Abstract
Circadian rhythms modulate nearly every mammalian physiological process. Chronic disruption of circadian timing in shift work or during chronic jet lag in animal models leads to a higher risk of several pathologies. Many of these conditions in both shift workers and experimental models share the common risk factor of inflammation. Here we show that experimentally-induced circadian disruption altered innate immune responses. Endotoxemic shock induced by LPS was magnified leading to hypothermia and death after 4 consecutive weekly 6h phase-advances of the light-dark schedule, with 89% mortality compared with 21% in unshifted control mice. This may be due to a heightened release of pro-inflammatory cytokines in response to LPS treatment in shifted animals. Isolated peritoneal macrophages harvested from shifted mice exhibited a similarly heightened response to LPS in vitro, indicating that these cells are a target for jet lag. Sleep deprivation and stress are known to alter immune function and are potential mediators of the effects we describe. However polysomnographic recording in mice exposed to the shifting schedule revealed no sleep loss, and stress measures were not altered in shifted mice. In contrast, we observed altered or abolished rhythms in the expression of clock genes in the central clock, liver, thymus and peritoneal macrophages in mice after chronic jet lag. We conclude that circadian disruption, but not sleep loss or stress, are associated with jet lag-related disregulation of the innate immune system. Such immune changes might be a common mechanism for the myriad negative health effects of shift work.
Keywords: innate immunity, circadian rhythms, jet lag, shift work, LPS, sepsis, stress, endotoxemic shock, Period 2, cytokines, peritoneal macrophages, peripheral clocks, SCN
INTRODUCTION
Nearly 15% of the US working population engage in some sort of shift work (1), loosely defined to include static night shifts, flex shifts, extended shifts, rotating shifts, and frequent international travel by airline flight crews. The sleep-wake cycle of the shift-worker represents a significant departure from the world in which we evolved where light and dark alternate reliably. The internal circadian clock system evolved as an adaptation to this predictable day-night geophysical pattern, and the biological consequences of living at odds with the normal environmental day-night cycle are just beginning to be appreciated. Exposure to non-traditional work schedules has been linked with increased risk of colorectal (2), breast (3), lymphatic (4) and prostate (5, 6) cancers, as well as gastric ulcers (7, 8), obesity (9), diabetes (10, 11), stroke (11), coronary heart disease, atherosclerosis and heart attack (11, 12), (13, 14). The mechanisms for these correlations between shift-work exposure and disease are unknown, however it is important to note that one common risk factor shared by many of these pathologies is inflammation.
Shift work is a complex lifestyle that to some degree includes exposure to circadian disruption, sleep disruption, altered phase-angle of entrainment and psychosocial stress. However, simple experimental jet lag in rodents reproduces some of the detrimental aspects of this lifestyle. Chronic jet lag (CJL), in which animals are housed in light-cycles which are shifted to mimic eastward or westward travel at regular intervals ranging from 2 days to 7 days has been linked to more rapid tumor growth (15), cardiomyopathy (16), increased sensitivity to the intestinal irritant dextran sodium sulfate (17), and non-specific death (18).
One potential common feature of the negative health consequences of circadian disruption may be the disregulation of the immune system. Alterations of the sleep-wake cycle affect the number of circulating lymphocytes, natural killer cells and antibody titers in humans (19) and rodents (20–22) and increased inflammatory cytokines such as IL-6, C-reactive protein, and TNF-α (23–26). When a challenge to the immune system is presented, the effects of these changes translate into impaired immune function. Rats that were sleep deprived showed spontaneously increased bacteremia (27). Humans also exhibited altered cellular immune responses after sleep disruption (28, 29). In many of the studies using sleep disruption, it is difficult to distinguish between effects on the immune system that could only be attributed to sleep loss versus effects of desynchrony or suppression of circadian rhythms. A comprehensive understanding of the effects of circadian disruption on immune function is lacking, but evidence does exist for a bidirectional relationship between the immune system and circadian timing. For example, immune stimulation by the bacterial cell wall component lipopolysaccharide (LPS) can alter circadian timing (30). LPS can shift the clock (31), and can alter the expression of several circadian clock genes such as Per1 (32, 33), Per2, and dbp (33). It has been suggested that the effects imposed on circadian timing by the innate immune system are mediated by pro-inflammatory cytokines in the brain (34).
Not only do immune signaling molecules affect circadian rhythms, the circadian system regulates the immune system. Circadian regulation is reported for many immune markers, including IL-2, IL-10, GM-CSF, CCR2, IL-6, IL-1β, TNF-α, MCP-1JE, IFN-γ, and IFN receptors (35–38). Genetic manipulations of circadian timing can modulate innate immunity. For example, the rhythm in IFN-γ seems to be absent in Per2 mutant mice (39) which are also deficient in their ability to produce IL-10 and IFN-γ in response to LPS (40). A dramatic phenotype of early aging and associated chronic inflammation results from the knockout of Bmal1, a uniquely non-redundant component of the circadian clock. Among other pathologies, Bmal1 KO mice develop progressive corneal inflammation and exhibit decreased numbers of lymphocytes (41). Additionally, mice lacking both clock genes Cry1 and Cry2 exhibit exacerbated cytokine production and joint swelling after arthritic induction (42). Importantly, macrophages display endogenous rhythms in clock gene expression (38, 43), phagocytosis (38) and LPS sensitivity (43).
Regulation of inflammation by the immune system may underlie the pathologies associated with circadian desynchrony. However, much remains unknown regarding the specific effects of circadian disruption on immune function and what in fact is the role of sleep deprivation in these effects. In the present study, we provide evidence which suggests that chronic circadian desynchrony targets the immune system and its ability to adequately regulate inflammation, and that this response is independent of sleep loss or stress.
MATERIALS & METHODS
Animals and housing conditions
Per2-luc knock-in mice (44), on a C57BL/6 background, 4 to 8 months of age from our colony at Morehouse School of Medicine were the subjects in this study. All animals were entrained to a LD 12:12 cycle from birth until the beginning of the experiments with water and food available ad libitum. During lighting manipulations and during the immune challenge, mice were singly-housed in polypropylene cages with no running wheel, inside light-tight boxes with overhead fluorescent lights of an intensity ranging from 200 to 400 lux at the level of the cage. All procedures and protocols were approved by the Morehouse School of Medicine Institutional Animal Care and Use Committee.
Core Body Temperature (CBT) and locomotor activity recording
At least three weeks before any light manipulations occurred, G2 E-mitters (Phillips-Respironics, OR) were implanted abdominally under isofluorane anesthesia. The telemeters allowed for continuous recording of general locomotor activity and core body temperature (Tb) before and during the immune challenge. Tb and locomotor activity recording was initiated 7 days before the LPS injection (the final week of the shift schedule), and continued for 7 days after the challenge was presented. Mice were not chronically recorded for the entire shift schedule due the limited number of telemetry receivers.
Chronic jet lag protocol
The six hour phase advance schedule is described in (18). Briefly, the 6 h phase advance was achieved by shortening the dark period on the day of the shift. The next shift is administered on the night of the 7th day following. To compare acute versus chronic effects of our lighting manipulation, some groups of mice were exposed to a single phase advance and allowed to acclimate to the new lights for 1, 2, or 4 weeks before challenge, while other sets of animals were exposed to the phase advance once every week during four consecutive weeks. After the last shift, all groups of mice remained in the same photoperiod for at least 7 days of resynchronization before presentation of the immune challenge.
In vivo immune challenge and cytokine measurements
Ultrapure LPS from E.coli (0111:B4; Invivogen, CA; 12.5 mg/Kg) diluted into sterile PBS was injected i.p. into shifted mice (both 1 and 4 shifts groups) at Zeitgeber Time (ZT) 3 (3 hrs after lights-on) on the 7th day after the last photoperiod change. Control mice were also injected with LPS at the same ZT. Body temperature, locomotor activity and survival were recorded during the 7 days following the injection in some mice, while others with identical light history were bled for cytokine measurements. Serum from shifted mice (4 shifts) and unshifted controls was separated from blood collected by retro-orbital bleeding under 2% isoflurane anesthesia at 90 min and 24h after the LPS injection. Blood was collected in vacutainer separator tubes (BD, NJ), allowed to clot for 30 minutes at room temperature, and then centrifuged at 9300 rpm for 15 minutes. Serum aliquots were collected and stored at −20°C until analyzed. Cytokines levels were determined using a Milliplex MAP (Millipore, MA) Kit on a Luminex xMAP platform according to the instructions of the manufacturer. Isolated IL-6 measurements from serum were obtained by an ELISA kit (R&D Systems, MN. Part # M6000B).
In a second experiment we compared the in vivo cytokine release in response to a smaller, 5mg/kg sublethal dose of LPS after 1 shift and 4 shifts, but varied the duration of time the mice were allowed to adjust to the phase shift. We used serum levels of 4 macrophage-derived cytokines, measured at 24h post-injection as our readout.
Macrophage (exudate) cultures
Shifted and control mice were sacrificed under 100% CO2 anesthesia, then soaked with 70% ethanol and a small incision in the medial abdominal section was made through the skin. Next, 10 ml of 5% FBS in ice cold PBS were injected into the abdominal cavity with a 26G needle allowing the cavity to seal itself when the syringe was retracted. The mouse was shaken vigorously for 30 seconds and then the peritoneal exudate cells were withdrawn in solution by extracting the FBS-PBS mix using a 21G needle. Cells were collected into 15 ml tubes, centrifuged at 1500 rpm for 5 minutes, washed two times with RPMI media containing 10% FBS and seeded into 35 mm dishes. The macrophages were allowed to adhere overnight inside an incubator at 37°C under 5% CO2 according to (45). On the next morning, cells were washed with PBS to discard dead and non adherent cells and fresh RPMI media containing 10% FBS and LPS (10 μg/ml) was added for stimulation. Supernatant (200μl) was collected at times 0, 3, 6, 12, 24 and 48h after LPS stimulation; and then frozen at −20°C for further IL-6 quantification by ELISA (R&D Systems, MN. Part # M6000B). Four high power fields (HPF; at 20X magnification) were taken from each dish to quantify macrophages during the course of the LPS stimulation. The IL-6 measurements were normalized against the average cell count per HPF.
Sleep recording during experimental jet lag
Electroencephalograph (EEG) and electromyograph (EMG) electrodes were surgically implanted in 6 mice for polysomnographic recording of sleep and wake states. Two stainless-steel recording screws (SmallParts Inc. Miami Lakes, FL) were positioned contralaterally to each other on the skull surface. The first was located 1 mm anterior to Bregma and 0.5 mm right of the central suture, whereas the second was located 0.5 mm posterior to Lambda and 1 mm left of the central suture. A subcutaneous pocket was created caudally along the dorsal surface using the incision for electrode implantation. A PhysioTel telemeter (Model F20-EET; Data Science International) was placed in this pocket. EEG leads from the telemeter were attached to the electrode screws with silver epoxy and the assembly covered with a small amount of dental acrylic. EMG leads from the telemeter were inserted bilaterally into the nuchal muscle. To aid the insertion, a 22 gauge needle was used as a trochar. Leads were sutured to the muscle tissue near the point of entry (6-0 braided silk). All mice were allowed to recover for at least 3 weeks prior to recording. First, one day of baseline sleep recording was performed. Mice were then placed on a CJL schedule consisting of a 6h phase advance every week during four weeks. During the schedule, sleep was recorded continuously during the first two weeks and during week four. Polysomnographic recordings were made via a receiver placed under the cage and connected to a PC with DataQuest ART hardware and software (Data Science International).
Behavioral and hormonal measurements of stress
2–6 month-old or 15 month-old c57bl/6j mice were housed in weekly phase-advancing light cycles. A group of young (n=4 shifted, 4 control) and aged (n=17 shifted, 19 control) were sacrificed after 4 weeks or 12 weeks of shifting, respectively. Mice were bled from the orbits under isoflurane anesthesia (aged), or from the trunk following decapitation with no anesthesia (young). Serum was isolated, diluted and assayed for corticosterone using enzyme immunoassay (IDS Inc., Scottsdale, AZ, USA) or elisa (B-Bridge International, Inc., Cupertino, CA, USA) according to the manufacturer’s instructions. Behavioral tests were performed on another set of mice (n=14 shifted, 14 control) on the eighth day after the fourth shift. First, total ambulatory distance was measured in an plexiglass open field apparatus with photobeam detectors (Med Associates Inc., St. Albans, VT, USA) for a total duration of 10 min. Half of the mice from shifted (n = 7) and control (n = 7) conditions were then tested in a open-fronted tail suspension apparatus, in which mice were suspended from their tails for 6 min by adhesive tape to a vertical metal bar equipped with a strain gauge (software and equipment from Med Associates Inc., St. Albans, VT, USA). The other half of the mice from each condition (n = 7 per condition) were then tested in a forced swim apparatus (Kinder Scientific, Poway, CA, USA) consisting of clear plexiglass cylinders filled with distilled water (23–25°C) and equipped with photobeam detectors. For both tail suspension and forced swim tests, immobility time was calculated as the total time of resting (no beam breaks) during the last 4 min of testing. Data were analyzed via independent samples t-tests using PASW Statistics 18 (SPSS, Inc. Chicago, IL, USA).
Gene expression measurements
Bioluminescence recording of circadian rhythms ex vivo
Both control and shifted mice (6 days after one or four shifts), were sacrificed at ZT 3 (the typical LPS injection time) and the suprachiasmatic nucleus (SCN), liver, spleen, and thymus were explanted and prepared for organotypic culture in a lumicycle apparatus (ACTIMETRICS, IL) as previously described (46). These data were analyzed for both phase (Figure 1B) and for other circadian parameters (Figure 6A&B; 4-week and control only).
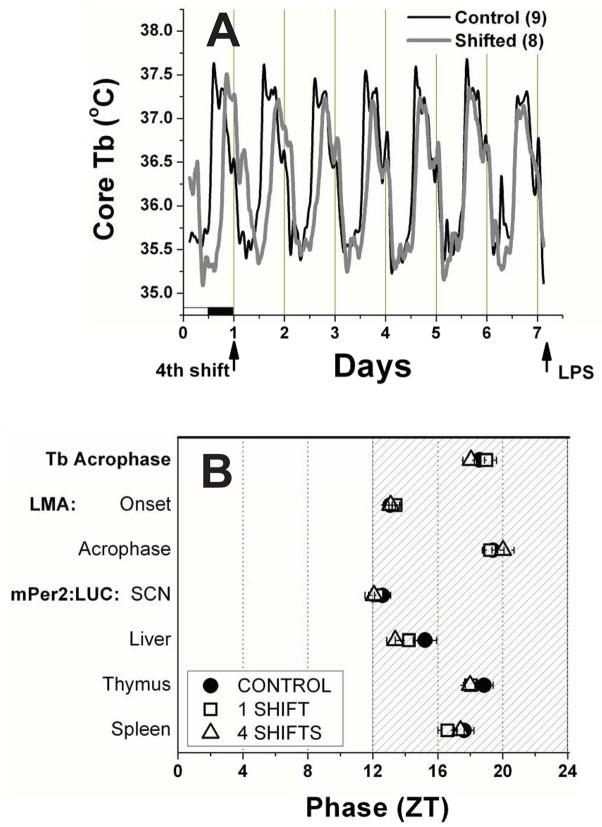
Figure 1. Resynchronization after 1 or 4 weekly 6h phase-advances.
A. Mean group core Tb from mice resynchronizing to a 4th 6h advance shift of the light-cycle, and control unshifted mice. The current light-dark cycle for both groups is shown at the bottom of Day 1. Note the initial 6h phase difference on Day 1, and the gradual leftward adjustment made by the shifters, until the two records overlay each other by the last day, indicating synchronization. B. A map of 7 phase markers in shifted and control mice. Shifted mice were analyzed for Day 6 after the first or the 4th advance shift. The LPS challenge occurred on Day 7. All measures except for locomotor activity (LMA) onset are the time of the rhythm peak.
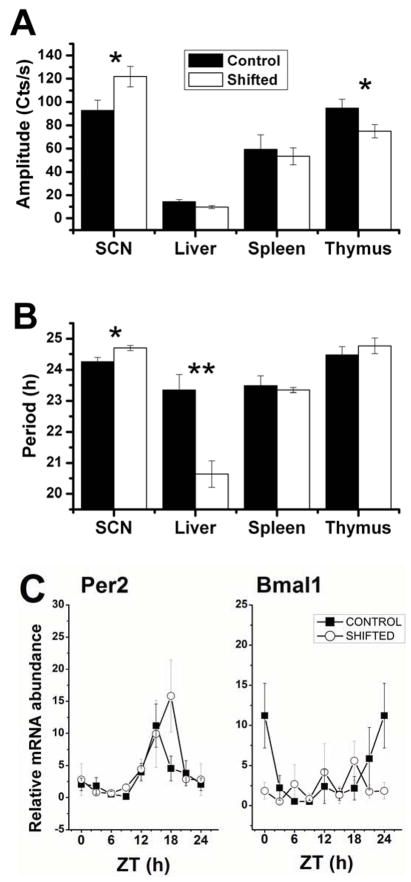
Figure 6. Altered molecular rhythms in central and peripheral tissues following CJL.
Mean (±SEM) circadian amplitude (A) and period (B) of mPer2:LUC rhythms for 4 tissues in vitro, harvested from 4-week shifted versus control mice. * p<0.05; ** p<0.01, Students t-test. C. Relative mRNA abundance of Period2 and Bmal1 in peritoneal macrophages harvested from shifted and control mice at 8 daily time points. ANOVA: Per2 Control p<0.01, Shifted: p<0.02; Bmal1 Control p=0.06, Shifted p=0.64; n=3–4 per time point for all groups.
Quantitative Real-time PCR
On the 7th day after the last photoperiod change, peritoneal macrophages were collected from shifted and control mice. RNA was promptly extracted in Trizol reagent (Invitrogen) and cDNA was prepared by using the RT2 EZ first strand cDNA kit from SABiosciences (Prod. No. C-09) according to the manufacturer’s instructions. cDNA was amplified on a BIORAD CFX-96 real time system using SYBR Green qPCR master mix from SABiosciences (Prod. No. PA-010). Specific primers against Per2 (Prod. No. PPM25497E), Bmal1 (PPM25679E) and 18S rRNA (PPM57735E) were also purchased from SABiosciences. Relative expression of clock genes was normalized to the 18S rRNA levels.
Data analysis
Acrophase of the diurnal rhythms of locomotor activity on Day 6 after the 1st and 4th shifts were calculated by a least squares cosine fit to the raw data in 10min bins (Cosinor analysis, El Temps 1.236, Barcelona, Sp; www.el-temps.com). Activity onset on Day 6 was the rising 24 mean crossing (50%) of daily activity counts. CBT peak time was the peak calculated by the SinFit (damped) algorithm in Lumicycle Analysis (Actimetrics). Phase values are all expressed in ZT.
Analysis of hypothermia during the first part of the immune challenge was achieved by collapsing temperature data into 6h averages from hours 6 to 30 after LPS injection. Significant differences were determined by repeated measures ANOVA. Statistical analysis of survival was performed using the Cox Proportional Hazard model in OriginPro 8 (Originlab, Northampton, MA). Cytokines levels are expressed as pg/ml and statistical differences were determined by Student’s t-test or one-way ANOVA. Sleep waveforms were classified by hand in 10-second epochs as wake (low-voltage, high-frequency EEG; high-amplitude EMG), NREM sleep (high-voltage, mixed-frequency EEG; low-amplitude EMG) or REM sleep (low-amplitude EEG with a predominance of theta activity (6–10 Hz); very low amplitude EMG), using Neuroscore software (Data Science International). Total sleep, vigilance states, sleep architecture, and sleep fragmentation were compared among groups by one-way ANOVA and Tukey’s post hoc test. Amplitude and phase values of ex vivo circadian rhythms were calculated by means of the SinFit (damped) algorithm in lumicycle analysis software. Statistical comparison of circadian rhythm amplitude and period among groups was carried out using Student’s T-test. RNA relative abundance was quantified by the comparative 2−ΔΔCt method as published by Livak and Schmittgen (47). To compare between groups (control vs. shifted) relative RNA levels of each individual sample was normalized to the 24h average value of the controls.
RESULTS
Circadian rhythms are resynchronized at the time of the immune challenge
Using a chronic jet lag (CJL) schedule of once-per-week 6h phase advances, we challenged CJL-exposed and control mice to an LPS immune challenge. Because the sensitivity to LPS in mice is dramatically affected by the phase at which the challenge is presented (48, 49), we needed to first explore whether 7 days after the last photoperiod change was enough time for mice to resynchronize diurnal rhythms before they were injected with LPS. The mean temperature curves for shifters and controls during resynchronization to the 4th shift of the LD cycle are shown in Figure 1A (Shifters (Sh) n=8; Controls (Con) n=9). The gradual leftward adjustment day over day is apparent in the shifting group, as they re-entrain to the original LD cycle, on which the control mice have been consistently housed. By the last day shown, just prior to the injection the following morning, the curves coincide, indicating that the body temperature rhythms of the shifted mice has fully re-entrained to the new light:dark cycle. Also, note that the amplitude and waveform of the body temperature curves throughout this record are highly similar between these two groups, suggesting thermoregulation is normal during re-entrainment.
A more comprehensive analysis of phase is provided in Figure 1B, where the phases of locomotor activity (Sh1: n=6; Sh4: n=8; Con: n=15), body temperature (Sh1: n=6 Sh4: n=8; Con: n=15), and Per2:luciferase rhythms from 4 tissues(Sh: n= 4–9 per group; Con: n=7–10) are all compared for Day 6 of the 1st and 4th shifts. All phase markers were statistically similar among shifted and unshifted mice on this day just prior to challenge. This was true for both 1 shift and 4 shifts, indicating that the chronic nature of the 4 week shifting schedule does not impair the capacity of mice to re-entrain to the shifted light:dark cycle,
LPS injection results in persistent hypothermia and arrhythmicity after 4 shifts
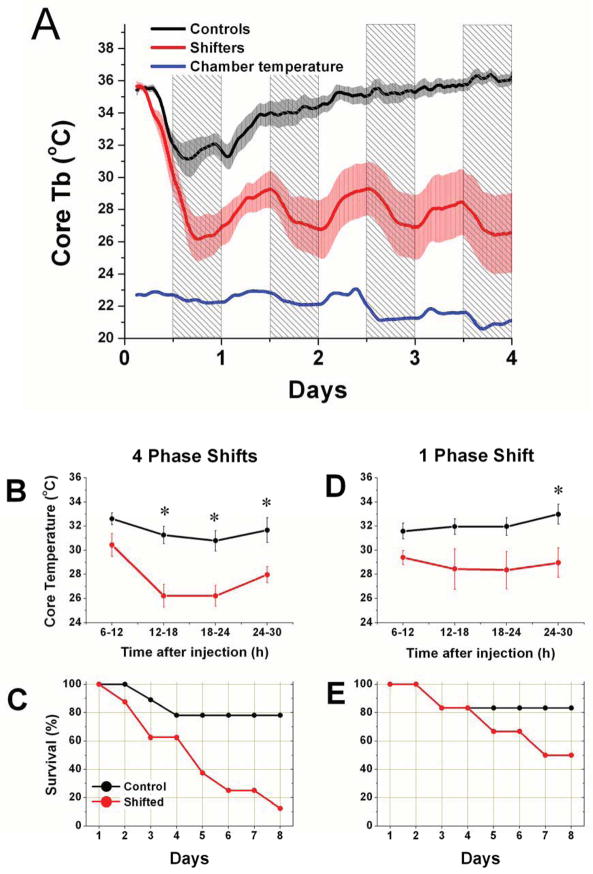
After 4 weeks of chronic jet lag, LPS challenge (12.5 mg/Kg) caused deep and persistent hypothermia, accompanied by loss of the nocturnal increase in body temperature (Fig. 2A; Shifters n=5). Control mice (n=7) became arrhythmic, and exhibited less pronounced and less persistent hypothermia. Shifters exhibited an apparently driven rhythm in body temperature, with a peak during the day that was coincident with the environmental temperature inside the chambers (Fig. 2A, blue line). This rhythm was present even after animals had perished. When collapsed over 6h intervals, body temperature in shifters was significantly lower than in controls (Tukey post-hoc pairwise comparisons, p<0.05) during the 2nd, 3rd and 4th intervals (Fig. 2b). Although the mice were recorded for 7 days after injection, later time points were not analyzed in this fashion since most of the shifters died. The survival curves for the 2 groups are shown in Fig. 2C, which indicates a remarkable difference in survival. The Cox Proportional Hazards Model (PHM) estimate of risk of death for shifters was 5.7x higher than controls (p=0.032).
Figure 2. The effects of LPS challenge after chronic jet lag.
A. Mean core body temperature (±SEM) after LPS challenge in control (black) and 4-week CJL-exposed (red) mice. Chamber temperature is shown in blue to indicate the increase in environmental temperature during the light phase of each day. The nighttime is indicated by shading. B. Average temperature in 6h time bins was calculated for each mouse for the 2 days after LPS injection, and then group means (±SEM) were compared. * p<0.05. C. Survival curve for 4-week shifters and controls after LPS challenge. D. 6h temperature comparison after LPS challenge for mice exposed to 1 phase shift, compared against control mice. Same conventions as B. E. Survival curve for LPS challenge after 1 shift. Same conventions as C.
A single phase shift also resulted in lower mean temperature relative to controls, but only at the 4th 6h time-point (Fig. 2D; shifters n=5; cont n=5). Survival appeared lower for shifters (Fig. 2E), but was not statistically different among the groups according to the Cox PHM or Chi square goodness-of-fit for Day 7.
Disregulation of inflammation during jetlag
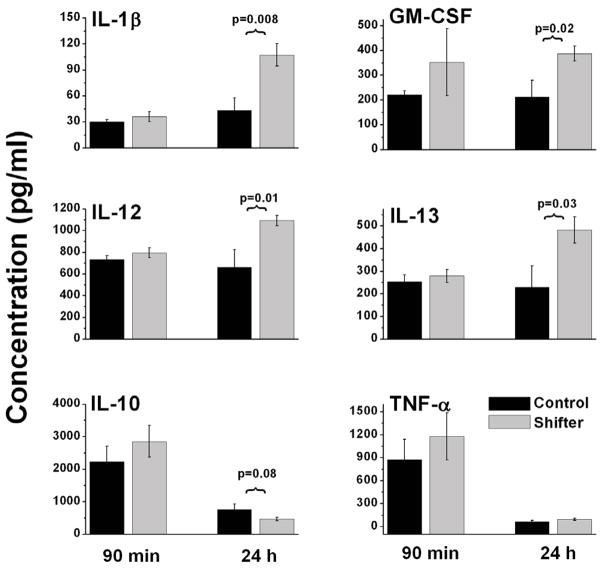
We next attempted to determine whether cytokine signaling was altered in 4-week shifters that were challenged with LPS compared with unshifted controls. We measured serum levels of a variety of both pro- and anti-inflammatory markers using xMAP multianalyte ELISA. Figure 3 shows systemic levels of six different cytokines measured at two time points during the inflammatory response to LPS. At 90 minutes following LPS challenge (12.5 mg/Kg), no significant difference was found in any of the cytokines analyzed between shifters and controls. However, at 24h, pro-inflammatory cytokines including IL-1β, GM-CSF, IL-12, and IL-13 were all significantly increased (p < 0.05, t-test, n=8 shifters; n=5 controls) compared to levels found in unshifted LPS-injected mice. One anti-inflammatory molecule, IL-10 trended lower (p = 0.08, t-test) in shifted mice 24 h after LPS injection, and TNF-α, an early stage pro-inflammatory cytokine was not different between control and shifted mice at either time point tested.
Figure 3. Disregulated response of the innate immune system during LPS challenge due to chronic jet lag.
Mice exposed to 4 weekly 6h advances of the light-cycle and unshifted control mice were injected with LPS, then bled at 90min or 24h. Cytokines were quantified in serum using a Milliplex MAP assay kit. IL-1β: Interleukin 1-beta; GM-CSF: Granulocyte-macrophage colony-stimulating factor; IL-12: Interleukin 12; IL-12: Interleukin 13; IL-10: Interleukin 10; TNF-α: Tumor necrosis factor alpha.
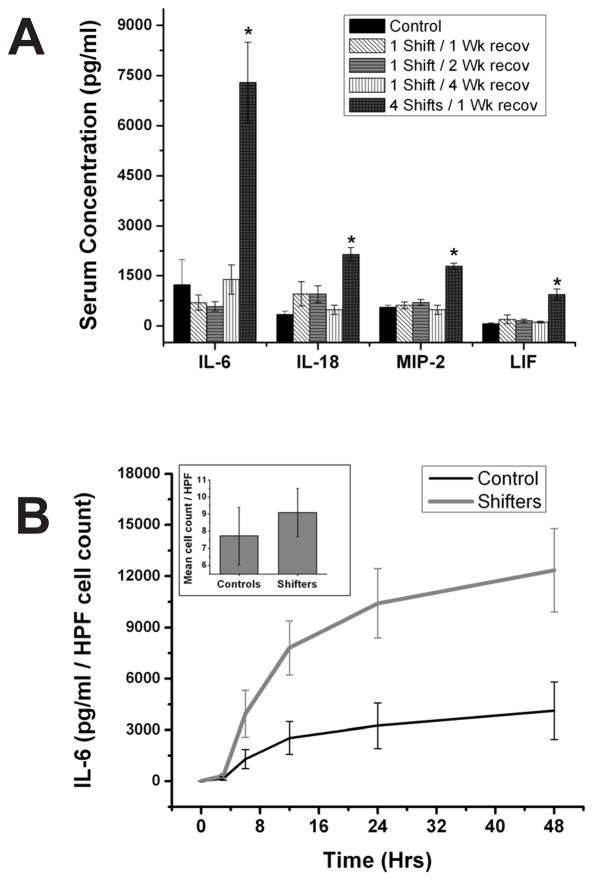
We next used a smaller LPS dose (5 mg/Kg) to investigate if the effects of a single shift of the light dark schedule were long lasting and contributed to the heightened immune response we observed after four consecutive shifts. The smaller dose was used to prevent severe sepsis and death, but still trigger a robust response. We found that when this dose was injected one week after a single phase-advance, shifted mice exhibited a similar IL-6 response to controls (Fig. 4A; n=5–8 per group). Furthermore, allowing 2 weeks or 4 weeks to pass after the single shift before challenging with LPS did not phenocopy the 4-shift response for any of 4 cytokines tested, confirming that multiple shifts are necessary for robust effects of circadian disruption on the immune system. Indeed, even with the smaller dose of LPS a robust increase in the response is seen between 4-week shifted mice and controls for IL-6, IL-18, MIP-2 and LIF.
Figure 4. Ontogeny and cellular targets of immune changes following CJL.
A. To determine whether 4 shifts are actually necessary to produce changes in the immune response, mice were shifted once, then allowed to recover for 1 week, 2 weeks or 4 weeks before being injected with 5mg/kg LPS. Control unshifted mice and 4 week-shifted mice are shown for comparison. A single shift produced no significant change in the serum responses of IL-6, IL-18, Macrophage inflammatory protein-2 (MIP-2) or leukemia inhibitory factor (LIF) following this smaller dose of LPS, after any recovery period. The 4-week shifted group exhibited increased levels of all of these factors after challenge relative to controls. B. Peritoneal macrophage quantity did not differ statistically (p > 0.05) among 4-week shifters versus controls (inset), but stimulation of these cultures with LPS induced higher IL-6 release in vitro from cells harvested from shifted mice. HPF: high-powered field (for cell counting).
Peritoneal macrophages in vitro
Since macrophages represent a primary source of cytokines involved in the initial response to LPS, we sought to determine if macrophages are a target for the effects of CJL on the immune response. Peritoneal exudate cells, comprised mostly of macrophages (45), were harvested and cultured from mice 7 days after the last of 4 weekly phase-advances. The number of macrophages we were able to collect was not statistically different between shifted and control unshifted mice (Fig. 4B, inset; p>0.05; n=8 shifters; n=8 controls). The in vitro cytokine response of those cells to LPS, even when normalized to cell number, was dramatically heightened in cultures from shifted mice (Fig. 4B, 2-way ANOVA; group p<0.01; time p<0.01; n=8 shifters; n=8 controls), indicating that CJL made macrophages more responsive to this bacterial product.
Acute or chronic jet lag does not result in sleep loss
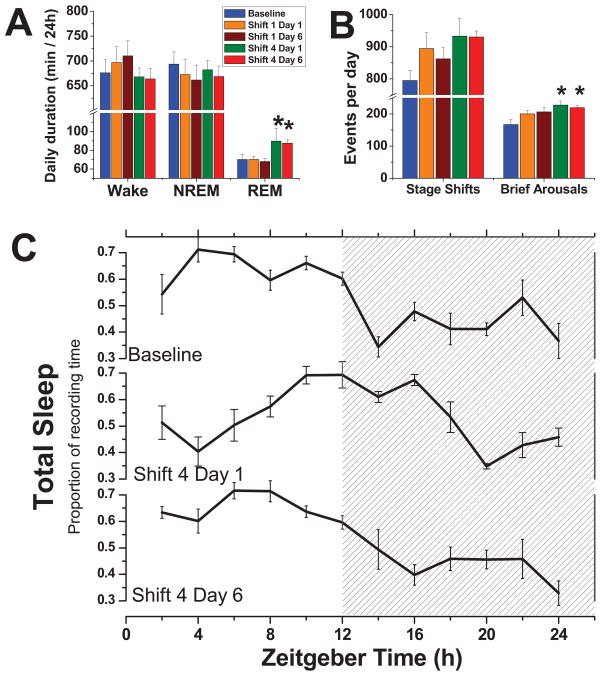
We performed polysomnography to determine if weekly 6h phase-advances results in sleep deprivation. During 24 hrs of baseline recording, mice exhibited spontaneous amounts of wake (676.2 ± 26.1 min), NREM sleep (693.8 ± 24.5 min), and REM sleep (70.2 ± 5.7 min) that were similar to previous reports (50, 51), suggesting that they began the protocol with optimal sleep/wake amounts. The circadian distribution of total sleep (457.2 ± 22.5 min during the light phase and 306.8 ± 14.3 min during the dark phase), sleep efficiency and sleep consolidation were also similar to previous reports. The 24h average quantity of NREM sleep and wake during the first and fourth phase-shift of Days 1 and 6 were similar to baseline (Fig 5A; Shifters n=6; Controls n=6). Rather than sleep loss, we observed an increase of REM sleep on Days 1 and 6 of Shift 4 (Holm-Sidak Post Hoc, p<0.05 vs. control). Two measures of sleep fragmentation, stage shifts and brief arousals, are presented in Figure 5B. When compared to baseline recordings on the same mice, only brief arousals (defined as a wake bout with duration <20s) were significantly increased on Days 1 and 6 of Shift 4 (Tukey’s Post Hoc, p<0.05). In Figure 5C, we illustrate the distribution of total sleep (sleep architecture) during the 24 h day during the course of resynchronization on Days 1 and 6 of Shift 4. In the middle panel (Day 1 in the newly advanced light-cycle), the phase of the sleep rhythm appears about 6h delayed relative to the baseline peak (top panel). By Day 6 (bottom panel), and still 24 h before LPS injections, the shift appears nearly complete. Supplemental Figure S1A depicts the same transitory phase shift in total sleep, but after just a single phase advance. Supplemental Figures S1B and S1C show the same data of total sleep after one and four shifts respectively, but both Days 1 and 6 have been adjusted as if the shift was completed to illustrate the absence of changes in sleep architecture (waveform) during resynchronization to an advanced LD cycle.
Figure 5. Chronic jet lag does not cause sleep deprivation.
A. Vigilance states were quantified for each day shown: baseline recording, the first and 6th day of Shift 1, and the first and 6th day of Shift 4. * p<0.05; Tukey post-hoc comparison. B. Fragmentation measures for each day. Same conventions as A. C. Mean (±SEM) waveform per group for total sleep for the baseline recording, and the first and 6th day of the 4th shift. Nighttime is indicated by shading
Chronic jet lag does not increase hormonal or behavioral measures of stress
In order to determine if our CJL paradigm induces stress, which can in turn impact immune function, we measured serum corticosterone and behavioral measures of anxiety and depression following CJL in 3 separate experiments (Figure S2). No significant differences were observed between shifted and control mice after 4 or 12 weeks of CJL in either hormonal or behavioral measures.
Central and peripheral rhythms are altered by CJL
The amplitude and period of the circadian rhythm in mPer2:luciferase was analyzed for 4 tissues (n=7–10 per tissue and group) from shifted (4 shifts) and control mice (Figure 6). Effects of CJL on circadian timing were tissue-specific in nature. While SCN amplitude was increased by CJL (p<0.05), thymus amplitude was decreased (p<0.05). Liver amplitude trended lower for shifters (P=0.09). Likewise, while SCN period was lengthened by ~0.5h in CJL mice (p<0.05), liver period was shortened dramatically by nearly 3h (p<0.01). Since ex vivo bioluminescence recording was unreliable from primary macrophage cultures due to the inconsistent effects of the isolation procedure on the recorded rhythms, we quantified relative mRNA abundance in these cells using qRT-PCR on discrete cohorts of mice killed at 8 times during the day and night. While Period2 mRNA was rhythmic in both groups (Figure 6C; 1-way ANOVA: Controls: p<0.01; Shifted: p<0.02), the peak was delayed by ~3h in the shifted mice (2-way factorial ANOVA Tukey Post-hoc for ZT 18: p=0.006). While the rhythms in Bmal1 mRNA in control mice was less reliable than those for Per2 (1-way ANOVA p=0.06), the apparent maxima in the controls was entirely absent in shifted mice (p=0.64) resulting in constitutively suppressed levels of Bmal1 transcript.
DISCUSSION
In this study we used the E. coli endotoxin lipopolysaccharide (LPS), at the relatively high doses of 12.5mg/Kg or 5mg/Kg to induce endotoxemic shock in mice. This is a severe challenge to the immune system that in many ways mimics sepsis (52), the tenth leading cause of death in the United States (53). We observed that chronic circadian disruption administered over 4 weeks with a weekly 6h phase advance resulted in a magnified response to the endotoxin. This change was not due to the shifters being at a different circadian phase than the controls at the time of challenge (48, 49), and indeed required multiple shifts, as 2 or 4 weeks of additional adjustment time to the advanced light cycle following a single shift did not produce the same result as 4 consecutive shifts.
Hypothermia in response to LPS was the prevalent response from both control and shifted mice. Cytokine signaling has been established as a mediator in the thermoregulatory response during experimental sepsis and endotoxemic shock (54, 55). Mild fever or hypothermia during infection can both serve as effective strategies for host defense (56), but both can also be dangerous if uncontrolled. Control animals were significantly better at emerging from hypothermia to survive the challenge in our study. In this regard, our initial dose of LPS (12.5 mg/Kg) tested the ability of the immune system to regulate and control the inflammatory response during endotoxemic shock. Our data indicate that TNF-α levels are similar among shifters and controls at both 90m and 24h after challenge. Since this protein is activated early in the toll-like receptor 4 – mediated signaling cascade after LPS stimulation, the data suggest that the initial response to LPS is unchanged between groups. It is possible however that measurement of TNF-α at an earlier stage could reveal an altered response, since macrophages from shifted mice do show a heightened IL-6 response to LPS stimulation in vitro at even earlier time points. At 24h after challenge we did observe higher circulating levels of other cytokines such as Interleukin-1β, GM-CSF, Interleukin-12 and Interleukin-13, which are all involved in activation of the system downstream of LPS binding to its receptor. In contrast, Interleukin-10, a potent anti-inflammatory mediator, was marginally reduced in shifters. Among other actions, IL-10 is known to inhibit IFN-γ, TNF-α, IL-12, IL-1 and IL-6 (57), promoting control of inflammation and eventual resolution of the challenge. In fact, presence of IL-10 in the circulation has a protective role against LPS-induced endotoxemia in mice (58). These results indicate that the exacerbated response to LPS following CJL is at least partially due to a disregulated cytokine response involving too much activation, and insufficient deactivation. Thus our results suggest the need to investigate how altered circadian rhythmicity may impinge on the mechanisms for resolution of endotoxemic shock.
Our results show that even a single experience of jet lag worsened the response to high-dose LPS challenge. This raises the possibility that outbreaks of illness during and immediately following trans-meridian travel (59) may be at least partially due to the effects of jet lag on the immune system. Immune system changes may also underlie the increased risks of disease in shift-working populations. Certainly, altered innate immune function could upset the balance between host and intestinal microbiota (60) and could exacerbate age-related pathologies such as heart disease, ulcers and cancers, all of which are more prevalent among shift workers (61). Importantly, our data with a still high, but milder LPS dose (5 mg/Kg), indicate that multiple shifts are required for a maximal response, further indicating that our results may be related to the health consequences of long-term shift work.
Our results provide a potential mechanism for earlier observations that showed jet lag-related increases in non-specific death (18, 62) and colitis in response to an intestinal irritant (17). Importantly, the observation that poor circadian lighting environments may exacerbate pathological immune responses suggests that intensive care units should at least minimize disruption of daily lighting conditions in order to reduce the risks associated with post-surgical or injury-related infections and sepsis.
One potential mediator of the relationship between the immune and circadian systems is the pineal hormone melatonin (63–67). Melatonin has well-known effects on the immune system, and may contribute to the control of cytokine action before and during the innate immune response (68) (69). However, since melatonin is not produced by the C57BL/6 mouse due to a mutation in serotonin N-acetyltransferase (70), our mouse model of CJL allows us to focus on the important mechanisms by which circadian disruption may directly alter the regulation of inflammation in the absence of melatonin.
We observed that isolated peritoneal macrophages harvested from shifted mice exhibited an enhanced response to LPS in vitro, identifying these immune cells as a specific target of CJL. A clock in these cells has been recently described which regulates gene expression (38, 43), phagocytosis (38) and LPS sensitivity (43). CJL may fundamentally alter this circadian regulation of macrophage function.
SLEEP AND STRESS
Sleep disruption can have profound effects on the immune system. Alterations of the sleep-wake cycle affect the number of circulating lymphocytes, natural killer cells, antibody titers, and levels of cytokines (19–26, 71), which translate into impaired immune function when an immune challenge is presented (27–29). Shift work may disrupt sleep and thereby lead to secondary effects on health; however, in many cases it is difficult to distinguish between effects attributed to sleep loss versus effects on circadian regulation.
In this study, we could not detect evidence of sleep deprivation as a result of our jet lag paradigm. Instead of sleep loss, the data indicate that mice may gradually adjust the phase of their sleep-wake rhythm in a manner very similar to that observed in records of core body temperature (as in Fig. 1a). Therefore, we do not attribute the exacerbated response to LPS evident in shifted mice to a loss of sleep. If anything we instead found an increase in REM sleep during adjustment to the 4th shift, which may account for the increase in brief arousals we observed on the same day (wake nearly always follows REM). This REM increase could be attributable to mild stress (72) associated with the CJL schedule, although our previous (18) and current work (Figure S2) strongly suggest that neither hormonal nor behavioral measures of stress are increased during or after chronic jet lag. Furthermore a recent study indicates that the peak value, rhythm amplitude and waveform of corticosterone excretion are not altered during the 2 weeks following a single phase advance (73). Altogether, these data strongly suggest that immune changes during and after CJL are due to a mechanism independent of sleep loss or stress.
RHYTHM ALTERATION BY CJL
To determine the extent to which circadian rhythms in the brain and peripheral tissues, including the immune system, were affected by chronic jet lag, we compared the rhythms of mPer2-luciferase in cultured explants taken from naïve and CJL-exposed mice. We observed tissue-specific effects of CJL on circadian parameters suggestive of dysfunction in systemic circadian organization. Phase was fully adjusted for SCN, liver, spleen and thymus on Day 6 after the 4th shift, and was therefore similar among shifted and control mice. However, amplitude of the mPer2:LUC rhythm was increased in the SCN, but suppressed in the thymus (and perhaps liver) by 4 weeks of CJL. Peripheral rhythm suppression during CJL observed here and in other studies (15) may indicate impaired communication with the SCN, an altered pattern of food intake, or an impairment in the local generation of coherent rhythmicity (perhaps via a loss of synchrony among hepatocytes and thymocytes). It is tempting to speculate that changes in rhythmicity due to CJL might impinge upon proper organ function during endotoxemic challenge, as altered liver function resulted from a liver-specific Bmal1 deletion (74). Since the liver contains the largest pool of macrophages in the body, it is responsible for clearance of endotoxin and is a major source of inflammatory mediators during the early stages of inflammation (75). Thus, the liver may be an important target for jet lag-related morbidity. Our data further indicate that peritoneal macrophages also exhibit significant changes in at least one important circadian clock gene: Bmal1. Relative abundance of Bmal1 is rhythmic in control mice (see also (38, 43)), but constitutively low in shifted mice. While mPer2 appears to still be rhythmic in shifted mice, the data indicate that the phase of the peak may be delayed by the CJL history. Selective loss of clock gene rhythms is not an unprecedented observation. Tissue-specific ablation of the endogenous clock in the liver by rev-erbα overexpression resulted in the loss of Bmal1 rhythmicity, but the retention of Per 2 rhythmicity, potentially due to the influence of afferent signals to the liver arising from rhythmic environmental signals or clocks in other loci (76). The specific loss of this gene leads to compromised immune function among other pathologies (41). Our environmental manipulation has resulted in at least a partial loss of circadian regulation in this cell population, and this may underlie the dramatic change in the cellular response to LPS that we have observed.
The circadian period of SCN explants was lengthened by CJL exposure, but liver period was dramatically shortened. Aftereffects of short and long T-cycles (experimental light-dark cycles different from a period of 24h) on behavioral and physiological rhythms are well-established (77, 78), and our CJL paradigm may share features with a short (22.83h) T-cycle, since mice are required to phase advance by 6h every 7 days in order to achieve entrainment before the next shift. Our data are consistent with intriguing but still unexplained data indicating that the isolated SCN expresses a T-cycle aftereffect that is opposite to that seen in behavior (79, 80).
The opposite direction of the period and amplitude effects of CJL on central versus peripheral targets may indicate a fundamental change in whole-animal circadian organization, the consequences of which may be an increased risk of pathology (41) (81) (82). The shorter period for liver would be expected to change the response of the liver clock to SCN or environmental (e.g. food) signals, thereby altering its ability to entrain to those signals.
We and others (83, 84) have observed a suppression of rhythmicity during sepsis. Our observations of altered rhythmic organization prior to the challenge in CJL-exposed mice may make the system more reactive, thereby exacerbating the pathological response. Importantly, it has been suggested that circadian organization during sepsis is functionally important to recovery (85). If so, one might predict that restoration of rhythmicity during the challenge will enhance recovery from systemic inflammatory states.
PERSPECTIVES
We propose that circadian desynchrony alters components of the innate immune system to affect their response to a challenge. However, we cannot rule out that the effect we observe could result from the chronic alteration of the lighting environment acting directly upon the immune system without the involvement of the circadian clock. Future studies will be needed to further clarify the role of the circadian system in the exacerbated response to LPS that we have described. For example, models in which clock function is altered genetically could be challenged with LPS with and without light manipulation. It seems likely that the clock system is involved, as earlier studies suggest that schedule parameters such as shift direction impinge upon the effects of CJL on health (18), and that generalized stress is likely not involved
Our data identify one important physiological target for chronic jet lag: the innate immune system and inflammatory processes. We specifically have identified peritoneal macrophages as a cellular target of this manipulation. However, these data do not rule out other systems that may also be negatively impacted by circadian disruption. Furthermore, our model of CJL in mice simulates some but not all aspects of human shift work. Dramatic changes in the inflammatory response in our model were evident without the contribution of sleep loss, melatonin suppression, and psychosocial stress in our model, all of which are known to alter immunity in humans. However, we suggest that our results point toward a common mechanism in which lack of adequate circadian regulation results in long-lasting and potentially fatal consequences during an immune challenge. Loss or alteration of clock components has been implicated recently in dozens of diseases in both animal models and human populations, but to our knowledge, this is the first report in which a purely environmental manipulation causes disregulation of inflammatory responses potentially mediated by loss or alteration of clock function. Further investigation into the health effects of circadian disruption is clearly warranted.
Supplementary Material
Acknowledgments
The authors wish to thank Lennisha Pinckney, M. Sc. Susana Contreras, Dr. Michael Powell, Dr. Kenkichi Baba, Dr. Jennifer Evans, Dr. Rosa Salazar Gonzalez, Dr. Michael Sellix, Dr. Gianluca Tosini and the MSM CLAR staff for technical assistance, advice and helpful discussions.
This work was supported by grants 5U54NS060659-020001 and P20CA132389 to AJD, GM086683 to KLG NIH/NCRR/RCMI Grant G12-RR03034, NIH/NCMHHD Grant 5S21MD000101-09, the Georgia Research Alliance, and the NSF Center for Behavioral Neuroscience.
References
- 1.Statistics, B. o. L. Workers on flexible and shift schedules in May 2004. United States Department of Labor; 2005. [Google Scholar]
- 2.Schernhammer ES, Laden F, Speizer FE, Willett WC, Hunter DJ, Kawachi I, Fuchs CS, Colditz GA. Night-shift work and risk of colorectal cancer in the nurses’ health study. J Natl Cancer Inst. 2003;95:825–828. doi: 10.1093/jnci/95.11.825. [DOI] [PubMed] [Google Scholar]
- 3.Schernhammer ES, Kroenke CH, Laden F, Hankinson SE. Night work and risk of breast cancer. Epidemiology. 2006;17:108–111. doi: 10.1097/01.ede.0000190539.03500.c1. [DOI] [PubMed] [Google Scholar]
- 4.Lahti TA, Partonen T, Kyyronen P, Kauppinen T, Pukkala E. Nighttime work predisposes to non-Hodgkin lymphoma. Int J Cancer. 2008;123:2148–2151. doi: 10.1002/ijc.23566. [DOI] [PubMed] [Google Scholar]
- 5.Kubo T, Ozasa K, Mikami K, Wakai K, Fujino Y, Watanabe Y, Miki T, Nakao M, Hayashi K, Suzuki K, Mori M, Washio M, Sakauchi F, Ito Y, Yoshimura T, Tamakoshi A. Prospective cohort study of the risk of prostate cancer among rotating-shift workers: findings from the Japan collaborative cohort study. Am J Epidemiol. 2006;164:549–555. doi: 10.1093/aje/kwj232. [DOI] [PubMed] [Google Scholar]
- 6.Conlon M, Lightfoot N, Kreiger N. Rotating shift work and risk of prostate cancer. Epidemiology. 2007;18:182–183. doi: 10.1097/01.ede.0000249519.33978.31. [DOI] [PubMed] [Google Scholar]
- 7.Segawa K, Nakazawa S, Tsukamoto Y, Kurita Y, Goto H, Fukui A, Takano K. Peptic ulcer is prevalent among shift workers. Dig Dis Sci. 1987;32:449–453. doi: 10.1007/BF01296025. [DOI] [PubMed] [Google Scholar]
- 8.Drake CL, Roehrs T, Richardson G, Walsh JK, Roth T. Shift work sleep disorder: prevalence and consequences beyond that of symptomatic day workers. Sleep. 2004;27:1453–1462. doi: 10.1093/sleep/27.8.1453. [DOI] [PubMed] [Google Scholar]
- 9.Karlsson B, Knutsson A, Lindahl B. Is there an association between shift work and having a metabolic syndrome? Results from a population based study of 27,485 people. Occup Environ Med. 2001;58:747–752. doi: 10.1136/oem.58.11.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morikawa Y, Nakagawa H, Miura K, Soyama Y, Ishizaki M, Kido T, Naruse Y, Suwazono Y, Nogawa K. Shift work and the risk of diabetes mellitus among Japanese male factory workers. Scand J Work Environ Health. 2005;31:179–183. doi: 10.5271/sjweh.867. [DOI] [PubMed] [Google Scholar]
- 11.Karlsson B, Alfredsson L, Knutsson A, Andersson E, Toren K. Total mortality and cause-specific mortality of Swedish shift- and dayworkers in the pulp and paper industry in 1952–2001. Scand J Work Environ Health. 2005;31:30–35. doi: 10.5271/sjweh.845. [DOI] [PubMed] [Google Scholar]
- 12.Tenkanen L, Sjoblom T, Harma M. Joint effect of shift work and adverse life-style factors on the risk of coronary heart disease. Scand J Work Environ Health. 1998;24:351–357. doi: 10.5271/sjweh.355. [DOI] [PubMed] [Google Scholar]
- 13.Haupt CM, Alte D, Dorr M, Robinson DM, Felix SB, John U, Volzke H. The relation of exposure to shift work with atherosclerosis and myocardial infarction in a general population. Atherosclerosis. 2008;201:205–211. doi: 10.1016/j.atherosclerosis.2007.12.059. [DOI] [PubMed] [Google Scholar]
- 14.Tuchsen F, Hannerz H, Burr H. A 12 year prospective study of circulatory disease among Danish shift workers. Occup Environ Med. 2006;63:451–455. doi: 10.1136/oem.2006.026716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Filipski E, Delaunay F, King VM, Wu MW, Claustrat B, Grechez-Cassiau A, Guettier C, Hastings MH, Levi F. Effects of Chronic Jet Lag on Tumor Progression in Mice. Cancer Res. 2004;64:7879–7885. doi: 10.1158/0008-5472.CAN-04-0674. [DOI] [PubMed] [Google Scholar]
- 16.Penev PD, Kolker DE, Zee PC, Turek FW. Chronic circadian desynchronization decreases the survival of animals with cardiomyopathic heart disease. Am J Physiol. 1998;275:H2334–2337. doi: 10.1152/ajpheart.1998.275.6.H2334. [DOI] [PubMed] [Google Scholar]
- 17.Preuss F, Tang Y, Laposky AD, Arble D, Keshavarzian A, Turek FW. Adverse effects of chronic circadian desynchronization in animals in a “challenging” environment. Am J Physiol Regul Integr Comp Physiol. 2008;295:R2034–2040. doi: 10.1152/ajpregu.00118.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davidson AJ, Sellix MT, Daniel J, Yamazaki S, Menaker M, Block GD. Chronic jet-lag increases mortality in aged mice. Curr Biol. 2006;16:R914–916. doi: 10.1016/j.cub.2006.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hui L, Hua F, Diandong H, Hong Y. Effects of sleep and sleep deprivation on immunoglobulins and complement in humans. Brain Behav Immun. 2007;21:308–310. doi: 10.1016/j.bbi.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 20.Renegar KB, Floyd R, Krueger JM. Effect of sleep deprivation on serum influenza-specific IgG. Sleep. 1998;21:19–24. [PubMed] [Google Scholar]
- 21.Everson CA. Clinical assessment of blood leukocytes, serum cytokines, and serum immunoglobulins as responses to sleep deprivation in laboratory rats. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1054–1063. doi: 10.1152/ajpregu.00021.2005. [DOI] [PubMed] [Google Scholar]
- 22.Palma BD, Gabriel A, Jr, Colugnati FA, Tufik S. Effects of sleep deprivation on the development of autoimmune disease in an experimental model of systemic lupus erythematosus. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1527–1532. doi: 10.1152/ajpregu.00186.2006. [DOI] [PubMed] [Google Scholar]
- 23.Mullington JM, Haack M, Toth M, Serrador JM, Meier-Ewert HK. Cardiovascular, inflammatory, and metabolic consequences of sleep deprivation. Prog Cardiovasc Dis. 2009;51:294–302. doi: 10.1016/j.pcad.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vgontzas AN, Zoumakis E, Bixler EO, Lin HM, Follett H, Kales A, Chrousos GP. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab. 2004;89:2119–2126. doi: 10.1210/jc.2003-031562. [DOI] [PubMed] [Google Scholar]
- 25.Shearer WT, Reuben JM, Mullington JM, Price NJ, Lee BN, Smith EO, Szuba MP, Van Dongen HP, Dinges DF. Soluble TNF-alpha receptor 1 and IL-6 plasma levels in humans subjected to the sleep deprivation model of spaceflight. J Allergy Clin Immunol. 2001;107:165–170. doi: 10.1067/mai.2001.112270. [DOI] [PubMed] [Google Scholar]
- 26.Meier-Ewert HK, Ridker PM, Rifai N, Regan MM, Price NJ, Dinges DF, Mullington JM. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol. 2004;43:678–683. doi: 10.1016/j.jacc.2003.07.050. [DOI] [PubMed] [Google Scholar]
- 27.Everson CA. Sustained sleep deprivation impairs host defense. Am J Physiol. 1993;265:R1148–1154. doi: 10.1152/ajpregu.1993.265.5.R1148. [DOI] [PubMed] [Google Scholar]
- 28.Born J, Lange T, Hansen K, Molle M, Fehm HL. Effects of sleep and circadian rhythm on human circulating immune cells. J Immunol. 1997;158:4454–4464. [PubMed] [Google Scholar]
- 29.Irwin M, McClintick J, Costlow C, Fortner M, White J, Gillin JC. Partial night sleep deprivation reduces natural killer and cellular immune responses in humans. Faseb J. 1996;10:643–653. doi: 10.1096/fasebj.10.5.8621064. [DOI] [PubMed] [Google Scholar]
- 30.Coogan AN, Wyse CA. Neuroimmunology of the circadian clock. Brain Res. 2008;1232:104–112. doi: 10.1016/j.brainres.2008.07.087. [DOI] [PubMed] [Google Scholar]
- 31.Marpegan L, Bekinschtein TA, Costas MA, Golombek DA. Circadian responses to endotoxin treatment in mice. J Neuroimmunol. 2005;160:102–109. doi: 10.1016/j.jneuroim.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 32.Takahashi S, Yokota S, Hara R, Kobayashi T, Akiyama M, Moriya T, Shibata S. Physical and inflammatory stressors elevate circadian clock gene mPer1 mRNA levels in the paraventricular nucleus of the mouse. Endocrinology. 2001;142:4910–4917. doi: 10.1210/endo.142.11.8487. [DOI] [PubMed] [Google Scholar]
- 33.Okada K, Yano M, Doki Y, Azama T, Iwanaga H, Miki H, Nakayama M, Miyata H, Takiguchi S, Fujiwara Y, Yasuda T, Ishida N, Monden M. Injection of LPS causes transient suppression of biological clock genes in rats. J Surg Res. 2008;145:5–12. doi: 10.1016/j.jss.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 34.Kwak Y, Lundkvist GB, Brask J, Davidson A, Menaker M, Kristensson K, Block GD. Interferon-gamma alters electrical activity and clock gene expression in suprachiasmatic nucleus neurons. J Biol Rhythms. 2008;23:150–159. doi: 10.1177/0748730407313355. [DOI] [PubMed] [Google Scholar]
- 35.Young MR, Matthews JP, Kanabrocki EL, Sothern RB, Roitman-Johnson B, Scheving LE. Circadian rhythmometry of serum interleukin-2, interleukin-10, tumor necrosis factor-alpha, and granulocyte-macrophage colony-stimulating factor in men. Chronobiol Int. 1995;12:19–27. doi: 10.3109/07420529509064496. [DOI] [PubMed] [Google Scholar]
- 36.Lundkvist GB, Robertson B, Mhlanga JD, Rottenberg ME, Kristensson K. Expression of an oscillating interferon-gamma receptor in the suprachiasmatic nuclei. Neuroreport. 1998;9:1059–1063. doi: 10.1097/00001756-199804200-00018. [DOI] [PubMed] [Google Scholar]
- 37.Takane H, Ohdo S, Baba R, Koyanagi S, Yukawa E, Higuchi S. Relationship between 24-hour rhythm in antiviral effect of interferon-beta and interferon-alpha/beta receptor expression in mice. Jpn J Pharmacol. 2002;90:304–312. doi: 10.1254/jjp.90.304. [DOI] [PubMed] [Google Scholar]
- 38.Hayashi M, Shimba S, Tezuka M. Characterization of the molecular clock in mouse peritoneal macrophages. Biol Pharm Bull. 2007;30:621–626. doi: 10.1248/bpb.30.621. [DOI] [PubMed] [Google Scholar]
- 39.Arjona A, Sarkar DK. The circadian gene mPer2 regulates the daily rhythm of IFN-gamma. J Interferon Cytokine Res. 2006;26:645–649. doi: 10.1089/jir.2006.26.645. [DOI] [PubMed] [Google Scholar]
- 40.Liu J, Malkani G, Shi X, Meyer M, Cunningham-Runddles S, Ma X, Sun ZS. The circadian clock Period 2 gene regulates gamma interferon production of NK cells in host response to lipopolysaccharide-induced endotoxic shock. Infect Immun. 2006;74:4750–4756. doi: 10.1128/IAI.00287-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kondratov RV, Kondratova AA, Gorbacheva VY, Vykhovanets OV, Antoch MP. Early aging and age-related pathologies in mice deficient in BMAL1, the core componentof the circadian clock. Genes Dev. 2006;20:1868–1873. doi: 10.1101/gad.1432206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hashiramoto A, Yamane T, Tsumiyama K, Yoshida K, Komai K, Yamada H, Yamazaki F, Doi M, Okamura H, Shiozawa S. Mammalian Clock Gene Cryptochrome Regulates Arthritis via Proinflammatory Cytokine TNF-{alpha} J Immunol. 2009 doi: 10.4049/jimmunol.0903284. [DOI] [PubMed] [Google Scholar]
- 43.Keller M, Mazuch J, Abraham U, Eom GD, Herzog ED, Volk HD, Kramer A, Maier B. A circadian clock in macrophages controls inflammatory immune responses. Proc Natl Acad Sci U S A. 2009;106:21407–21412. doi: 10.1073/pnas.0906361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, Menaker M, Takahashi JS. INAUGURAL ARTICLE: PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci U S A. 2004;101:5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Handel-Fernandez ME, Lopez DM. Isoaltion of macrophages from tissues, fluids, and immune response sites. In: Paulnock DM, editor. Macrophages: A Practical Approach. Oxford University Press; New York: 2000. pp. 1–29. [Google Scholar]
- 46.Davidson AJ, Castanon-Cervantes O, Leise TL, Molyneux PC, Harrington ME. Visualizing jet lag in the mouse suprachiasmatic nucleus and peripheral circadian timing system. Eur J Neurosci. 2009;29:171–180. doi: 10.1111/j.1460-9568.2008.06534.x. [DOI] [PubMed] [Google Scholar]
- 47.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 48.Halberg F, Johnson EA, Brown BW, Bittner JJ. Susceptibility rhythm to E. coli endotoxin and bioassay. Proc Soc Exp Biol Med. 1960;103:142–144. doi: 10.3181/00379727-103-25439. [DOI] [PubMed] [Google Scholar]
- 49.Marpegan L, Leone MJ, Katz ME, Sobrero PM, Bekinstein TA, Golombek DA. Diurnal variation in endotoxin-induced mortality in mice: correlation with proinflammatory factors. Chronobiol Int. 2009;26:1430–1442. doi: 10.3109/07420520903408358. [DOI] [PubMed] [Google Scholar]
- 50.Franken P, Malafosse A, Tafti M. Genetic variation in EEG activity during sleep in inbred mice. Am J Physiol. 1998;275:R1127–1137. doi: 10.1152/ajpregu.1998.275.4.R1127. [DOI] [PubMed] [Google Scholar]
- 51.Huber R, Deboer T, Tobler I. Effects of sleep deprivation on sleep and sleep EEG in three mouse strains: empirical data and simulations. Brain Res. 2000;857:8–19. doi: 10.1016/s0006-8993(99)02248-9. [DOI] [PubMed] [Google Scholar]
- 52.Poli-de-Figueiredo LF, Garrido AG, Nakagawa N, Sannomiya P. Experimental models of sepsis and their clinical relevance. Shock. 2008;30(Suppl 1):53–59. doi: 10.1097/SHK.0b013e318181a343. [DOI] [PubMed] [Google Scholar]
- 53.Kochanek KD, Smith BL. Deaths: preliminary data for 2002. Natl Vital Stat Rep. 2004;52:1–47. [PubMed] [Google Scholar]
- 54.Kluger MJ, Kozak W, Leon LR, Conn CA. The use of knockout mice to understand the role of cytokines in fever. Clin Exp Pharmacol Physiol. 1998;25:141–144. doi: 10.1111/j.1440-1681.1998.tb02193.x. [DOI] [PubMed] [Google Scholar]
- 55.Leon LR. Invited review: cytokine regulation of fever: studies using gene knockout mice. J Appl Physiol. 2002;92:2648–2655. doi: 10.1152/japplphysiol.01005.2001. [DOI] [PubMed] [Google Scholar]
- 56.Romanovsky AA, Szekely M. Fever and hypothermia: two adaptive thermoregulatory responses to systemic inflammation. Med Hypotheses. 1998;50:219–226. doi: 10.1016/s0306-9877(98)90022-6. [DOI] [PubMed] [Google Scholar]
- 57.Rongione AJ, Kusske AM, Ashley SW, Reber HA, McFadden DW. Interleukin-10 prevents early cytokine release in severe intraabdominal infection and sepsis. J Surg Res. 1997;70:107–112. doi: 10.1006/jsre.1997.5071. [DOI] [PubMed] [Google Scholar]
- 58.Howard M, Muchamuel T, Andrade S, Menon S. Interleukin 10 protects mice from lethal endotoxemia. J Exp Med. 1993;177:1205–1208. doi: 10.1084/jem.177.4.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Silverman D, Gendreau M. Medical issues associated with commercial flights. Lancet. 2009;373:2067–2077. doi: 10.1016/S0140-6736(09)60209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yamamoto-Furusho JK, Podolsky DK. Innate immunity in inflammatory bowel disease. World J Gastroenterol. 2007;13:5577–5580. doi: 10.3748/wjg.v13.i42.5577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Knutsson A. Health disorders of shift workers. Occup Med (Lond) 2003;53:103–108. doi: 10.1093/occmed/kqg048. [DOI] [PubMed] [Google Scholar]
- 62.Davidson AJ, Yamazaki S, Arble DM, Menaker M, Block GD. Resetting of central and peripheral circadian oscillators in aged rats. Neurobiol Aging. 2008;29:471–477. doi: 10.1016/j.neurobiolaging.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Freeman DA, Kampf-Lassin A, Galang J, Wen JC, Prendergast BJ. Melatonin acts at the suprachiasmatic nucleus to attenuate behavioral symptoms of infection. Behav Neurosci. 2007;121:689–697. doi: 10.1037/0735-7044.121.4.689. [DOI] [PubMed] [Google Scholar]
- 64.Prendergast BJ, Hotchkiss AK, Nelson RJ. Photoperiodic regulation of circulating leukocytes in juvenile Siberian hamsters: mediation by melatonin and testosterone. J Biol Rhythms. 2003;18:473–480. doi: 10.1177/0748730403258486. [DOI] [PubMed] [Google Scholar]
- 65.Prendergast BJ. Behavioral tolerance to endotoxin is enhanced by adaptation to winter photoperiods. Psychoneuroendocrinology. 2008;33:540–545. doi: 10.1016/j.psyneuen.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Prendergast BJ, Hotchkiss AK, Bilbo SD, Kinsey SG, Nelson RJ. Photoperiodic adjustments in immune function protect Siberian hamsters from lethal endotoxemia. J Biol Rhythms. 2003;18:51–62. doi: 10.1177/0748730402239676. [DOI] [PubMed] [Google Scholar]
- 67.Navara KJ, Trainor BC, Nelson RJ. Photoperiod alters macrophage responsiveness, but not expression of Toll-like receptors in Siberian hamsters. Comp Biochem Physiol A Mol Integr Physiol. 2007;148:354–359. doi: 10.1016/j.cbpa.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 68.Guerrero JM, Reiter RJ. Melatonin-immune system relationships. Curr Top Med Chem. 2002;2:167–179. doi: 10.2174/1568026023394335. [DOI] [PubMed] [Google Scholar]
- 69.Carrillo-Vico A, Guerrero JM, Lardone PJ, Reiter RJ. A review of the multiple actions of melatonin on the immune system. Endocrine. 2005;27:189–200. doi: 10.1385/ENDO:27:2:189. [DOI] [PubMed] [Google Scholar]
- 70.Roseboom PH, Namboodiri MA, Zimonjic DB, Popescu NC, Rodriguez IR, Gastel JA, Klein DC. Natural melatonin ‘knockdown’ in C57BL/6J mice: rare mechanism truncates serotonin N-acetyltransferase. Brain Res Mol Brain Res. 1998;63:189–197. doi: 10.1016/s0169-328x(98)00273-3. [DOI] [PubMed] [Google Scholar]
- 71.Majde JA, Krueger JM. Links between the innate immune system and sleep. J Allergy Clin Immunol. 2005;116:1188–1198. doi: 10.1016/j.jaci.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 72.Pawlyk AC, Morrison AR, Ross RJ, Brennan FX. Stress-induced changes in sleep in rodents: models and mechanisms. Neurosci Biobehav Rev. 2008;32:99–117. doi: 10.1016/j.neubiorev.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kiessling S, Eichele G, Oster H. A Role for adrenal glucocorticoids in the circadian resynchronization during jet lag in mice. Journal of Clinical Investigation. 2010 doi: 10.1172/JCI41192. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lamia KA, Storch KF, Weitz CJ. Physiological significance of a peripheral tissue circadian clock. Proc Natl Acad Sci U S A. 2008 doi: 10.1073/pnas.0806717105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Szabo G, Romics L, Jr, Frendl G. Liver in sepsis and systemic inflammatory response syndrome. Clin Liver Dis. 2002;6:1045–1066. doi: 10.1016/s1089-3261(02)00058-2. [DOI] [PubMed] [Google Scholar]
- 76.Kornmann B, Schaad O, Bujard H, Takahashi JS, Schibler U. System-driven and oscillator-dependent circadian transcription in mice with a conditionally active liver clock. PLoS Biol. 2007;5:e34. doi: 10.1371/journal.pbio.0050034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Matsumoto S, Basil J, Jetton AE, Lehman MN, Bittman EL. Regulation of the phase and period of circadian rhythms restored by suprachiasmatic transplants. J Biol Rhythms. 1996;11:145–162. doi: 10.1177/074873049601100207. [DOI] [PubMed] [Google Scholar]
- 78.Page TL, Wassmer GT, Fletcher J, Block GD. Aftereffects of entrainment on the period of the pacemaker in the eye of the mollusk Bulla gouldiana. J Biol Rhythms. 1997;12:218–225. doi: 10.1177/074873049701200303. [DOI] [PubMed] [Google Scholar]
- 79.Aton SJ, Block GD, Tei H, Yamazaki S, Herzog ED. Plasticity of circadian behavior and the suprachiasmatic nucleus following exposure to non-24-hour light cycles. J Biol Rhythms. 2004;19:198–207. doi: 10.1177/0748730404264156. [DOI] [PubMed] [Google Scholar]
- 80.Molyneux PC, Dahlgren MK, Harrington ME. Circadian entrainment aftereffects in suprachiasmatic nuclei and peripheral tissues in vitro. Brain Res. 2008;1228:127–134. doi: 10.1016/j.brainres.2008.05.091. [DOI] [PubMed] [Google Scholar]
- 81.Fu L, Pelicano H, Liu J, Huang P, Lee C. The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell. 2002;111:41–50. doi: 10.1016/s0092-8674(02)00961-3. [DOI] [PubMed] [Google Scholar]
- 82.Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR, Eckel RH, Takahashi JS, Bass J. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308:1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Carlson DE, Chiu WC, Scalea TM. Cecal ligation and puncture in rats interrupts the circadian rhythms of corticosterone and adrenocortical responsiveness to adrenocorticotrophic hormone. Crit Care Med. 2006;34:1178–1184. doi: 10.1097/01.CCM.0000207340.24290.3C. [DOI] [PubMed] [Google Scholar]
- 84.Mundigler G, Delle-Karth G, Koreny M, Zehetgruber M, Steindl-Munda P, Marktl W, Ferti L, Siostrzonek P. Impaired circadian rhythm of melatonin secretion in sedated critically ill patients with severe sepsis. Crit Care Med. 2002;30:536–540. doi: 10.1097/00003246-200203000-00007. [DOI] [PubMed] [Google Scholar]
- 85.Carlson DE, Chiu WC. The absence of circadian cues during recovery from sepsis modifies pituitary-adrenocortical function and impairs survival. Shock. 2008;29:127–132. doi: 10.1097/shk.0b013e318142c5a2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.