Abstract
Extensive research within the last two decades has revealed that most chronic illnesses, including cancer, diabetes, and cardiovascular and pulmonary diseases, are mediated through chronic inflammation. Thus, suppressing chronic inflammation has the potential to delay, prevent, and even treat various chronic diseases, including cancer. Various nutraceuticals from fruits, vegetables, vitamins, spices, legumes, and traditional Chinese and Ayurvedic medicine have been shown to safely suppress proinflammatory pathways; however, their low bioavailability in vivo limits their use in preventing and treating cancer. We describe here the potential of nanotechnology to fill this gap. Several nutraceuticals, including curcumin, green tea polyphenols, coenzyme Q, quercetin, thymoquinone and others, have been packaged as nanoparticles and proven to be useful in “nano-chemoprevention” and “nano-chemotherapy.”
Keywords: Inflammation, diabetes, cancer, NF-κB, curcumin, nutraceuticals, nanotechnology
1. Introduction
The natural products are valuable sources of bioactive compounds [1], and have been considered the single most successful discovery of modern medicine [2]. In recent years, natural dietary agents have drawn a great deal of attention both from researchers and the general public because of their potential ability to suppress cancers as well as reduce the risk of cancer development [3]. However, a large number of natural products have never been replicated by synthetic medicinal chemistry, which illustrates the importance of drug discovery to identify active compounds and define novel pharmacophores [4]. Also because of their low solubility, many phytochemicals are poorly absorbed by human body, thus one of the most important and interesting applications for encapsulation of phytochemicals is to enhance the bioavailability of phytochemicals by changing the pharmacokinetics and biodistribution [5].
In the past decade, tremendous advancement has been made toward making nanoparticle-based therapeutic products and formulations commercially available. A 2006 European Technological Observatory survey showed that more than 150 pharmaceutical companies were developing nanoscale therapeutics [6] The idea of controlled drug delivery has been shown to improve the therapeutic index of drugs by increasing their localization to specific tissues, organs, or cells [7, 8]. This approach tends to decrease potential side effects by leaving the normal sensitive cells unharmed. Contemporary systemically administered chemotherapeutic agents are extremely toxic to cancer cells, but can also harm normal cells leading to serious side effects [9]. Controlled drug delivery systems administer the required amount of the drug to the target site and prevent it from circulating until its half-life finishes. The career system associated with site-specific drug delivery can be modulated for better pharmacokinetics and drug bioavailability [5]. More than half of the chemotherapeutic agents currently being administered either are plant derived or semi-synthetic in nature. The inevitable share of botanicals, therefore, in the development of modern chemotherapeutics is beyond doubt. Several different classes of active natural products have been documented.
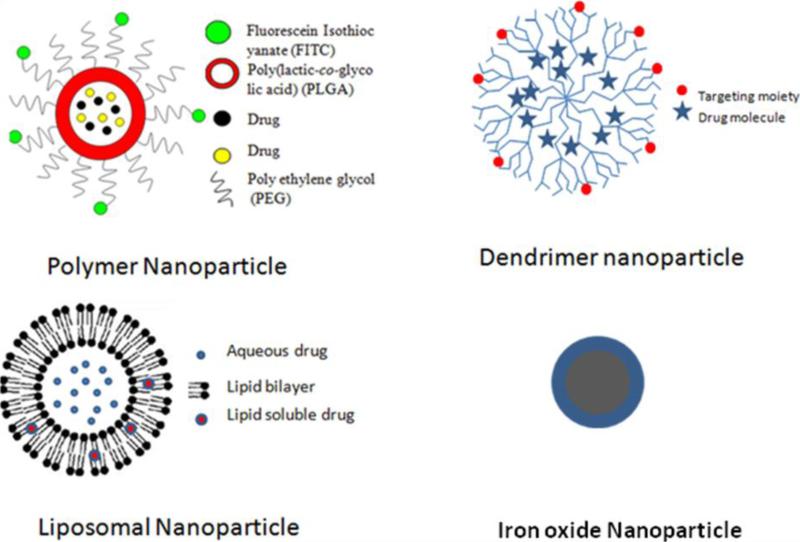
According to the National Nanotechnology Initiative (NNI; http://www.nano.gov) nanotechnologic structures should be only 1-100 nm in at least one dimension. This size requirement can be achieved through various rational designs, including top-down and bottom-up approaches. Nanocarriers have the potential to modify modern drugs by increasing their efficacy, stability, and solubility; decreasing their toxicity; and sustaining their release [10]. Nanoparticulate drug delivery systems using liposomes and biodegradable polymers have attracted increasing attention in recent years. In addition to liposomes and biodegradable polymers, the most common materials for nanocarriers include dendrimers, virus nanoparticles, and magnetic nanoparticles[11] (Fig. 1). Some of the commonly used methods to characterize the nanoparticles are depicted in Fig. 2. The most noticeable nanotechnologic applications in medicine have been related to oncology [12, 13]. In this review, we discuss the recent advances made in approaches for targeting anticancer bioactive natural products in both basic research and clinical trials.
Fig.1. Different types of nanoparticles.
Diagrammatic representation of some commonly nanocarriers used for the delivery of neutraceuticals. These include dendrimers, virus nanoparticles, and magnetic nanoparticles.
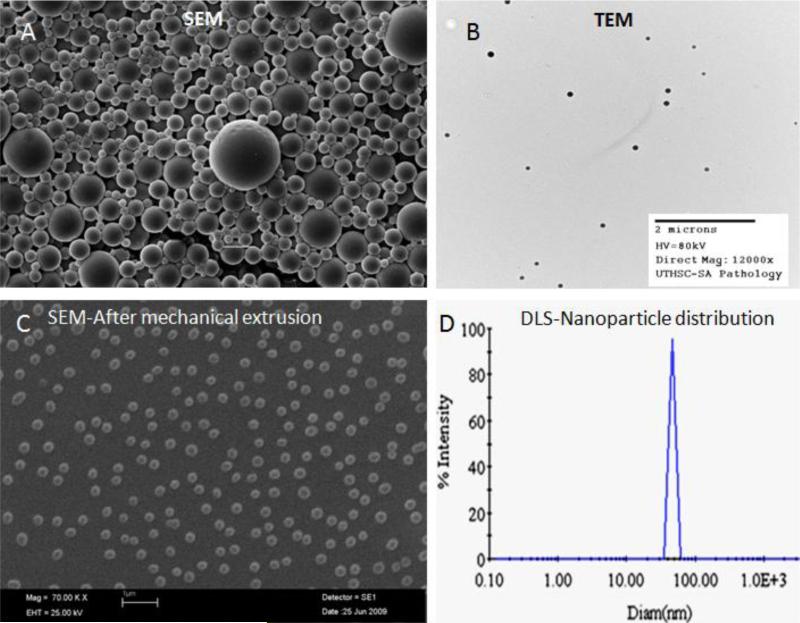
Figure 2. Physical characterization of the nanoparticles.
Some of the commonly used methods to characterize the nanoparticles are depicted in the figure. A- Scanning electron microscopy (SEM) image of nanoparticles; B- Transmission electron microscopy (TEM) image; C- SEM image of nanoparticles after mechanical extrusion; D – Determination of size distribution of nanoparticles by use of dynamic light scattering (DLS).
2. Inflammation, chronic diseases and cancer
A growing body of evidence suggests that many neoplasms are initiated by infections [14]. Some recent reviews have discussed intimate connection between inflammation and cancer [15-17]. Inflammation is known to contribute to physiological and pathological processes such as wound healing and infection by the activation and directed migration of leukocytes from the venous system to sites of damage[14]. Inflammation functions at all three stages of tumor development: initiation, progression, and metastasis. Tumor cells produce various cytokines and chemokines that attract leukocytes. The inflammatory component of a developing neoplasm may include a diverse leukocyte population that includes neutrophils, dendritic cells, macrophages, eosinophils, and mast cells [18]. It is believed that tumor-associated macrophages are a significant component of inflammatory infiltrates in neoplastic tissues and are derived from monocytes that are recruited largely by monocyte chemotactic protein (MCP) chemokines. The proinflammatory cytokines, including tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6), induce direct effects on stromal and neoplastic cells in addition to their roles in regulating leukocyte recruitment. The direct evidence for the association of chronic inflammation with malignant diseases is in colon carcinogenesis in individuals with inflammatory bowel diseases [19, 20]. The emerging concept in clinical trials reveals that inflammation is a potential therapeutic target in cancer treatment and nonsteroidal antiinflammatory drugs are thereby potentially useful as adjuvant therapy [21].
Inflammation also plays a key role in the physiology of arthritis, diabetes, heart disease, irritable bowel syndrome, Alzheimer's disease, Parkinson's disease, and many other illnesses. In Type I diabetes, the immune system attacks the cells that make insulin. Children who have allergies are less likely to develop Type I diabetes. Several spices, vegetables, and fruits have potent antiinflammatory properties. For example, curcumin (diferuloylmethane) is a major constituent of the yellow spice turmeric, derived from the rhizomes of Curcuma longa. It is safe and nontoxic and has demonstrable antitumor, antiinflammatory, apoptotic, and antioxidant properties. We have shown previously that curcumin inhibits tumor metastasis, invasion, and angiogenesis [22]. Beside curcumin, the potential of red chili (capsaicin), cloves (eugenol), ginger (zerumbone), fennel (anethole), kokum (gambogic acid), fenugreek (diosgenin), and black cumin (thymoquinone) in cancer prevention has been established [23].
3. Antiinflammatory nutraceuticals
A wide variety of nutraceuticals, the most common of which are shown in Table 1, are known to possess antiinflammatory properties. Extensive research within the last two decades has revealed that curcumin exhibits antioxidative, antiinflammatory, antiapoptotic, antiproliferative, antiinvasive, and antiangiogenic activity [24]. Animal studies have revealed that curcumin can prevent carcinogen-induced tumorigenesis and inhibit the growth of implanted human tumors [25]. Such studies have led to clinical trials of curcumin in patients with colon cancer [25-27], familial adenomatous polyposis [28], pancreatic cancer[29] , and multiple myeloma [30]. Unfortunately, these studies revealed that one of the major limitations of curcumin is its low oral bioavailability in vivo. Traditionally, turmeric is delivered orally as an emulsion in oil or milk, perhaps because of the hydrophobic nature of its bioactive constituents, such as curcumin and turmeric oil. Curcumin has indeed been shown to interact with phospholipids [31-34], surfactants[35], proteins [36], and cyclodextrin [37]. Various methods have been tried to enhance curcumin delivery, including its incorporation into liposomes [38, 39] and into phospholipid vesicles [40]. The latter was used to deliver curcumin intravenously to bone morrow and splenic macrophages. Another way to solve the problem of lack of water solubility and poor oral bioavailability is the use of polymer-based nanoparticles [41]. Studies from our laboratory showed that serum levels were almost twice as high and half-life was substantially longer in curcumin-nanoparticle than in free curcumin [42].
Table 1.
Common nutraceuticals that show anti-inflammatory properties
| Nutraceutical | Major Activities |
|---|---|
| Apigenin | Antioxidative, vasoprotective, antiinflammatory, hypocholesterolemic |
| Naringin | Vasoprotective, antiinflammatory, hypocholesterolemic |
| Ursolic acid | Antioxidative, antimicrobial, antiinflammatory |
| Hesperidin | Vasoprotective, antiinflammatory |
| Piperine | Antiinflammatory, respiratory diseases, digestive, absorptive |
| Eucalyptol | Gastroprotective, antiinflammatory, antioxidative, hepatoprotective |
| Curcumin | Antioxidative, antiinflammatory, anticarcinogenic |
| Eugenol | Antioxidative, antiinflammatory |
| Diosgenin | Antioxidative, antiinflammatory, anticarcinogenic |
| Diallyl sulfide | Antioxidative, antiinflammatory, anticarcinogenic |
| Gingerol | Antioxidative, antiinflammatory, cardioprotective, antimicrobial |
| Thymoquinone | Antiinflammatory, anticancer |
| Garcinol | Antioxidative, antiinflammatory, anticarcinogenic |
| Capsaicin | Antioxidative, antiinflammatory |
| Rosmarinic acid | Antiinflammatory, respiratory diseases |
| Gossypin | Antioxidant, antinociceptive, antiinflammatory, anticarcinogenic |
Many other spice-derived nutraceuticals have been found to play a role in reducing inflammation. These include ajoene, allicin, allyl isothiocyanate, anethole, apigenin, capsaicin, carnosol, caryophyllene, cinnamaldehyde, diallyl sulfide, eugenol, [6]-gingerol, humulene, kaempferol, limonene, myrcene, [6]-paradol, perillyl alcohol, phytic acid, piperine, quercetin, sulforaphane, ursolic acid, and zinger-one. Many of these nutraceuticals target the transcription factor NF-κB, leading to its down-regulation [43]. Other flavonoids, such as resveratrol, butein, cardamomin, chalcone, silibinin, xanthohumol, fisetin, epigallocatechin gallate (EGCG), etc. derived from fruits, vegetables, legumes, spices, and nuts also suppress the proinflammatory cell signaling pathways and thus can prevent and even treat the cancer [44].
4. Nanotechnology
Therapeutic uses of nanotechnology typically involve the delivery of small-molecule drugs, peptides, proteins, and nucleic acids. Nanoparticles have advanced pharmacological effects compared with the therapeutic entities they contain. Active intracellular delivery and improved pharmacokinetics and pharmacodynamics of drug nanoparticles depend on various factors, including their size and surface properties. Nanoparticle therapeutics is an emerging treatment modality in cancer and other inflammatory disorders. The National Cancer Institute has recognized nanotechnology as an emerging field with the potential to revolutionize modern medicine for detection, treatment, and prevention of cancer [45]. More selective therapies, such as angiogenesis inhibitors, vascular disrupting agents, and estrogen- and HER-2-targeted therapies have been developed to treat cancer. These approaches have increased patient survival because of treatment efficacy [25]. Most solid tumors possess unique features, including enhanced angiogenesis, defective vasculature and lymphatics, and increased vascular permeability, which stimulate their growth. Rationally designed nanoparticles can take advantage of these tumor features to deliver chemotherapeutics selectively and specifically. Some commonly used materials for preparation of nanopartcles and their advantages are shown in Table 2. The key properties of nanoparticles that can be used for delivering anticancer drugs are discussed in the following sections:
Table 2.
Commonly used biosynthetic polymers for drug delivery and their properties
| Carrier | Properties |
|---|---|
| Polyethylene Glycol (PEG) | Increases bioavailability, biocompatibility, and circulation time |
| poly(lactic-co-glycolic acid (PLGA) | Increases biocompatibility, decreases cytotoxicity |
| Ployvinyl alocohol (PVA) | Reduces aggregation, increases hydrophilicity and strength |
| Dextran | Decreases agglomeration, reduces 'stealth'nessness (increases opsonization) |
| Polypeptide | Increases targeting |
| Polyacrylate | Increases biostability, , biocompatibility |
| Chitosan | Increases biocompatibility, hydrophilicity, and opsonization |
| Hyaluronic acid | Enhances bioadhesion and targeting |
| Galactose | Increases liver targeting |
| Polysaccharide modifications | Improve cancer targeting |
| Sialic acid | Decreases macrophage uptake |
| Poly-N-isopropylacrylamide (PNIPAm) | For temperature-sensitive drug delivery |
| Poly(N-vinyl pyrrolidone) | Increases opsonization and decreases bioavailability |
| Polyglycolides (PGA) | Increase biocompatibility and thermostability |
| Polyanhydrides | Increase biostability and biocompatibility |
| Polyorthoesters | Increase biocompatibility |
Nanoparticle size
: The National Cancer Institute currently recommends that the size of the nanoparticles be 10-100 nm. Even though the upper limit of nanoparticle size is not strictly defined, the lower limit (10 nm) is fixed based on the threshold for first-pass elimination by the kidneys [46]. Size is a key factor in the biodistribution of long-circulating nanoparticles on the basis of physiological parameters such as hepatic filtration, tissue extravasation, tissue diffusion, and kidney excretion [47]. Tumor-targeting nanoparticles must resist hydrostatic and biophysical/biochemical barriers, overcome cellular resistance to treatment, resist biotransformation, and resist sudden degradation, immediate clearance, and enhanced distribution even in poorly perused areas [48].
Surface characteristics
The surface properties of nanoparticles determine their interaction with the local environment. Sterically stabilized nanoparticles exhibit minimal self and non-self interactions [13]. These particles keep slightly high or low negative or positive charges on their surfaces, which leads to an increased reticuloendothelial clearance; therefore, minimizing nonspecific interactions and controlling surface charge by steric stabilization helps to prevent nanoparticle loss in undesired locations to a certain extent. Nanoparticles have high surface-to-volume ratios, which can be manipulated by rational design. The surface properties of the nanoparticles will determine their solubility, stability, and clearance. It has been shown that polymer drug or antibody conjugates have superior half-lives, which can improve the pharmacokinetics of the drug [49]. Increased opsonization associated with nanoparticles’ surface during circulation can trigger substantial hepatic agglomeration. Peggylation has been shown to reduce protein absorption. In general, peggylated nanoparticles have longer circulation times and higher levels of tumor accumulation than non-PEGylated nanoparticles [50]
Targeting
Any ideal therapy to treat a disease should destroy specifically diseased or affected cells in an organ while conserving normal cells. Ultimately, targeting will enable the cancer cells to receive the pharmacologically required concentration of the drug molecules. Various targeting approaches, including passive and active targeting of drug molecules, are well characterized and studied. A large body of evidence suggests that these targeting strategies could overcome drug resistance and side effects to the vital organs and could minimize systemic drug administration.
Passive targeting
Passive targeting can be achieved by changing the physiochemical characteristics, pH, or hydrophobicity of nanoparticles. Passive targeting of nanoparticles utilizes the EPR of tumor blood vessels. Nanoparticles that escape the reticuloendothelial system (RES) can circulate for longer times in the bloodstream and have a greater chance of reaching the targeted tumor tissues. To supply oxygen and nutrients to the tumor, rapidly-growing cancer cells have enhanced neovascularization [51]. This imbalance of angiogenic regulators such as growth factors and matrix metalloproteinases makes tumor vessels highly disorganized and inflated with enlarged gap junctions between endothelial cells and improper lymphatic drainage [52]. Table 3 lists several of the passively targeted drug candidates currently approved by the Food and Drug Administration (FDA) or being considered by pharmaceutical companies.
Table 3.
List of passively targeted drug candidates currently approved by the Food and Drug Administration
| Formulation | Manufacturer | Disease indication |
|---|---|---|
| Liposomal cytarabine | Skyepharma | Malignant lymphomatous meningitis |
| Liposomal morphine | Skyepharma | Postsurgical anesthesia |
| Liposomal daunorubicin | Gilead Sciences | HIV-related Kaposi's sarcoma |
| Liposomal amphotericin B | Enzon | Various fungal infections |
| Liposomal-peggylated doxorubicin | Ortho Biotech/Schering-Plough | Metastatic breast and ovarian cancer |
| Liposomal estradiol | Novavax | Multiple indications symptoms associated with menopause |
| Methoxy PEG-PLA paclitaxel | Samyang | Metastatic breast cancer |
| PEG-GCSF | Amgen | Chemotherapy-associated neutropenia |
| PEG-L-asparaginase | Enzon | Acute lymphoblatic leukemia |
| Nanoctrystalline aprepitant | Elan, Merck | Antiemetic |
Superparamagnetic dextran-coated iron oxide nanoparticles (SDIONs) and superparamagnetic iron oxide nanoparticles (SPIONs) are widely used to achieve passive targeting. SDION conjugates could protect the DNA inside the body and prevent nanoparticle carrier/gene compounds from being dissociated and nonspecifically distributed. Moreover, compared with viral vectors and liposomes, tiny SDIONs could effectively evade phagocytosis by macrophages, thus prolonging the drug's circulation time in the body and increasing its transfection efficiency, and hence playing an important role in locating and targeting [53]. SPIONs are widely accepted magnetic resonance imaging (MRI) contrast agents for clinical use and have several important advantages over traditional gadolinium-based MRI contrast agents: lower toxicity, stronger enhancement of proton relaxation, and lower detection limits [54].
Active Targeting
Cancer biomarkers include a variety of molecules, such as mutant genes, RNAs, proteins, lipids, carbohydrates, and small metabolite molecules. The identification of such biomarkers is very important for individualized cancer treatment. Active targeting of cancer cells by multifunctionalization, in which drugs and contrast agents are attached, involves a corona of polymeric material that improves biokinetics and biodistribution and a ligand that adds specificity for cancer biomarker molecular recognition and attaches to cancer cells [8, 55]. One major advantage is the nanoparticles’ surface functionality, which allows for the selective coupling of imaging agents and targeting of ligands and/or other components to increase tumor specificity. Adding a targeting moiety onto the surface of nanoparticles can increase selective cellular binding and internalization through receptor-mediated endocytosis. The ideal active-targeting nanoparticles should meet the following criteria: specificity to exclusively target cancer cells, faster uptake through receptor-mediated endocytosis, faster delivery to tumors, stability of ligands until the drug reaches the target, stealth nature or neutral surface coating, and increased retention through compartmentalization. Therapeutic nanoparticles that are already in preclinical and clinical trials are shown in Table 4.
Table 4.
Active targeting of nanoparticle therapeutics in preclinical and clinical trials
| Formulation | Manufacturer | Disease indications |
|---|---|---|
| PEG-anti-TNFα antibody fragment | Nektar | Crohn's desease, Rheumatoid arthritis |
| Rhenium-labeled doxorubicin | Azaya Therapeutics | Metastatic breast cancer |
| Methotrexate-folic acid Ployamidomine (PAMAM) dendrimers | Michigan Nanotechnology Institute, University of Michigan | Epithelial cancers |
| PEGylated hyaluronidase | Halozyme Therapeutics | Solid tumors |
| Prostate specific membrane antigen (PSMA) dendrimers | BIND Biosciences | Prostate cancer |
| Transferrin-diphtheria toxin conjugate | Xenova (KS Biomedix) | High-grade glioma |
Structures such as antibodies, antibody fragments, proteins, small molecules, aptamers, and peptides have all demonstrated the ability to induce nanoparticle targeting of cancer cells. Ferrari [8] showed that silicon and silica are emerging as interesting candidates for injectable nanovectors. Porosified silicon is biodegradable with kinetics of degradation that are much more rapid (minutes to hours) than those of the other biodegradable polymers (weeks to months), and therefore release drugs with previously unattainable time profiles. Metal-based nanovectors include nanoshells [56], which comprise a gold layer over a silica core and are considered to be highly selective, externally activated therapeutic agents. The relative distribution of cancer signatures and markers associated with the tumor microenvironment have been detected by multifunctional nanoparticles such as cross-linked iron oxide nanoparticles which were conjugated to annexin-V, which recognizes the phosphatidylserine that is present on apoptotic cells and were used for MRI identification of camptothecin-induced apoptosis of Jurkat T cells in vitro [57]. Apart from HER2, transferring receptor, prostate specific antigen (PSA), and prostate specific membrane antigen (PSMA), another protein that has shown potential for targeted cancer therapy is the urokinase plasminogen activator,(uPA), a natural ligand for the urokinase plasminogen activator receptor for targeting the overexpressed receptors on colon and breast cancers [58]. In a recent study, hydrophobically modified carboxymethylated chitosan nanoparticles were used for the targeted delivery of paclitaxel. The surface of the nanoparticles was modified by the covalent attachment of folic acid by simple carbodiimide reaction to achieve tumor cell-targeting properties [59]. The hybrid systems consisting of anticancer drugs such as methotrexate or 5-fluorouracil and a two-dimensional inorganic delivery carrier like layered double hydroxide (LDH) were described by Choi et al. [60]. The advantage of the LDH nanoparticles, such as 5-fluorouracil-LDH, is that they rapidly excrete from the body and do not accumulate in the organs after administration. Therefore, the hybrid system is a promising anticancer chemotherapy agent for tumor targeting with biocompatibility.
As mentioned earlier in Section 3, even though natural products are potential candidates for treating many dreaded diseases, the amount of the drugs needed usually impedes the further development of those drugs as single agents and results in the search for semisynthetic or synthetic scaled-up strategies. Potential approaches to overcoming this scenario include targeted delivery of these drugs. In a recent study, genistein was covalently attached to Fe3O4 nanoparticles coated by cross-linked carboxymethylated chitosan (CMCH) to create a new, multifunctional, tumor-targeting drug delivery system [61]. The results from this study indicate that the Fe3O4-CMCH-genistein nanoconjugate significantly enhanced inhibition of SGC-7901 cancer cells compared to genistein alone. This drug delivery system may be promising for a future multifunctional chemotherapeutic application that combines drug release and magnetic hyperthermia [61].
5. Formulation technologies
Efficient delivery of bioactive agents and peptides and drugs to the systemic circulation and then to a target cell or organ has received considerable attention in medicine because of recent advances in biotechnology.
Nanoprecipitation
This method of formulating nanopharmaceuticals involves the nanoprecipitation of a preformed polymer from an organic solution in which it is held by the diffusion of the organic solvent in the aqueous medium in the presence of a surfactant. This method is basically applicable to lipophilic drugs because of the miscibility of the solvent with the aqueous phase [62]. Briefly, the poly(lactic-co-glycolic acid) (PLGA) and the drug are dissolved in acetone or other organic solvents. The organic phase is then poured into water containing PluronicF-68 as a surfactant. The organic solvent is immediately removed from the colloidal suspension by rotaevaporation under reduced pressure. The resulting particle suspension is filtered through a 1.0-μm cellulose nitrate membrane filter, adjusted in size by mechanical extrusion, and concentrated to a desired volume by the removal of water under the same conditions. The amount of drug present in the nanoparticles is determined as the difference between the total amount of the drug used to prepare the nanoparticles and the amount of the drug present in the aqueous medium. The drug present in the aqueous medium analyzed by directly injecting into a high-performance liquid chromatography system under standard conditions.
Thymoquinone (TQ), derived from the medicinal spice Nigella sativa, has been shown to exhibit antiinflammatory and anticancer activity. TQ nanoparticles prepared by nanoprecipitation have shown improved effectiveness and bioavailability [63]. Our previous study also showed that curcumin-loaded PLGA nanoparticles prepared by nanoprecipitation enhanced cellular uptake and increased bioactivity in vitro and superior bioavailability in vivo over free curcumin [42].
Nanoemulsion techniques
Another method for formulating nanoparticles is nanoemulsion, a heterogeneous mixture of oil mixtures of very-small-diameter oil droplets in water (20-500 nm). They are used in various chemical, pharmaceutical, and cosmetic applications and have been widely tested for transdermal applications. The advantages of nanoemulsions include an opportunity to solubilize hydrophobic compounds in the oil phase, modify the surface of the oil droplets with polymers to extend circulation times, and passively target tumors and/or actively target ligands [64]. The oil-containing nanoemulsions are prepared by coarse homogenization followed by high-energy ultrasonication as previously described [65, 66]. Briefly, the aqueous phase is prepared by adding egg lecithin to the deionized water, and stirred at high speed. The drug of interest is dissolved in a suitable organic solvent and dispersed in oil. The mixture is then heated to 70-75° C to evaporate the aqueous phase. This oil phase that contains entrapped drug, is then added gradually to the aqueous phase and homogenized to produce the coarse oil-in-water emulsion [64]. The coarse emulsion is then ultrasonicated to obtain the nanosized oil droplets.
The peculiarity of nanoemulsions, making them prime techniques for nanoparticle engineering, lies in the long-term kinetic stability of their droplet suspension Nanoemulsions last for months and withstand dilution and moderate temperature changes. Two widely accepted methods are used to prepare nanoemulsions: high-energy emulsification and low-energy emulsification. High-energy emulsification utilizes high (mechanical) energy; this common method is particularly exploited in nanoemulsion polymerization [64]. The mechanical processes that generate nanometric emulsions include initial drop creation, followed by the deformation and disruption of these macrometric initial droplets, and surfactant absorption at their interface to ensure steric stabilization [67]. On the other hand, low-energy emulsification uses the rapid diffusion of a water-soluble solvent, solubilized first in the organic phase, and again in the aqueous phase, when the two phases are mixed. Current literature on spontaneous emulsification emphasizes the solvent displacement method, also called the “Ouzo effect [64, 68, 69], which consists of nanoemulsion formulation due to the specific and very rapid diffusion of an organic solvent (e.g., acetone or ethanol) from the oil phase to the aqueous phase.
Nanoemulsion of caffeine (1,3,7-trimethylhanthine), found in tea leaves, coffee, cocoa, guarana, and kola nuts, has been widely studied and has tremendous potential, since caffeine could protect the skin from ultraviolet light-induced skin cancer [70].
Reverse-phase evaporation
The reverse-phase evaporation method is widely used to prepare liposomes for various drug delivery purposes [71-73]. Several phospholipids, either pure or mixed with other lipids such as cholesterol or long-chain alcohols, may be used. The lipid mixture is added to the organic solvent and then the solvent is removed under reduced pressure by rotaevaporation. The system is then purged with nitrogen and the lipids are re-dissolved in the organic phase, in which the reverse-phase vesicles are formed. When the lipids have low solubility in ether, chloroform or methanol can be added to increase their solubility. The system is kept continuously under nitrogen and the aqueous phase and the resulting two-phase system is sonicated briefly (2-5 min) until the mixture becomes either a clear one-phase dispersion or a homogeneous opalescent dispersion that does not separate for at least 30 min after sonication. The organic solvent is then removed under reduced pressure by rotaevaporation. As the majority of the solvent is removed, the material first forms a viscous gel, then subsequently (within 5-10 min) becomes an aqueous suspension. The preparation is then either dialyzed or centrifuged to remove nonencapsulated material and residual organic solvent [71].
6. Role of nanotechnology for nutraceuticals
Nano formulations of nutraceuticals essentialy follow the general principles of nanotechnology. Therefore, the nanotechnology platforms are widely being used create delivery systems for bioactive natural products and nutraceuticals with poor water solubility. The market projections for these technologies suggest a multifold increase in their commercial potential over the next 5 years. Table 5 summarizes a list of nutraceuticals, the materials used for preparing nanoparticles, the size of the nanoparticles, and their possible tissue/cancer targets. Some of the extensively studied nutraceutical nanoparticles are discussed below.
Table 5.
List of nutraceuticals that have been formulated as nanoparticles and their characteristics
| Phytochemicals | Materials | Size (nm; average) | Targets | References |
|---|---|---|---|---|
| EGCG | PLA-PEG | 260 | Pancreatic cancer | [74] |
| β-Lapachone | Gold | 47 | Lung, colon cancer cells | [75] |
| PLA-PEG | 29.6 | Lung, prostate, breast cancer cells | [76] | |
| Curcumin | PLGA | 80.9 | Leukemia, colon, breast, prostate, cancer cells | [42] |
| 77 | NPD in mice | [77] | ||
| 45 | Prostate cancer cells | [78] | ||
| Alginate-chitosan | 100 ± 20 | Cervical cancer cells | [79] | |
| Poly(butyl) cyanoacrylate | 164–281 | Neuroblastoma cells | [80] | |
| Silk | 100 | Breast cancer cells | [81] | |
| Casein | 10~20 | Cervical cancer cells | [82] | |
| NIPAAM/VP/PEG-A | 50 | Pancreatic cancer cells | [83] | |
| Daidzein | PEGylated phospholipid | 126 | Cardiovascular system | [84] |
| Dibenzoylmethane | Polylactic acid | 77-96 | Cervical cancer cells | [85] |
| Dihydroartemisinin | Polyvinyl pyrrolidone K30 | 30 | in vitro antimalarial | [86] |
| Ellagic acid | PLGA-polycaprolactone | 120 | Kidney | [87] |
| Epigallocatechin | Bovine serum albumin | 200 | Prostate cancer cells | [88] |
| Eugenol | Chitosan | 235 | Bacteria | [89] |
| Ferulic acid | BSA | 100-200 | Liver | [90] |
| Bovine serum albumin | 100-200 | Liver | [91] | |
| Gambogic acid | Fe3O4 | N.A. | Lymphoma cells | [92] |
| Genistein | Egg lecithin – MCT or ODD | 230-280 | Skin (topical target) | [93] |
| Honokiol | PEG-poly(epsilon-caprolactone)-PEG | 33 | Pleural tumors | [94] |
| Naringenin | Polyvinyl acetate | 66 | Liver | [95] |
| Nobliletin | N.A. | 15.5 ± 2.9 | Brain, kidney | [96] |
| Quercetin | Polylactide | 50 | Brain | [97] |
| PLGA | 270 | Brain, liver | [98] | |
| Glyceryl monoste | 20~500 | Stomach, intestine | [99] | |
| Resveratrol | Compritol® 888ATO-Phospholipon80H-Lutrol | 180 ± 8 | Keratinocyte | [100] |
| Poly-caprolactone-PEG | <100 | Neuronal cell line | [101] | |
| mPEG–PCL | 78.3 ± 7.9 | Glioma cells | [102] | |
| Simvastatin | Solutol® HS-15-Tween 20-oleic acid | 39.4,78.1 | Intestine | [103] |
| Compritol® 888 ATO- Lutrol® F68 | ~100 | Mammary cells | [104] | |
| Glyceryl monooleate/poloxamer 407 | 100~150 | Plasma | [105] | |
| Thymoquinone | PLGA | 150~200 | Leukemic cells | [63] |
| Triptolide | Poly(D, L-lactic acid) | 149.7 | Skin (paw edema) | [106] |
| Compritol ATO 888-tricaprylic glyceride | 116.1 | Kidney, liver | [107] | |
| Tristearin glyceride-lecithin- PEG400MS | 123 ± 0.9 | Skin | [108] | |
| Tristearin glyceride | 116.1 | Liver | [109] | |
| Toxifolin | Polyvinylpirrolidone | 150 | [110] | |
| Ursolic acid | Soybean phospholipid-poloxamer 188 | 273.23 ± 2.3 | liver | [111] |
Curcumin, for example, has minimal systemic bioavailability, but its biologic activity and bioavailability have been tremendously increased via various nanoparticle formulations. Although many published studies have proposed curcumin, with its antiinflammatory and anticancer properties, as safe for cancer therapy and chemoprevention, the compound has by no means been embraced by the cancer community. A phase I study of colorectal cancer patients found that the systemic bioavailability of postoperatively administered curcumin is low in humans [29]. In this study, patients with hepatic colorectal cancer metastases were administered 3,600 mg of oral curcumin daily, and levels of curcumin and its metabolites were measured by high-performance liquid chromatography in portal and peripheral blood. Curcumin was poorly available following oral administration, with low nanomolar levels of the parent compound and its glucuronide and sulphate conjugates found in the peripheral or portal circulation [112]. Bisht et al. have developed nanoparticulate curcumin using cross-linked polymeric nanoparticles comprised of N-isopropylacrylamide, N-vinyl-2-pyrrolidinone, and PEG acrylate, which has been tested in various pancreatic cancer cell lines. In this study, nanoparticulate curcumin showed superior cytotoxicity and downregulated multiple proinflammatory markers in a dose-dependent manner [83]. Another study revealed that nanoparticle encapsulation improves the oral bioavailability of curcumin by at least 9-fold when compared to curcumin administered with piperine as an absorption enhancer. In this study, curcumin-encapsulated nanoparticles were prepared by emulsion, and a particle size of 264 nm showed enhanced in vivo pharmacokinetics with a 9-fold increase in oral bioavailability compared to curcumin administered with piperine as an absorption enhancer [113]. In another study, piperine has been found to enhance the bioavailability of curcumin in both preclinical and clinical studies [114]. It has been suggested that piperine can inhibit curcumin-metabolizing enzymes and thereby circumvent first-pass metabolism[113]. Ursolic acid (UA), another poorly soluble natural product, is a triterpenoid with a wide variety of antitumor activities [115]. To achieve a high bioavailability, targeting effect, stability, and intravenous administration, UA phospholipid nanopowders have been prepared by solvent emulsification-evaporation and ultrasonic dispersion [111]. However, limited biological testing of these particles has been reported. Lee et al. [116] have tested the ability of nanoparticles to deliver bioactive agents by preparing amphiphilic self-assembled nanoparticles composed of chitosan and UA for protein delivery to the skin. Topical treatment of skin diseases has the advantage that high drug levels can be achieved at the site of disease and systemic side effects can be reduced compared to oral or parenteral drug administration. Topical drug administration is still a pharmaceutical challenge because of the difficulties of controlling and determining the exact amount of the drug that reaches the different skin layers [117]. Natural products like curcumin and UA are potential candidates for treating various skin conditions, namely atopic eczema, psoriasis, acne, skin mycosis, and inflammations. Nanoformulations will make these candidate drugs much more valuable in the pharmaceutical market in the coming decade. It has been shown that it is possible to enhance percutaneous absorption with lipid nanoparticles. These carriers may even allow drugs to target the skin or even its substructures. Thus, they might have the potential to improve the benefit-to-risk ratio of topical drug therapy [117].
Triptolide is a purified compound of a traditional Chinese medicine with antiinflamatory, immunosuppressive, antifertility, and antineoplastic activity [118]. Mei et al. showed that solid lipid nanoparticles prepared for transdermal delivery increased triptolide penetration into the skin and its antiinflammatory activity [119]. It is assumed that )his strategy improves the drug's bioavailability at the site of action, reduces the required dose, and reduces dose-dependent side effects like irritation and stinging. Epigallocatechin gallate (EGCG), an abundant catechin found most notably in tea, is among other plants, and is also a potent antioxidant that may have therapeutic properties for many disorders, including cancer [120]. A recent study showed that EGCG nanoparticles ameliorated cyclosporine-induced nephrotoxicity in rats at a dose three times lower than the dose at which an oral solution produced the same effect and enhanced oral bioavailability [121].
Polyphenols are another group of nutraceuticals that has proven anti-inflammatory properties and thus has high potentials for cancer therapy. The challenge of using polyphenols to treat cancer is their potentially low bioavailability and short half-life. One alternative to using free compounds is to use polyphenol-loaded nanoparticles [122]. However, additional challenges are faced while encapsulating the polyphenols due to their varying structures, moderate solubility, and fast oxidation under basic conditions [123, 124. ]. Encapsulated EGCG nanoparticles have been limited to a PLGA nanoparticulate formulation used for in vivo evaluation of the antioxidant efficacy of EGCG in a rat model [125] and chitosan-tripolyphosphate nanoparticles for the encapsulation of green tea catechin extracts. A very interesting study reported EGCG encapsulation using gelatin-based 200- to 300- nm nanoparticles consisting of a soft gel-like interior and a surrounding shell of polyelectrolytes (polystyrene sulfonate/polyallylamine hydrochloride [PSS/PAH]), polyglutamic acid/poly-l-lysine (PGA/PLL), dextran sulfate/protamine sulfate (DexS/ProtS), and carboxymethyl cellulose/gelatin, type A (CMC/GelA) assembled using the layer-by-layer technique [124]. In this study, two combinations of polyanion/polycation pairs were used to form a layer-by-layer coating around 300-nm gelatin nanoparticles, PSS/PAH (strong polyanion/weak polycation), and PGA/PLL (weak polyanion/strong polycation), as well as two combinations of polyanion/protein, DexS/ProtS (strong polyanion/strongly positively charged polypeptide) and CMC/GelA (weak polyanion/weakly positively charged protein). While these particles showed promising results in vitro, no in vivo data were reported. Another research group introduced the concept of nanochemoprevention aimed at using nanotechnology to enhance the outcome of chemoprevention [74]. In this study, PLA-PEG nanoparticles of EGCG (nano-EGCG) exhibited a >10-fold dose advantage over nonencapsulated EGCG [74].
Studies from our laboratory have shown that curcumin nanoparticles were more active than free curcumin for inhibiting TNFα-induced NF-κB activation and suppressing NF-κB-regulated proteins involved in cell proliferation (cyclin D1), invasion (matrix metalloproteinase-9), and angiogenesis (vascular endothelial growth factor (VEGF)). In mice, curcumin nanoparticles were more bioavailable and had a longer half-life than free curcumin [42, 126]. We used biodegradable nanoparticulate formulation based on PLGA and a stabilizer, PEG-5000, for these studies. In a similar, more recent study, we showed that TQ exhibits antiinflammatory and anticancer activity and encapsulation of TQ enhances its biological activity and bioavailability [63]. In that study, we observed that TQ nanoparticles were also more potent for suppressing proliferation of colon cancer, breast cancer, prostate cancer, and multiple myeloma cells. Esterase staining for plasma membrane integrity revealed that TQ nanoparticles were more potent than free TQ for sensitizing leukemic cells to TNFα- and paclitaxel-induced apoptosis [63].
Resveratrol (trans-3,4,5-trihydroxystilbene) is a natural polyphenolic compound abundant in grapes, peanuts, red wine, and a variety of other food sources, and has been reported to elicit many cellular responses, including cell cycle arrest, differentiation, and apoptosis, and to inhibit the growth of several types of cancer, such as prostate and colon cancers [102, 127]. However, its wide array of activity has been compromised by intrinsic features that lead to low bioavailability, low water-solubility, and instability. Active targeting of resveratrol was tested by formulating chitosan nanoparticles with amine-free surfaces for appropriate ligand conjugation was prepared by sodium chloride precipitation. The absence of amine on the surfaces allows for the design of an active target drug delivery system.
7. In vivo pharmacokinetics
Site-specific drug delivery is an important area of research that is anticipated to increase the efficacy of drugs and reduce their potential side effects. Biodegradable polymers are currently being used as drug carriers because of their inherent properties of controlled release, enhanced distribution and overall pharmacokinetic availability. The release of loaded drugs from nanoparticles may be controlled in response to changes in environmental conditions, such as temperature and pH. Biodistribution profiles and anticancer efficacy of nanonutraceuticals in vivo might differ depending upon their size, surface charge, PEGylation, and other biophysical properties. Micro- and nanoparticulate systems formulated with these polymers have shown wide applicability for oral, subcutaneous, or intravenous delivery of lipophilic and hydrophilic drugs. Wang et al. showed that t1/2 beta and area under the curve of a paclitaxel micelle formulation were 4.0- and 2.2-fold higher than that of a Taxol injection. Their study of biodistribution in mice showed that the paclitaxel micelle formulation not only decreased drug uptake by the liver but also prolonged drug retention in the blood and increased drug distribution in the kidneys, spleen, ovaries, and uterus, suggesting that this formulation could be a useful drug carrier for intravenous administration of paclitaxel [128]. In a different study of the controlled release of insulin to the lungs, insulin-loaded PLGA nanoparticles fabricated by a double emulsion method by the aid of hydroxypropyl-beta-cyclodextrin (HPbetaCD) significantly reduced blood glucose levels as a function of the administered dose in animal models [129]. In vivo data show that PLGA/HPbetaCD/insulin nanoparticles can reach alveoli and release insulin, which is absorbed in its bioactive form [129]. Another study described the development of biodegradable nanoparticles with a core-shell structure to formulate superparamagnetic iron oxide (CSNPSPIO) for MRI [130]. The developed nanoparticles were composed of a hydrophobic PLGA core and a positively charged glycol chitosan shell. In this study, a high level of radioactivity was observed in the liver shortly after the intravenous administration of the 99-mTc-labeled CSNPSPIO. Studies from our group and others have shown curcumin as a promising bioactive agent with antiinflammatory, antiproliferative, antiangiogenic, antihelmintic, and wound-healing properties; however, the major challenge of curcumin is its poor oral bioavailability in vivo. Shaikh et al. showed that the in vivo pharmacokinetics of curcumin-entrapped nanoparticles demonstrate at least a 9-fold increase in oral bioavailability when compared to curcumin administered with piperine as an absorption enhancer [113].
The rational design of nanoparticles is very important for altering the pharmacokinetics of the encapsulated drug [7, 131] The shape and size of the particles are key determinants in governing the biodistribution and bioavailability of the cargo [132] Attaining steric stabilization of particles with a methoxy-PEG surface brush layer is commonly utilized to create long-circulating particles. PEGylation reduces the absorption of the reticuloendothelial system. Tumor accumulation of the nanoparticles is a function of both the rate of extravasation from the blood to the tumor space and the rate of clearance from the tumor. Hydrostatic pressure in a tumor mass typically decreases from the center to the periphery, so particles that enter into the tumor vasculature by convection are more likely to be cleared; this washout can be reduced by restricting the movement of particles through the extracellular tumor matrix [130].
8. Conclusion
Overall, these studies indicate that nanotechnology has great potential for delivering nutraceuticals. To fully realize this potential, more clinical trials are needed with nano-formulated nutraceuticals. Abraxan, protein-bound paclitaxel with a mean particle size of approximately 130 nm for injectable suspension, has been approved by the Food and Drug Administration for patients with metastatic breast cancer, but it requires intravenous delivery. Oral delivery of nutraceuticals, however, is preferred. The fate of the carrier materials used for the entrapment of nutraceuticals is also unclear at present. The short-term and long-term effects of the carrier material remain to be understood. Another concern is that properties of a nanoscale material may differ from the bulk-material, and it may alter absorption, digestion, metabolism, or excretion of nanodrugs in the body. Furthermore, because there is little information available about the potential health risk of nanoparticles, more research on the toxicology of nanoparticles is warranted[131-133]. Chemoprevention requires the administration of nutraceuticals to normal and healthy individuals; thus, safety and cost are other points of concern.
Acknowledgments
We would like to thank Markeda Wade and Stephanie Deming for carefully editing the manuscript and providing valuable comments. We also thank Dr. Chitra Sundaram for assisting with references. This work was supported by MD Anderson's Cancer Center Support Grant from the National Institutes of Health (NIH CA-16 672), a program project grant from the National Institutes of Health (NIH CA-124787-01A2), and a grant from the Center for Targeted Therapy at The University of Texas MD Anderson Cancer Center, where Dr. Aggarwal is the Ransom Horne, Jr., Professor of Cancer Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Molinski TF. Developments in marine natural products. Receptor-specific bioactive compounds. J Nat Prod. 1993;56:1–8. doi: 10.1021/np50091a001. [DOI] [PubMed] [Google Scholar]
- 2.Grabley S, Thiericke R. Bioactive agents from natural sources: trends in discovery and application. Adv Biochem Eng Biotechnol. 1999;64:101–54. doi: 10.1007/3-540-49811-7_4. [DOI] [PubMed] [Google Scholar]
- 3.Amin AR, Kucuk O, Khuri FR, Shin DM. Perspectives for cancer prevention with natural compounds. J Clin Oncol. 2009;27:2712–25. doi: 10.1200/JCO.2008.20.6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leach AR, Gillet VJ, Lewis RA, Taylor R. Three-dimensional pharmacophore methods in drug discovery. J Med Chem. 53:539–58. doi: 10.1021/jm900817u. [DOI] [PubMed] [Google Scholar]
- 5.Huang Q, Yu H, Ru Q. Bioavailability and delivery of nutraceuticals using nanotechnology. J Food Sci. 75:R50–7. doi: 10.1111/j.1750-3841.2009.01457.x. [DOI] [PubMed] [Google Scholar]
- 6.Wagner V, Dullaart A, Bock AK, Zweck A. The emerging nanomedicine landscape. Nat Biotechnol. 2006;24:1211–7. doi: 10.1038/nbt1006-1211. [DOI] [PubMed] [Google Scholar]
- 7.Riehemann K, Schneider SW, Luger TA, Godin B, Ferrari M, Fuchs H. Nanomedicine--challenge and perspectives. Angew Chem Int Ed Engl. 2009;48:872–97. doi: 10.1002/anie.200802585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferrari M. Cancer nanotechnology: opportunities and challenges. Nat Rev Cancer. 2005;5:161–71. doi: 10.1038/nrc1566. [DOI] [PubMed] [Google Scholar]
- 9.American Cancer Society; 2010. Facts and Figures. [Google Scholar]
- 10.Merisko-Liversidge EM, Liversidge GG. Drug nanoparticles: formulating poorly water-soluble compounds. Toxicol Pathol. 2008;36:43–8. doi: 10.1177/0192623307310946. [DOI] [PubMed] [Google Scholar]
- 11.Koning GA, Krijger GC. Targeted multifunctional lipid-based nanocarriers for image-guided drug delivery. Anticancer Agents Med Chem. 2007;7:425–40. doi: 10.2174/187152007781058613. [DOI] [PubMed] [Google Scholar]
- 12.Mousa SA, Bharali DJ, Armstrong D. From nutraceuticals to pharmaceuticals to nanopharmaceuticals: a case study in angiogenesis modulation during oxidative stress. Mol Biotechnol. 2007;37:72–80. doi: 10.1007/s12033-007-0064-7. [DOI] [PubMed] [Google Scholar]
- 13.Davis ME, Chen ZG, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discov. 2008;7:771–82. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- 14.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balkwill F, Mantovani A. Cancer and inflammation: implications for pharmacology and therapeutics. Clin Pharmacol Ther. 87:401–6. doi: 10.1038/clpt.2009.312. [DOI] [PubMed] [Google Scholar]
- 16.Mantovani A, Garlanda C, Allavena P. Molecular pathways and targets in cancer-related inflammation. Ann Med. 42:161–70. doi: 10.3109/07853890903405753. [DOI] [PubMed] [Google Scholar]
- 17.Grivennikov SI, Karin M. Inflammation and oncogenesis: a vicious connection. Curr Opin Genet Dev. 20:65–71. doi: 10.1016/j.gde.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol. 2007;127:514–25. doi: 10.1038/sj.jid.5700701. [DOI] [PubMed] [Google Scholar]
- 19.Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am J Physiol Gastrointest Liver Physiol. 2004;287:G7–17. doi: 10.1152/ajpgi.00079.2004. [DOI] [PubMed] [Google Scholar]
- 20.Danese S, Mantovani A. Inflammatory bowel disease and intestinal cancer: a paradigm of the Yin-Yang interplay between inflammation and cancer. Oncogene. 29:3313–23. doi: 10.1038/onc.2010.109. [DOI] [PubMed] [Google Scholar]
- 21.Turini ME, DuBois RN. Cyclooxygenase-2: a therapeutic target. Annu Rev Med. 2002;53:35–57. doi: 10.1146/annurev.med.53.082901.103952. [DOI] [PubMed] [Google Scholar]
- 22.Kamat AM, Tharakan ST, Sung B, Aggarwal BB. Curcumin potentiates the antitumor effects of Bacillus Calmette-Guerin against bladder cancer through the downregulation of NF-kappaB and upregulation of TRAIL receptors. Cancer Res. 2009;69:8958–66. doi: 10.1158/0008-5472.CAN-09-2045. [DOI] [PubMed] [Google Scholar]
- 23.Aggarwal BB, Kunnumakkara AB, Harikumar KB, Tharakan ST, Sung B, Anand P. Potential of spice-derived phytochemicals for cancer prevention. Planta Med. 2008;74:1560–9. doi: 10.1055/s-2008-1074578. [DOI] [PubMed] [Google Scholar]
- 24.Sagar SM, Yance D, Wong RK. Natural health products that inhibit angiogenesis: a potential source for investigational new agents to treat cancer-Part 1. Curr Oncol. 2006;13:14–26. [PMC free article] [PubMed] [Google Scholar]
- 25.Anand P, Sundaram C, Jhurani S, Kunnumakkara AB, Aggarwal BB. Curcumin and cancer: an “old-age” disease with an “age-old” solution. Cancer Lett. 2008;267:133–64. doi: 10.1016/j.canlet.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 26.Sharma RA, McLelland HR, Hill KA, Ireson CR, Euden SA, Manson MM, et al. Pharmacodynamic and pharmacokinetic study of oral Curcuma extract in patients with colorectal cancer. Clin Cancer Res. 2001;7:1894–900. [PubMed] [Google Scholar]
- 27.Sharma RA, Euden SA, Platton SL, Cooke DN, Shafayat A, Hewitt HR, et al. Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin Cancer Res. 2004;10:6847–54. doi: 10.1158/1078-0432.CCR-04-0744. [DOI] [PubMed] [Google Scholar]
- 28.Cruz-Correa M, Shoskes DA, Sanchez P, Zhao R, Hylind LM, Wexner SD, et al. Combination treatment with curcumin and quercetin of adenomas in familial adenomatous polyposis. Clin Gastroenterol Hepatol. 2006;4:1035–8. doi: 10.1016/j.cgh.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 29.Dhillon N, Aggarwal BB, Newman RA, Wolff RA, Kunnumakkara AB, Abbruzzese JL, et al. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin Cancer Res. 2008;14:4491–9. doi: 10.1158/1078-0432.CCR-08-0024. [DOI] [PubMed] [Google Scholar]
- 30.Bhardwaj A, Sethi G, Vadhan-Raj S, Bueso-Ramos C, Takada Y, Gaur U, et al. Resveratrol inhibits proliferation, induces apoptosis, and overcomes chemoresistance through down-regulation of STAT3 and nuclear factor-kappaB-regulated antiapoptotic and cell survival gene products in human multiple myeloma cells. Blood. 2007;109:2293–302. doi: 10.1182/blood-2006-02-003988. [DOI] [PubMed] [Google Scholar]
- 31.Began G, Sudharshan E, Sankar KU, Rao AG. Interaction of curcumin with phosphatidylcholine: A spectrofluorometric study. J Agric Food Chem. 2000;48:576. doi: 10.1021/jf991308g. [DOI] [PubMed] [Google Scholar]
- 32.Maiti K, Mukherjee K, Gantait A, Saha BP, Mukherjee PK. Curcumin-phospholipid complex: Preparation, therapeutic evaluation and pharmacokinetic study in rats. Int J Pharm. 2007;330:155–63. doi: 10.1016/j.ijpharm.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 33.Kunwar A, Barik A, Pandey R, Priyadarsini KI. Transport of liposomal and albumin loaded curcumin to living cells: an absorption and fluorescence spectroscopic study. Biochim Biophys Acta. 2006;1760:1513–20. doi: 10.1016/j.bbagen.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 34.Marczylo TH, Verschoyle RD, Cooke DN, Morazzoni P, Steward WP, Gescher AJ. Comparison of systemic availability of curcumin with that of curcumin formulated with phosphatidylcholine. Cancer Chemother Pharmacol. 2007;60:171–7. doi: 10.1007/s00280-006-0355-x. [DOI] [PubMed] [Google Scholar]
- 35.Tonnesen HH. Solubility, chemical and photochemical stability of curcumin in surfactant solutions. Studies of curcumin and curcuminoids, XXVIII. Pharmazie. 2002;57:820–4. [PubMed] [Google Scholar]
- 36.Kumar V, Lewis SA, Mutalik S, Shenoy DB, Venkatesh, Udupa N. Biodegradable microspheres of curcumin for treatment of inflammation. Indian J Physiol Pharmacol. 2002;46:209–17. [PubMed] [Google Scholar]
- 37.Salmaso S, Bersani S, Semenzato A, Caliceti P. New cyclodextrin bioconjugates for active tumour targeting. J Drug Target. 2007;15:379–90. doi: 10.1080/10611860701349752. [DOI] [PubMed] [Google Scholar]
- 38.Li L, Braiteh FS, Kurzrock R. Liposome-encapsulated curcumin: in vitro and in vivo effects on proliferation, apoptosis, signaling, and angiogenesis. Cancer. 2005;104:1322–31. doi: 10.1002/cncr.21300. [DOI] [PubMed] [Google Scholar]
- 39.Takahashi M, Kitamoto D, Imura T, Oku H, Takara K, Wada K. Characterization and bioavailability of liposomes containing a ukon extract. Biosci Biotechnol Biochem. 2008;72:1199–205. doi: 10.1271/bbb.70659. [DOI] [PubMed] [Google Scholar]
- 40.Sou K, Inenaga S, Takeoka S, Tsuchida E. Loading of curcumin into macrophages using lipid-based nanoparticles. Int J Pharm. 2008;352:287–93. doi: 10.1016/j.ijpharm.2007.10.033. [DOI] [PubMed] [Google Scholar]
- 41.Kim K, Kim JH, Park H, Kim YS, Park K, Nam H, et al. Tumor-homing multifunctional nanoparticles for cancer theragnosis: Simultaneous diagnosis, drug delivery, and therapeutic monitoring. J Control Release. doi: 10.1016/j.jconrel.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 42.Anand P, Nair HB, Sung B, Kunnumakkara AB, Yadav VR, Tekmal RR, et al. Design of curcumin-loaded PLGA nanoparticles formulation with enhanced cellular uptake, and increased bioactivity in vitro and superior bioavailability in vivo. Biochem Pharmacol. 79:330–8. doi: 10.1016/j.bcp.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43.Aggarwal BB, Van Kuiken ME, Iyer LH, Harikumar KB, Sung B. Molecular targets of nutraceuticals derived from dietary spices: potential role in suppression of inflammation and tumorigenesis. Exp Biol Med (Maywood) 2009;234:825–49. doi: 10.3181/0902-MR-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prasad S, Phromnoi K, Yadav VR, Chaturvedi MM, B.B. A. Targeting Inflammatory Pathways by Flavonoids for Prevention and Treatment of Cancer. Planta Medica. 2010 doi: 10.1055/s-0030-1250111. In press. [DOI] [PubMed] [Google Scholar]
- 45.Kawasaki ES, Player A. Nanotechnology, nanomedicine, and the development of new, effective therapies for cancer. Nanomedicine. 2005;1:101–9. doi: 10.1016/j.nano.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 46.Silva LF, da Boit KM. Nanominerals and nanoparticles in feed coal and bottom ash: implications for human health effects. Environ Monit Assess. doi: 10.1007/s10661-010-1449-9. [DOI] [PubMed] [Google Scholar]
- 47.Alexis F, Pridgen E, Molnar LK, Farokhzad OC. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol Pharm. 2008;5:505–15. doi: 10.1021/mp800051m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jain RK. Transport of molecules in the tumor interstitium: a review. Cancer Res. 1987;47:3039–51. [PubMed] [Google Scholar]
- 49.Duncan R, Vicent MJ, Greco F, Nicholson RI. Polymer-drug conjugates: towards a novel approach for the treatment of endrocine-related cancer. Endocr Relat Cancer. 2005;12(Suppl 1):S189–99. doi: 10.1677/erc.1.01045. [DOI] [PubMed] [Google Scholar]
- 50.Pasche S, Voros J, Griesser HJ, Spencer ND, Textor M. Effects of ionic strength and surface charge on protein adsorption at PEGylated surfaces. J Phys Chem B. 2005;109:17545–52. doi: 10.1021/jp050431+. [DOI] [PubMed] [Google Scholar]
- 51.Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989;49:6449–65. [PubMed] [Google Scholar]
- 52.Connor KM, Krah NM, Dennison RJ, Aderman CM, Chen J, Guerin KI, et al. Quantification of oxygen-induced retinopathy in the mouse: a model of vessel loss, vessel regrowth and pathological angiogenesis. Nat Protoc. 2009;4:1565–73. doi: 10.1038/nprot.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Simberg D, Duza T, Park JH, Essler M, Pilch J, Zhang L, et al. Biomimetic amplification of nanoparticle homing to tumors. Proc Natl Acad Sci U S A. 2007;104:932–6. doi: 10.1073/pnas.0610298104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Caravan P. Protein-targeted gadolinium-based magnetic resonance imaging (MRI) contrast agents: design and mechanism of action. Acc Chem Res. 2009;42:851–62. doi: 10.1021/ar800220p. [DOI] [PubMed] [Google Scholar]
- 55.Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2:751–60. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 56.Prestidge CA, Barnes TJ, Lau CH, Barnett C, Loni A, Canham L. Mesoporous silicon: a platform for the delivery of therapeutics. Expert Opin Drug Deliv. 2007;4:101–10. doi: 10.1517/17425247.4.2.101. [DOI] [PubMed] [Google Scholar]
- 57.Schellenberger EA, Bogdanov A, Jr., Hogemann D, Tait J, Weissleder R, Josephson L. Annexin V-CLIO: a nanoparticle for detecting apoptosis by MRI. Mol Imaging. 2002;1:102–7. doi: 10.1162/15353500200202103. [DOI] [PubMed] [Google Scholar]
- 58.Mazar AP. Urokinase plasminogen activator receptor choreographs multiple ligand interactions: implications for tumor progression and therapy. Clin Cancer Res. 2008;14:5649–55. doi: 10.1158/1078-0432.CCR-07-4863. [DOI] [PubMed] [Google Scholar]
- 59.Sahu SK, Maiti S, Maiti TK, Ghosh SK, Pramanik P. Hydrophobically modified carboxymethyl chitosan nanoparticles targeted delivery of paclitaxel. J Drug Target. doi: 10.3109/10611861003733987. [DOI] [PubMed] [Google Scholar]
- 60.Choi SJ, Oh JM, Choy JH. Biocompatible nanoparticles intercalated with anticancer drug for target delivery: pharmacokinetic and biodistribution study. J Nanosci Nanotechnol. 10:2913–6. doi: 10.1166/jnn.2010.1415. [DOI] [PubMed] [Google Scholar]
- 61.Si HY, Li DP, Wang TM, Zhang HL, Ren FY, Xu ZG, et al. Improving the anti-tumor effect of genistein with a biocompatible superparamagnetic drug delivery system. J Nanosci Nanotechnol. 10:2325–31. doi: 10.1166/jnn.2010.1913. [DOI] [PubMed] [Google Scholar]
- 62.Rawat MK, Jain A, Mishra A, Muthu MS, Singh S. Development of repaglinide loaded solid lipid nanocarrier: selection of fabrication method. Curr Drug Deliv. 7:44–50. doi: 10.2174/156720110790396472. [DOI] [PubMed] [Google Scholar]
- 63.Ravindran J, Nair HB, Sung B, Prasad S, Tekmal RR, Aggarwal BB. Thymoquinone poly (lactide-co-glycolide) nanoparticles exhibit enhanced anti-proliferative, anti-inflammatory, and chemosensitization potential. Biochem Pharmacol. 79:1640–7. doi: 10.1016/j.bcp.2010.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 64.Anton N, Benoit JP, Saulnier P. Design and production of nanoparticles formulated from nano-emulsion templates-a review. J Control Release. 2008;128:185–99. doi: 10.1016/j.jconrel.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 65.Ganta S, Amiji M. Coadministration of Paclitaxel and curcumin in nanoemulsion formulations to overcome multidrug resistance in tumor cells. Mol Pharm. 2009;6:928–39. doi: 10.1021/mp800240j. [DOI] [PubMed] [Google Scholar]
- 66.Ganta S, Sharma P, Paxton JW, Baguley BC, Garg S. Pharmacokinetics and pharmacodynamics of chlorambucil delivered in long-circulating nanoemulsion. J Drug Target. 2009 doi: 10.3109/10611860903244199. [DOI] [PubMed] [Google Scholar]
- 67.Villamizar RA, Braun J, Gompf B, Dressel M, Rius FX. Morphological and electrical characteristics of biofunctionalized layers on carbon nanotubes. Biosens Bioelectron. 2009;25:161–6. doi: 10.1016/j.bios.2009.06.034. [DOI] [PubMed] [Google Scholar]
- 68.Bouchemal K, Briancon S, Perrier E, Fessi H. Nano-emulsion formulation using spontaneous emulsification: solvent, oil and surfactant optimisation. Int J Pharm. 2004;280:241–51. doi: 10.1016/j.ijpharm.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 69.Francois G, Katz JL. Nanoparticles and nanocapsules created using the Ouzo effect: spontaneous emulisification as an alternative to ultrasonic and high-shear devices. Chemphyschem. 2005;6:209–16. doi: 10.1002/cphc.200400527. [DOI] [PubMed] [Google Scholar]
- 70.Shakeel F, Ramadan W. Transdermal delivery of anticancer drug caffeine from water-in-oil nanoemulsions. Colloids Surf B Biointerfaces. 75:356–62. doi: 10.1016/j.colsurfb.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 71.Szoka F, Jr., Papahadjopoulos D. Procedure for preparation of liposomes with large internal aqueous space and high capture by reverse-phase evaporation. Proc Natl Acad Sci U S A. 1978;75:4194–8. doi: 10.1073/pnas.75.9.4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim S, Martin GM. Preparation of cell-size unilamellar liposomes with high captured volume and defined size distribution. Biochim Biophys Acta. 1981;646:1–9. doi: 10.1016/0005-2736(81)90264-9. [DOI] [PubMed] [Google Scholar]
- 73.Meure LA, Foster NR, Dehghani F. Conventional and dense gas techniques for the production of liposomes: a review. AAPS PharmSciTech. 2008;9:798–809. doi: 10.1208/s12249-008-9097-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Siddiqui IA, Adhami VM, Bharali DJ, Hafeez BB, Asim M, Khwaja SI, et al. Introducing nanochemoprevention as a novel approach for cancer control: proof of principle with green tea polyphenol epigallocatechin-3-gallate. Cancer Res. 2009;69:1712–6. doi: 10.1158/0008-5472.CAN-08-3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jeong SY, Park SJ, Yoon SM, Jung J, Woo HN, Yi SL, et al. Systemic delivery and preclinical evaluation of Au nanoparticle containing beta-lapachone for radiosensitization. J Control Release. 2009;139:239–45. doi: 10.1016/j.jconrel.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 76.Blanco E, Bey EA, Dong Y, Weinberg BD, Sutton DM, Boothman DA, et al. Beta-lapachone-containing PEG-PLA polymer micelles as novel nanotherapeutics against NQO1-overexpressing tumor cells. J Control Release. 2007;122:365–74. doi: 10.1016/j.jconrel.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cartiera MS, Ferreira EC, Caputo C, Egan ME, Caplan MJ, Saltzman WM. Partial correction of cystic fibrosis defects with PLGA nanoparticles encapsulating curcumin. Mol Pharm. 7:86–93. doi: 10.1021/mp900138a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mukerjee A, Vishwanatha JK. Formulation, characterization and evaluation of curcumin-loaded PLGA nanospheres for cancer therapy. Anticancer Res. 2009;29:3867–75. [PubMed] [Google Scholar]
- 79.Das RK, Kasoju N, Bora U. Encapsulation of curcumin in alginate-chitosan-pluronic composite nanoparticles for delivery to cancer cells. Nanomedicine. 6:153–60. doi: 10.1016/j.nano.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 80.Mulik RS, Monkkonen J, Juvonen RO, Mahadik KR, Paradkar AR. ApoE3 Mediated Poly(butyl) Cyanoacrylate Nanoparticles Containing Curcumin: Study of Enhanced Activity of Curcumin against Beta Amyloid Induced Cytotoxicity Using In Vitro Cell Culture Model. Mol Pharm. doi: 10.1021/mp900306x. [DOI] [PubMed] [Google Scholar]
- 81.Gupta V, Aseh A, Rios CN, Aggarwal BB, Mathur AB. Fabrication and characterization of silk fibroin-derived curcumin nanoparticles for cancer therapy. Int J Nanomedicine. 2009;4:115–22. doi: 10.2147/ijn.s5581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sahu A, Kasoju N, Bora U. Fluorescence study of the curcumin-casein micelle complexation and its application as a drug nanocarrier to cancer cells. Biomacromolecules. 2008;9:2905–12. doi: 10.1021/bm800683f. [DOI] [PubMed] [Google Scholar]
- 83.Bisht S, Feldmann G, Soni S, Ravi R, Karikar C, Maitra A. Polymeric nanoparticle-encapsulated curcumin (“nanocurcumin”): a novel strategy for human cancer therapy. J Nanobiotechnology. 2007;5:3. doi: 10.1186/1477-3155-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gao Y, Gu W, Chen L, Xu Z, Li Y. The role of daidzein-loaded sterically stabilized solid lipid nanoparticles in therapy for cardio-cerebrovascular diseases. Biomaterials. 2008;29:4129–36. doi: 10.1016/j.biomaterials.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 85.Contreras J, Xie J, Chen YJ, Pei H, Zhang G, Fraser CL, et al. Intracellular uptake and trafficking of difluoroboron dibenzoylmethane-polylactide nanoparticles in HeLa cells. ACS Nano. 4:2735–47. doi: 10.1021/nn901385y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chingunpitak J, Puttipipatkhachorn S, Chavalitshewinkoon-Petmitr P, Tozuka Y, Moribe K, Yamamoto K. Formation, physical stability and in vitro antimalarial activity of dihydroartemisinin nanosuspensions obtained by co-grinding method. Drug Dev Ind Pharm. 2008;34:314–22. doi: 10.1080/03639040701662388. [DOI] [PubMed] [Google Scholar]
- 87.Sonaje K, Italia JL, Sharma G, Bhardwaj V, Tikoo K, Kumar MN. Development of biodegradable nanoparticles for oral delivery of ellagic acid and evaluation of their antioxidant efficacy against cyclosporine A-induced nephrotoxicity in rats. Pharm Res. 2007;24:899–908. doi: 10.1007/s11095-006-9207-y. [DOI] [PubMed] [Google Scholar]
- 88.Zu YG, Yuan S, Zhao XH, Zhang Y, Zhang XN, Jiang R. [Preparation, activity and targeting ability evaluation in vitro on folate mediated epigallocatechin-3-gallate albumin nanoparticles]. Yao Xue Xue Bao. 2009;44:525–31. [PubMed] [Google Scholar]
- 89.Chen F, Shi Z, Neoh KG, Kang ET. Antioxidant and antibacterial activities of eugenol and carvacrol-grafted chitosan nanoparticles. Biotechnol Bioeng. 2009;104:30–9. doi: 10.1002/bit.22363. [DOI] [PubMed] [Google Scholar]
- 90.Li FQ, Su H, Chen X, Qin XJ, Liu JY, Zhu QG, et al. Mannose 6-phosphate-modified bovine serum albumin nanoparticles for controlled and targeted delivery of sodium ferulate for treatment of hepatic fibrosis. J Pharm Pharmacol. 2009;61:1155–61. doi: 10.1211/jpp/61.09.0004. [DOI] [PubMed] [Google Scholar]
- 91.Li FQ, Su H, Wang J, Liu JY, Zhu QG, Fei YB, et al. Preparation and characterization of sodium ferulate entrapped bovine serum albumin nanoparticles for liver targeting. Int J Pharm. 2008;349:274–82. doi: 10.1016/j.ijpharm.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 92.Liang YQ, Chen BA, Wu WW, Gao F, Xia GH, Shao ZY, et al. Effects of magnetic nanoparticle of Fe3O4 on apoptosis induced by Gambogic acid in U937 leukemia cells. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 18:67–73. [PubMed] [Google Scholar]
- 93.Silva AP, Nunes BR, De Oliveira MC, Koester LS, Mayorga P, Bassani VL, et al. Development of topical nanoemulsions containing the isoflavone genistein. Pharmazie. 2009;64:32–5. [PubMed] [Google Scholar]
- 94.Fang F, Gong C, Qian Z, Zhang X, Gou M, You C, et al. Honokiol nanoparticles in thermosensitive hydrogel: therapeutic effects on malignant pleural effusion. ACS Nano. 2009;3:4080–8. doi: 10.1021/nn900785b. [DOI] [PubMed] [Google Scholar]
- 95.Yen FL, Wu TH, Lin LT, Cham TM, Lin CC. Naringenin-loaded nanoparticles improve the physicochemical properties and the hepatoprotective effects of naringenin in orally-administered rats with CCl(4)-induced acute liver failure. Pharm Res. 2009;26:893–902. doi: 10.1007/s11095-008-9791-0. [DOI] [PubMed] [Google Scholar]
- 96.Yao J, Zhou JP, Ping QN. [Characteristics of nobiletin-loaded nanoemulsion and its in vivo distribution in mice]. Yao Xue Xue Bao. 2007;42:663–8. [PubMed] [Google Scholar]
- 97.Das S, Mandal AK, Ghosh A, Panda S, Das N, Sarkar S. Nanoparticulated quercetin in combating age related cerebral oxidative injury. Curr Aging Sci. 2008;1:169–74. doi: 10.2174/1874609810801030169. [DOI] [PubMed] [Google Scholar]
- 98.Ghosh A, Mandal AK, Sarkar S, Panda S, Das N. Nanoencapsulation of quercetin enhances its dietary efficacy in combating arsenic-induced oxidative damage in liver and brain of rats. Life Sci. 2009;84:75–80. doi: 10.1016/j.lfs.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 99.Li H, Zhao X, Ma Y, Zhai G, Li L, Lou H. Enhancement of gastrointestinal absorption of quercetin by solid lipid nanoparticles. J Control Release. 2009;133:238–44. doi: 10.1016/j.jconrel.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 100.Teskac K, Kristl J. The evidence for solid lipid nanoparticles mediated cell uptake of resveratrol. Int J Pharm. 390:61–9. doi: 10.1016/j.ijpharm.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 101.Lu X, Ji C, Xu H, Li X, Ding H, Ye M, et al. Resveratrol-loaded polymeric micelles protect cells from Abeta-induced oxidative stress. Int J Pharm. 2009;375:89–96. doi: 10.1016/j.ijpharm.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 102.Shao J, Li X, Lu X, Jiang C, Hu Y, Li Q, et al. Enhanced growth inhibition effect of resveratrol incorporated into biodegradable nanoparticles against glioma cells is mediated by the induction of intracellular reactive oxygen species levels. Colloids Surf B Biointerfaces. 2009;72:40–7. doi: 10.1016/j.colsurfb.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 103.Zhang Z, Bu H, Gao Z, Huang Y, Gao F, Li Y. The characteristics and mechanism of simvastatin loaded lipid nanoparticles to increase oral bioavailability in rats. Int J Pharm. doi: 10.1016/j.ijpharm.2010.04.039. [DOI] [PubMed] [Google Scholar]
- 104.Ali H, Shirode AB, Sylvester PW, Nazzal S. Preparation, characterization, and anticancer effects of simvastatin-tocotrienol lipid nanoparticles. Int J Pharm. 389:223–31. doi: 10.1016/j.ijpharm.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 105.Lai J, Chen J, Lu Y, Sun J, Hu F, Yin Z, et al. Glyceryl monooleate/poloxamer 407 cubic nanoparticles as oral drug delivery systems: I. In vitro evaluation and enhanced oral bioavailability of the poorly water-soluble drug simvastatin. AAPS PharmSciTech. 2009;10:960–6. doi: 10.1208/s12249-009-9292-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu M, Dong J, Yang Y, Yang X, Xu H. Anti-inflammatory effects of triptolide loaded poly(D,L-lactic acid) nanoparticles on adjuvant-induced arthritis in rats. J Ethnopharmacol. 2005;97:219–25. doi: 10.1016/j.jep.2004.10.031. [DOI] [PubMed] [Google Scholar]
- 107.Xiong FL, Chen HB, Chang XL, Yang YJ, Xu HB, Yang XL. Research progress of triptolide-loaded nanoparticles delivery systems. Conf Proc IEEE Eng Med Biol Soc. 2005;5:4966–9. doi: 10.1109/IEMBS.2005.1615589. [DOI] [PubMed] [Google Scholar]
- 108.Mei Z, Wu Q, Hu S, Li X, Yang X. Triptolide loaded solid lipid nanoparticle hydrogel for topical application. Drug Dev Ind Pharm. 2005;31:161–8. doi: 10.1081/ddc-200047791. [DOI] [PubMed] [Google Scholar]
- 109.Mei Z, Li X, Wu Q, Hu S, Yang X. The research on the anti-inflammatory activity and hepatotoxicity of triptolide-loaded solid lipid nanoparticle. Pharmacol Res. 2005;51:345–51. doi: 10.1016/j.phrs.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 110.Shikov AN, Pozharitskaya ON, Miroshnyk I, Mirza S, Urakova IN, Hirsjarvi S, et al. Nanodispersions of taxifolin: impact of solid-state properties on dissolution behavior. Int J Pharm. 2009;377:148–52. doi: 10.1016/j.ijpharm.2009.04.044. [DOI] [PubMed] [Google Scholar]
- 111.Zhou XJ, Hu XM, Yi YM, Wan J. Preparation and body distribution of freeze-dried powder of ursolic acid phospholipid nanoparticles. Drug Dev Ind Pharm. 2009;35:305–10. doi: 10.1080/03639040802302165. [DOI] [PubMed] [Google Scholar]
- 112.Garcea G, Jones DJ, Singh R, Dennison AR, Farmer PB, Sharma RA, et al. Detection of curcumin and its metabolites in hepatic tissue and portal blood of patients following oral administration. Br J Cancer. 2004;90:1011–5. doi: 10.1038/sj.bjc.6601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shaikh J, Ankola DD, Beniwal V, Singh D, Kumar MN. Nanoparticle encapsulation improves oral bioavailability of curcumin by at least 9-fold when compared to curcumin administered with piperine as absorption enhancer. Eur J Pharm Sci. 2009;37:223–30. doi: 10.1016/j.ejps.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 114.Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, Srinivas PS. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998;64:353–6. doi: 10.1055/s-2006-957450. [DOI] [PubMed] [Google Scholar]
- 115.Kress CL, Konopleva M, Martinez-Garcia V, Krajewska M, Lefebvre S, Hyer ML, et al. Triterpenoids display single agent anti-tumor activity in a transgenic mouse model of chronic lymphocytic leukemia and small B cell lymphoma. PLoS One. 2007;2:e559. doi: 10.1371/journal.pone.0000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lee SB, Cho K, Shim JK. Amphiphilic Self-assembled Nanoparticles Composed of Chitosan and Ursolic Acid for Protein Delivery on the Skin. Nanotechnology. 2006;2 Boston, May 7-11, 2006. [Google Scholar]
- 117.Schafer-Korting M, Mehnert W, Korting HC. Lipid nanoparticles for improved topical application of drugs for skin diseases. Adv Drug Deliv Rev. 2007;59:427–43. doi: 10.1016/j.addr.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 118.Chen BJ. Triptolide, a novel immunosuppressive and anti-inflammatory agent purified from a Chinese herb Tripterygium wilfordii Hook F. Leuk Lymphoma. 2001;42:253–65. doi: 10.3109/10428190109064582. [DOI] [PubMed] [Google Scholar]
- 119.Mei Z, Chen H, Weng T, Yang Y, Yang X. Solid lipid nanoparticle and microemulsion for topical delivery of triptolide. Eur J Pharm Biopharm. 2003;56:189–96. doi: 10.1016/s0939-6411(03)00067-5. [DOI] [PubMed] [Google Scholar]
- 120.Hwang JT, Ha J, Park IJ, Lee SK, Baik HW, Kim YM, et al. Apoptotic effect of EGCG in HT-29 colon cancer cells via AMPK signal pathway. Cancer Lett. 2007;247:115–21. doi: 10.1016/j.canlet.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 121.Siddiqui IA, Mukhtar H. Nanochemoprevention by bioactive food components: a perspective. Pharm Res. 27:1054–60. doi: 10.1007/s11095-010-0087-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Barras A, Mezzetti A, Richard A, Lazzaroni S, Roux S, Melnyk P, et al. Formulation and characterization of polyphenol-loaded lipid nanocapsules. Int J Pharm. 2009;379:270–7. doi: 10.1016/j.ijpharm.2009.05.054. [DOI] [PubMed] [Google Scholar]
- 123.Barik A, Priyadarsini KI, Mohan H. Photophysical studies on binding of curcumin to bovine serum albumins. Photochem Photobiol. 2003;77:597–603. doi: 10.1562/0031-8655(2003)077<0597:psoboc>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 124.Shutava TG, Balkundi SS, Vangala P, Steffan JJ, Bigelow RL, Cardelli JA, et al. Layer-by-Layer-Coated Gelatin Nanoparticles as a Vehicle for Delivery of Natural Polyphenols. ACS Nano. 2009 doi: 10.1021/nn900451a. [DOI] [PubMed] [Google Scholar]
- 125.Italia JL, Datta P, Ankola DD, Kumar MNVR. Nanoparticles Enhance Per Oral Bioavailability of Poorly Available Molecules: Epigallocatechin Gallate Nanoparticles Ameliorates Cyclosporine Induced Nephrotoxicity in Rats at Three Times Lower Dose Than Oral Solution. J Biomed Nanotechnol. 2008;4:304–12. [Google Scholar]
- 126.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4:807–18. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 127.Jiang H, Zhang L, Kuo J, Kuo K, Gautam SC, Groc L, et al. Resveratrol-induced apoptotic death in human U251 glioma cells. Mol Cancer Ther. 2005;4:554–61. doi: 10.1158/1535-7163.MCT-04-0056. [DOI] [PubMed] [Google Scholar]
- 128.Wang Y, Li Y, Wang Q, Fang X. Pharmacokinetics and biodistribution of polymeric micelles of paclitaxel with pluronic P105/poly(caprolactone) copolymers. Pharmazie. 2008;63:446–52. [PubMed] [Google Scholar]
- 129.Ungaro F, d'Emmanuele di Villa Bianca R, Giovino C, Miro A, Sorrentino R, Quaglia F, et al. Insulin-loaded PLGA/cyclodextrin large porous particles with improved aerosolization properties: in vivo deposition and hypoglycaemic activity after delivery to rat lungs. J Control Release. 2009;135:25–34. doi: 10.1016/j.jconrel.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 130.Lee PW, Hsu SH, Wang JJ, Tsai JS, Lin KJ, Wey SP, et al. The characteristics, biodistribution, magnetic resonance imaging and biodegradability of superparamagnetic core-shell nanoparticles. Biomaterials. 31:1316–24. doi: 10.1016/j.biomaterials.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 131.Maynard AD, Aitken RJ, Butz T, Colvin V, Donaldson K, Oberdorster G, et al. Safe handling of nanotechnology. Nature. 2006;444:267–9. doi: 10.1038/444267a. [DOI] [PubMed] [Google Scholar]
- 132.Nagahara LA, Lee JS, Molnar LK, Panaro NJ, Farrell D, Ptak K, et al. Strategic Workshops on Cancer Nanotechnology. Cancer Res. doi: 10.1158/0008-5472.CAN-09-3716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Srinivas PR, Philbert M, Vu TQ, Huang Q, Kokini JL, Saltos E, et al. Nanotechnology research: applications in nutritional sciences. J Nutr. 140:119–24. doi: 10.3945/jn.109.115048. [DOI] [PMC free article] [PubMed] [Google Scholar]