J, Young SH, Rozengurt E. Protein kinase C nu/protein kinase D3 nuclear localization, catalytic activation, and intracellular redistribution in response to G protein-coupled receptor agonists. J Biol Chem. 2003;278:23773–23785. doi: 10.1074/jbc.M300226200. [DOI] [PubMed] [Google Scholar]
- 33.Rozengurt E, Rey O, Waldron RT. Protein kinase D signaling. J Biol Chem. 2005;280:13205–13208. doi: 10.1074/jbc.R500002200. [DOI] [PubMed] [Google Scholar]
- 34.Johannes FJ, Prestle J, Dieterich S, Oberhagemann P, Link G, Pfizenmaier K. Characterization of activators and inhibitors of protein kinase C mu. Eur J Biochem. 1995;227:303–307. doi: 10.1111/j.1432-1033.1995.tb20389.x. [DOI] [PubMed] [Google Scholar]
- 35.Matthews SA, Pettit GR, Rozengurt E. Bryostatin 1 induces biphasic activation of protein kinase D in intact cells. J Biol Chem. 1997;272:20245–20250. doi: 10.1074/jbc.272.32.20245. [DOI] [PubMed] [Google Scholar]
- 36.Paolucci L, Rozengurt E. Protein kinase D in small cell lung cancer cells: rapid activation through protein kinase C. Cancer Res. 1999;59:572–577. [PubMed] [Google Scholar]
- 37.Yuan JZ, Bae D, Cantrell D, Nel AE, Rozengurt E. Protein kinase D is a downstream target of protein kinase C theta. Biochem Biophys Res Commun. 2002;291:444–452. doi: 10.1006/bbrc.2002.6469. [DOI] [PubMed] [Google Scholar]
- 38.Storz P, Doppler H, Toker A. Protein kinase Cdelta selectively regulates protein kinase D-dependent activation of NF-kappaB in oxidative stress signaling. Mol Cell Biol. 2004;24:2614–2626. doi: 10.1128/MCB.24.7.2614-2626.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rey O, Young SH, Cantrell D, Rozengurt E. Rapid protein kinase D translocation in response to G protein-coupled receptor activation. Dependence on protein kinase C. J Biol Chem. 2001;276:32616–32626. doi: 10.1074/jbc.M101649200. [DOI] [PubMed] [Google Scholar]
- 40.Rey O, Sinnett-Smith J, Zhukova E, Rozengurt E. Regulated nucleocytoplasmic transport of protein kinase D in response to G protein-coupled receptor activation. J Biol Chem. 2001;276:49228–49235. doi: 10.1074/jbc.M109395200. [DOI] [PubMed] [Google Scholar]
- 41.Rey O, Zhukova E, Sinnett-Smith J, Rozengurt E. Vasopressin-induced intracelluar redistribution of protein kinase D in intestinal epithelial cells. J Cell Physiol. 2003;196:483–492. doi: 10.1002/jcp.10323. [DOI] [PubMed] [Google Scholar]
- 42.Rozengurt E. Neuropeptides as growth factors for normal and cancer cells. Trends Endocrinol Metabol. 2002;13:128–134. doi: 10.1016/s1043-2760(01)00544-6. [DOI] [PubMed] [Google Scholar]
- 43.Dorsam RT, Gutkind JS. G-protein-coupled receptors and cancer. Nat Rev Cancer. 2007;7:79–94. doi: 10.1038/nrc2069. [DOI] [PubMed] [Google Scholar]
- 44.Ryder NM, Guha S, Hines OJ, Reber HA, Rozengurt E. G protein-coupled receptor signaling in human ductal pancreatic cancer cells: neurotensin responsiveness and mitogenic stimulation. J Cell Physiol. 2001;186:53–64. doi: 10.1002/1097-4652(200101)186:1<53::AID-JCP1004>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 45.Guha S, Lunn JA, Santiskulvong C, Rozengurt E. Neurotensin stimulates protein kinase C-dependent mitogenic signaling in human pancreatic carcinoma cell line PANC-1. Cancer Res. 2003;63:2379–2387. [PubMed] [Google Scholar]
- 46.Kisfalvi K, Guha S, Rozengurt E. Neurotensin and EGF induce synergistic stimulation of DNA synthesis by increasing the duration of ERK signaling in ductal pancreatic cancer cells. J Cell Physiol. 2005;202:880–890. doi: 10.1002/jcp.20187. [DOI] [PubMed] [Google Scholar]
- 47.Kisfalvi K, Eibl G, Sinnett-Smith J, Rozengurt E. Metformin disrupts crosstalk between G protein-coupled receptor and insulin receptor signaling systems and inhibits pancreatic cancer growth. Cancer Res. 2009;69:6539–6545. doi: 10.1158/0008-5472.CAN-09-0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guha S, Eibl G, Kisfalvi K, Fan RS, Burdick M, Reber H, et al. Broad-spectrum G protein-coupled receptor antagonist, (D-Arg1,D-Trp5,7,9,Leu11)SP: a dual inhibitor of growth and angiogenesis in pancreatic cancer. Cancer Res. 2005;65:2738–2745. doi: 10.1158/0008-5472.CAN-04-3197. [DOI] [PubMed] [Google Scholar]
- 49.Elek J, Pinzon W, Park KH, Narayanan R. Relevant genomics of neurotensin receptor in cancer. Anticancer Res. 2000;20:53–58. [PubMed] [Google Scholar]
- 50.Wang L, Friess H, Zhu Z, Graber H, Zimmermann A, Korc M, et al. Neurotensin receptor-1 mRNA analysis in normal pancreas and pancreatic disease. Clin Cancer Res. 2000;6:566–571. [PubMed] [Google Scholar]
- 51.Reubi JC, Waser B, Friess H, Bèuchler M, Laissue J. Neurotensin receptors: a new marker for human ductal pancreatic adenocarcinoma. Gut. 1998;42:546–550. doi: 10.1136/gut.42.4.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arafat HA, Gong Q, Chipitsyna G, Rizvi A, Saa CT, Yeo CJ. Antihypertensives as novel antineoplastics: angiotensin-I-converting enzyme inhibitors and angiotensin II type 1 receptorblockers in pancreatic ductal adenocarcinoma. J Am Coll Surg. 2007;204:996–1005. doi: 10.1016/j.jamcollsurg.2007.01.067. [DOI] [PubMed] [Google Scholar]
- 53.Kisfalvi K, Rey O, Young SH, Sinnett-Smith J, Rozengurt E. Insulin potentiates Ca2+ signaling and phosphatidylinositol 4,5-bisphosphate hydrolysis induced by Gq protein-coupled receptor agonists through an mTOR-dependent pathway. Endocrinology. 2007;148:3246–3257. doi: 10.1210/en.2006-1711. [DOI] [PubMed] [Google Scholar]
- 54.Zhukova E, Sinnett-Smith J, Rozengurt E. Protein kinase D potentiates DNA synthesis and cell proliferation induced by bombesin, vasopressin, or phorbol esters in Swiss 3T3 Cells. J Biol Chem. 2001;276:40298–40305. doi: 10.1074/jbc.M106512200. [DOI] [PubMed] [Google Scholar]
- 55.Sinnett-Smith J, Zhukova E, Hsieh N, Jiang X, Rozengurt E. Protein kinase D potentiates DNA synthesis induced by Gq-coupled receptors by increasing the duration of ERK signaling in Swiss 3T3 cells. J Biol Chem. 2004;279:16883–16893. doi: 10.1074/jbc.M313225200. [DOI] [PubMed] [Google Scholar]
- 56.Sinnett-Smith J, Zhukova E, Rey O, Rozengurt E. Protein kinase D2 potentiates MEK/ERK/RSK signaling, c-Fos accumulation and DNA synthesis induced by bombesin in Swiss 3T3 cells. J Cell Physiol. 2007;211:781–789. doi: 10.1002/jcp.20984. [DOI] [PubMed] [Google Scholar]
- 57.Sinnett-Smith J, Jacamo R, Kui R, Wang YM, Young SH, Rey O, et al. Protein kinase D mediates mitogenic signaling by Gq-coupled Receptors through protein kinase C-independent regulation of activation loop Ser744 and Ser748 phosphorylation. J Biol Chem. 2009;284:13434–13445. doi: 10.1074/jbc.M806554200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rozengurt E. Signal transduction pathways in the mitogenic response to G proteincoupled neuropeptide receptor agonists. J Cell Physiol. 1998;177:507–517. doi: 10.1002/(SICI)1097-4652(199812)177:4<507::AID-JCP2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 59.Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- 60.Meloche S, Pouyssegur J. The ERK1/2 mitogen-activated protein kinase pathway as a master regulator of the G1- to S-phase transition. Oncogene. 2007;26:3227–3239. doi: 10.1038/sj.onc.1210414. [DOI] [PubMed] [Google Scholar]
- 61.Marshall CJ. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- 62.Pouyssegur J, Lenormand P. Fidelity and spatio-temporal control in MAP kinase (ERKs) signalling. Eur J Biochem. 2003;270:3291–3299. doi: 10.1046/j.1432-1033.2003.03707.x. [DOI] [PubMed] [Google Scholar]
- 63.Murphy LO, Smith S, Chen RH, Fingar D, Blenis J. Molecular interpretation of ERK signal duration by immediate early gene products. Nat Cell Biol. 2002;4:556–564. doi: 10.1038/ncb822. [DOI] [PubMed] [Google Scholar]
- 64.Murphy LO, MacKeigan JP, Blenis J. A network of immediate early gene products propagates subtle differences in mitogen-activated protein kinase signal amplitude and duration. Mol. Cell. Biol. 2004;24:144–153. doi: 10.1128/MCB.24.1.144-153.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kisfalvi K, Hurd C, Guha S, Rozengurt E. Induced overexpression of protein kinase D1 stimulates mitogenic signaling in human pancreatic carcinoma PANC-1 cells. J Cell Physiol. 2010;223:309–316. doi: 10.1002/jcp.22036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bagowski CP, Stein-Gerlach M, Choidas A, Ullrich A. Cell-type specific phosphorylation of threonines T654 and T669 by PKD defines the signal capacity of the EGF receptor. EMBO J. 1999;18:5567–5576. doi: 10.1093/emboj/18.20.5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hurd C, Waldron RT, Rozengurt E. Protein kinase D complexes with C-Jun N-terminal kinase via activation loop phosphorylation and phosphorylates the C-Jun N-terminus. Oncogene. 2002;21:2154–2160. doi: 10.1038/sj.onc.1205290. [DOI] [PubMed] [Google Scholar]
- 68.Hurd C, Rozengurt E. Protein kinase D is sufficient to suppress EGF-induced c-Jun Ser 63 phosphorylation. Biochem Biophys Res Commun. 2001;282:404–408. doi: 10.1006/bbrc.2001.4591. [DOI] [PubMed] [Google Scholar]
- 69.Wagner EF, Nebreda AR. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat Rev Cancer. 2009;9:537–549. doi: 10.1038/nrc2694. [DOI] [PubMed] [Google Scholar]
- 70.Kolch W. Coordinating ERK/MAPK signalling through scaffolds and inhibitors. Nat Rev Mol Cell Biol. 2005;6:827–837. doi: 10.1038/nrm1743. [DOI] [PubMed] [Google Scholar]
- 71.Ebisuya M, Kondoh K, Nishida E. The duration, magnitude and compartmentalization of ERK MAP kinase activity: mechanisms for providing signaling specificity. J Cell Sci. 2004;118:2997–3002. doi: 10.1242/jcs.02505. [DOI] [PubMed] [Google Scholar]
- 72.Wang Y, Waldron RT, Dhaka A, Patel A, Riley MM, Rozengurt E, et al. The RAS effector RIN1 directly competes with RAF and is regulated by 14-3-3 proteins. Mol Cell Biol. 2002;22(3):916–926. doi: 10.1128/MCB.22.3.916-926.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liljedahl M, Maeda Y, Colanzi A, Ayala I, Van Lint J, Malhotra V. Protein kinase D regulates the fission of cell surface destined transport carriers from the trans-Golgi network. Cell. 2001;104:409–420. doi: 10.1016/s0092-8674(01)00228-8. [DOI] [PubMed] [Google Scholar]
- 74.Yeaman C, Ayala MI, Wright JR, Bard F, Bossard C, Ang A, et al. Protein kinase D regulates basolateral membrane protein exit from trans-Golgi network. Nat Cell Biol. 2004;6:106–112. doi: 10.1038/ncb1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hausser A, Storz P, Martens S, Link G, Toker A, Pfizenmaier K. Protein kinase D regulates vesicular transport by phosphorylating and activating phosphatidylinositol-4 kinase III[beta] at the Golgi complex. Nat Cell Biol. 2005;7:880–886. doi: 10.1038/ncb1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ghanekar Y, Lowe M. Signalling for secretion. Nat Cell Biol. 2005;7:851–853. doi: 10.1038/ncb0905-851. [DOI] [PubMed] [Google Scholar]
- 77.Prigozhina NL, Waterman-Storer CM. Protein kinase D-mediated anterograde membrane trafficking is required for fibroblast motility. Curr Biol. 2004;14:88–98. doi: 10.1016/j.cub.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 78.Woods AJ, White DP, Caswell PT, Norman JC. PKD1/PKCmicro promotes alphavbeta3 integrin recycling and delivery to nascent focal adhesions. EMBO J. 2004;23:2531–2543. doi: 10.1038/sj.emboj.7600267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Qiang YW, Yao L, Tosato G, Rudikoff S. Insulin-like growth factor I induces migration and invasion of human multiple myeloma cells. Blood. 2004;103:301–308. doi: 10.1182/blood-2003-06-2066. [DOI] [PubMed] [Google Scholar]
- 80.Bowden ET, Barth M, Thomas D, Glazer RI, Mueller SC. An invasion-related complex of cortactin, paxillin and PKCmu associates with invadopodia at sites of extracellular matrix degradation. Oncogene. 1999;18:4440–4449. doi: 10.1038/sj.onc.1202827. [DOI] [PubMed] [Google Scholar]
- 81.Eiseler T, Doppler H, Yan IK, Kitatani K, Mizuno K, Storz P. Protein kinase D1 regulates cofilin-mediated F-actin reorganization and cell motility through slingshot. Nat Cell Biol. 2009;11:545–556. doi: 10.1038/ncb1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Eiseler T, Hausser A, De Kimpe L, Van Lint J, Pfizenmaier K. Protein kinase D controls actin polymerization and cell motility through phosphorylation of cortactin. J Biol Chem. 2010;285:18672–18683. doi: 10.1074/jbc.M109.093880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li J, O’Connor KL, Hellmich MR, Greeley GH, Jr, Townsend CM, Jr, Evers BM. The role of protein kinase D in neurotensin secretion mediated by protein kinase C-{alpha}/-{delta} and Rho/Rho kinase. J Biol Chem. 2004;279:28466–28474. doi: 10.1074/jbc.M314307200. [DOI] [PubMed] [Google Scholar]
- 84.Iglesias T, Cabrera-Poch N, Mitchell MP, Naven TJ, Rozengurt E, Schiavo G. Identification and cloning of Kidins220, a novel neuronal substrate of protein kinase D. J Biol Chem. 2000;275:40048–40056. doi: 10.1074/jbc.M005261200. [DOI] [PubMed] [Google Scholar]
- 85.Cabrera-Poch N, Sanchez-Ruiloba L, Rodriguez-Martinez M, Iglesias T. Lipid raft disruption triggers protein kinase C and Src-dependent protein kinase D activation and Kidins220 phosphorylation in neuronal cells. J Biol Chem. 2004;279:28592–28602. doi: 10.1074/jbc.M312242200. [DOI] [PubMed] [Google Scholar]
- 86.Li J, Chen LA, Townsend CM, Jr, Evers BM. PKD1, PKD2, and their substrate Kidins220 regulate neurotensin secretion in the BON human endocrine cell line. J Biol Chem. 2008;283:2614–2621. doi: 10.1074/jbc.M707513200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.von Wichert G, Edenfeld T, von Blume J, Krisp H, Krndija D, Schmid H, et al. Protein kinase D2 regulates chromogranin A secretion in human BON neuroendocrine tumour cells. Cell Signal. 2008;20:925–934. doi: 10.1016/j.cellsig.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 88.Romero DG, Welsh BL, Gomez-Sanchez EP, Yanes LL, Rilli S, Gomez-Sanchez CE. Angiotensin II-mediated protein kinase D activation stimulates aldosterone and cortisol secretion in H295R human adrenocortical cells. Endocrinology. 2006;147(12):6046–6055. doi: 10.1210/en.2006-0794. [DOI] [PubMed] [Google Scholar]
- 89.Chang H-W, Chu T-S, Huang H-Y, Chueh S-C, Wu V-C, Chen Y-M, et al. Down-regulation of D2 dopamine receptor and increased protein kinase C{micro} phosphorylation in aldosterone-producing adenoma play roles in aldosterone overproduction. J Clin Endocrinol Metab. 2007;92(5):1863–1870. doi: 10.1210/jc.2006-2338. [DOI] [PubMed] [Google Scholar]
- 90.Sumara G, Formentini I, Collins S, Sumara I, Windak R, Bodenmiller B, et al. Regulation of PKD by the MAPK p38delta in insulin secretion and glucose homeostasis. Cell. 2009;136:235–248. doi: 10.1016/j.cell.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rozengurt E, Sinnett-Smith J, Kisfalvi K. Crosstalk between insulin/insulin-like growth factor-1 receptors and G protein-coupled receptor signaling systems: a novel target for the antidiabetic drug metformin in pancreatic cancer. Clin Cancer Res. 2010;16:2505–2511. doi: 10.1158/1078-0432.CCR-09-2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bisbal M, Conde C, Donoso M, Bollati F, Sesma J, Quiroga S, et al. Protein kinase d regulates trafficking of dendritic membrane proteins in developing neurons. J Neurosci. 2008;28:9297–9308. doi: 10.1523/JNEUROSCI.1879-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yin DM, Huang YH, Zhu YB, Wang Y. Both the establishment and maintenance of neuronal polarity require the activity of protein kinase D in the Golgi apparatus. J Neurosci. 2008;28:8832–8843. doi: 10.1523/JNEUROSCI.1291-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Suzuki A, Ohno S. The PAR-aPKC system: lessons in polarity. J Cell Sci. 2006;19:979–987. doi: 10.1242/jcs.02898. [DOI] [PubMed] [Google Scholar]
- 95.Watkins JL, Lewandowski KT, Meek SE, Storz P, Toker A, Piwnica-Worms H. Phosphorylation of the Par-1 polarity kinase by protein kinase D regulates 14-3-3 binding and membrane association. Proc Natl Acad Sci USA. 2008;105:18378–18383. doi: 10.1073/pnas.0809661105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yuan J, Rozengurt E. PKD, PKD2, and p38 MAPK mediate Hsp27 serine-82 phosphorylation induced by neurotensin in pancreatic cancer PANC-1 cells. J Cell Biochem. 2008;103:648–662. doi: 10.1002/jcb.21439. [DOI] [PubMed] [Google Scholar]
- 97.Widmann C, Gibson S, Jarpe MB, Johnson GL. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol Rev. 1999;79:143–180. doi: 10.1152/physrev.1999.79.1.143. [DOI] [PubMed] [Google Scholar]
- 98.Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- 99.Maizels ET, Peters CA, Kline M, Cutler RE, Jr, Shanmugam M, Hunzicker-Dunn M. Heat-shock protein-25/27 phosphorylation by the delta isoform of protein kinase C. Biochem J. 1998;332(Pt 3):703–712. doi: 10.1042/bj3320703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Doppler H, Storz P, Li J, Comb MJ, Toker A. A phosphorylation state-specific antibody recognizes Hsp27, a novel substrate of protein kinase D. J Biol Chem. 2005;280:15013–15019. doi: 10.1074/jbc.C400575200. [DOI] [PubMed] [Google Scholar]
- 101.Waldron RT, Rozengurt E. Oxidative stress induces protein kinase D activation in intact cells–involvement of Src and dependence on protein kinase C. J Biol Chem. 2000;275:17114–17121. doi: 10.1074/jbc.M908959199. [DOI] [PubMed] [Google Scholar]
- 102.Waldron RT, Rey O, Zhukova E, Rozengurt E. Oxidative stress induces protein kinase C mediated activation loop phosphorylation and nuclear redistribution of protein kinase D. J Biol Chem. 2004;279:27482–27493. doi: 10.1074/jbc.M402875200. [DOI] [PubMed] [Google Scholar]
- 103.Liu P, Scharenberg AM, Cantrell DA, Matthews SA. Protein kinase D enzymes are dispensable for proliferation, survival and antigen receptor-regulated NFkappaB activity in vertebrate B-cells. FEBS Lett. 2007;581:1377–1382. doi: 10.1016/j.febslet.2007.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hassan S, Biswas MH, Zhang C, Du C, Balaji KC. Heat shock protein 27 mediates repression of androgen receptor function by protein kinase D1 in prostate cancer cells. Oncogene. 2009;28:4386–4396. doi: 10.1038/onc.2009.291. [DOI] [PubMed] [Google Scholar]
- 105.Wong C, Jin ZG. Protein kinase C-dependent protein kinase D activation modulates ERK signal pathway and endothelial cell proliferation by vascular endothelial growth factor. J Biol Chem. 2005;280:33262–33269. doi: 10.1074/jbc.M503198200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Qin L, Zeng H, Zhao D. Requirement of protein kinase D tyrosine phosphorylation for VEGF-A165-induced angiogenesis through its interaction and regulation of phospholipase C{gamma} phosphorylation. J Biol Chem. 2006;281:32550–32558. doi: 10.1074/jbc.M604853200. [DOI] [PubMed] [Google Scholar]
- 107.Mottet D, Bellahcene A, Pirotte S, Waltregny D, Deroanne C, Lamour V, et al. Histone deacetylase 7 silencing alters endothelial cell migration, a key step in angiogenesis. Circ Res. 2007;101:1237–1246. doi: 10.1161/CIRCRESAHA.107.149377. [DOI] [PubMed] [Google Scholar]
- 108.Ha CH, Jhun BS, Kao HY, Jin ZG. VEGF Stimulates HDAC7 phosphorylation and cytoplasmic accumulation modulating matrix metalloproteinase expression and angiogenesis. Arterioscler Thromb Vasc Biol. 2008;28:1782–1788. doi: 10.1161/ATVBAHA.108.172528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ha CH, Wang W, Jhun BS, Wong C, Hausser A, Pfizenmaier K, et al. Protein kinase ddependent phosphorylation and nuclear export of histone deacetylase 5 mediates vascular endothelial growth factor-induced gene expression and angiogenesis. J Biol Chem. 2008;283:14590–14599. doi: 10.1074/jbc.M800264200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Altschmied J, Haendeler J. A new kid on the block: PKD1: a promising target for antiangiogenic therapy? Arterioscler Thromb Vasc Biol. 2008;28:1689–1690. doi: 10.1161/ATVBAHA.108.174250. [DOI] [PubMed] [Google Scholar]
- 111.Storz P, Toker A. Protein kinase D mediates a stress-induced NF-kappaB activation and survival pathway. Embo J. 2003;22:109–120. doi: 10.1093/emboj/cdg009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mihailovic T, Marx M, Auer A, Van Lint J, Schmid M, Weber C, et al. Protein kinase D2 mediates activation of nuclear factor {kappa}B by Bcr-Abl in Bcr-Abl+ human myeloid leukemia cells. Cancer Res. 2004;64:8939–8944. doi: 10.1158/0008-5472.CAN-04-0981. [DOI] [PubMed] [Google Scholar]
- 113.Storz P, Doppler H, Toker A. Activation loop phosphorylation controls protein kinase D dependent activation of nuclear factor kappaB. Mol Pharmacol. 2004;66:870–879. doi: 10.1124/mol.104.000687. [DOI] [PubMed] [Google Scholar]
- 114.Chiu TT, Leung WY, Moyer MP, Strieter RM, Rozengurt E. Protein kinase D2 mediates lysophosphatidic acid-induced interleukin 8 production in non-transformed human colonic epithelial cells through NF-{kappa}B. Am J Physiol Cell Physiol. 2007;292:C767–C777. doi: 10.1152/ajpcell.00308.2006. [DOI] [PubMed] [Google Scholar]
- 115.Song J, Li J, Qiao J, Jain S, Mark Evers B, Chung DH. PKD prevents H2O2-induced apoptosis via NF-[kappa]B and p38 MAPK in RIE-1 cells. Biochem Biophys Res Commun. 2009;378:610–614. doi: 10.1016/j.bbrc.2008.11.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yuan J, Lugea A, Zheng L, Gukovsky I, Edderkaoui M, Rozengurt E, et al. Protein kinase D1 mediates NF-kappaB activation induced by cholecystokinin and cholinergic signaling in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol. 2008;295:G1190–G1201. doi: 10.1152/ajpgi.90452.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Storz P, Doppler H, Toker A. Protein kinase D mediates mitochondrion-to-nucleus signaling and detoxification from mitochondrial reactive oxygen species. Mol Cell Biol. 2005;25:8520–8530. doi: 10.1128/MCB.25.19.8520-8530.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Doppler H, Storz P. A novel tyrosine phosphorylation site in protein kinase D contributes to oxidative stress-mediated activation. J Biol Chem. 2007;282:31873–31881. doi: 10.1074/jbc.M703584200. [DOI] [PubMed] [Google Scholar]
- 119.Singh R, Czaja MJ. Regulation of hepatocyte apoptosis by oxidative stress. J Gastroenterol Hepatol. 2007;22 Suppl 1:S45–S48. doi: 10.1111/j.1440-1746.2006.04646.x. [DOI] [PubMed] [Google Scholar]
- 120.Storz P. Mitochondrial ROS - radical detoxification, mediated by protein kinase D. Trends Cell Biol. 2007;17:13–18. doi: 10.1016/j.tcb.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 121.Song J, Li J, Lulla A, Evers BM, Chung DH. Protein kinase D protects against oxidative stress-induced intestinal epithelial cell injury via Rho/ROK/PKC-{delta} pathway activation. Am J Physiol Cell Physiol. 2006;290:C1469–C1476. doi: 10.1152/ajpcell.00486.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lee EG, Boone DL, Chai S, Libby SL, Chien M, Lodolce JP, et al. Failure to Regulate TNF-induced NF-kappa B and cell death responses in A20-deficient mice. Science. 2000;289:2350–2354. doi: 10.1126/science.289.5488.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Coornaert B, Carpentier I, Beyaert R. A20: central gatekeeper in inflammation and immunity. J Biol Chem. 2009;284:8217–8221. doi: 10.1074/jbc.R800032200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liuwantara D, Elliot M, Smith MW, Yam AO, Walters SN, Marino E, et al. Nuclear factor-{kappa}B regulates {beta}-cell death: a critical role for A20 in {beta}-cell protection. Diabetes. 2006;55:2491–2501. doi: 10.2337/db06-0142. [DOI] [PubMed] [Google Scholar]
- 125.Trauzold A, Schmiedel S, Sipos B, Wermann H, Westphal S, Roder C, et al. PKCmu prevents CD95-mediated apoptosis and enhances proliferation in pancreatic tumour cells. Oncogene. 2003;22:8939–8947. doi: 10.1038/sj.onc.1207001. [DOI] [PubMed] [Google Scholar]
- 126.Harikumar KB, Kunnumakkara AB, Ochi N, Tong Z, Deorukhkar A, Sung B, et al. A novel small-molecule inhibitor of protein kinase D blocks pancreatic cancer growth in vitro and in vivo. Mol Cancer Ther. 2010;9:1136–1146. doi: 10.1158/1535-7163.MCT-09-1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ehlers RA, Zhang Y, Hellmich MR, Evers BM. Neurotensin-mediated activation of MAPK pathways and AP-1 binding in the human pancreatic cancer cell line, MIA PaCa-2. Biochem Biophys Res Commun. 2000;269:704–708. doi: 10.1006/bbrc.2000.2335. [DOI] [PubMed] [Google Scholar]
- 128.Mori-Iwamoto S, Kuramitsu Y, Ryozawa S, Mikuria K, Fujimoto M, Maehara S, et al. Proteomics finding heat shock protein 27 as a biomarker for resistance of pancreatic cancer cells to gemcitabine. Int J Oncol. 2007;31:1345–1350. [PubMed] [Google Scholar]
- 129.Martiny-Baron G, Kazanietz MG, Mischak H, Blumberg PM, Kochs G, Hug H, et al. Selective inhibition of protein kinase C isozymes by the indolocarbazole Go 6976. J Biol Chem. 1993;68:9194–9197. [PubMed] [Google Scholar]
- 130.Evans IM, Bagherzadeh A, Charles M, Raynham T, Ireson C, Boakes A, et al. Characterisation of biological effects of a novel protein kinase D inhibitor in endothelial cells. Biochem J. 2010;429:565–572. doi: 10.1042/BJ20100578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sharlow ER, Giridhar KV, LaValle CR, Chen J, Leimgruber S, Barrett R, et al. Potent and selective disruption of protein kinase D functionality by a benzoxoloazepinolone. J Biol Chem. 2008;283:33516-2. doi: 10.1074/jbc.M805358200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Torres-Marquez E, Sinnett-Smith J, Guha S, Kui R, Waldron RT, Rey O, et al. CID755673 enhances mitogenic signaling by phorbol esters, bombesin and EGF through a protein kinase D-independent pathway. Biochem Biophys Res Commun. 2010;391:63–68. doi: 10.1016/j.bbrc.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.LaValle C, Bravo-Altamirano K, Giridhar K, Chen J, Sharlow E, Lazo J, et al. Novel protein kinase D inhibitors cause potent arrest in prostate cancer cell growth and motility. BMC Chem Biol. 2010;10(1):5. doi: 10.1186/1472-6769-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Monovich L, Vega RB, Meredith E, Miranda K, Rao C, Capparelli M, et al. A novel kinase inhibitor establishes a predominant role for protein kinase D as a cardiac class IIa histone deacetylase kinase. FEBS Lett. 2010;584:631–637. doi: 10.1016/j.febslet.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 135.Jacamo R, Sinnett-Smith J, Rey O, Waldron RT, Rozengurt E. Sequential protein kinase C [PKC]-dependent and PKC-independent protein kinase D catalytic activation via Gq-coupled receptors: differential regulation of activation loop Ser[744] and Ser[748] phosphorylation. J Biol Chem. 2008;283:12877–12887. doi: 10.1074/jbc.M800442200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Brandlin I, Hubner S, Eiseler T, Martinez-Moya M, Horschinek A, Hausser A, et al. Protein kinase C (PKC)eta-mediated PKC mu activation modulates ERK and JNK signal pathways. J Biol Chem. 2002;277:6490–6496. doi: 10.1074/jbc.M106083200. [DOI] [PubMed] [Google Scholar]
- 137.Matthews SA, Liu P, Spitaler M, Olson EN, McKinsey TA, Cantrell DA, et al. Essential role for protein kinase D family kinases in the regulation of class II histone deacetylases in B lymphocytes. Mol Cell Biol. 2006;26:1569–1577. doi: 10.1128/MCB.26.4.1569-1577.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yoo J, Chung C, Slice L, Sinnett-Smith J, Rozengurt E. Protein kinase D mediates synergistic expression of COX-2 induced by TNF-{alpha} and bradykinin in human colonic myofibroblasts. Am J Physiol Cell Physiol. 2009;297:C1576–C1587. doi: 10.1152/ajpcell.00184.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Fielitz J, Kim MS, Shelton JM, Qi X, Hill JA, Richardson JA, et al. Requirement of protein kinase D1 for pathological cardiac remodeling. Proc Natl Acad Sci USA. 2008;105:3059–3063. doi: 10.1073/pnas.0712265105. [DOI] [PMC free article] [PubMed] [Google Scholar]