Abstract
Levels of preexisting antibodies to the hemagglutinin of pandemic influenza A(H1N1) 2009 (hereafter pandemic H1N1) virus positively correlate with age. The impact of contemporary seasonal influenza vaccines on establishing immunity to other pandemic H1N1 proteins is unknown. We measured serum antibodies to the neuraminidase (NA) of pandemic H1N1 in adults prior to and after vaccination with seasonal trivalent inactivated influenza vaccines. Serum antibodies to pandemic H1N1 NA were observed in all age groups; however, vaccination elevated levels of pandemic H1N1 NA antibodies predominately in elderly individuals (age,⩾60 years). Therefore, contemporary seasonal vaccines likely contribute to reduction of pandemic H1N1-associated disease in older individuals.
The degree of immunity to pandemic influenza A(H1N1) 2009 (hereafter pandemic H1N1) in humans positively correlates with age [1, 2]. Although previous vaccination with a 1976 swine influenza A(H1N1) vaccine elevated serum neutralizing antibodies to the hemagglutinin (HA) of pandemic H1N1 virus, recent seasonal influenza vaccines did not, suggesting that the latter are unable to elicit immunity against pandemic H1N1 [1, 3]. Nevertheless, contemporary seasonal vaccines can induce immunity against influenza A(H5N1) viruses in mice [4], which suggests that their role in protection from pandemic H1N1 warrants further evaluation. Furthermore, the capacity of contemporary seasonal influenza vaccines to generate immunity to non-HA proteins of pandemic H1N1 virus has not yet been elucidated. Studies have revealed that the neuraminidase (NA) of various influenza subtypes elicits immunity to heterologous influenza strains [5, 6]. For example, anti-N2 serum antibodies confer protection against genetically and antigenically distinct H1N1 viruses [5]. In addition, antibodies to the NA of contemporary H1N1 viruses in humans afford partial immunity against antigenically distinct influenza viruses in mice [6], further demonstrating that antibodies raised against NA can provide protection from pandemic H1N1.
Despite the knowledge that a proportion of humans aged ⩾60 years have preexisting antibodies to pandemic H1N1 HA [1], the extent of preexisting antibodies to pandemic H1N1 NA circulating in the human population has not been addressed. Moreover, the age distribution and effect of contemporary seasonal influenza vaccines on immunity to pandemic H1N1 NA is unknown. To better define the breadth of preexisting antibodies to pandemic H1N1 virus, we analyzed human serum samples from young and old adults prior to and after vaccination with influenza vaccines from the 2007–2008 or 2008–2009 seasons and determined the level of serum antibodies to pandemic H1N1 NA.
Methods. Wild-type seasonal influenza A/Solomon Islands/ 3/06(H1N1) (hereafter Solomon), wild-type seasonal influenza A/Brisbane/59/2002(H1N1) (hereafter Brisbane), and wild-type pandemic influenza A/Tennessee/1–560/2009(H1N1) (hereafter Tennessee) were obtained from World Health Organization influenza collaboration laboratories. The rg-A/Tennessee/1–560/ 2009 7+1 virus, with 7 internal genes from influenza A/Puerto Rico/8/1934 and the NA gene segment from the Tennessee virus, was generated using the 8-plasmid reverse genetic method [7]. The Tennessee, Solomon, and Brisbane viruses were either grown in Madin-Darby canine kidney cells (American Type Culture Collection) or propagated in the allantoic cavities of 10day-old embryonic chicken eggs.
We received serum samples from a prospective study of 605 adults aged 20–40 years (median age, 29 years) or 60–93 years (median age, 74 years) who were recruited in the Greater Vancouver area of British Columbia, Canada, or in the vicinity of the Greater Hartford area of Connecticut during the 2007–2008 and 2008–2009 influenza seasons. Written informed consent was obtained from all participants, and all study protocols were approved by the University of British Columbia and the institutional review board of the University of Connecticut. All participants received the standard dose of the licensed trivalent split-inactivated virus (TIV) seasonal influenza vaccine, which contained A/Solomon Islands/3/2006(H1N1)-like, A/Wisconsin/67/ 2005(H3N2)-like, and B/Malaysia/2506/2004-like viruses in the 2007–2008 season and A/Brisbane/59/2007(H1N1)-like, A/Brisbane/10/2007(H3N2)-like, and B/Florida/4/2006-like viruses in the 2008–2009 season. Serum samples were collected from each participant before and 4 weeks after vaccination.
For each serological assay, serum samples were used at a starting dilution of 1:10. A subset of prevaccination and postvaccination serum samples (117 samples) was tested for inhibition of NA activity against the Brisbane, Solomon, and rg-Tennessee viruses by use of a miniaturized or conventional format of the NA assay [8]. NA inhibition titers were expressed as the reciprocal of the highest serum dilution that caused 50% inhibition of NA activity. Seroconversion was defined as a titer that went from negative to positive or a 4-fold increase in the titer (response to the vaccine). Samples that did not exhibit a detectable titer against pandemic H1N1 NA (<10 samples) were assigned a number of 0. For HA inhibition assays, serum samples were treated with receptor-destroying enzyme (Denka Seiken) overnight and then tested for HA inhibition titers against the Brisbane, Solomon, and whole inactivated Tennessee viruses with the use of 0.5% turkey red blood cells.
For statistical analysis, the effect of age on antibodies against the Tennessee, Solomon, or Brisbane virus was assessed by logistic regression. Individuals were grouped in 4–10-year age intervals, and geometric mean titers (GMTs) were compared between groups by use of analysis of variance and the Tukey multiple comparisons test. All statistical analyses of NA and HA inhibition titers were performed with R software (version 2.9.0; http://www.R-project.org). An increase in the GMT of total immunoglobulin G (IgG) to whole Tennessee virus was analyzed by use of the Student t test.
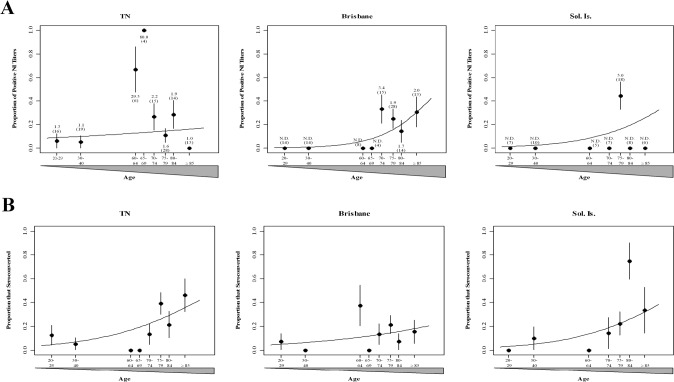
Results. An NA inhibition assay [8] was used to measure prevaccination NA inhibition titers in serum samples from participants aged 20–29, 30–40, or 60–84 years (in 4-year age intervals) or ⩾85 years (Figure 1 A). We examined the effect of age on the extent of preexisting antibodies to pandemic H1N1 NA (Tennessee virus) or the NA of seasonal influenza strains from the 2008–2009 season (Brisbane virus) and 2007–2008 season (Solomon virus). Samples from individuals either 20- 29 or 30–40 years old displayed a low proportion of positive NA inhibition titers to the Tennessee virus and undetectable titers to the seasonal viruses. Of note, samples from older individuals aged 70–84 or ⩾85 years were more likely to exhibit NA inhibition titers against the Brisbane virus (P⩽ .012) but not the Solomon virus (P⩽ .12) or the Tennessee virus (P⩽ .42). The peak GMTs for all 3 strains were in samples from adults ⩽60 years old. Therefore, the amount of serum antibodies to pandemic H1N1 NA in samples from nonvaccinated adults was minimal in most age intervals.
We next addressed whether vaccination elevated the level of immunity to pandemic H1N1 NA. The TIV vaccine failed to elicit a statistically significant increase in the rate of seroconversion to all 3 strains in participants aged 20–29 or 30–40 years (Figure 1 B). The fraction of individuals who seroconverted to pandemic H1N1 NA after vaccination with either the Brisbane virus or the Solomon virus increased with age (P⩽ .001). However, the choice of H1N1 component of the TIV vaccine was not a predictor of elevated titers to pandemic H1N1 NA (P⩽ .289). The increase in the rate of seroconversion to pandemic H1N1 NA occurred mainly in adults aged ⩾70 years, and the peak GMT (4.7) was observed in individuals aged ⩾85 years. As with the prevaccination samples, the highest GMT for all 3 viruses after vaccination was in samples from adults ⩾60 years old. Age contributed to increased NA inhibition titers to the Solomon virus (P⩽ .025) but not the Brisbane virus (P⩽ .131) in samples from participants who were vaccinated with the corresponding strain. To ensure that the age-related increase in pandemic H1N1 NA titers were not due to HA-specific antibody interference, a subset of serum samples from individuals ⩾62 years old were incubated with monospecific goat anti-A/Puerto Rico/8/34 HA antibody. There was no statistically significant difference in the mean (± standard deviation) NA inhibition titers in the presence (48.75 ± 27.99) or absence (56.2 ± 27.2) of anti-HA antibody (P = .595). Therefore, recent recipients of the TIV vaccine are capable of increasing the amount of antibodies to pandemic H1N1 NA in predominately elderly populations.
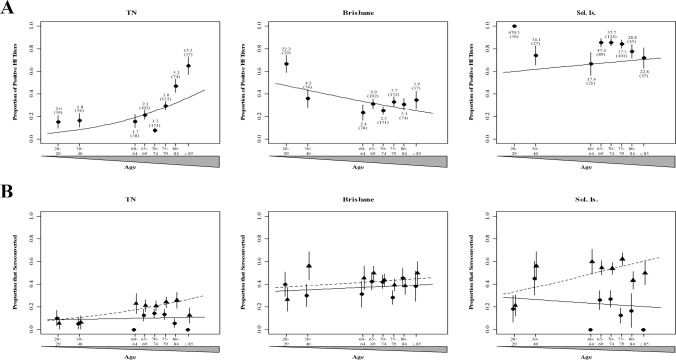
In a proof of concept study, we assessed the amount of pandemic H1N1 HA antibodies in a larger cohort of 605 adults, including 117 whose samples were tested by means of NA inhibition. Using HA inhibition assays, we measured HA inhibition titers in samples from younger participants (age, 20–29 or 30–40 years) and older participants (age, 60–84 years [grouped in 4-year age intervals] or ⩾85 years) (Figure 2 A). Since older individuals (age, ⩾60 years) had the highest levels of preexisting pandemic H1N1 HA antibodies [1], we further stratified this group by measuring the level of serum antibodies in samples from in individuals aged 60–64, 65–69, 70–74, 75- 79, 80–84, and ⩾85 years. As found in previous studies [1], we found that samples from older participants were more likely to exhibit positive titers against pandemic H1N1 HA. The age groups with the highest proportion of positive pandemic H1N1 HA titers were 80–84 and ⩾85 years (P< .001). We also discovered a statistically significant difference in GMT between individuals aged ⩾80 years and those aged <40 years (data not shown) (P< .001). Conversely, titers against the Brisbane virus or the Solomon virus were not greater in older individuals. There was no age-related difference in preexisting titers to the Solomon virus in adults immunized with that strain (P ⩽ .18). Our data reveal an age-dependent increase in the proportion of older individuals with preexisting antibody titers to pandemic H1N1 HA, which confirms the findings of others.
To determine whether vaccination with current influenza strains enhanced preexisting immunity to pandemic H1N1 HA in the elderly, as others have found [1], we compared rates of seroconversion among the different age groups. Rates of seroconversion to pandemic H1N1 HA increased for 60–84-yearold adults in all age intervals and for individuals ⩾85 years old after vaccination with the Solomon virus (P< .001) but not the Brisbane virus (P> .05) (Figure 2 B). These results contrast other reported results that neither contemporary vaccine statistically significantly enhances levels of pandemic H1N1 HA antibodies in older participants [1]. Unexpectedly, the increase in titers to the corresponding vaccine strain was statistically significantly higher in samples from participants in our study who were vaccinated with the Solomon virus (P< .001) but not in those who were vaccinated with the Brisbane virus, which may be attributable to general differences in vaccine formulation or viral antigenicity. Thus, the TIV vaccine with the Solomon virus enhanced pandemic H1N1 HA responses in older adults (age, ⩾60 years).
Discussion. The purpose of the present study was to define the extent of immunity to pandemic H1N1 among adults of different ages. The TIV vaccine containing either the Brisbane virus or the Solomon virus provided a benefit to older individuals by enhancing preexisting pandemic H1N1 NA titers. Immunization also increased the levels of antibodies to other pandemic H1N1 proteins. This conclusion is supported by our enzyme-linked immunosorbent assay results, which show that the Brisbane virus elevated levels of preexisting IgG antibodies to inactivated whole pandemic H1N1 virus (P ⩽ .001 ; data not shown). Presumably, these antibodies reacted with nonneutralizing conserved epitopes found on whole pandemic virus [9]. Although we observed an increase in the levels of pandemic H1N1 NA-specific antibodies after vaccination, we cannot determine the effect that prior exposure to antigenically distinct N subtypes, as demonstrated with influenza A(H1N2) infection [10] or natural infection with contemporary seasonal viruses (Brisbane or Solomon), had on the level of reactive antibodies.
There is likely little antigenic similarity between pandemic H1N1 NA and the NA of recent seasonal influenza viruses because of their genetic divergence. Our data show the TIV vaccine boosts pandemic H1N1 NA antibodies and strongly suggest that some epitopes are in common [3]. The dependency of the level of pandemic H1N1 NA antibodies on age is also suggestive that pandemic H1N1 NA may be more antigenically related to earlier influenza strains in humans (eg, pandemic influenza A[H1N1] 1918) than to contemporary viruses in humans. This is somewhat surprising because unlike the pandemic H1N1 HA, which is derived from the same lineage as that of the 1918 pandemic virus, the pandemic H1N1 NA is of the Eurasian avian lineage [3]. Nevertheless, due to its more recent introduction into humans, the 1918 NA protein is closer to the avian consensus than is the NA protein from contemporary seasonal H1N1 viruses. Alternatively, increasing age results in more exposure to influenza viruses and thus a more diverse and cross-reactive response. Analysis of the antigenic profiles of N1 proteins may elucidate these underlying mechanisms.
Similar to earlier findings [1], we found statistically significant levels of preexisting serum pandemic H1N1 HA antibodies in older adults (age, ⩾60 years) as well as in adults of much older age (⩾85 years). We attribute these findings to preexisting responses to early strains of the pandemic influenza A(H1N1) 1918 virus or related H1N1 strains. This may explain why this age population is at lower risk of getting infected with the current pandemic H1N1 strain [3]. In contrast to the earlier study [1], in this study we show that TIV vaccines are capable of elevating HA inhibition titers to pandemic H1N1 HA in older adults. This discrepancy may be attributable to several factors: (1) geographical population differences—a subset of samples from the previous study [1] were collected in Europe, whereas participants in our study were from either the United States or Canada; (2) as described in the earlier study [1], only a modest boost was observed in older adults (age, ⩾60 years) because this age group already had high preexisting GMTs to pandemic H1N1 HA [1]; and (3) sample size—our present study tested a much larger cohort of individuals >64 years old and was more stratified by age.
Although the antibody response to HA may predominate [11], sufficient evidence demonstrates the importance of other viral components in generating immunity against diverse influenza subtypes [6, 12–14]. NA may be a suitable component for future vaccine formulations for the elderly but would require standardization of NA concentrations in each vaccine preparation, as is currently done for HA. Still, long-term retrospective studies in the elderly are required to detect a correlation between protective immunity to pandemic H1N1 and NA inhibition titers to pandemic H1N1 NA. It has been shown that anti-N2 antibodies correlate inversely with disease severity of influenza A(H3N2) in humans [13].
Several limitations exist with measuring antibody responses to merely HA. HA inhibition or microneutralization assays may severely underestimate the degree of immunity by omitting the detection of antibodies raised against other viral proteins [15]. In our studies, NA inhibition titers did not positively correlate with HA inhibition titers (P ⩽ .84), nor were they associated with IgG titers to whole virus (data not shown), illustrating the complexity of the antibody response to influenza. We conclude that measuring serum responses to NA, in addition to measuring those to HA, may be a more reliable marker to assess the scope of immunity to pandemic H1N1 in certain aged populations.
Figure 1.
Proportion of study participants showing neuraminidase inhibition (NI) titers. Serum samples from younger (age, 20–40 years) and older (age, 60–84 or ⩾85 years) adults were collected prior to and 4 weeks after vaccination with trivalent inactivated seasonal influenza vaccines with H1N1 components from A/Brisbane/59/2007 (Brisbane; 2008–2009 season) or A/Solomon Islands/3/2006 (Sol. Is.; 2007–2008 season) and tested against pandemic influenza A/Tennessee/1–560/2009 (TN) or corresponding seasonal vaccines. A, Predicted (curves) and actual (points) proportion of individuals in each age group with measurable neuraminidase inhibition titers prior to vaccination. The geometric mean titer and sample size (in parentheses) are indicated for each age group. B, Predicted (curves) and actual (points) proportion of individuals in each age group who seroconverted to either pandemic or seasonal influenza A(H1N1) strains after vaccination with the corresponding vaccine. Error bars show the standard error. N.D., not detected (titer below the detectable limit).
Figure 2.
Proportion of study participants showing hemagglutinin inhibition (HI) titers. Serum samples from younger (age, 20–40 years) and older (age, 60–84 or ⩾85 years) adults were collected prior to and 4 weeks after vaccination with trivalent inactivated seasonal influenza vaccines with H1N1 components from A/Brisbane/59/2007 (Brisbane; 2008–2009 season) or A/Solomon Islands/3/2006 (Sol. Is.; 2007–2008 season) and tested against pandemic influenza A/Tennessee/1–560/2009 (TN) or corresponding seasonal vaccines. A, Predicted (curves) and actual (points) proportion of individuals in each age group with measurable hemagglutinin inhibition titers prior to vaccination. The geometric mean titer and sample size (in parentheses) are indicated for each age group. B, Predicted (curves) and actual (points) proportion of individuals in each age group who seroconverted to either pandemic or seasonal influenza A(H1N1) strains after vaccination with A/Brisbane/59/2007 (solid lines and circles) or A/Solomon Islands/3/ 2006 (dashed lines and triangles). Error bars show the standard error.
Acknowledgments
We acknowledge Adrianus C.M. Boon for editorial assistance and the research teams supporting the influenza projects at the University of Connecticut Health Center and Vancouver Coastal Health Research Institute. We thank the World Health Oranization Global Influenza Surveillance Network for providing the H1N1 viruses.
Footnotes
Potential conflicts of interest: none reported.
Financial support: National Institute of Allergy and Infectious Diseases (contract HHSN266200700005C and grant R01 AI68265); Canadian Institutes of Health Research; American Lebanese Syrian Associated Charities; GlaxoSmithKline (investigator-initiated contract to J.E.M.).
References
- 1.Hancock K, eguilla V, Lu X, et al. Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N Engl J Med. 2009;361:1945–1952. doi: 10.1056/NEJMoa0906453. [DOI] [PubMed] [Google Scholar]
- 2.Itoh Y, Shinya K, Kiso M, et al. In vitro and in vivo characterization of new swine-origin H1N1 influenza viruses. Nature. 2009;460:1021–1025. doi: 10.1038/nature08260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team. Dawood FS, Jain S, et al. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009;360:2605–2615. doi: 10.1056/NEJMoa0903810. [DOI] [PubMed] [Google Scholar]
- 4.van Maurik A, Sabarth N, Dacho H, et al. Seasonal influenza vaccine elicits heterosubtypic immunity against H5N1 that can be further boosted by H5N1 vaccination. Vaccine. 2010;28:1778–1785. doi: 10.1016/j.vaccine.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 5.Chen Z, Kadowaki S, Hagiwara Y, et al. Cross-protection against a lethal influenza virus infection by DNA vaccine to neuraminidase. Vaccine. 2000;18:3214–3222. doi: 10.1016/s0264-410x(00)00149-3. [DOI] [PubMed] [Google Scholar]
- 6.Sandbulte MR, Jimenez GS, Boon AC, Smith LR, Treanor JJ, Webby RJ. Cross-reactive neuraminidase antibodies afford partial protection against H5N1 in mice and are present in unexposed humans. PLoS Med. 2007;4:59. doi: 10.1371/journal.pmed.0040059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoffmann E, Krauss S, Perez D, Webby R, Webster RG. Eight-plasmid system for rapid generation of influenza virus vaccines. Vaccine. 2002;20:3165–3170. doi: 10.1016/s0264-410x(02)00268-2. [DOI] [PubMed] [Google Scholar]
- 8.Sandbulte MR, Gao J, Straight TM, Eichelberger MC. A miniaturized assay for influenza neuraminidase-inhibiting antibodies utilizing reverse genetics-derived antigens. Influenza Other Respi Viruses. 2009;3:233–240. doi: 10.1111/j.1750-2659.2009.00094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xing Z, Cardona CJ. Preexisting immunity to pandemic (H1N1) 200. Emerg Infect Dis. 2009;15:1847–1849. doi: 10.3201/eid1511.090685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Reeth K, Gregory V, Hay A, Pensaert M. Protection against a European H1N2 swine influenza virus in pigs previously infected with H1N1 and/or H3N2 subtypes. Vaccine. 2003;21:1375–1381. doi: 10.1016/s0264-410x(02)00688-6. [DOI] [PubMed] [Google Scholar]
- 11.Johansson BE, Moran TM, Kilbourne ED. Antigen-presenting B cells and helper T cells cooperatively mediate intravirionic antigenic competition between influenza A virus surface glycoproteins. Proc Natl AcadSci U S A. 1987;84:6869–6873. doi: 10.1073/pnas.84.19.6869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johansson BE, Grajower B, Kilbourne ED. Infection-permissive immunization with influenza virus neuraminidase prevents weight loss in infected mice. Vaccine. 1993;11:1037–1039. doi: 10.1016/0264-410x(93)90130-p. [DOI] [PubMed] [Google Scholar]
- 13.Murphy BR, Kasel JA, Chanock RM. Association of serum anti-neuraminidase antibody with resistance to influenza in man. N Engl J Med. 1972;286:1329–1332. doi: 10.1056/NEJM197206222862502. [DOI] [PubMed] [Google Scholar]
- 14.Jimenez GS, Planchon R, Wei Q, et al. Vaxfectin-formulated influenza DNA vaccines encoding NP and M2 viral proteins protect mice against lethal viral challenge. Hum Vaccin. 2007;3:157–164. doi: 10.4161/hv.3.5.4175. [DOI] [PubMed] [Google Scholar]
- 15.Hancock EJ, Pot K, Puterman ML, Tingle AJ. Lack of association between titers of HAI antibody and whole-virus ELISA values for patients with congenital rubella syndrome. J Infect Dis. 1986;154:1031–1033. doi: 10.1093/infdis/154.6.1031. [DOI] [PubMed] [Google Scholar]