Abstract
Leishmania major aquaglyceroporin LmAQP1 allows adventitious passage of antimonite, an activated form of the drug Pentostam, which is used as the first line treatment for leishmaniasis. The extracellular C-loop of an aquaglyceroporin confers substrate specificity. Alteration of Glu125 to serine in the Plasmodium falciparum aquaglyceroporin PfAQP has been shown to selectively affect water but not glycerol permeability. The C-loop of LmAQP1 is twelve residues longer than PfAQP, and Ala163 is at an equivalent position as Glu125 of PfAQP. The role of Ala163 in LmAQP1 solute permeability was investigated. Alteration of Ala163 to serine or threonine did not significantly affect conduction of solutes. However, alteration to aspartate, glutamate, and glutamine blocked passage of water, glycerol, and other organic solutes. While LmAQP1 is a mercurial insensitive water channel, mutation of the adjacent threonine (Thr164) to cysteine led to inhibition of water passage by Hg2+. This inhibition could be reversed upon addition of β-mercaptoethanol. These data suggest that, unlike Glu125 (PfAQP), Ala163 is not involved in stabilization of the C-loop and selective solute permeability. Ala163 is located near the pore mouth of the channel, and replacement of Ala163 by bulkier residue sterically hinders the passage of solutes. Alteration of Ala163 to serine or threonine affected metalloid uptake in the order, wild-type > A163S > A163T. Metalloid conduction was near completely blocked when Ala163 was mutagenized to aspartate, glutamate, or glutamine. Mutations such as A163S and A163T that reduced the permeability to antimonite, without a significant loss in water or solute conductivity raises the possibility that, subtle changes in the side chain of the amino acid residue in position 163 of LmAQP1 may play a role in drug resistance.
Keywords: alanine, antimonite, aquaglyceroporin, drug resistance, Leishmania, LmAQP1, C-loop, pore mouth
Introduction
Leishmaniasis is a protozoan parasitic infection ranging from self healing cutaneous lesions to non-healing mucocutaneous and visceral ailments. The disease is endemic in parts of 88 countries across five continents - the majority of the affected countries are in the tropics and subtropics. Approximately 12 million people worldwide are affected by leishmaniasis and 2 million new cases are considered to occur annually. The Leishmania parasite exists in two morphologically distinct forms: promastigotes and amastigotes. The promastigotes reside in the intestinal tract of the sandfly vector and have a slipper like body with an anterior flagellum. Inside the mammalian host, the promastigote forms of the parasites are transformed into amastigotes that appear as small, oval-shaped, aflagelleted structures, and reside in macrophages and other mononuclear phagocytes. The first line of treatment against all forms of leishmaniasis are the pentavalent antimony [Sb(V)] containing drugs, sodium stibogluconate (Pentostam) and meglumine antimonate (Glucantime).
The Leishmania major aquaglyceroporin (LmAQP1) is adventitiously permeable to antimonite [Sb(III)], an activated form of Pentostam or Glucantime [1]. Besides the two metalloids arsenite [As(III)] and Sb(III), LmAQP1 is also permeable to water; its water conduction capacity is 65% of that of the classical water channel, human AQP1 [2]. In contrast to Plasmodium and Trypanosome aquaglyceroporins (AQPs) that are inhibited by mercurials, water movement through LmAQP1 is not inhibited by mercuric chloride, and it is therefore a mercurial independent water channel. LmAQP1 also conducts glycerol, glyceraldehyde, dihydroxyacetone, and sugar alcohols. Expression of LmAQP1 is limited exclusively to the flagellum of promastigotes, while in amastigotes it is found in the flagellar pocket, rudimentary flagellum, and contractile vacuoles. LmAQP1 has a physiological role in water and solute transport, volume regulation and osmotaxis [2]. Disruption of one of the two LmAQP1 alleles in L. major conferred a 10-fold increase in resistance to Sb(III) [1]. LmAQP1 mRNA levels are significantly less in either the Sb(III) or As(III) resistant L. major and Leishmania tarentolae cells, indicating that downregulation of LmAQP1 leads to drug resistance [3]. Therefore, LmAQP1 plays a major role in both Leishmania cellular physiology and drug resistance.
Genome sequencing of protozoan parasites have led to the identification of several aquaporin genes. Apicomplexan parasites such as the Plasmodium species and Toxoplasma gondii carry a single aquaporin gene, whereas the genomes of the kinetoplastida Trypanosoma brucei and L. major code for three and five aquaporins, respectively [4]. Protozoan AQPs conduct both glycerol and water at high rates. This is in contrast to Escherichia coli aquaglyceroporin GlpF, which is a glycerol channel with low water permeability. Structural comparison of GlpF [5] and the Plasmodium falciparum aquaglyceroporin PfAQP [6] shows that, although the protein cores are highly similar, the connecting loops, in particular the C-loop is markedly different. In GlpF, the C-loop connecting the adjacent transmembrane spans 4 and 5 has two short helices. This is different from the C-loop of PfAQP, which begins with a short helix, while the remainder lacks any secondary structure. The C-loop in both proteins dips deep into the protein core towards the conduction channel and forms a vestibule at the outer channel mouth. Beitz et al [7] have shown that the C-loop Glu125 is critically responsible for the high water permeability of PfAQP. Alteration of Glu125 to serine eliminates the stabilization of the C-loop, which in turn disrupts the hydrogen bonding between Arg196 and Trp124, resulting in increased solvent interaction of Arg196 and a higher barrier for passage of water. In contrast, the hydroxyl groups of glycerol have lower polarity than in water, and are not held back similarly [6].
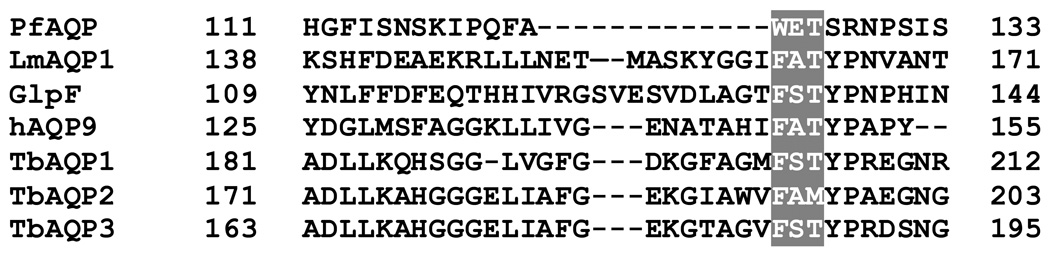
LmAQP1 shares ~32% sequence identity and ~50% similarity with either GlpF or PfAQP (Supplementary Fig. S1). The C-loop region of LmAQP1 is similar in length to GlpF and 12-residues longer than PfAQP (Fig. 1). Sequence alignment indicates that Ala163 in LmAQP1 is at an equivalent position to Glu125 of PfAQP (Fig. 1). This alanine is also conserved in T. brucei aquaglyceroporin TbAQP2 and human aquaglyceroporin hAQP9 (Fig. 1), and both proteins conduct glycerol and water at high rates [8, 9]. Homology models of LmAQP1 were generated based on either the structure of GlpF or PfAQP, and in both models Ala163 lays in an unstructured region of the C-loop (Supplementary Fig. S2). Despite the information provided by the crystal structures of GlpF and PfAQP, the contribution of individual residues in the conduction channel to solute permeability is not yet fully understood. We have undertaken site directed mutagenesis studies to gain insights into the solute selectivity of LmAQP1 and have earlier shown that alteration of Glu152 of LmAQP1 to alanine selectively abrogates metalloid but not glycerol permeability [10]. The objective of this work was to examine the role of Ala163 in solute permeability of LmAQP1.
Fig. 1.
Multiple sequence alignment of the C-loop region of various protozoan AQPs. The shaded box marks a conserved amino acid triad that has been shown to be close to the conserved arginine in the selectivity filter of GlpF and PfAQP. Multiple sequence alignment was performed with ClustalW [24] and shading by BOXSHADE. Abbreviations: H, Homo sapiens; Lm, Leishmania major; Pf, Plasmodium falciparum; Tb, Trypanosoma brucei.
2. Materials and methods
2.1. Strains and media
L. donovani strain LdBob was a kind gift from Professor Stephen M. Beverley, Washington University School of Medicine. Leishmania promastigotes and amastigotes were grown in culture media as described earlier [11]. Promastigotes were grown at 25°C while the axenic amastigotes were cultivated at 37°C with 5% CO2. Human leukemia monocyte cell line THP1 (ATCC) was maintained in RPMI-1640 medium with 10% fetal bovine serum (Invitrogen) and 50 µM β-mercaptoethanol (Sigma, cell culture grade) at 37°C with 5% CO2. Xenopus laevis were maintained in our animal facility and oocytes were harvested periodically.
2.2. Oligonucleotide-directed mutagenesis
The cloning of wild-type LmAQP1 into pGEM-T Easy vector (LmAQP1/pGEMEasy) has been described previously [1]. Mutations in LmAQP1 were introduced by site-directed mutagenesis using the QuikChange Site-Directed Mutagenesis Procedure (Stratagene), as described previously [12]. The mutagenic oligonucleotides used for both strands and the respective changes introduced (underlined) are as follows: A163C, 5’-CCAAGTACGGCGGAATCTTCTGCACATACCCTAATGTTGC-3’ (sense) and 5’-GCAACATTAGGGTATGTGCAGAAGATTCCGCCGTACTTGG-3’ (antisense); A163D, 5’-GTACGGCGGAATCTTCGACACATACCCTAATGTTG-3’ (sense) and 5’-CAACATTAGGGTATGTGTCGAAGATTCCGCCGTAC-3’ (antisense); A163E, 5’-GTACGGCGGAATCTTCGAGACATACCCTAATGTTG-3’ (sense) and 5’-CAACATTAGGGTATGTCTCGAAGATTCCGCCGTAC-3’ (antisense); A163Q, 5’-GTACGGCGGAATCTTCCAAACATACCCTAATGTTG-3’ (sense) and 5’-CAACATTAGGGTATGTTTGGAAGATTCCGCCGTAC-3’ (antisense); A163S, 5’-GTACGGCGGAATCTTCTCCACATACCCTAATGTTG-3’ (sense) and 5’-CAACATTAGGGTATGTGGAGAAGATTCCGCCGTAC-3’ (antisense); A163T, 5’-GTACGGCGGAATCTTCACCACATACCCTAATGTTG-3’ (sense) and 5’-CAACATTAGGGTATGTGGTGAAGATTCCGCCGTAC-3’ (antisense); T164C, 5’-CGGCGGAATCTTCGCCTGCTACCCTAATGTTGCAAACACC-3’ (sense) and 5’-GGTGTTTGCAACATTAGGGTAGCAGGCGAAGATTCCGCCG-3’ (antisense). Each mutation was confirmed by sequencing the entire gene using a CEQ2000 DNA sequencer (Beckman Coulter).
2.3. Expression of LmAQP1 in oocytes
For expression in Xenopus oocytes, wild-type and mutant LmAQP1 in pGEM-T Easy vector were subcloned into the EcoRI site of the pL2-5 vector [13] (a generous gift from Professor Scott M. Landfear, Oregon Health Sciences University). Following linearization with SmaI, cRNAs were transcribed in vitro using the mMessage mMachine kit (Ambion). Stage V- and VI-defolliculated X. laevis oocytes were injected with 50 nl of water (control), 50 nl of water containing 10 ng of LmAQP1-cRNA (wild-type, A163C, A163D, A163E, A163Q, A163S, A163T or T164C), 50 nl of water containing 5 ng of AQP1-cRNA, or a combination of 10 ng of LmAQP1-cRNA (wild-type, A163C, A163D, A163Q, A163S, A163T or T164C) and 5 ng of AQP1-cRNA. The oocytes were maintained at 16°C for 3 days in ND96 buffer containing 5 mM HEPES, pH 7.4, 96 mM NaCl, 2 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, 2.5 mM sodium pyruvate, 0.5 mM theophylline, and 2 µg ml−1 of gentamicin sulphate.
2.4. Cloning of LmAQP1 in Leishmania expression vector pSP72αhygroα
pSP72αhygroα was created on the backbone of pSP72 (Promega) and pGEM7αhygroα vectors. pGEM7αhygroα was created as described previously [1, 14]. Either the wild-type or mutant LmAQP1/pGEM-T Easy was digested with XbaI and HindIII and cloned into the same sites of pSP72αhygroα
2.5. Transfection of LmAQP1 in Leishmania
Transfection of pSP72αhygroα bearing either the wild-type or mutant LmAQP1 gene into the LdBob promastigotes was accomplished as described previously [15]. LdBob was cycled between the promastigote and axenic amastigote forms using an established protocol [11]. All transfectants were maintained in the presence of 0.3 mg ml−1 hygromycin (Invitrogen).
2.6. Cell lysates and Western blot
Flagellar membrane fraction from LdBob promastigotes transfected with either the vector or altered LmAQP1 were isolated as described previously [2]. Total protein content of the flagellar fraction was estimated using BCA protein assay kit (Pierce). For oocytes expressing either wild-type or altered LmAQP1, 15 oocytes from each category was suspended in 150 µl of ice-cold lysis buffer (7.5 mM Na2HPO4 (pH 7.4), 1 mM EDTA, and Complete Mini Protease Inhibitor cocktail (Roche)), and lysed by pipetting. All procedures were performed at 4°C. The lysate was centrifuged at 500 g for 5 minutes. The supernatant fraction was centrifuged at 100,000 g for 1 h, and the pellet containing the membrane fraction was suspended in 50 µl of a buffer consisting of 25 mM Tris-HCl (pH 7.4), 0.2 mM EDTA, 5 mM MgCl2, 12 mM β-mercaptoethanol, and 0.32 M sucrose. The total protein content of the oocyte membrane fraction was estimated by BCA protein assay kit (Pierce). Thirty microgram of the flagellar and 10 µg of oocyte protein samples were analyzed by 12% SDS-PAGE [16]. The proteins were transferred to a nitrocellulose membrane (Whatman) and probed with the LmAQP1 antipeptide antibody at 1:300 dilution as described previously [2].
2.7. Oocyte swelling assays
Oocyte swelling assays were performed as described by Hansen et al [17]. Water permeability was measured by transferring oocytes into 1:3 diluted ND96 medium. Solute permeability was measured in an isoosmotic ND96 medium in which 65 mM NaCl was substituted with 130 mM of a non-ionic test solute. While determining the permeability of methylglyoxal, NaCl was entirely replaced with 115 mM methylglyoxal to maintain buffer isoosmoticity. To test the effect of HgCl2 on LmAQP1 activity, oocytes were pre-incubated with 0.3 mM HgCl2 for 5 min, washed twice with ND96 medium, followed by swelling assays. Reversal of HgCl2 inhibition was performed by incubating the HgCl2-treated oocytes with 3 mM β-mercaptoethanol for 5 min before subjecting them to swelling assay. The swelling assays were performed at room temperature and video-monitored. The relative oocyte volume was calculated from the covered area. Osmotic water permeability (Pf, µm s−1) and solute permeability (Ps) were calculated using the following equation:
| (1) |
[17]
| (2) |
[18] where, S is the oocyte surface area (= 0.045 cm2), V0 is the initial oocyte volume (= 9 × 10−4 cm3), Vw represents the molecular water volume (= 18 cm3 mol−1), osmin − osmout is the osmotic gradient, osmtotal is the total osmolarity of the system (200 mOsm), solout − solin is the osmotic solute gradient, and d(V/V0)/dt in s−1 is measured from the initial slope of the relative volume increase.
2.8. Determination of EC50 of metalloids in promastigotes
Log phase promastigote cultures were diluted to 106 cells ml−1 in a culture medium containing various concentrations of As(III) in the form of sodium arsenite, or Sb(III) in the form of potassium antimonyl tartrate [1]. Following 5 days of incubation, cell growth was monitored from the absorbance at 600 nm using a microplate reader (Spectramax 340, Molecular Devices). Percentage survival was plotted against metalloid concentrations and EC50 was determined using SigmaPlot 11.0. Each assay was performed at least three times and the results are represented as mean ± SE.
2.9. Determination of EC50 of antimonate in amastigotes
L. donovani strain LdBob promastigotes were used to infect the human leukemia cell line THP-1, as described previously [19], with some minor modifications. THP-1 cells were differentiated with phorbol myristate acetate and infected with L. donovani promastigotes at a ratio of 20:1 for 4 h. Noninternalized parasites were washed away and the infected macrophages were treated with varying concentrations of potassium hexahydroxoantimonate [Sb(V)] (Sigma). After 4 days of culture, wells containing adherent differentiated THP-1 cells were washed, and luciferase activity was determined as described [19], using a microtiter plate luminometer (LMaxII, Molecular Devices). EC50 was calculated from the sigmoidal analysis of percentage light emitted (compared to untreated macrophages) versus antimonate concentrations using SigmaPlot 11.0. Each assay was performed at least three times and the results are represented as mean ± SE.
2.10. Uptake assays
Log phase Leishmania promastigotes or axenic amastigotes were washed twice with phosphate-buffered saline (PBS), pH 7.4 (Invitrogen) and suspended in PBS at a density of 108 cells ml−1. Either promastigotes or amastigotes were then incubated with 10 µM As(III) or Sb(III) for 10 min at room temperature. A 0.2 ml portion was filtered through a 0.22-µm nitrocellulose filter and the filter washed once with 5 ml of ice-cold PBS. The filters were digested with 0.5 ml of concentrated HNO3 (69–70%) (EM Science) for 1 h at 70°C, allowed to cool to room temperature, and diluted with high pressure liquid chromatography grade water (Sigma) to produce a final concentration of HNO3 of approximately 3%, and then analyzed by a PerkinElmer SCIEX ELAN® DRC-e inductively coupled plasma mass spectrometer. Standard solutions were made in the range of 0.5–10 p.p.b. in 3% HNO3 using arsenic and antimony standards (Ultra Scientific). Each transport experiment was repeated at least three times with duplicate samples. Error bars were calculated from the mean ± SE.
2.11. Molecular modeling
The models were generated using SWISS-MODEL [20]. Crystallographic Object-Oriented Toolkit (COOT) was used for molecular modeling and to superimpose structures [21]. The final models were subjected to idealization using REFMAC [22] and the graphical images were rendered using PYMOL (http://www.pymol.org). The channel was calculated using CAVER [23].
3. Results
3.1. Effect of alteration of Ala163 on water and solute permeability
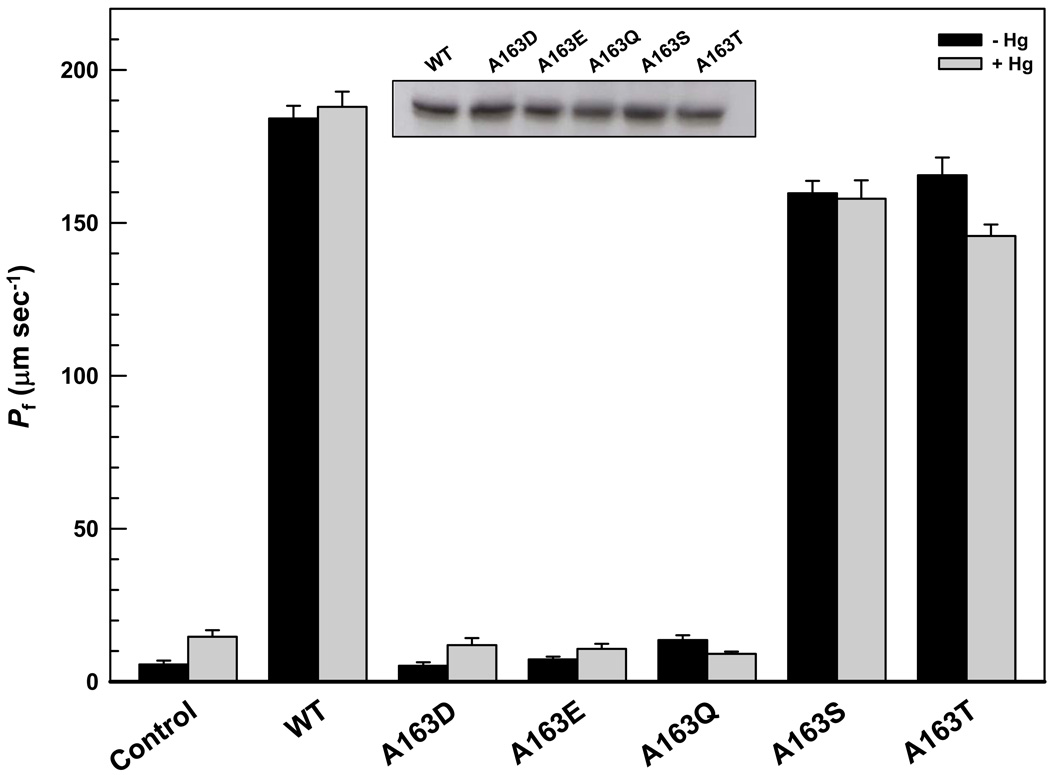
Ala163 in the C-loop was individually changed to aspartate, glutamate, glutamine, serine, and threonine by site-directed mutagenesis to produce LmAQP1 derivatives A163D, A163E, A163Q, A163S, and A163T, respectively. The choices of altered residues (or their conservative replacements) included those observed in other parasitic protozoan aquaglyceroporins, for example, PfAQP contains a glutamate in the equivalent position, whereas GlpF, TbAQP1, and TbAQP3 have a serine residue (Fig. 1). Xenopus laevis oocytes were injected with the mutant LmAQP1 cRNA, and expression of the altered proteins was monitored by Western blotting with a rabbit polyclonal antibody raised against a synthetic peptide corresponding to amino acids 139–152 of LmAQP1. Each of the point mutants of Ala163 were expressed in oocyte membranes at similar levels as that of wild-type LmAQP1 (Fig. 2, inset).
Fig. 2.
Swelling rates of Xenopus oocytes expressing LmAQP1 with C-loop mutations. Oocytes were injected with water (control), wild-type, A163D, A163E, A163Q, A163S and A163T LmAQP1-cRNA. Swelling rates of oocytes were determined before and after 5 min incubation in ND96 medium with 0.3 mM HgCl2 as described in Materials and Methods. Inset: Western blot analysis of membrane fractions of Xenopus oocytes expressing wild type and altered LmAQP1.
The water permeability of both wild-type and altered LmAQP1 was evaluated by expression in Xenopus oocytes. After 3 days of incubation at 16°C, oocytes expressing either the wild-type or altered LmAQP1 were subjected to hypotonic shock in 1:3 diluted ND96 medium, which established an outwardly directed osmotic gradient of 140 mOsm. Oocytes injected with wildtype LmAQP1 cRNA exhibited a ~20-fold (Pf ≈ 200 µm s−1) increase in swelling rate compared with the water-injected control (Fig. 2). Alteration of Ala163 to serine showed only <20% decline in water permeability compared to the wild-type (Fig. 2). Replacement of Ala163 with threonine also showed a similar effect as the A163S mutant (Fig. 2). In contrast, A163D, A163E, and A163Q LmAQP1 exhibited negligible water permeability that was similar as the water-injected control (Fig. 2).
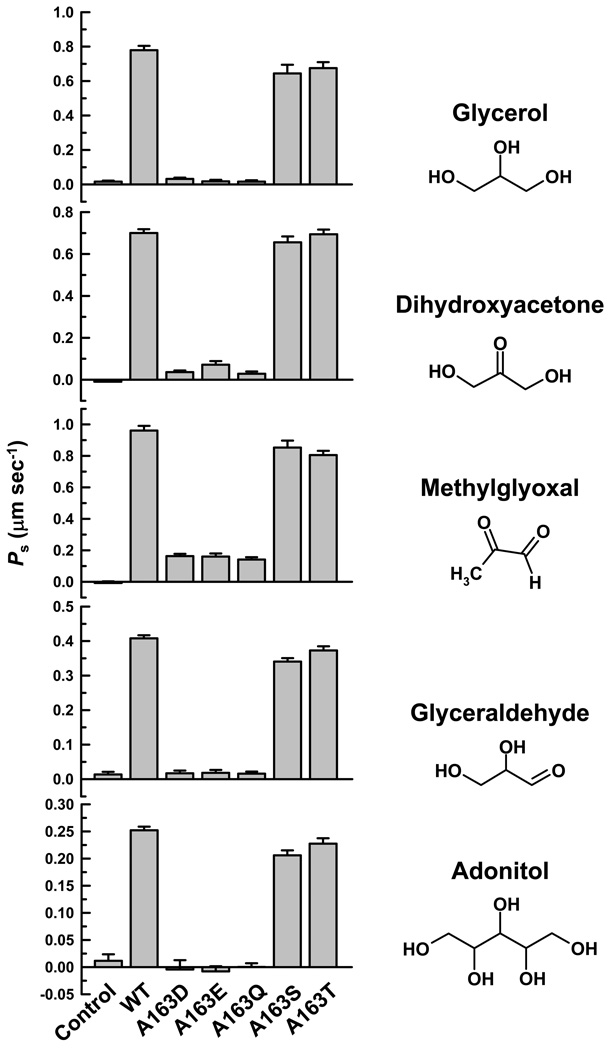
We have previously shown that LmAQP1-expressing oocytes also transport a variety of solutes such as adonitol, dihydroxyacetone, glyceraldehyde, glycerol, and methylglyoxal [2]. Glycerol permeability of the LmAQP1 mutants was determined by an isosmotic swelling assay with a 130 mM inwardly directed gradient of the solutes. This concentration gradient leads to an influx of solutes, resulting in a secondary influx of water, and consequent swelling of the oocytes. Injection of oocytes with either the wild-type or A163S or A163T LmAQP1 showed similar permeability for glycerol, methylglyoxal, dihydroxyacetone, glyceraldehyde and adonitol (Fig. 3). However, A163D, A163E, and A163Q LmAQP1 exhibited negligible permeability to glycerol, glyceraldehyde, and adonitol as the water-injected control, and showed >85% reduction in dihydroxyacetone and methylglyoxal permeability than the wild-type.
Fig. 3.
Solute permeability of Xenopus oocytes expressing altered LmAQP1. Oocytes were injected with water (control), wild-type, A163D, A163E, A163Q, A163S and A163T LmAQP1-cRNA. Solute permeability was measured as described in Materials and Methods.
3.2. Effect of alteration of Ala163 on metalloid sensitivity and uptake
Both wild-type and altered LmAQP1s were cloned individually into the Leishmania expression vector pSP72αhygroα. L. donovani strain LdBob was transfected with the resulting plasmids as described in the Materials and methods section. Ideally, an LmAQP1 null mutant should present a perfect background for expression of altered and wild type LmAQP1, and provide the best platform to study metalloid sensitivity and transport. However, despite several attempts we have not been able to generate the null mutant, which supports the idea of LmAQP1 being an essential gene for Leishmania survival and growth (R. Mukhopadhyay and M. Ouellette, unpublished observation).
Wild-type LmAQP1 is specifically localized to the flagellum of Leishmania promastigotes [2]. Flagellar membrane was isolated from Leishmania promastigotes expressing each of the altered proteins and the protein expression was monitored by Western analysis. Each of the point mutants of Ala163 were expressed at levels similar to that of wild-type LmAQP1 (Supplementary Fig. S3). As(III) and Sb(III) sensitivity was examined in Leishmania promastigotes while Sb(V) sensitivity was studied in intracellular amastigotes. Note that Sb(V) is taken up by macrophages, and is reduced to Sb(III), which is then transported into Leishmania amastigotes by LmAQP1. When compared with vector alone control, each of the transfected strains showed varying levels of sensitivity to As(III), Sb(III), and Sb(V) (Table 1). Promastigotes expressing A163S LmAQP1 were about 1.5- and 3-fold more resistant to As(III) and Sb(III), respectively, than cells expressing wild-type LmAQP1. Intracellular amastigotes expressing A163S LmAQP1 were 1.5-fold resistant to Sb(V) than wild-type. Promastigotes expressing A163T LmAQP1 were 6- and 12-fold more resistant to As(III) and Sb(III), respectively, whereas amastigotes expressing A163T LmAQP1 were 2.5-fold resistant to Sb(V) than wild-type. In contrast, promastigotes expressing A163D, A163E, and A163Q mutants showed near similar resistance to metalloids as the vector alone control, and were about 75- and 225-fold more resistant to As(III) and Sb(III), respectively, than cells expressing wild-type LmAQP1. Likewise, amastigotes expressing A163D, A163E, and A163Q LmAQP1 showed nearly similar resistance to metalloids as the vector alone control, and were 5-fold more resistant to Sb(V) than cells expressing wild-type LmAQP1.
Table 1.
Metalloid sensitivity of L. donovani expressing altered LmAQP1
| LmAQP1 | EC50 | ||
|---|---|---|---|
| [As(III)] µM | [Sb(III)] µM | [Sb(V)] µg/ml | |
| vector only | 14.5 ± 1.8 | 135 ± 8 | 300 ± 10 |
| wild type | 0.2 ± 0.03 | 0.6 ± 0.05 | 48 ± 2 |
| A163D | 15.8 ± 1.1 | 117 ± 15 | 260 ± 34 |
| A163E | 13.6 ± 0.7 | 110 ± 18 | 248 ± 22 |
| A163Q | 11.4 ± 0.5 | 128 ± 8 | 265 ± 21 |
| A163S | 0.3 ± 0.03 | 2 ± 0.2 | 70 ± 2 |
| A163T | 1.3 ± 0.03 | 7 ± 0.2 | 120 ± 5 |
EC50 values for As(III) and Sb(III) were determined in L. donovani promastigotes transfected with pSP72αhygroα vector bearing either the wild-type or mutant LmAQP1 gene. Values for Sb(V) were determined for intracellular amastigotes.
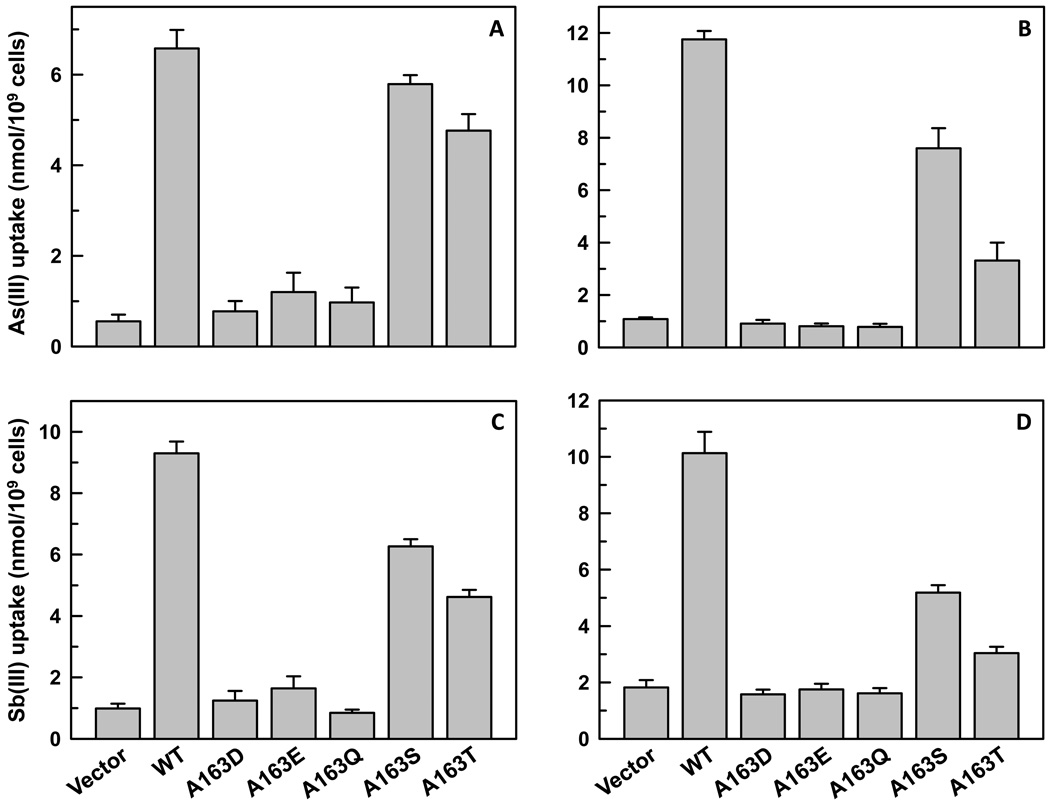
The permeability of Ala163 mutants to As(III) and Sb(III) was also investigated. Promastigotes expressing A163S LmAQP1 showed 85% and 65% of As(III) and Sb(III) uptake, respectively, than cells expressing the wild-type protein (Fig. 4A, 4C). Axenic amastigotes expressing A163S LmAQP1 showed 65% and 50% of As(III) and Sb(III) uptake, respectively, than cells expressing wild-type LmAQP1 (Fig. 4B, 4D). Promastigotes expressing A163T LmAQP1 showed a 30% decline in As(III) uptake and a 50% reduction in Sb(III) uptake, whereas, amastigotes showed a 70% turn down in As(III) uptake and a similar decline in Sb(III) permeability than cells expressing wild-type LmAQP1 (Fig. 4). In congruence with the EC50 data, alteration of Ala163 to aspartate, glutamine or glutamate resulted in negligible metalloid uptake in either promastigotes or amastigotes that was similar as the vector alone control (Fig. 4).
Fig. 4.
Metalloid uptake in L. donovani cells expressing wild-type or altered LmAQP1. (A) and (B) depict As(III) uptake in L. donovani promastigotes and amastigotes, respectively; (C) and (D) depict Sb(III) uptake in L. donovani promastigotes and amastigotes, respectively. Cells were transfected with wild-type, A163D, A163E, A163Q, A163S, A163T LmAQP1 or vector control.
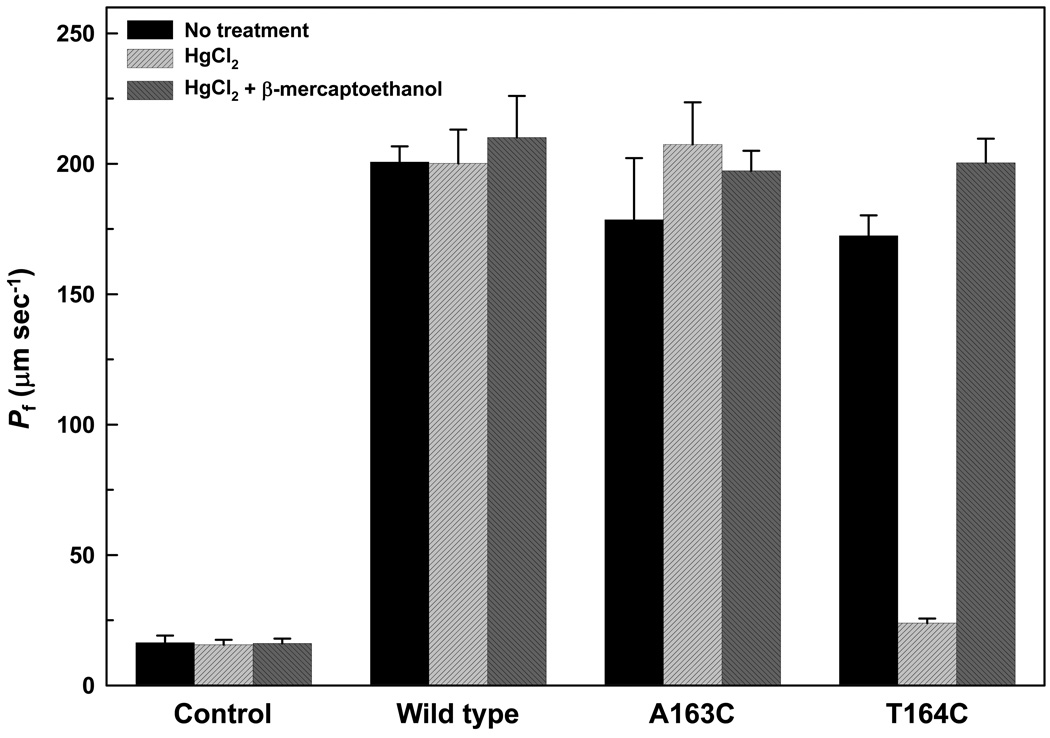
3.3. Effect of HgCl2 on the water permeability of A163C and T164C LmAQP1
We have earlier shown that treatment with HgCl2 does not affect the water permeability of oocytes injected with wild-type LmAQP1 cRNA, and consequently LmAQP1 is a mercurial-insensitive water channel [2]. Treatment with 0.3 mM HgCl2 did not affect the water permeability of oocytes injected with either A163S or A163T LmAQP1-cRNA (Fig. 2). The effect of Hg2+ on oocytes injected with A163D, A163E, or A163Q LmAQP1-cRNA was inconclusive as the untreated samples showed negligible water permeability (Fig. 2). To determine whether Ala163 is located near the pore mouth of LmAQP1, either Ala163 or its neighboring residue, Thr164, was individually altered to cysteine by site directed mutagenesis. Both mutants were expressed in Xenopus oocytes and were expressed in similar amounts as the wild-type (data not shown). Both A163C and T164C LmAQP1 exhibited similar water permeability as the wild-type (Fig. 5). Treatment with 0.3 mM HgCl2 did not affect the water permeability of oocytes injected with A163C LmAQP1-cRNA (Fig. 5). However, water passage through the channel was inhibited when oocytes injected with T164C LmAQP1 were treated with 0.3 mM HgCl2 for 5 min (Fig. 5). This inhibition was reversed upon addition of 3 mM β-mercaptoethanol (Fig. 5). These results indicate that Thr164 resides near the pore mouth of the channel. The results also indicate that LmAQP1 can be converted to a mercurial-sensitive water channel upon a single threonine to cysteine substitution.
Fig. 5.
Effect of Hg2+ on water permeation of A163C and T164C LmAQP1. Swelling rates of Xenopus oocytes were determined in the absence of Hg2+, after 5 min incubation with 0.3 mM Hg2+, and 5 min incubation with 3 mM β-mercaptoethanol following Hg2+ treatment. Oocytes were injected with water (control), wild-type, A163C, and T164C LmAQP1-cRNA.
4. Discussion
In the absence of a crystal structure for LmAQP1, we have used the information provided by the structure of PfAQP [6] to identify the contribution of individual residues at or near the channel pore, and tested their role in selective solute permeability. Beitz et al [7] have shown that the C-loop Glu125 is critically responsible for the high water permeability of PfAQP, and that alteration of Glu125 to serine selectively abolished water but not glycerol permeability. Sequence alignment indicates that Ala163 of LmAQP1 is located at an equivalent position as Glu125 in PfAQP (Fig. 1). Our objective was to determine, first, whether alteration of Ala163 of LmAQP1 selectively affects water or glycerol permeability; second, whether Ala163 resides near the pore mouth; and third, whether alteration of Ala163 effects the conduction of metalloid.
The role of Ala163 in LmAQP1 was investigated by mutating the alanine to aspartate, glutamate, glutamine, serine or threonine. Each of the altered proteins was expressed in normal amounts in Xenopus oocytes (Fig. 2, inset) and the flagellum of Leishmania promastigotes (Supplementary Fig. S3). Alteration of alanine to aspartate, glutamate or glutamine severely affected the solute permeability of the channel, and each of the mutants was impermeable to both water (Fig. 2) and glycerol (Fig. 3). We interpret this observation as Ala163 being uninvolved in stabilization of the C-loop of LmAQP1. This hypothesis is supported by the homology modeling of LmAQP1 based on the structure of either GlpF or PfAQP. Both models show that Ala163 is located in an unstructured region of the C-loop (Supplementary Fig. S2). Homology modeling also shows that Ala163 is not within short hydrogen bonding distance (<3.2 Å) with residues in equivalent position as Ser200 and Ser212 of PfAQP (Supplementary Fig. S4). The carbonyl oxygen of the adjacent phenylalanine residue (Phe162) of LmAQP1 forms two short hydrogen bonds (2.9 Å) with Arg230 (Supplementary Fig. S4). Also, the third hydrogen bond between Arg196 and Ser200 in PfAQP is not observed in this model, as LmAQP1 lacks the serine in the equivalent position and contains a proline (Pro234) instead (Supplementary Fig. S4). Whether Pro234, which is also conserved in other AQPs (Supplementary Fig. S1), participates in hydrogen bond formation with Arg230 needs to be examined. We hypothesize that alteration of Ala163 does not affect the hydrogen bonding network around Arg230. If so, we would have expected to observe selective permeability to glycerol.
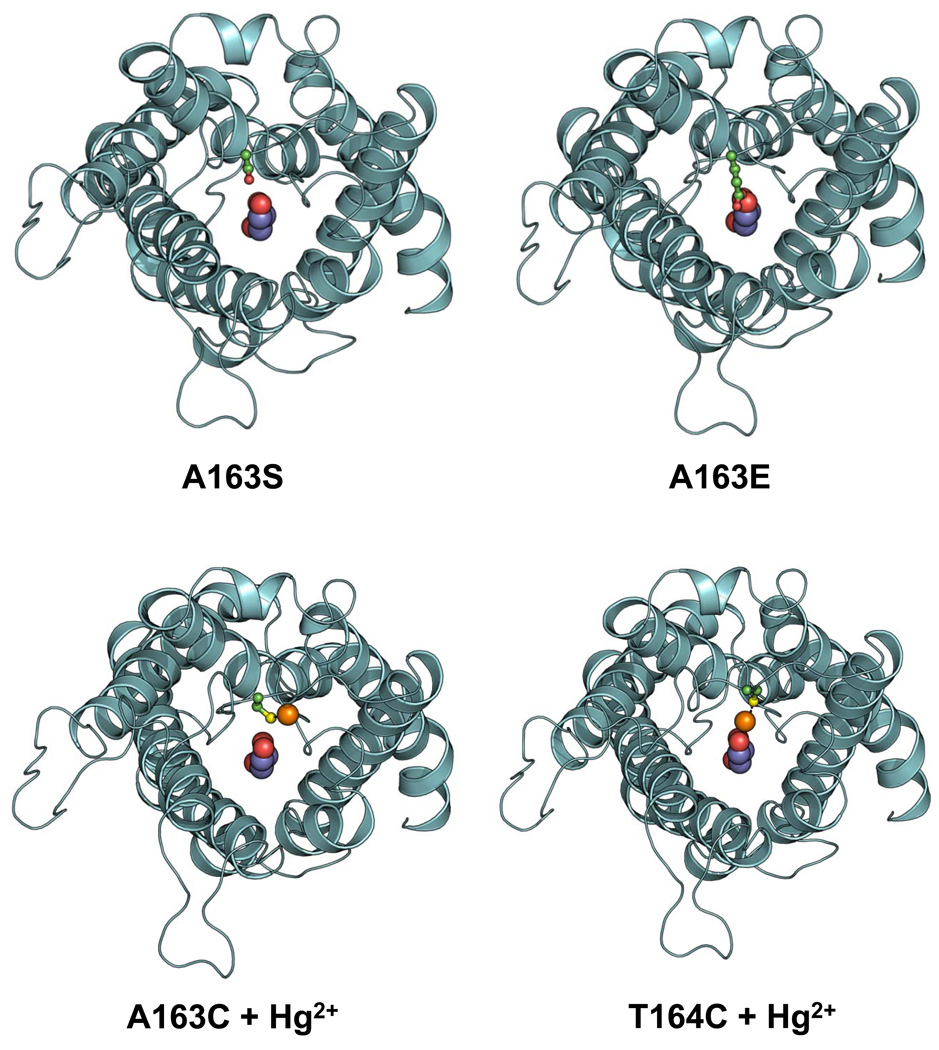
The homology model of LmAQP1 indicates that the side chain of Ala163 is pointed away from the channel, similar to Glu125 of PfAQP (Supplementary Fig. S4). However, mutational analyses show that, while alteration of Ala163 to either serine or threonine does not significantly affect the channel properties, mutation of Ala163 to aspartate, glutamate, or glutamine disrupts water and solute conductance. To determine whether Ala163 is located near the pore mouth, both Ala163 and its neighboring residue Thr164 was individually altered to cysteine by site directed mutagenesis. Water passage through A163C LmAQP1 was unaffected upon treatment with Hg2+ (Fig. 5). The possibility of Cys163 being buried inside the protein and therefore inaccessible to Hg2+ cannot be ruled out. However, while the T164C mutant showed similar permeability to water as wild type LmAQP1, water passage was completely blocked upon treatment with Hg2+. This blockage could be reversed upon treatment with β-mercaptoethanol, suggesting that Thr164 resides near the channel orifice (Fig. 5). Our interpretation is that Thr164, as well as Ala163, reside near the pore mouth. Since the C-loop of LmAQP1 is 12-residues longer than PfAQP, it provides added flexibility, allowing the side-chains of bulkier residues such as aspartate, glutamate, or glutamine to be directed towards the pore, causing steric hindrance, thereby affecting channel permeability (Fig. 6). Another interpretation would be that the loop is shifted in its position to accommodate the bulkier residues and that the shift occludes the pore. In contrast, serine and threonine substitutions, as well as the mercurated A163C mutant, are either pointed away or are not bulky enough to occlude the pore (Fig. 6). In conclusion, the data presented here suggests that, unlike Glu125 (PfAQP), Ala163 is not involved in stabilization of the C-loop and selective solute permeability. Ala163 is located near the pore mouth of the channel, and replacement of Ala163 by bulkier residue sterically hinders the passage of solutes.
Fig. 6.
Occlusion of the pore mouth upon alteration of Ala163 with bulky residue or upon Hg2+ treatment of T164C LmAQP1. Ribbon representations of A163S, A163E, and either Hg2+ treated A163C or T164C LmAQP1 are based on the structure of PfAQP, and viewed from the periplasmic side. The side chain of Ser163, Glu163, Cys163, and Cys164 is shown as ball-and-stick model with the carbon, oxygen, and sulfur atoms colored in green, red, and yellow respectively. A glycerol molecule is shown at the centre of the channel as a spherical model with the oxygen and carbon atoms colored as red and magenta, respectively. Hg2+ is shown as an orange sphere.
Leishmania promastigotes and amastigotes expressing A163S LmAQP1 showed 35% and 50% decline in Sb(III) uptake, respectively, than cells expressing wild type LmAQP1 (Fig. 4). Promastigotes expressing A163T LmAQP1 showed a 50% reduction in Sb(III) uptake, whereas, amastigotes showed a 70% decline in Sb(III) permeability than cells expressing wild-type LmAQP1. Since LmAQP1 appears to be an essential gene for Leishmania survival and growth, it is unlikely that mutations such as A163D, A163E, and A163Q that ablate channel permeability could play a role in resistance to metalloids. However, more moderate mutations such as A163S and A163T that reduce the permeability of LmAQP1 to As(III) and Sb(III), without any significant loss of water or solute conductivity might play a role in drug resistance. Such a possibility will be tested on antimony-resistant field isolates.
The study of protozoan AQPs may serve as a promising target for chemotherapy. An in-depth understanding of the extracellular pore mouth and delineation of the subtle differences in channel properties of LmAQP1 from that of human AQPs would lead to the development of small molecule inhibitors that can selectively target protozoan AQPs. We have previously shown that alteration of Glu152 to alanine selectively abrogates metalloid but not glycerol permeability [10]. Identification of other residues that play a role in water or solute conductance as well as metalloid permeability is currently in progress.
Supplementary Material
Acknowledgements
This work was supported by National Institutes of Health grants GM55425 and AI58170.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gourbal B, Sonuc N, Bhattacharjee H, Legare D, Sundar S, Ouellette M, Rosen BP, Mukhopadhyay R. Drug uptake and modulation of drug resistance in Leishmania by an aquaglyceroporin. J Biol Chem. 2004;279:31010–31017. doi: 10.1074/jbc.M403959200. [DOI] [PubMed] [Google Scholar]
- 2.Figarella K, Uzcategui NL, Zhou Y, Lefurgey A, Ouellette M, Bhattacharjee H, Mukhopadhyay R. Biochemical characterization of Leishmania major aquaglyceroporin LmAQP1: possible role in volume regulation and osmotaxis. Mol Microbiol. 2007;65:1006–1017. doi: 10.1111/j.1365-2958.2007.05845.x. [DOI] [PubMed] [Google Scholar]
- 3.Marquis N, Gourbal B, Rosen BP, Mukhopadhyay R, Ouellette M. Modulation in aquaglyceroporin AQP1 gene transcript levels in drug-resistant Leishmania. Molecular Microbiology. 2005;57:1690–1699. doi: 10.1111/j.1365-2958.2005.04782.x. [DOI] [PubMed] [Google Scholar]
- 4.Beitz E. Aquaporin water and solute channels from malaria parasites and other pathogenic protozoa. ChemMedChem. 2006;1:587–592. doi: 10.1002/cmdc.200500105. [DOI] [PubMed] [Google Scholar]
- 5.Fu D, Libson A, Miercke LJ, Weitzman C, Nollert P, Krucinski J, Stroud RM. Structure of a glycerol-conducting channel and the basis for its selectivity. Science. 2000;290:481–486. doi: 10.1126/science.290.5491.481. [DOI] [PubMed] [Google Scholar]
- 6.Newby ZE, O'Connell J, III, Robles-Colmenares Y, Khademi S, Miercke LJ, Stroud RM. Crystal structure of the aquaglyceroporin PfAQP from the malarial parasite Plasmodium falciparum. Nat Struct Mol Biol. 2008;15:619–625. doi: 10.1038/nsmb.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beitz E, Pavlovic-Djuranovic S, Yasui M, Agre P, Schultz JE. Molecular dissection of water and glycerol permeability of the aquaglyceroporin from Plasmodium falciparum by mutational analysis. Proc Natl Acad Sci USA. 2004;101:1153–1158. doi: 10.1073/pnas.0307295101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uzcategui NL, Szallies A, Pavlovic-Djuranovic S, Palmada M, Figarella K, Boehmer C, Lang F, Beitz E, Duszenko M. Cloning, heterologous expression, and characterization of three aquaglyceroporins from Trypanosoma brucei. J Biol Chem. 2004;279:42669–42676. doi: 10.1074/jbc.M404518200. [DOI] [PubMed] [Google Scholar]
- 9.Tsukaguchi H, Weremowicz S, Morton CC, Hediger MA. Functional and molecular characterization of the human neutral solute channel aquaporin-9. Am J Physiol. 1999;277:F685–F696. doi: 10.1152/ajprenal.1999.277.5.F685. [DOI] [PubMed] [Google Scholar]
- 10.Uzcategui NL, Zhou Y, Figarella K, Ye J, Mukhopadhyay R, Bhattacharjee H. Alteration in glycerol and metalloid permeability by a single mutation in the extracellular C-loop of Leishmania major aquaglyceroporin LmAQP1. Mol Microbiol. 2008;70:1477–1486. doi: 10.1111/j.1365-2958.2008.06494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goyard S, Segawa H, Gordon J, Showalter M, Duncan R, Turco SJ, Beverley SM. An in vitro system for developmental and genetic studies of Leishmania donovani phosphoglycans. Mol Biochem Parasitol. 2003;130:31–42. doi: 10.1016/s0166-6851(03)00142-7. [DOI] [PubMed] [Google Scholar]
- 12.Mukhopadhyay R, Zhou Y, Rosen BP. Directed evolution of a yeast arsenate reductase into a protein-tyrosine phosphatase. J Biol Chem. 2003;278:24476–24480. doi: 10.1074/jbc.M302610200. [DOI] [PubMed] [Google Scholar]
- 13.Seyfang A, Kavanaugh MP, Landfear SM. Aspartate 19 and glutamate 121 are critical for transport function of the myo-inositol/H+ symporter from Leishmania donovani. J Biol Chem. 1997;272:24210–24215. doi: 10.1074/jbc.272.39.24210. [DOI] [PubMed] [Google Scholar]
- 14.Papadopoulou B, Roy G, Ouellette M. A novel antifolate resistance gene on the amplified H circle of Leishmania. Embo J. 1992;11:3601–3608. doi: 10.1002/j.1460-2075.1992.tb05444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ouellette M, Fase-Fowler F, Borst P. The amplified H circle of methotrexate-resistant Leishmania tarentolae contains a novel P-glycoprotein gene. EMBO J. 1990;9:1027–1033. doi: 10.1002/j.1460-2075.1990.tb08206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sambrook J, Fritsch EF, Maniatis T. Molecular cloning, a laboratory manual. New York: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 17.Hansen M, Kun JF, Schultz JE, Beitz E. A single, bi-functional aquaglyceroporin in blood-stage Plasmodium falciparum malaria parasites. J Biol Chem. 2002;277:4874–4882. doi: 10.1074/jbc.M110683200. [DOI] [PubMed] [Google Scholar]
- 18.Carbrey JM, Gorelick-Feldman DA, Kozono D, Praetorius J, Nielsen S, Agre P. Aquaglyceroporin AQP9: solute permeation and metabolic control of expression in liver. Proc Natl Acad Sci USA. 2003;100:2945–2950. doi: 10.1073/pnas.0437994100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou Y, Messier N, Ouellette M, Rosen BP, Mukhopadhyay R. Leishmania major LmACR2 is a pentavalent antimony reductase that confers sensitivity to the drug pentostam. J Biol Chem. 2004;279:37445–37451. doi: 10.1074/jbc.M404383200. [DOI] [PubMed] [Google Scholar]
- 20.Arnold K, Bordoli L, Kopp J, Schwede T. The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics. 2006;22:195–201. doi: 10.1093/bioinformatics/bti770. [DOI] [PubMed] [Google Scholar]
- 21.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 22.Murshudov GN, Vagin AA, Dodson EJ. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr D Biol Crystallogr. 1997;53:240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- 23.Petrek M, Otyepka M, Banas P, Kosinova P, Koca J, Damborsky J. CAVER: a new tool to explore routes from protein clefts, pockets and cavities. BMC Bioinformatics. 2006;7:316. doi: 10.1186/1471-2105-7-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.