Abstract
Naturally occurring regulatory T (nTreg) cells express Foxp3 and were originally discovered as immune suppressors critical for self-tolerance and immune homeostasis. Through yet-to-be-defined mechanisms, nTreg cells were recently shown to convert into proinflammatory cells. Particularly, attenuation of Foxp3 expression led to Th2 conversion of nTreg cells in vivo. In this paper, we demonstrated an nTreg-specific mechanism controlling their Th2 conversion. We found that wild-type nTreg cells expressing reduced levels of Foxp3 but not those expressing no Foxp3 produced the Th2 cytokine IL-4. Intriguingly, IL-4 production by converted nTreg cells is required for Th2 differentiation of coexisting naive CD4 T cells in vivo, suggesting that Th2 conversion of nTreg cells might be critical for directing Th2 immune responses. Th2 conversion of nTreg cells was not due to their inability to become Th1 cells, because IFN-γ was produced by Foxp3-low–expressing cells when IL-4/STAT-6 signaling was abrogated. Surprisingly, however, unlike naive CD4 T cells whose IL-4 production is dependent on STAT-6, Foxp3-low–expressing cells generated IL-4 independent of STAT-6, indicating an intrinsic mechanism that favors nTreg-to-Th2 differentiation. Indeed, compared with naive CD4 T cells, nTreg expressed elevated levels of GATA-3 independent of STAT-6. And GATA-3 was required for nTreg-to-Th2 conversion. Foxp3 may account for this GATA-3 upregulation in nTreg cells, because ectopic expression of Foxp3 preferentially promoted GATA-3 but not T-bet expression. Thus, we have identified an intrinsic mechanism that imposes a Th2/Th1 imbalance and predisposes Foxp3-expressing cells to IL-4 production independent of STAT-6 signaling.
Naturally occurring regulatory T (nTreg) cells were originally discovered as immune suppressors critical for self-tolerance and immune homeostasis (1, 2). Foxp3, an X-linked transcription factor highly and specifically expressed in nTreg cells, is regarded as the master regulator and genetic marker for these cells (3–5). nTreg cells were traditionally thought to be terminally differentiated and fully committed lineage specializing in immune suppression. However, accumulating evidence suggest that nTreg cells have much more diverse functions than immune suppression. In fact, nTreg cells are “plastic,” being able to convert into other types of Th cells to promote immune response (6–9). Four major types of Th cells, Th1, Th2, Th17, and follicular helper T (Tfh) cells, have been described previously (10–13). nTreg cells may convert into all these four cell types under distinct conditions. In vivo, nTreg cells became Tfh cells in Peyer's patches upon transfer into T cell-deficient CD3ε−/− mice (6) and IFN-γ–producing Th1 and IL-17A–producing Th17 cells under homeostatic and autoimmune conditions (7). Such Tfh, Th1, and Th17 conversion of nTreg cells occurred concomitant with the loss of Foxp3 expression. Nevertheless, Foxp3 expression and nTreg-to-Th conversion are not mutually exclusive. In FILIG mice, where Foxp3 expression was attenuated but not lost because of the insertion of an internal ribosome entry site (IRES)-luciferase–IRES-EGFP expression cassette into 3′-untranslated region (UTR) of endogenous Foxp3 gene, Foxp3-expressing cells preferentially converted into IL-4–producing Th2 cells (8). In addition, nTreg cells deficient in Runx1-Cbfβ transcription complex produced high levels of IL-4 but not IFN-γ or IL-17A with modest reduction of Foxp3 expression (14). These studies suggest that, upon reduction but not loss of Foxp3 expression, nTreg cells are biased toward Th2 conversion in vivo.
nTreg cells are now known to be able to convert into effector T cells to promote immune response. The underlying mechanisms, however, remain to be defined. In this paper, we revealed an intrinsic mechanism controlling the Th2 conversion of Foxp3-expressing cells in vivo. We found that only cells expressing reduced levels of Foxp3 but not those expressing no Foxp3 produced the Th2 cytokine IL-4. Intriguingly, IL-4 production by converted nTreg cells was required for Th2 differentiation of coexisting naive CD4 T cells, suggesting that Th2 conversion of nTreg cells might be critical for directing Th2 immune response. Th2 conversion of nTreg cells was not due to their inability to become Th1 cells, because IFN-γ was produced by Foxp3-expressing cells when IL-4/STAT-6 signaling was abrogated. Surprisingly, however, unlike naive CD4 T cells whose Th2 differentiation is dependent on STAT-6, Foxp3-expressing cells generated IL-4 independent of STAT-6. Compared with naive CD4 T cells, nTreg cells expressed elevated levels of GATA-3 but not T-bet independent of STAT-6. GATA-3 was critical for Th2 conversion of nTreg cells as GATA-3–deficient Foxp3-expressing T cells failed to produce Th2 cytokines. The specific increase of GATA-3 expression in nTreg cells may be due to Foxp3 expression, because ectopic expression of Foxp3 preferentially promoted GATA-3 but not T-bet expression. Collectively, we have identified an IL-4/STAT-6–independent and GATA-3–dependent mechanism intrinsic to nTreg cells for the Th2 conversion.
Materials and Methods
Mice and adoptive transfer assays
FIR, FILIG, 4GET, IL-4−/−, IL-4−/−-IFN-γ−/− [4-γ double knockout (DKO)], STAT6−/−, CD4Cre, GATA3flox/flox, Rag1−/−, and CD45.1 mice are on C57BL/6 background and were kept under specific pathogen-free conditions in the animal care facility at the University of North Carolina (Chapel Hill, NC). Special mouse strains used in this study and their properties are described in Table I. All mouse experiments were approved by Institutional Animal Care and Use Committee of the University of North Carolina or Yale University (New Haven, CT). For adoptive transfer assays, FACS-sorted cells were either transferred alone or were mixed at different ratios as elaborated in the texts or figure legends. A total of 2 × 105 cells were transferred into Rag1−/− mice via retro-orbital injection. Eight weeks after transfer, T cells were recovered from recipient mice for further analysis.
Table I.
Special mouse strains used in this study and their properties
| Strain Name | Genetic Manipulation | CD4 T Cell Property |
|---|---|---|
| FIR/Y | IRES-mRFP knocked into 3′-UTR of endogenous Foxp3 locus. | Normal |
| FIR-4GET | IRES-mRFP knocked into 3′-UTR of endogenous Foxp3 locus. | Normal |
| IRES-EGFP knocked into 3′-UTR of endogenous IL-4 locus. | ||
| FIR-4-γ DKO | IRES-mRFP knocked into 3′-UTR of endogenous Foxp3 locus. | Grossly normal but fail to express IL-4 and IFN-γ |
| Deletion of IL-4 gene. | ||
| Deletion of IFN-γ gene. | ||
| FILIG/Y | IRES-luciferase-IRES-EGFP knocked into 3′-UTR of endogenous Foxp3 locus. | 5- to 10-fold reduction of Foxp3 expression Displayed activated phenotype |
| Dominant Th2 cytokine production by Foxp3+ and Foxp3– CD4 T cells. | ||
| IL-4–/–-FILIG/Y | IRES-luciferase-IRES-EGFP knocked into 3′-UTR of endogenous Foxp3 locus. | 5- to 10-fold reduction of Foxp3 expression Displayed activated phenotype |
| Deletion of IL-4 gene. | Th1 and Th2 cytokine production by Foxp3+ and Foxp3– CD4 T cells. | |
| STAT-6–/–-FILIG/Y | IRES-luciferase-IRES-EGFP knocked into 3′-UTR of endogenous Foxp3 locus. | 5- to 10-fold reduction of Foxp3 expression Displayed activated phenotype |
| Deletion of STAT-6 gene. | Foxp3+ T cells produce Th1 and Th2 cytokine; Foxp3– CD4 T cells produce Th1 cytokine. | |
| STAT-6–/–-FIR/Y | IRES-mRFP knocked into 3′-UTR of endogenous Foxp3 locus. | Grossly normal but deficient in STAT-6 |
| Deletion of STAT-6 gene. | ||
| CD4Cre-GATA3flox/flox-FIR/Y | IRES-mRFP knocked into 3′-UTR of endogenous Foxp3 locus. | Reduced CD4 T cell numbers. |
| Deletion of GATA-3 gene in T cells. |
Flow cytometry
Lymphocytes were isolated from the lymph nodes and spleen from 8- to 12-wk-old mice. Abs for FACS analysis were purchased from eBioscience (San Diego, CA). Surface and intracellular staining was performed per manufacturer's protocols (eBioscience). Stained cells were analyzed on a LSRII station (BD Biosciences, San Jose, CA) and CyAn (DakoCytomation, Carpinteria, CA; Beckman Coulter, Fullerton, CA) or sorted on a MoFlow cell sorter (DakoCytomation; Beckman Coulter).
T cell culture, activation, and retroviral transduction
T cells were cultured in Bruff's medium supplemented with 10% FBS and 1% penicillin/streptomycin. T cells were activated with soluble 2 μg/ml anti-CD3 and 1 μg/ml anti-CD28 for long-term culturing or with 40 ng/ml PMA and 1 μM ionomycin to stimulate cytokine production. To ectopically express genes of interest in T cells, mouse stem cell virus (MSCV)-mediated gene transfer was performed. recombinant MSCV viral constructs encoding EGFP (MSCV-IRES–enhanced GFP [MIG]) and EGFP/ Foxp3 (MIG-Foxp3) were provided by Dr. A. Rudensky (Memorial Sloan– Kettering Cancer Center, New York, NY) and Dr. S. Ziegler (Benaroya Research Institute, Seattle, WA). These constructs were transfected into Phoenix-Eco packaging cells using Lipofectamine 2000 per manufacturer's protocols (Invitrogen, Carlsbad, CA), and recombinant retroviruses were harvested 48 and 72 h after transfection. Foxp3− CD4 T cells were sorted from FIR mice (Fig. 6) or from FIR mice deficient in STAT-6 (Supplemental Fig. 6). Purified cells were activated for 48 h under culturing conditions and then spin-inoculated with MIG or MIG-Foxp3 viruses. The expression of the genes of interest was assessed by flow cytometry analysis 72 h posttransduction.
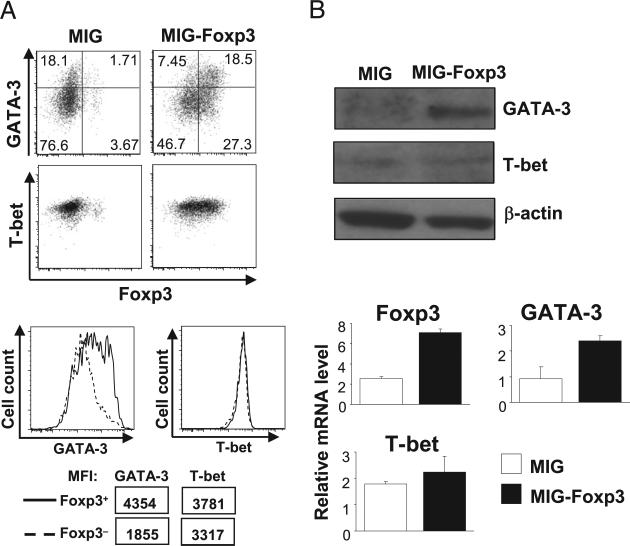
FIGURE 6.
Ectopic expression of Foxp3 promoted GATA-3 but not T-bet expression. Foxp3− (RFP−) CD4 T cells were sorted from FIR mice. Purified cells were then stimulated and subsequently transduced with MIG and MIG-Foxp3 retroviruses. A, The expression of Foxp3, GATA-3, and T-bet was assessed by intracellular staining and flow cytometric analysis. After MIG-Foxp3 transduction, expression levels of GATA-3 and T-bet were compared between Foxp3+ (solid lines) and Foxp3− (dashed lines) CD4 T cells that coexisted in the same culture. MFIs of GATA-3 and T-bet staining are also shown. Results are representative of at least three experiments. B, The protein and mRNA levels of GATA-3 and T-bet in MIG- or MIG-Foxp3–transduced CD4 T cells were compared by immunoblotting and quantitative RT-PCR. Results are representative of three experiments. The differences observed in Foxp3 and GATA-3 expression were statistically significant.
Quantitative RT-PCR and immunoblotting
RNA was extracted using TRIzol reagent and cDNA was synthesized by Superscript II reverse transcriptase per the manufacturer's protocols (Invitrogen). Quantitative PCR was performed on the ABI 7900HT Real-time PCR system using primer/probe sets purchased from Applied Bio-systems (Foster City, CA). Abs against GATA-3, T-bet, and β-actin (Santa Cruz Biotechnology, Santa Cruz, CA) were used for immunoblotting per manufacturer's protocols.
Statistical analysis
Data from at least three sets of samples were used for statistical analysis. Statistical significance was calculated by Student t test. A p value <0.05 was considered significant.
Results
Downregulation of Foxp3 expression in nTreg cells in vivo
Foxp3-expressing cells have been reported to convert into Th2 cells either in FILIG mice where the Foxp3 expression was substantially attenuated (8) or in mice deficient in Runx1-Cbfβ transcription complex, where Foxp3 expression was reduced modestly (14). It, however, remains unknown as whether wild-type nTreg cells can attenuate Foxp3 expression and convert into IL-4–producing Th2 cells in vivo.
The existence of small numbers of T cells in immune-deficient lymphopenic hosts may lead to Th2 immune diseases (15, 16). We therefore hypothesized that transferring nTreg cells into lymphopenic hosts could result in the downregulation of Foxp3 expression and nTreg-to-Th2 conversion. To test this hypothesis, we purified RFP+ cells, wild-type nTreg equivalent from FIR mice where an IRES-mRFP expression cassette was knocked into 3′-UTR of endogenous Foxp3 gene (17). These purified nTreg cells were then transferred into Rag1−/− mice. Eight weeks post-transfer, the Foxp3 expression in recovered T cells was assessed by intracellular staining. More than 50% of recovered cells showed no Foxp3 expression (Foxp3−), whereas ~40% of them maintained Foxp3 expression (Foxp3+) (Fig. 1A). However, the expression levels of Foxp3 in recovered Foxp3+ cells were modestly reduced when compared with freshly isolated nTreg cells (Fig. 1B). The degree of Foxp3 expression reduction was similar to that found in mice deficient in Runx1-Cbfβ transcription complex (14, 18).
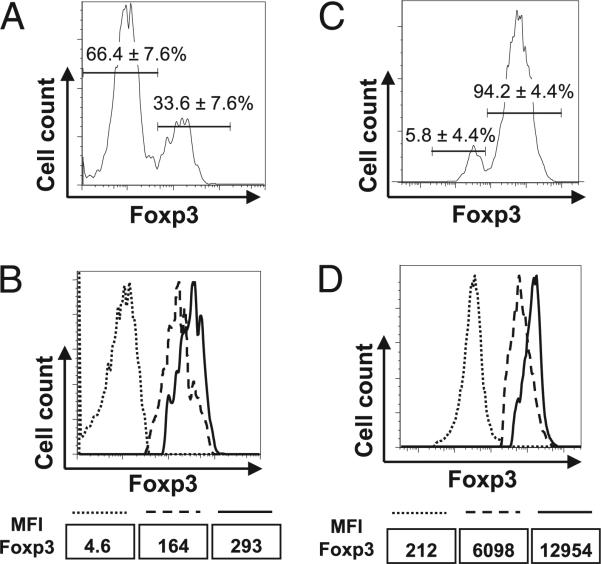
FIGURE 1.
Foxp3 downregulation in Treg cells in vivo. Sorted RFP+ nTreg cells from FIR/Y mice bearing CD45.2 congenic marker were transferred into Rag1−/− mice either alone (A, B) or together with equal numbers of naive CD4 T cells (CD4+CD25−CD45RBhigh) sorted from wild-type CD45.1 mice (C, D). Eight weeks after transfer, CD4 T cells were recovered from the spleens of recipient mice. Foxp3 expression of transferred CD45.2+ cells was assessed by intracellular staining. A and C, The percentages of Foxp3+ and Foxp3− cells among donor cells (CD45.2+) recovered from Rag1−/− recipient mice are shown with mean value ± SD from three experiments. B and D, Foxp3 expression levels in Foxp3+ donor cells (CD45.2+) recovered from Rag1−/− recipient mice (dashed lines) were compared with those in freshly purified Foxp3+ (RFP+) cells (solid lines) and Foxp3− (RFP−) cells (dotted lines) from FIR/Y mice by Foxp3 intracellular staining. MFIs of Foxp3 staining are also shown. Representative results from at least three experiments are shown. MFI, mean fluorescence intensity.
Although a small fraction of nTreg cells lost Foxp3 expression under homeostatic conditions (7), a great number of nTreg cells lost Foxp3 expression when they were transferred into T cell-deficient CD3ε−/− mice (6). The high percentage of Foxp3− regulatory T (Treg) cells found in the lymphopenic CD3ε−/− and Rag1−/− recipient mice could be due to the absence of coexisting naive CD4 T cells. We therefore examined whether the presence of naive CD4 T cells might affect Foxp3 expression in transferred nTreg cells. CD4 nTreg cells (RFP+)sorted from FIR mice bearing CD45.2 congenic marker were mixed at a ratio of 1:1 with naive CD4 T cells (CD4+CD25−CD45RBhigh) sorted from wild-type mice bearing the CD45.1 congenic marker. The cell mixtures were then transferred into Rag1−/− mice. Eight weeks posttransfer, the cells recovered from different donor origins were distinguished by congenic markers, and the Foxp3 expression was assessed by intracellular staining. The coexistence of naive CD4 T cells largely prevented the generation of Foxp3− Treg cells; on average, 5% of Foxp3− Treg cells was recovered (Fig. 1C), a percentage similar to what had been reported under homeostatic conditions (7). Nevertheless, the expression levels of Foxp3 in recovered Foxp3+ cells remained modestly reduced when they were compared with freshly isolated nTreg cells (Fig. 1D). Thus, wild-type nTreg cells can indeed attenuate Foxp3 expression under lymphopenic condition, regardless the presence or absence of naive CD4 T cells.
Th2 conversion of Foxp3-expressing cells and its impact on Th2 differentiation of coexisting naive CD4 T cells
We next examined whether nTreg cells with reduced Foxp3 expression could convert into IL-4–producing Th2 cells or other types of effector T cells. To this end, we generated FIR-4GET mice where Foxp3-expressing cells were marked by mRFP expression from FIR allele (17), and IL-4–expressing cells were marked by EGFP expression from 4GET alleles (19). RFP+ cells were purified from FIR-4GET mice and transferred into lymphopenic Rag1−/− hosts. Foxp3 and IL-4 expression in CD4 T cells recovered from the recipients was assessed by RFP and GFP expression, respectively. Although little IL-4 was expressed by Foxp3− CD4 T cells, ~10% of Foxp3+ cells expressed IL-4 (Fig. 2A). Further analysis revealed that Foxp3+ cells produced minimum amounts of IFN-γ or IL-17A (Supplemental Fig. 1). In contrast to Foxp3+ cells, Foxp3− cells recovered from the recipients adopted a Th1 phenotype with IFN-γ but not IL-17A being produced (Supplemental Fig. 1), agreeing with a previous report (7). The presence of naive CD4 T cells did not prevent Th2 conversion of nTreg cells, because ~4% of Foxp3+ cells expressed IL-4 when naive CD4 T cells were cotransferred (Fig. 2B). These findings suggest that, with reduced Foxp3 expression, wild-type nTreg cells could indeed convert into IL-4–producing Th2 cells.
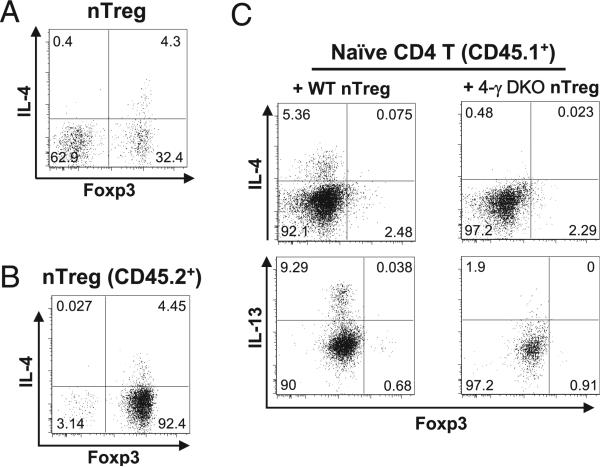
FIGURE 2.
IL-4 production in Foxp3+ cells and its involvement in directing Th2 differentiation of coexisting naive CD4 T cells. A and B, RFP+ nTreg cells were sorted from FIR-4GET mice bearing CD45.2 congenic marker and then transferred into Rag1−/− mice either alone (A) or together with equal numbers of naive CD4 T cells (CD4+CD25− CD45RBhigh) sorted from wild-type CD45.1 mice (B). IL-4 expression in transferred nTreg cells was assessed by the GFP expression from 4GET alleles. Representative results from at least three experiments are shown. C, Sorted wild-type naive CD4 T cells (CD4+CD25−CD45RBhigh) were mixed with equal numbers of RFP+ nTreg cells purified either from FIR mice (+WT nTreg) or from FIR mice lacking both IL-4 and IFN-γ genes (+ 4-γ DKO nTreg). Naive CD4 T and nTreg cells expressed congenic markers CD45.1 and CD45.2, respectively. Cell mixtures were then transferred into Rag1−/− recipient mice. Eight weeks posttransfer, CD4 T cells were recovered from the recipient mice. The expression of IL-4, IL-13, and Foxp3 in wild-type naive CD4 T cells (CD45.1+) was assessed by intracellular staining. Results representative of three experiments are shown. WT, wild-type.
Intrigued by above findings, we speculated that Th conversion of nTreg cells may impact the differentiation of coexisting naive CD4 T cells. To investigate whether IL-4 and IFN-γ production by converted nTreg cells could affect the differentiation of coexisting naive CD4 T cells, we transferred wild-type naive CD4 T cells together with either wild-type nTreg cells or nTreg cells lacking both IL-4 and IFN-γ genes (4-γ DKO). Naive CD4 T and nTreg cells were distinguished by the congenic markers CD45.1 and CD45.2, respectively. Although IL-4 and IL-13 were produced by wild-type naive CD4 T cells when wild-type nTreg cells were cotransferred, such a production was virtually abolished when 4-γ DKO nTreg cells were cotransferred (Fig. 2C). Large numbers of naive CD4 T cells produced IFN-γ when wild-type nTreg cells were cotransferred; such a production was modestly decreased when 4-γ DKO Treg cells were cotransferred (Supplemental Fig. 2). Thus, IL-4 and, to a lesser extent, IFN-γ production by converted nTreg cells was important for the differentiation of coexisting naive CD4 T cells, underscoring a potential role of dysregulated nTreg cells in directing differentiation of coexisting naive CD4 T cells.
Foxp3-expressing cells were permissive for Th1 conversion upon abrogation of IL-4/STAT-6 signaling
High level of Foxp3 expression suppresses the production of effector cytokines in T cells (3). Th2 conversion of nTreg cells upon Foxp3 attenuation could be attributed to at least two explanations. One is that high levels of Foxp3 expression are required to suppress IL-4 expression, but low levels of Foxp3 are sufficient to suppress IFN-γ expression. Thus only IL-4 but not IFN-γ could be produced by nTreg cells when Foxp3 expression is reduced. The other is that high levels of Foxp3 expression are required to suppress both IL-4 and IFN-γ expression; IL-4 and IFN-γ can be expressed in nTreg cells when Foxp3 expression is decreased. Nevertheless, mechanisms exist in nTreg cells to preferentially express IL-4 but not IFN-γ to promote Th2 conversion. To investigate the mechanisms underlying the Th2 bias of Foxp3-expressing cells, we tested these two possibilities.
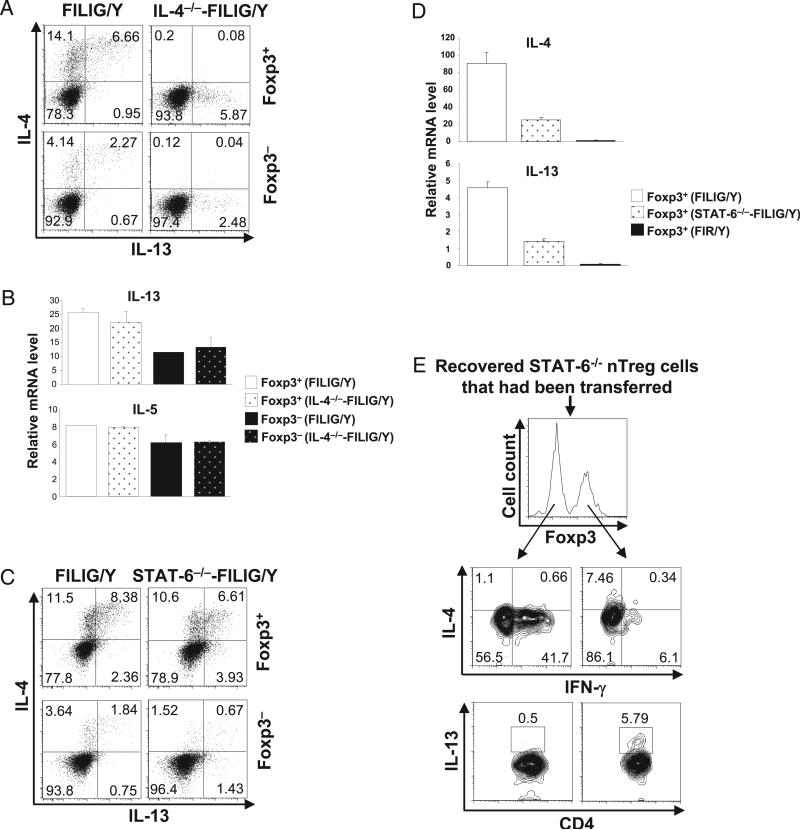
As previously described, when an IRES-luciferase-IRES-EGFP expression cassette was knocked into the 3′-UTR of endogenous Foxp3 gene, Foxp3 expression was decreased 5- to 10-fold in FILIG nTreg cells when compared with wild-type nTreg cells (8). The suppressive activity of Foxp3-expressing cells from FILIG mice was greatly impaired, leading to the activation of T cells and the development of an aggressive autoimmune syndrome in FILIG/Y mice (8). In addition, both Foxp3+ and Foxp3− CD4 T cells from FILIG/Y mice predominantly produced Th2 signature cytokine IL-4 (Supplemental Fig. 3) (8). IL-4/STAT-6 signaling axis is critical for Th2 differentiation of T cells (20–22). To test whether Foxp3+ cells are able to convert into effector cells other than Th2 cells in FILIG/Y mice, we abrogated the IL-4/STAT-6 signaling pathways in T cells in FILIG/Y mice by deleting IL-4 or STAT-6 genes. Deletion of IL-4 or STAT-6 gene in control FIR/Y mice did not perturb the normal T cell phenotype (data not shown). Compared with wild-type nTreg cells, Foxp3+ cells from IL-4−/−-FILIG/Y and STAT-6−/−-FILIG/Y mice expressed 5- to 10-fold less Foxp3. Similar to what had been observed in FILIG/Y mice, Foxp3− CD4 T cells displayed activated T cell phenotypes in IL-4−/−-FILIG/Y and STAT-6−/−-FILIG/Y (Supplemental Fig. 4).
We assessed cytokines production in Foxp3-expressing T cells from IL-4−/−-FILIG/Y and STAT-6−/−-FILIG/Y mice by intracellular staining. nTreg cells from control FIR mice deficient in IL-4 or STAT-6 produced minimal amounts of IFN-γ or IL-17A (data not shown). However, IFN-γ but not IL-17A was produced by a large percentage of Foxp3+ T cells in IL-4−/−-FILIG/Y mice (Fig. 3A). To gain more information on the Th conversion of Foxp3-expressing cells in these mice, we purified Foxp3+ CD4 T cells from FILIG/Y and IL-4−/−-FILIG/Y mice, based on GFP expression and assessed mRNA levels of cytokines and related transcription factors. In agreement with the results of intracellular staining and flow cytometry analysis, compared with Foxp3+ CD4 T cells from FILIG/Y mice, Foxp3+ CD4 T cells from IL-4−/−-FILIG/Y mice expressed higher mRNA levels of the Th1 signature cytokine IFN-γ and specifying transcription factor T-bet (23), lower levels of the Th2-specifying factor GATA-3 (Fig. 3B) (24, 25), and comparable levels of the Th17 signature cytokine IL-17A and specifying factor ROR-γt (Fig. 3B) (26).
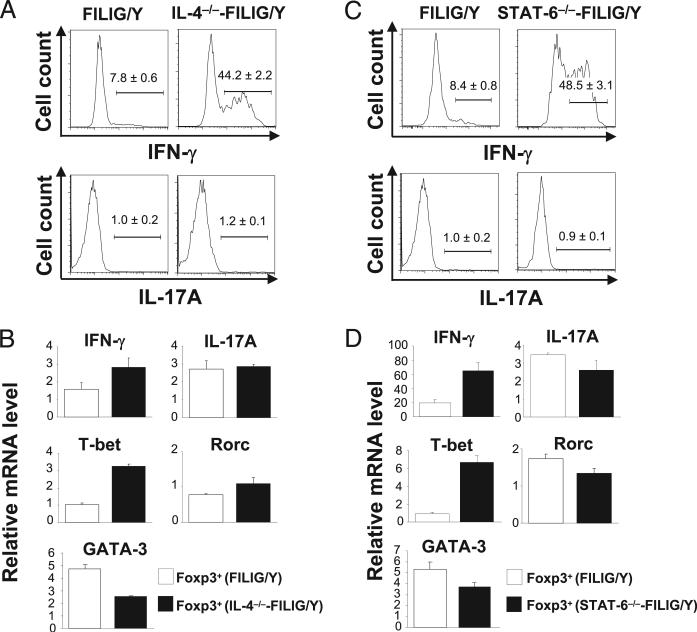
FIGURE 3.
Th1 but not Th17 conversion of Foxp3-expressing cells upon ablation of IL-4/STAT-6 signaling. A, Foxp3+ CD4 T cells from FILIG/Y mice (FILIG/Y) and IL-4–deficient FILIG/Y mice (IL-4−/−-FILIG/Y) were identified by intracellular staining. The percentages of IFN-γ– and IL-17A–producing cells in Foxp3+ T cells were determined by intracellular staining and are shown with mean value ± SD from three experiments. B, Relative mRNA levels of IFN-γ, IL-17A, T-bet, Rorc, and GATA-3 in Foxp3+ (GFP+) CD4 T cells sorted from FILIG/Y mice and IL-4–deficient FILIG/Y mice were determined by quantitative RT-PCR. Data are mean value ± SD of triplicates done in one experiment representative of three. The differences observed in IFN-γ, T-bet, and GATA-3 expression were statistically significant. C, Foxp3+ CD4 T cells from FILIG/Y mice (FILIG/Y) and STAT-6–deficient FILIG/Y mice (STAT-6−/−-FILIG/Y) were identified by intracellular staining. The percentages of IFN-γ– and IL-17A–producing cells in Foxp3+ T cells were determined by intracellular staining and are shown with mean value ± SD from three experiments. D, Relative mRNA levels of IFN-γ, IL-17A, T-bet, Rorc, and GATA-3 in Foxp3+ (GFP+) CD4 T cells sorted from FILIG/Y mice and STAT-6–deficient FILIG/Y mice were assessed by quantitative RT-PCR. Data are mean value ± SD of triplicates done in one experiment representative of three. The differences observed in IFN-γ, T-bet, and GATA-3 expression were statistically significant.
Similarly, IFN-γ but not IL-17A was produced by a large percentage of Foxp3+ CD4 T cells from STAT-6−/−-FILIG/Y mice (Fig. 3C). Compared with Foxp3+ CD4 T cells from FILIG/Y mice, Foxp3+ CD4 T cells from STAT6−/−-FILIG/Y mice expressed higher levels of IFN-γ and T-bet, lower levels of GATA-3, and similar levels of IL-17A and ROR-γt (Fig. 3D). Thus, Treg cells are permissive for converting into Th1 cells in the absence of IL-4/STAT-6 signaling.
Persistent Th2 conversion of Foxp3-expressing cells in the absence of IL-4/STAT-6 signaling
Th1 and Th2 differentiation programs antagonize each other in a reciprocal fashion. Once Th1 differentiation is initiated, Th1 cells reinforce their differentiation by both promoting Th1 program and shutting down Th2 program. Thus, Th1-Th2 differentiation is generally thought to be mutually exclusive (27). Unexpectedly, however, we found that Th2 differentiation of Treg cells persisted even when IL-4/STAT-6 signaling was abrogated.
Although IL-13 was minimally produced by CD4 T cells from FIR/Y, IL-4−/−-FIR/Y,and STAT-6−/−-FIR/Y mice (data not shown), it was substantially upregulated in Foxp3+ and Foxp3− CD4 Tcells in FILIG/Y mice (Fig. 4A). Interestingly, in IL-4−/−-FILIG/Y mice, although IL-4 deficiency led to a dramatic increase of IFN-γ production by Foxp3+ CD4 T cells (Fig. 3A), IL-13 production by these cells was virtually unchanged (Fig. 4A), suggesting that Th2 differentiation occurred when IL-4 production was absent, and IFN-γ production was dominant. Consistent with this, CD4 T cells (Foxp3+ or Foxp3−) purified from FILIG/Y and IL-4−/−-FILIG/Y mice expressed similar levels of IL-13 and IL-5 mRNA (Fig. 4B). More strikingly, in STAT6−/−-FILIG/Y mice, although IL-4 and IL-13 production in Foxp3− CD4 T cells was nearly abolished, a great number of Foxp3+ CD4 T cells produced IL-4 and IL-13 (Fig. 4C), albeit at reduced levels when compared with IL-4 and IL-13 produced by Foxp3+ CD4 T cells from FILIG/Y mice (Fig. 4C). In addition, we noticed that the frequencies of IL-4–producing cells among Foxp3+ CD4 cells did not decline with age in STAT6−/−-FILIG/Y mice. In fact, similar percentages of Foxp3+ cells produced IL-4 in 3- and 10-wk-old STAT6−/−-FILIG/Y mice (Supplemental Fig. 5). Consistent with these findings, we detected substantial amounts of IL-4 and IL-13 mRNA produced by Foxp3+ T cells isolated from STAT6−/−-FILIG/Y (Fig. 4D). However, compared with Foxp3+ isolated from FILIG/Y mice, Foxp3+ T cells isolated from STAT6−/−-FILIG/Y produced fewer amounts of IL-4 and IL-13 mRNA. Collectively, these findings suggest that Foxp3 reduction leads to Th2 conversion of nTreg cells independent of STAT-6, and STAT-6 is nevertheless important for the optimal/maximal IL-4 production by converted Tcells.
FIGURE 4.
Persistent Th2 conversion of Foxp3-expressing cells in the absence of IL-4/STAT-6 signaling. A, IL-4 and IL-13 production by Foxp3+ (GFP+) and Foxp3− (GFP−) CD4 T cells sorted from FILIG/Y mice (FILIG/Y) and IL-4–deficient FILIG/Y mice (IL-4−/−-FILIG/Y) were assessed by intracellular staining. Results representative of two experiments are shown. B, Relative mRNA levels of IL-13 and IL-5 in Foxp3+ (GFP+) and Foxp3− (GFP−) CD4 T cells sorted from FILIG/Y mice and IL-4–deficient FILIG/Y mice were determined by quantitative RT-PCR. Data are mean value ± SD of triplicates done in one experiment representative of three. C, IL-4 and IL-13 production by Foxp3+ (GFP+) and Foxp3− (GFP−) CD4 T cells sorted from FILIG/Y mice (FILIG/Y) and STAT-6–deficient FILIG/Y mice (STAT-6−/−-FILIG/Y) were assessed by intracellular staining. Results representative of two experiments are shown. D, Relative mRNA levels of IL-4 and IL-13 in GFP+ (Foxp3+) CD4 T cells sorted from FILIG/Y mice and STAT-6–deficient FILIG/Y mice and RFP+ (Foxp3+) CD4 T cells sorted from FIR/Y mice were determined by quantitative RT-PCR. Data are mean value ± SD of triplicates done in one experiment representative of three. E, STAT-6−/− nTreg cells (RFP+) sorted from STAT-6–deficient FIR mice were transferred into Rag1−/− mice. Eight weeks later, the expression of IL-4, IFN-γ, and IL-13 in Foxp3+ and Foxp3− cells recovered from the recipient mice was assessed. Representative results of three experiments are shown.
We next investigated whether nTreg cells without genetically forced attenuation of Foxp3 can indeed convert into IL-4–producing cells independent of STAT-6 in vivo. To this end, nTreg cells were purified from STAT6−/−-FIR/Y mice and transferred into Rag1−/− mice. Eight weeks after transfer, the expression of Foxp3, IL-4, IL-13, and IFN-γ in recovered T cells was assessed by intracellular staining. In accordance with the abovementioned findings, Foxp3+ but not Foxp3− cells produced IL-4 and IL-13 in the absence of STAT-6 (Fig. 4E). Our findings demonstrated that, rather surprisingly, Th2 conversion of Treg cells is independent of IL-4/STAT-6. These results suggested that, unlike naive CD4 T cells whose Th2 differentiation is dependent on IL-4/STAT-6 signaling (20–22), IL-4/STAT-6–independent signaling pathway(s) exists in nTreg cells to program their Th2 conversion.
GATA-3 is upregulated in Foxp3-expressing cells and required for Th2 conversion of nTreg cells
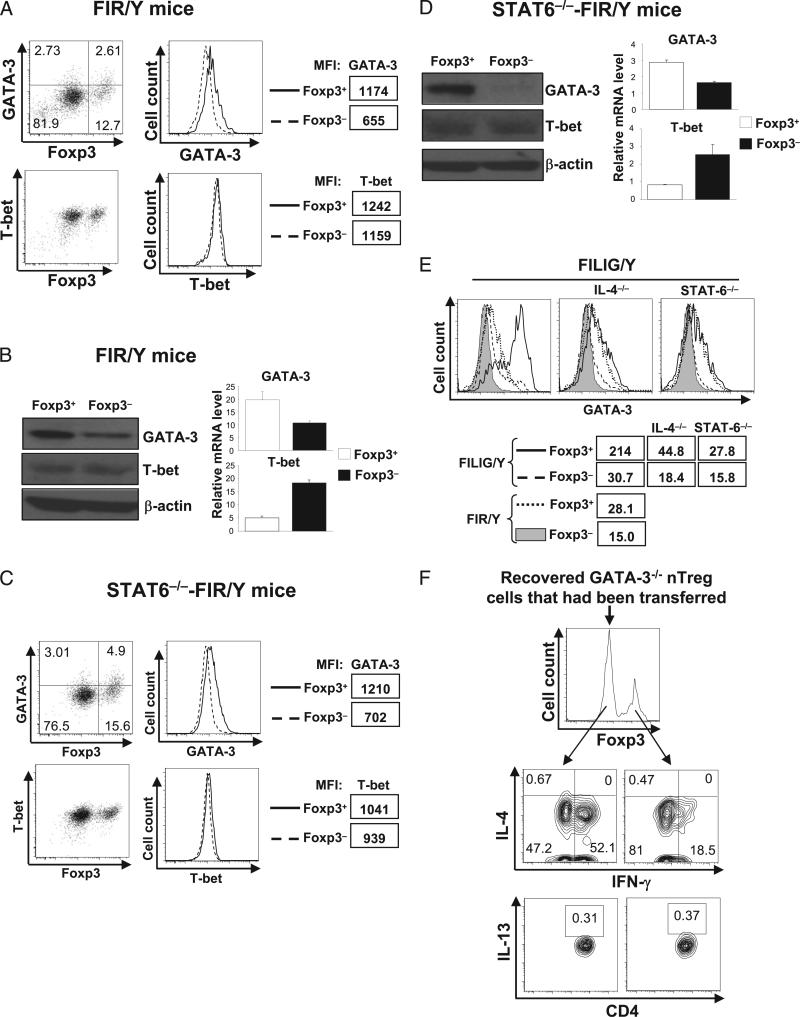
GATA-3 and T-bet are recognized as the master regulators to promote Th2 (24, 25) and Th1 (23) differentiation, respectively. In a reciprocal fashion, GATA-3 and T-bet antagonize each other in controlling Th1/2 differentiation by regulating genes largely in common (27, 28). Thus, the relative expression levels of GATA-3 and T-bet dictate Th2-Th1 differentiation. We hypothesized that an imbalance between GATA-3 and T-bet expression existed in Foxp3-expressing cells to favor Th2 program. To test this hypothesis, we investigated the expression of GATA-3 and T-bet in naive CD4 T cells and nTreg cells in wild-type FIR/Y mice. By intracellular staining assays, we observed that, compared with Foxp3– CD4 T cells, Foxp3+ T cells expressed higher levels of GATA-3 and similar levels of T-bet (Fig. 5A). To confirm this finding, we purified Foxp3− and Foxp3+ from FIR/Y mice and measured the expression of GATA-3 and T-bet by immunoblotting and quantitative RT-PCR. Consistent with the results of intracellular-staining, compared with Foxp3− CD4 T cells, Foxp3+ CD4 T cells expressed more GATA-3 and similar amounts of T-bet at the protein level and expressed higher levels of GATA-3 mRNA but lower levels of T-bet mRNA (Fig. 5B). Therefore, wild-type nTreg cells preferentially upregulated GATA-3 but not T-bet expression, resulting in an imbalance biasing toward Th2 conversion.
FIGURE 5.
GATA-3 expression is elevated in nTreg cells and required for nTreg-to-Th2 conversion. A, GATA-3 and T-bet expression in Foxp3+ and Foxp3− CD4 T cells in wild-type mice were assessed by intracellular staining and flow cytometry analysis. MFIs of GATA-3 and T-bet intracellular staining are also shown. Results are representative of at least five experiments. B, GATA-3 and T-bet expression in purified RFP+ (Foxp3+) and RFP− (Foxp3−) CD4 T cells from FIR mice were assessed by immunoblotting and quantitative RT-PCR. Data for quantitative RT-PCR are mean value ± SD of triplicates done in one experiment. Representative results from three experiments are shown. The differences observed in GATA-3 and T-bet mRNA expression levels were statistically significant. C, GATA-3 and T-bet expression in Foxp3+ and Foxp3− CD4 T cells in STAT-6−/− mice assessed by flow cytometry. MFIs of GATA-3 and T-bet staining are also shown. Results are representative of three experiments. D, GATA-3 and T-bet expression in sorted RFP+ (Foxp3+) and RFP− (Foxp3−) CD4 T cells from STAT-6–deficient FIR mice were assessed by immunoblotting and quantitative RT-PCR. Data for quantitative RT-PCR are mean value ± SD of triplicates done in one experiment. Representative results from three experiments are shown. The differences observed in GATA-3 and T-bet mRNA expression levels were statistically significant. E, GATA-3 expression in Foxp3+ (solid lines) and Foxp3− (dashed lines) CD4 T cells purified from FILIG/Y mice, IL-4–deficient FILIG/Y mice (IL-4−/−), and STAT-6–deficient FILIG/Y mice (STAT-6−/−) was assessed by flow cytometry and compared with that in Foxp3+ (dotted lines) and Foxp3− (shaded area) CD4 T cells purified from FIR/Y mice. MFI of GATA-3 intracellular staining is also shown. Results are representative of three experiments. F, GATA3−/− nTreg cells (RFP+) sorted from GATA-3–deficient FIR mice (CD4Cre-GATA3flox/flox-FIR/Y) were transferred into Rag1−/− mice. Eight weeks posttransfer, the expression of IL-4, IFN-γ, and IL-13 in Foxp3+ and Foxp3− cells recovered from the recipient mice was assessed. Representative results of three experiments are shown.
STAT-6 is essential to promote GATA-3 expression in T cells (29). We then investigated whether increased GATA-3 expression observed in nTreg cells may be due to STAT-6 signaling during nTreg development or homeostasis. We assessed the expression of GATA-3 and T-bet in Foxp3− and Foxp3+ CD4 T cells in STAT-6– deficient FIR/Y mice. By intracellular staining assays, we observed that, in STAT-6−/−-FIR mice, compared with Foxp3– CD4 T cells, Foxp3+ CD4 T cells expressed higher levels of GATA-3 and similar levels of T-bet (Fig. 5C). To confirm this finding, we purified Foxp3+ and Foxp3− CD4 T cells from STAT-6−/−-FIR mice and then measured the protein and mRNA levels of GATA-3 and T-bet by immunoblotting and quantitative RT-PCR, respectively. In the absence of STAT-6, compared with Foxp3− CD4 T cells, Foxp3+ CD4 T cells expressed higher levels of GATA-3 protein and mRNA, but similar levels of T-bet protein and decreased levels of T-bet mRNA (Fig. 5D). These findings suggest that increased expression of GATA-3 is an intrinsic property of nTreg cells independent of STAT-6 signaling.
We further investigated how GATA-3 expression was regulated in Foxp3+ and Foxp3− CD4 T cells under Th2-type inflammatory conditions in FILIG/Y mice. Compared with Foxp3+ cells from FIR/Y mice, Foxp3+ cells from FILIG/Y mice expressed increased levels of GATA-3. Compared with Foxp3− CD4 T cells from FIR/Y mice, Foxp3− CD4 T cells from FILIG/Y mice also expressed increased levels of GATA-3 (Fig. 5, left panel). Such observation is in accordance with elevated IL-4 production in both Foxp3+ and Foxp3− CD4 T cells in FILIG/Y mice (Supplemental Fig. 3) (8). Upon deletion of IL-4 or STAT-6 in FILIG/Y mice, the further increase of GATA-3 in both Foxp3+ and Foxp3− CD4 T cells were blunted (Fig. 5E, middle and right panels). Nevertheless, we found that Foxp3+ cells consistently expressed higher levels of GATA-3 than coexisting Foxp3− CD4 T cells in these mice. Collectively, these observations suggest that, although IL-4/STAT-6–dependent signaling pathway remains functional in converted Foxp3+ cells to further promote GATA-3 expression, IL-4/STAT-6–independent mechanisms exist in Foxp3+ cells to elevate GATA-3 expression.
GATA-3 plays critical roles for Th2 differentiation (25) through STAT-6–independent autoactivation mechanisms (30). Thus, elevated GATA-3 expression in nTreg cells may explain their tendency toward Th2 conversion. Nevertheless, in light of above finding that nTreg cells may use mechanisms distinct from naive CD4 T cells to engage in IL-4 production and Th2 conversion, we further investigated whether GATA-3 is required for Th2 conversion of nTreg cells. We deleted GATA-3 specifically in T cells by crossing mice bearing floxed GATA-3 alleles (31) with mice transgenic for the gene encoding Cre recombinase driven by the Cd4 promoter (32) and then with FIR mice (17) to obtain CD4Cre-GATA-3flox/flox-FIR mice. GATA-3 was efficiently deleted in CD4 T cells from these mice (data not shown) and led to reduced numbers of CD4 T cells in the periphery as reported previously (33). Nevertheless, sufficient numbers of Foxp3+ (RFP+) cells were purified from these mice and transferred into Rag1−/− recipients, resulting in the downregulation of Foxp3 expression in these cells (Fig. 5F). Although IL-4 and IFN-γ was minimally produced by freshly isolated GATA-3–deficient nTreg cells before the transfer (data not shown), large numbers of CD4 T cells recovered from the recipient mice produced IFN-γ but not IL-4 or IL-13 (Fig. 5F). Therefore, GATA-3 is essential for Th2 conversion of nTreg cells in vivo.
Ectopic expression of Foxp3 specifically promoted the expression of GATA-3 but not T-bet
In a STAT-6–independent manner, GATA-3 but not T-bet expression was found elevated specifically in Foxp3+ but not Foxp3– CD4 T cells (Fig. 5C, 5D), suggesting that intrinsic mechanisms exist in Treg cells to promote GATA-3 expression. We then sought to address whether Foxp3 expression may differentially affect the expression of GATA-3 and T-bet in T cells. To this end, we ec-topically expressed Foxp3 in activated T cells and assessed its impact on GATA-3 and T-bet expression. Retrovirus-mediated expression of Foxp3 increased GATA-3 but not T-bet expression in activated CD4 T cells, which was confirmed by intracellular staining, immunoblotting, and quantitative RT-PCR assays (Fig. 6). This Foxp3-driven GATA-3 upregulation was independent of STAT-6 signaling as ectopic expression of Foxp3 elevated GATA-3 but not T-bet expression in STAT-6–deficient CD4 T cells (Supplemental Fig. 6).
Collectively, we have demonstrated that, without genetic manipulation, wild-type nTreg cells could indeed attenuate Foxp3 expression to result in Th2 conversion of Foxp3-expressing cells and to promote Th2-differentiation of coexisting naive CD4 T cells in vivo. In addition, we identified an intrinsic mechanism in nTreg cells that controls Th2 conversion in a STAT-6–independent manner. GATA-3 was found to be the central component of this mechanism, because its expression was found elevated in nTreg cells, and it was required for nTreg-to-Th2 conversion in vivo (Table I). Moreover, we found that Foxp3 expression could account for the specific upregulation of GATA-3 expression in nTreg cells as ectopic expression of Foxp3 promoted GATA-3 but not T-bet expression in T cells. Thus, this study revealed an intrinsic mechanism predisposing Foxp3-expressing nTreg cells to Th2 conversion in vivo.
Discussion
Th cells were traditionally thought to be terminally differentiated and fully committed lineages. Emerging evidence suggests that Th cells are “plastic” and can convert into other types of Th cells. Treg cells appear to possess the most diversity as they were reported to convert into Th1, Th2, Th17, and Tfh cells in vivo. The loss of Foxp3 expression led to Th1, Th17, and Tfh conversion of nTreg cells (6, 7). Reduction but not loss of Foxp3 expression led to Th2 conversion of nTreg cells in genetically modified mouse models (8, 14). Nevertheless, the question remains as to whether wild-type nTreg cells can indeed attenuate Foxp3 expression and become Th2 cells in vivo. In this paper, we showed that wild-type nTreg cells downregulated Foxp3 expression in lymphopenic hosts and those Foxp3-expressing cells converted into IL-4–producing Th2 cells. In addition, we found that the IL-4 production by Treg cells was important for the Th2 differentiation of coexisting naive CD4 T cells. Reconstitution with limited T cells into lymphopenic mice induced a Th2-type immune disease (15, 16). Our results thus suggest that Th2 conversion of Treg cells may be involved in these diseases. In addition, drastic attenuation of Foxp3 expression in Treg cells was found in human patients with chronic asthma (34), which may result in the Th2 conversion of Treg cells to contribute to asthma development. Nevertheless, whether and how nTreg-to-Th2 conversion is involved in Th2-type immune disorders needs to be investigated in the future.
nTreg-to-Th conversion has gained much attention recently in immunology research. The underlying mechanism through which Treg cells become other types of Th cells is yet to be defined. Treg is a unique T cell lineage phenotypically distinct from naive CD4 T cells even when Foxp3 expression was absent (35). Thus, nTreg and naive CD4 T cells may use different mechanisms to engage in the Th2 program. Indeed, we have identified an intrinsic mechanism favoring Th2 conversion of nTreg cells. Exogenous IL-4 plays a dominant role in directing Th2 differentiation in naive CD4 T cells by activating STAT-6 and subsequently GATA-3 to initiate and to stabilize Th2 phenotype. STAT-6 and GATA-3 are essential for Th2 differentiation of naive CD4 T cells (22, 25). We found that, in the absence of STAT-6, whereas the Th2 differentiation of naive CD4 T cells was abolished, the Th2 conversion of Treg cells persisted. Thus, Th2 conversion of Treg cells is controlled by STAT-6–independent mechanisms. Nevertheless, STAT-6–dependent pathways were found to be critical for the optimal IL-4 production in converted nTreg cells, because IL-4 and IL-13 production and GATA-3 expression in FILIG/Y mice decreased when STAT-6 was deficient. Therefore, both STAT-6–independent and –dependent mechanisms are operating during Th2 conversion of nTreg cells. So it appeared that, during nTreg-to-Th2 conversion, IL-4 production and Th2 program could be initiated through a STAT-6–independent mechanism and be further enhanced and stabilized through a STAT-6– dependent mechanism via a IL-4/STAT-6/GATA-3 feed-forward loop. GATA-3 may serve as the converging point for the pathways controlling nTreg-to-Th2 conversion. We found that, compared with naive CD4 T cells, nTreg cells upregulated GATA-3 but not T-bet, potentially because of the expression of Foxp3. More importantly, nTreg cells failed to become Th2 cells when GATA-3 was deficient. Thus, nTreg cells convert into Th2 cells in a STAT-6–independent and a GATA-3–dependent fashion. IL-4 production and Th2 immune response were observed in STAT6−/− mice (36, 37), albeit at a much reduced degree when compared with wild-type mice. However, whether Treg or non-Treg cells account for such observations was not investigated in these studies. In light of our findings, the involvement of Treg-to-Th2 conversion under those conditions warrants investigation.
Multiple mechanisms exist in Th cells to ensure their proper differentiation and function. Th1 and Th2 differentiation is dictated by the relative expression levels of GATA-3 and T-bet. Dominant GATA-3 activities not only promote the Th2 but also suppress the Th1 program. The converse is true of T-bet for Th1 differentiation (27). Foxp3 expression suppresses the production of both Th1 and Th2 effector cytokines in T cells (3). Although we found that GATA-3 expression was elevated in Foxp3-expressing cells, wild-type nTreg cells produced minimal amount of IL-4 and IL-13, suggesting that the suppressive function of Foxp3 overrides the Th2-promoting function of GATA-3 under normal conditions. However, through undefined mechanisms, when Treg function is dysregulated and Foxp3 expression is attenuated, Foxp3-mediated suppression on cytokine production is relieved. Under this circumstance, elevated expression of GATA-3 but not T-bet in Foxp3-expressing cells becomes functionally relevant and leads to Th2 conversion of nTreg cells. Of interest, activation of IL-4/ STAT-6/GATA-3 signaling pathway was found to inhibit Foxp3 expression in TGF-β–induced Treg cells (38, 39). One implication for these findings is that, upon initiation of Th2 conversion, Foxp3 expression in nTreg cell may be further inhibited by ensuing IL-4/ STAT-6/GATA-3 signaling to reinforce Th2 differentiation of converted cells and to prevent regaining nTreg functions of converted cells. Therefore, it warrants further investigation as to whether nTreg cells that have converted into Th2 are able to return to normal nTreg phenotype.
We demonstrated that ectopic expression of Foxp3 upregulated GATA-3 expression at the protein and mRNA level independent of STAT-6. Such an effect of Foxp3 on GATA-3 expression is likely to be through a direct binding of Foxp3 to the GATA-3 promoter or enhancers. Using a genome-wide ChIP-on-Chip tiling array analysis, a previous study has shown that Foxp3 directly binds to the GATA-3 promoter, although the exact binding site(s) was not revealed (40). Such a binding was correlated with the increased rather than decreased GATA-3 expression. Therefore, our results agreed with these findings and furthermore suggest that Foxp3 expression is sufficient to cause the preferential upregulation of GATA-3 expression, providing an explanation for the intrinsic ability of Treg cells to convert into Th2 cells. Numerous mechanisms exist to promote Th2 differentiation of naive CD4 T cells (41); Foxp3-promoted GATA-3 expression may not be the only mechanism involved in Treg-to-Th2 conversion. Further investigation is needed to reveal additional mechanisms controlling Th2 conversion of Treg cells.
Supplementary Material
Acknowledgments
We thank A. Rudensky and S. Ziegler for providing recombinant MSCV constructs. We are grateful to L. Zenewicz for critical reading of the manuscript and helpful comments.
This work was supported by a K99/R00 award from the National Institutes of Health/National Institute of Allergy and Infectious Diseases (to Y.Y.W.). R.A.F. is an investigator of the Howard Hughes Medical Institute.
Abbreviations used in this paper
- DKO
double knockout
- IRES
internal ribosome entry site
- MFI
mean fluorescence intensity
- MIG
MSCV-IRES–enhanced GFP
- MSCV
mouse stem cell virus
- nTreg
naturally occurring regulatory T
- Tfh
T follicular helper
- Treg
regulatory T
- UTR
untranslated region
- WT
wild-type
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 2.Shevach EM. Regulatory T cells in autoimmmunity*. Annu. Rev. Immunol. 2000;18:423–449. doi: 10.1146/annurev.immunol.18.1.423. [DOI] [PubMed] [Google Scholar]
- 3.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 4.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 5.Zheng Y, Rudensky AY. Foxp3 in control of the regulatory T cell lineage. Nat. Immunol. 2007;8:457–462. doi: 10.1038/ni1455. [DOI] [PubMed] [Google Scholar]
- 6.Tsuji M, Komatsu N, Kawamoto S, Suzuki K, Kanagawa O, Honjo T, Hori S, Fagarasan S. Preferential generation of follicular B helper T cells from Foxp3+ T cells in gut Peyer's patches. Science. 2009;323:1488–1492. doi: 10.1126/science.1169152. [DOI] [PubMed] [Google Scholar]
- 7.Zhou X, Bailey-Bucktrout SL, Jeker LT, Penaranda C, Martínez-Llordella M, Ashby M, Nakayama M, Rosenthal W, Bluestone JA. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat. Immunol. 2009;10:1000–1007. doi: 10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wan YY, Flavell RA. Regulatory T-cell functions are subverted and converted owing to attenuated Foxp3 expression. Nature. 2007;445:766–770. doi: 10.1038/nature05479. [DOI] [PubMed] [Google Scholar]
- 9.Lund JM, Hsing L, Pham TT, Rudensky AY. Coordination of early protective immunity to viral infection by regulatory T cells. Science. 2008;320:1220–1224. doi: 10.1126/science.1155209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 11.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 13.King C. New insights into the differentiation and function of T follicular helper cells. Nat. Rev. Immunol. 2009;9:757–766. doi: 10.1038/nri2644. [DOI] [PubMed] [Google Scholar]
- 14.Kitoh A, Ono M, Naoe Y, Ohkura N, Yamaguchi T, Yaguchi H, Kitabayashi I, Tsukada T, Nomura T, Miyachi Y, et al. Indispensable role of the Runx1-Cbfβ transcription complex for in vivo-suppressive function of FoxP3+ regulatory T cells. Immunity. 2009;31:609–620. doi: 10.1016/j.immuni.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Sommers CL, Park CS, Lee J, Feng C, Fuller CL, Grinberg A, Hildebrand JA, Lacaná E, Menon RK, Shores EW, et al. A LAT mutation that inhibits T cell development yet induces lymphoproliferation. Science. 2002;296:2040–2043. doi: 10.1126/science.1069066. [DOI] [PubMed] [Google Scholar]
- 16.Milner JD, Ward JM, Keane-Myers A, Paul WE. Lymphopenic mice reconstituted with limited repertoire T cells develop severe, multiorgan, Th2-associated inflammatory disease. Proc. Natl. Acad. Sci. USA. 2007;104:576–581. doi: 10.1073/pnas.0610289104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wan YY, Flavell RA. Identifying Foxp3-expressing suppressor T cells with a bicistronic reporter. Proc. Natl. Acad. Sci. USA. 2005;102:5126–5131. doi: 10.1073/pnas.0501701102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rudra D, Egawa T, Chong MM, Treuting P, Littman DR, Rudensky AY. Runx-CBFb complexes control expression of the transcription factor Foxp3 in regulatory T cells. Nat. Immunol. 2009;10:1170–1177. doi: 10.1038/ni.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohrs M, Shinkai K, Mohrs K, Locksley RM. Analysis of type 2 immunity in vivo with a bicistronic IL-4 reporter. Immunity. 2001;15:303–311. doi: 10.1016/s1074-7613(01)00186-8. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan MH, Schindler U, Smiley ST, Grusby MJ. Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity. 1996;4:313–319. doi: 10.1016/s1074-7613(00)80439-2. [DOI] [PubMed] [Google Scholar]
- 21.Le Gros G, Ben-Sasson SZ, Seder R, Finkelman FD, Paul WE. Generation of interleukin 4 (IL-4)–producing cells in vivo and in vitro: IL-2 and IL-4 are required for invitro generation of IL-4–producing cells. J. Exp. Med. 1990;172:921–929. doi: 10.1084/jem.172.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shimoda K, van Deursen J, Sangster MY, Sarawar SR, Carson RT, Tripp RA, Chu C, Quelle FW, Nosaka T, Vignali DA, et al. Lack of IL-4–induced Th2 response and IgE class switching in mice with disrupted Stat6 gene. Nature. 1996;380:630–633. doi: 10.1038/380630a0. [DOI] [PubMed] [Google Scholar]
- 23.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 24.Zheng W, Flavell RA. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 1997;89:587–596. doi: 10.1016/s0092-8674(00)80240-8. [DOI] [PubMed] [Google Scholar]
- 25.Zhu J, Min B, Hu-Li J, Watson CJ, Grinberg A, Wang Q, Killeen N, Urban JF, Jr., Guo L, Paul WE. Conditional deletion of Gata3 shows its essential function in T(H)1-T(H)2 responses. Nat. Immunol. 2004;5:1157–1165. doi: 10.1038/ni1128. [DOI] [PubMed] [Google Scholar]
- 26.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 27.Szabo SJ, Sullivan BM, Peng SL, Glimcher LH. Molecular mechanisms regulating Th1 immune responses. Annu. Rev. Immunol. 2003;21:713–758. doi: 10.1146/annurev.immunol.21.120601.140942. [DOI] [PubMed] [Google Scholar]
- 28.Jenner RG, Townsend MJ, Jackson I, Sun K, Bouwman RD, Young RA, Glimcher LH, Lord GM. The transcription factors T-bet and GATA-3 control alternative pathways of T-cell differentiation through a shared set of target genes. Proc. Natl. Acad. Sci. USA. 2009;106:17876–17881. doi: 10.1073/pnas.0909357106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurata H, Lee HJ, O'Garra A, Arai N. Ectopic expression of activated Stat6 induces the expression of Th2-specific cytokines and transcription factors in developing Th1 cells. Immunity. 1999;11:677–688. doi: 10.1016/s1074-7613(00)80142-9. [DOI] [PubMed] [Google Scholar]
- 30.Ouyang W, Löhning M, Gao Z, Assenmacher M, Ranganath S, Radbruch A, Murphy KM. Stat6-independent GATA-3 autoactivation directs IL-4-independent Th2 development and commitment. Immunity. 2000;12:27–37. doi: 10.1016/s1074-7613(00)80156-9. [DOI] [PubMed] [Google Scholar]
- 31.Amsen D, Antov A, Jankovic D, Sher A, Radtke F, Souabni A, Busslinger M, McCright B, Gridley T, Flavell RA. Direct regulation of Gata3 expression determines the T helper differentiation potential of Notch. Immunity. 2007;27:89–99. doi: 10.1016/j.immuni.2007.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee PP, Fitzpatrick DR, Beard C, Jessup HK, Lehar S, Makar KW, Pérez-Melgosa M, Sweetser MT, Schlissel MS, Nguyen S, et al. A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity. 2001;15:763–774. doi: 10.1016/s1074-7613(01)00227-8. [DOI] [PubMed] [Google Scholar]
- 33.Pai SY, Truitt ML, Ting CN, Leiden JM, Glimcher LH, Ho IC. Critical roles for transcription factor GATA-3 in thymocyte development. Immunity. 2003;19:863–875. doi: 10.1016/s1074-7613(03)00328-5. [DOI] [PubMed] [Google Scholar]
- 34.Provoost S, Maes T, van Durme YM, Gevaert P, Bachert C, Schmidt-Weber CB, Brusselle GG, Joos GF, Tournoy KG. Decreased FOXP3 protein expression in patients with asthma. Allergy. 2009;64:1539–1546. doi: 10.1111/j.1398-9995.2009.02056.x. [DOI] [PubMed] [Google Scholar]
- 35.Gavin MA, Rasmussen JP, Fontenot JD, Vasta V, Manganiello VC, Beavo JA, Rudensky AY. Foxp3-dependent programme of regulatory T-cell differentiation. Nature. 2007;445:771–775. doi: 10.1038/nature05543. [DOI] [PubMed] [Google Scholar]
- 36.Jankovic D, Kullberg MC, Noben-Trauth N, Caspar P, Paul WE, Sher A. Single cell analysis reveals that IL-4 receptor/Stat6 signaling is not required for the in vivo or in vitro development of CD4+ lymphocytes with a Th2 cytokine profile. J. Immunol. 2000;164:3047–3055. doi: 10.4049/jimmunol.164.6.3047. [DOI] [PubMed] [Google Scholar]
- 37.Blease K, Schuh JM, Jakubzick C, Lukacs NW, Kunkel SL, Joshi BH, Puri RK, Kaplan MH, Hogaboam CM. Stat6-deficient mice develop airway hyperresponsiveness and peribronchial fibrosis during chronic fungal asthma. Am. J. Pathol. 2002;160:481–490. doi: 10.1016/S0002-9440(10)64867-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takaki H, Ichiyama K, Koga K, Chinen T, Takaesu G, Sugiyama Y, Kato S, Yoshimura A, Kobayashi T. STAT6 Inhibits TGF-beta1-mediated Foxp3 induction through direct binding to the Foxp3 promoter, which is reverted by retinoic acid receptor. J. Biol. Chem. 2008;283:14955–14962. doi: 10.1074/jbc.M801123200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mantel PY, Kuipers H, Boyman O, Rhyner C, Ouaked N, Rückert B, Karagiannidis C, Lambrecht BN, Hendriks RW, Crameri R, et al. GATA3-driven Th2 responses inhibit TGF-β1–induced FOXP3 expression and the formation of regulatory T cells. PLoS Biol. 2007;5:e329. doi: 10.1371/journal.pbio.0050329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng Y, Josefowicz SZ, Kas A, Chu TT, Gavin MA, Rudensky AY. Genome-wide analysis of Foxp3 target genes in developing and mature regulatory T cells. Nature. 2007;445:936–940. doi: 10.1038/nature05563. [DOI] [PubMed] [Google Scholar]
- 41.Amsen D, Spilianakis CG, Flavell RA. How are T(H)1 and T(H)2 effector cells made? Curr. Opin. Immunol. 2009;21:153–160. doi: 10.1016/j.coi.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.