Abstract
Nerve agent-induced seizures cause neuronal damage in brain limbic and cortical circuits leading to persistent behavioral and cognitive deficits. Without aggressive anticholinergic and benzodiazepine therapy, seizures can be prolonged and neuronal damage progresses for extended periods of time. The objective of this study was to determine the effects of the nerve agent soman on expression of cyclooxygenase-2 (COX-2), the initial enzyme in the biosynthetic pathway of the proinflammatory prostaglandins and a factor that has been implicated in seizure initiation and propagation. Rats were exposed to a toxic dose of soman and scored behaviorally for seizure intensity. Expression of COX-2 was determined throughout brain from 4 hr to 7 days after exposure by immunohistochemistry and immunoblotting. Microglial activation and astrogliosis were assessed microscopically over the same time-course. Soman increased COX-2 expression in brain regions known to be damaged by nerve agents (e.g., hippocampus, amygdala, piriform cortex and thalamus). COX-2 expression was induced in neurons, and not in microglia or astrocytes, and remained elevated through 7 days. The magnitude of COX-2 induction was correlated with seizure intensity. COX-1 expression was not changed by soman. Increased expression of neuronal COX-2 by soman is a late-developing response relative to other signs of acute physiological distress caused by nerve agents. COX-2-mediated production of prostaglandins is a consequence of the seizure-induced neuronal damage, even after survival of the initial cholinergic crisis is assured. COX-2 inhibitors should be considered as adjunct therapy in nerve agent poisoning to minimize nerve agent-induced seizure activity.
Keywords: soman, COX-2, neurons, microglia, astrocytes, neurodegeneration
1. Introduction
Nerve agents (NAs) such as soman, sarin and tabun are organophosphate inhibitors of acetylcholinesterase (AChE). Exposure to NAs leads to a constellation of symptoms that reflect rapid and irreversible inactivation of AChE. Significant physiological effects can be observed within minutes of exposure and generally progress from hypersecretions to fasciculations, tremor, seizures and respiratory distress. Left untreated, NA exposure is rapidly fatal in humans and in animal models of NA toxicity (Kadar et al., 1995; Lee, 2003; McDonough and Shih, 1997). NA are part of the military chemical/biological arsenal and they have also been used by terrorists against civilian targets (e.g., 1995 Tokyo subway attack). Therefore, it is very important to understand their mechanisms of action so that optimal countermeasures and therapies are available.
NA toxicity is generally attributed to the synaptic buildup of exceedingly high levels of acetylcholine (Taylor, 2001). A combined regimen of prophylaxis and therapy has been designed mechanistically to counteract the immediate effects of NAs. First, carbamate cholinesterase inhibitors such as pyridostigmine are administered to shield a fraction of the body's AChE from irreversible inactivation (Berry and Davies, 1970; Dirnhuber et al., 1979). Second, an anti-muscarinic (e.g., atropine) is given to block excessive stimulation by rapidly increasing levels of ACh at muscarinic synapses. Third, an oxime such as pralidoxime chloride (2-PAM Cl) is administered to reactivate NA-inhibited AChE molecules before they become “aged” and are no longer amenable to reactivation (Moore et al., 1995). Finally, a benzodiazepine drug such as diazepam is used to counteract seizure activity in cases of NA poisoning (Kadar et al., 1995; Moore et al., 1995). This combination therapy has proved quite effective in preventing NA-induced lethality. However, it does not always control the development of seizures. Prevention of seizures is important because their duration and intensity have been linked directly to ensuing brain damage after NA exposure (McDonough et al., 1995; Shih et al., 2003). Benzodiazepines in sufficient doses are very effective in preventing or arresting NA-induced seizures and they also limit the extent of brain damage (McDonough et al., 1989; McDonough et al., 1999; McDonough et al., 2000; McDonough et al., 2009; Shih et al., 1999). However, for both practical and theoretical reasons, the timing of benzodiazepine therapy is extremely critical. If benzodiazepine therapy is delayed, seizures can become refractory or reemerge at later times after an initial suppression, raising concerns of brain damage (Grauer et al., 2008; McDonough et al., 2009).
Shih and McDonough (Shih and McDonough, 1997) have proposed a temporal model linking NA-induced seizures to ensuing neuropathology. The initial seizure phase (0-5 min after NA exposure) reflects excess cholinergic tone and is responsive to anti-muscarinics. Shortly thereafter (5-40 min after NA exposure), glutamate excitatory systems are recruited and seizures in this phase are responsive to benzodiazepines. Other mechanisms may emerge well after passage through this second phase and could include inflammatory processes. The expression of numerous proinflammatory genes is increased after NA exposure and these effects are seen hours-to-days later (Chapman et al., 2006; Dhote et al., 2007; Dillman et al., 2009; Grauer et al., 2008; Williams et al., 2003). If non-cholinergic and non-glutamatergic systems are part of a late phase response to NAs, the possibility exists that additional therapies should be considered to extend protection of the brain against seizure-induced damage. In light of results showing that prostaglandins (PGEs) can mediate neuronal excitation (Bezzi et al., 1998; Nishihara et al., 1995; Sekiyama et al., 1995) and are involved in various aspects of seizure initiation and propagation (Cole-Edwards and Bazan, 2005; Takemiya et al., 2003), we have examined the effects of soman on expression of cyclooxygenase-2 (COX-2). Cyclooxygenases are the rate-limiting enzymes in PGE biosynthesis. COX-1 is expressed constitutively whereas COX-2 is the inducible isoform (Smith et al., 2000). We report presently that COX-2 protein expression is increased substantially after soman exposure. COX-2 levels are increased most in brain areas known to be damaged by NAs and the extent of COX-2 up-regulation is correlated with seizure intensity. COX-1 levels are not changed by soman. The present results suggest that COX-2 may be a target for therapeutic intervention in late-occurring or in recurrent seizures after NA exposure.
2. Materials and Methods
2.1. Materials
Soman was obtained from the US Army Edgewood Chemical Biological Center (Aberdeen Proving Ground, MD). Primary antibodies against COX-1 and COX-2 were obtained from Cayman Chemical (Ann Arbor, MI). These antibodies are highly COX-isoform specific and do not show antigen-crossreactivity (Thomas and Kuhn, 2005). Antibodies against the neuronal marker NeuN were purchased from Chemicon (Bedford, MA) and the astrocyte marker GFAP were obtained from Neomarkers (Fremont, CA). Tris-glycine polyacrylamide gels and Alexa-conjugated reagents were from Invitrogen (Carlsbad, CA). Vectastain ABC kits, DAB peroxidase substrate kits, and biotinylated secondary antibodies were obtained from Vector Labs (Burlingame, CA). The BCA Protein Reagent Assay Kit was obtained from ThermoScientific (Rockford, IL). Nitrocellulose membrane was from Whatman (Piscataway, NJ). Western Lightning™ Plus-ECL substrate was procured from PerkinElmer (Waltham, MA). Biomax MR film was from Kodak (Rochester, NY). Isolectin B4, Citrosolv, and all other buffers and reagents were purchased from Sigma-Aldrich (St. Louis, MO).
2.2. Treatment of animals with soman
Male Sprague-Dawley rats weighing 250-300g (Charles River Laboratories, Wilmington, MA) were challenged with phosphate buffered saline (PBS, control) or soman (133 μg/kg sc; 1.2 X LD50). The peripherally-acting anti-muscarinic atropine methyl nitrate (2 mg/kg, im) was given 1 min after soman to protect from NA-induced respiratory problems and immediate lethality (McDonough and Shih, 1997; Shih et al., 1999). This treatment regimen results in seizure activity in ~80% of animals tested. Rats were scored for the presence of the following symptoms 1 hr after soman treatment and just prior to sacrifice at 4, 8, 12, 24, 48 hours or 7 days after treatment as previously described (Shih and McDonough, 1997; Shih and McDonough, 1999): motor signs (0=normal, 1=fasciculations, 2=tremors, 3=convulsions/seizures); coordination (0=normal, 1= mild uncoordinated, 2=impaired movement, 3=prostrate [i.e., no righting reflex]); salivation (0=absent, 1=present); lacrimation (0=absent, 1=present); eyes (0=normal, 1=nystagmus). For immunohistochemistry studies, rats were injected with an overdose of sodium pentobarbital (75 mg/kg, ip) and then perfused with PBS followed by 4% buffered paraformaldehyde. Brains were stored at 4°C until they were processed for immunohistochemistry. For immunoblotting studies, rats were anesthetized with isoflurane and then sacrificed by decapitation and brains were rapidly dissected into hippocampus, piriform cortex, and amygdala. Tissues were frozen and stored at -80°C until they were processed for immunoblotting. All research protocols were approved by the institutional IACUC and conducted in compliance with the Animal Welfare Act and other federal statutes and regulations relating to animals.
2.3. Immunohistochemistry
Sections (50 μm) were cut throughout the entire rostro-caudal axis of the brain and subjected to immunohistochemical analysis using antibodies against COX-2 and COX-1. COX-2 and COX-1-positive cells were viewed and photographed under bright-field conditions using an Olympus BX51 fluorescence microscope. COX-2-positive cells were counted in a 0.38 mm2 area of the hippocampus by a person blinded to the treatment conditions. To identify COX-2-containing cells as neurons, microglia, or astrocytes, sections were subjected to double labeling immunohistochemistry. In all cases, COX-2-positive cells were identified using Alexa Fluor 488-conjugated secondary antibodies (green fluorophore). Neurons were identified using antibodies against NeuN and astrocytes were identified with antibodies against GFAP. Alexa Fluor 555 (orange fluorophore) or 647 (red fluorophore)-conjugated secondary antibodies were used with NeuN and GFAP primary antibodies, respectively. Activated microglia were identified with the use of Alexa Fluor 568-conjugated ILB4 (red fluorophore). Fluorescence was viewed using the appropriate filters and photographed using an Olympus BX51 fluorescence microscope. Captured images were merged to ascertain if COX-2 positive cells were neurons (NeuN-positive), astrocytes (GFAP-positive) or activated microglia (ILB4-positive). Detailed descriptions of all immunohistochemical methods and cell counting are presented elsewhere (Thomas et al., 2008; Thomas and Kuhn, 2005).
2.4. Immunoblotting
Frozen tissues were sonicated in 1% SDS at 95°C. Homogenates were centrifuged at 15000 × g for 15 min at 4°C. Equal amounts of protein from all treatment groups were loaded in duplicate and gels were run under denaturing and reducing conditions. All gels contained samples from controls and soman-treated rats. Resolved proteins were transferred to nitrocellulose and COX-2 and COX-1 levels were determined by immunoblotting as previously described (Thomas and Kuhn, 2005). Relative levels of protein expression were determined by scanning developed films with a Sony CCD-Iris-RGB video camera to obtain pixel densities for each band.
2.5. Data analysis
Individual treatment groups were compared to appropriate controls for COX-2 and COX-1 positive cell counts (immunohistochemistry) as well as for COX protein expression (immunoblotting) with one-way ANOVA. Post hoc analyses were carried out using Tukey's Multiple Comparison Test. A correlation analysis was performed to determine the relationship between COX protein expression and seizure intensity. All statistical analyses were carried out using GraphPad Prism version 5.02 for Windows, GraphPad Software, San Diego, CA, www.graphpad.com.
3. Results
3.1. Soman causes a time-dependent increase in COX-2 expression
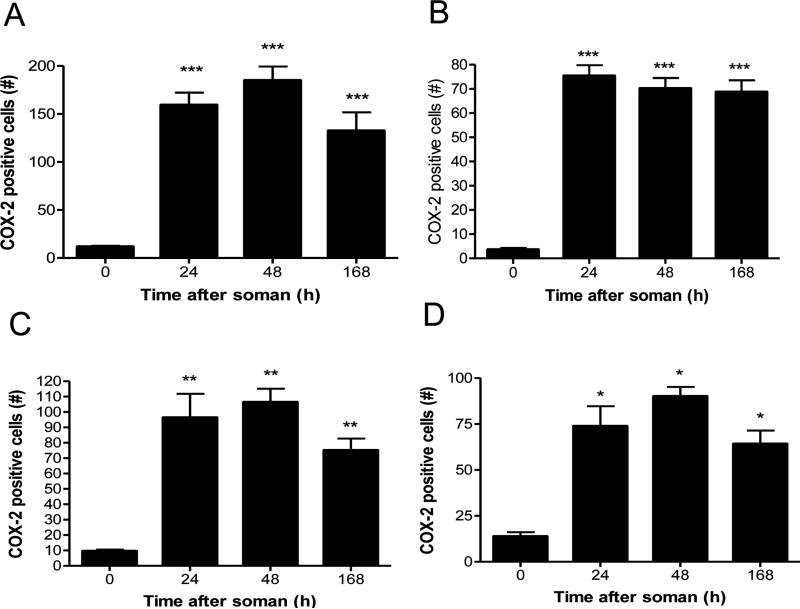
The effects of soman on COX-2 protein expression were examined by immunohistochemical analysis over a broad time course (i.e., 4 hr to 7 days after soman). Counts of COX-2 positive cells revealed a somewhat delayed response to soman (Fig 1). Very few cells expressing COX-2 were seen from 4-12 hr after soman treatment (data not shown). By 24-48 hr, large numbers of COX-2 positive cells were seen in hippocampal CA3 regions and especially the dentate gyrus (Fig. 1A and 1B) as well as in the amygdala (Fig. 1C) and piriform cortex (Fig. 1D). By 7 days COX-2 levels declined slightly below those seen at 48 hr in each brain region, but remained significantly elevated over control. Increases in COX-2 immunoreactivity were observed in cingulate cortex and ventral thalamus as well (data not shown).
Fig 1.
Soman increases COX-2 expression in brain. Rats (N= 5 per group) were treated with soman and brains were processed for immunohistochemistry at the indicated times after treatment using antibodies against COX-2 as described in Materials and Methods. COX-2 positive cells were counted in dentate gyrus (A), CA3 (B), amygdala (C) and piriform cortex (D). Very few COX-2 positive cells were seen from 0-12 hours after soman so these data are not shown. Results are presented as number of COX-2 positive cells in each brain region ± SEM. *p < 0.05; **p < 0.01; ***p < 0.001, Tukey's multiple comparison test by comparison to controls.
3.2. Soman increases COX-2 expression at the cellular level in a highly circumscribed manner as revealed by immunohistochemistry
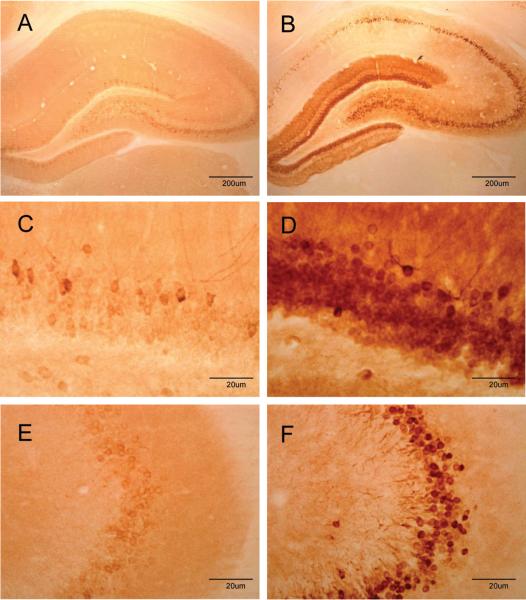
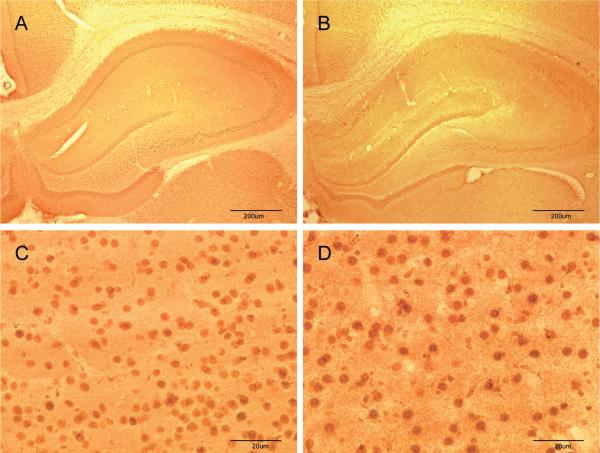
Immunohistochemical analyses revealed the highly circumscribed effect of soman on COX-2 expression at the cellular level. After treatment with soman (48 hr), COX-2 positive cells essentially define the anatomical facets of the dentate gyrus, CA3 and CA1 regions of the hippocampus (Fig 2). Cells expressing COX-2 immunoreactivity were small and uniformly round. The piriform cortex and amygdala also showed substantial increases in the number of COX-2 immunoreactive cells after soman (Fig 3). These cells were somewhat more diffuse in the piriform cortex and many displayed an extensive axonal network. COX-2 positive cells were more densely packed in the amygdala of soman-treated animals by comparison to the piriform cortex (see Fig 3). Soman did not change the expression of COX-1 at any time (4 hr to 7 days) in any brain region examined (Fig 4). COX-1 immunoreactivity was very weak in hippocampus of controls (Fig. 4A) and soman treated rats (Fig. 4B). COX-1 containing cells were also seen throughout the amygdala with no apparent alteration by soman in their number or in the intensity of their staining for COX-1 (Fig. 4C and 4D).
Fig 2.
Soman increases COX-2 expression in the hippocampus. Rats (N= 5 per group) were treated with soman and brains were prepared for immunohistochemistry 48 hr after treatment using antibodies against COX-2. Few COX-2 positive cells are seen in controls (A) whereas the dentate gyrus, CA1 and CA3 are heavily labeled with COX-2 after soman treatment (B). Higher magnification images of the dentate gyrus shows sparse labeling of cells in controls (C) and densely packed COX-2-positive cells in soman-treated rats (D). Similarly, higher magnification images from the CA3 region of controls shows few COX-2-positive cells in controls (E) whereas soman treatment causes a large increase in COX-2 levels (F).
Fig 3.
Soman increases COX-2 expression in the piriform cortex and amygdala. Rats (N= 5 per group) were treated with soman and brains were prepared for immunohistochemistry 48 hr after treatment using antibodies against COX-2. Few COX-2 positive cells are seen in the piriform cortex of controls (A) whereas numerous cells and processes are COX-2 positive after soman treatment (B). Soman also leads to large increases in COX-2 expression in the amygdala (D) as compared to controls (C).
Fig 4.
Soman does not change COX-1 expression in brain. Rats (N= 5 per group) were treated with soman and brains were processed for immunohistochemistry 48 hr after treatment using antibodies against COX-1 as described in Materials and Methods. Few COX-1 positive cells are seen in the hippocampus of controls (A) or soman (B) treated rats at low power magnification. Images collected from the amygdala at higher magnification showed numerous COX-1 positive cells in controls (C) but soman treatment (D) did not increase the number of these cells.
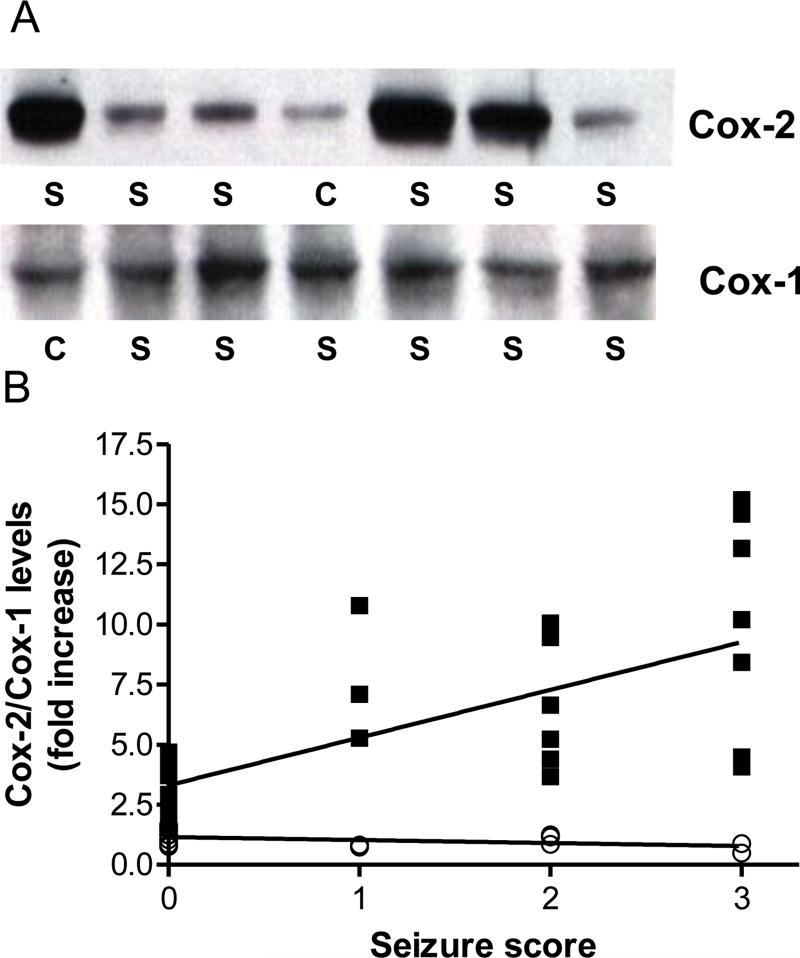
3.2. Soman-induced increases COX-2 protein levels are correlated with seizure intensity
Immunoblot analysis provided independent confirmation of soman effects on COX-2 expression. Soman caused increases in hippocampal COX-2 that varied considerably (Fig 5A). Because all rats were injected with the same soman dose (i.e., 1.2 X LD50), these results suggest that the changes in COX-2 were not linked to soman per se. All rats were scored for seizures as described in Materials and Methods and behavioral scores were plotted versus the fold-increase in COX-2 immunoreactivity on western blots. This analysis indicated that COX-2 expression was positively correlated with seizure intensity (Fig 5B). Soman-treated rats showing no fasciculations, tremors or seizure activity (behavioral score of 0) showed slight increases in COX-2 (~1.5-2 fold over controls). Animals showing mild fasciculations (behavioral score of 1) and tremor (behavioral score of 2) showed increases in COX-2 expression that increased by 4-10 fold. By far, the largest increase in COX-2 expression (7-15-fold) was seen in rats showing the most intense seizures (behavioral score of 3). Similar results for COX-2 expression were seen in other brain regions (data not shown). Immunoblot analyses also confirmed that hippocampal COX-1 protein levels were not altered by soman at any seizure intensity (see Figs 5A and 5B).
Fig 5.
Soman-induced increases in COX-2 expression are correlated with seizure intensity. Rats (N= 5-8 per group) were treated with soman and scored behaviorally for seizure intensity 1 hr after treatment as described in Materials and Methods. At 48 hr after soman, brains were processed for immunoblotting for COX-2 and COX-1 in the hippocampus. Panel A shows immunoblotting results for COX-2 and COX-1 from controls (C) and soman treated rats (S). The fold-increase in COX-2 (■) and COX-1 (○) expression caused by soman was plotted versus seizure intensity in panel B. The relationship between changes in COX-2 levels and seizure intensity was significant (r=0.73, p < 0.0001). COX-1 levels were not changed by soman treatment.
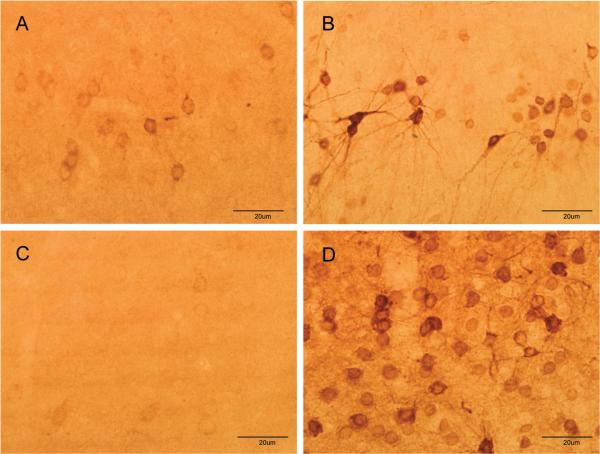
3.3. Soman increases COX-2 expression in neurons and not in microglia or astrocytes
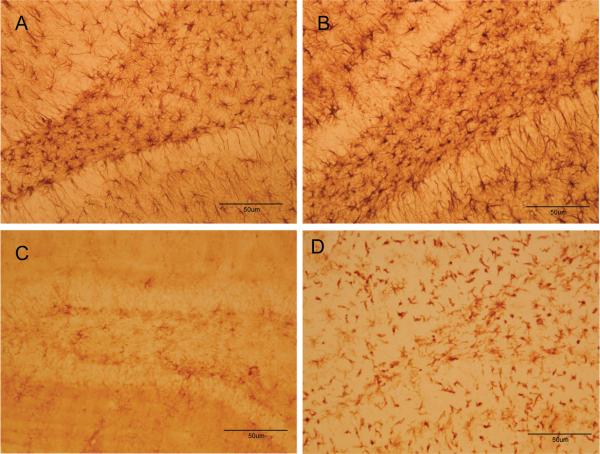
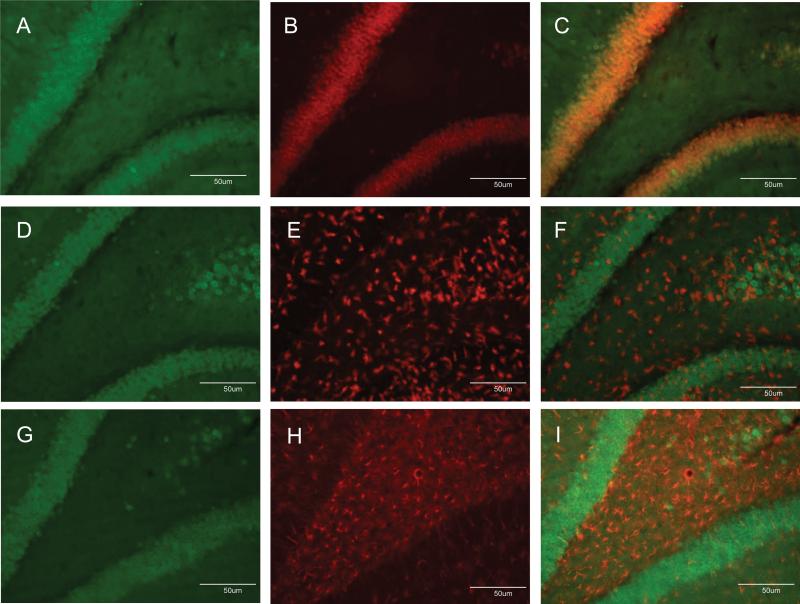
COX-2 can be expressed in neurons and by activated microglia and astrocytes (Minghetti and Levi, 1998) so efforts were made to identify the cell-type in which COX-2 expression was increased by soman. First, hippocampus was examined 48 hr after soman exposure for changes in astrocyte and microglial reactivity using GFAP and Isolectin B4, respectively. The density and staining intensity of astrocytes were increased substantially in hippocampus after soman (Fig. 6B) by comparison to controls (Fig. 6A). Microglial activation in hippocampus was also increased dramatically by soman (Fig. 6D) by comparison to controls (Fig. 6C). In light of this soman-induced gliosis in hippocampus, brain sections were labeled with COX-2 antibodies followed by co-labeling with antibodies against either NeuN to identify neurons, antibodies against GFAP to identify astrocytes, or ILB4 to identify activated microglia. Patterns of COX-2 (Fig. 7A) and NeuN (Fig. 7B) fluorescence in hippocampus were very similar and when merged, a near-total overlap of cells that are immuno-positive for both COX-2 and NeuN was evident (Fig 7C). The soman-induced microglial activation is evident throughout the hippocampus (Fig. 7E) and it is clear from the merged image (Fig 7F) that the pattern of COX-2 fluorescence staining shows no overlap with that of microglia. Finally, intense GFAP reactivity is evident after soman treatment in the area between CA3 and the dentate gyrus of the hippocampus (Fig 7H), and the lack of overlap of COX-2 containing cells with astrocytes is apparent in the merged image (Fig 7I). The morphology of COX-2 containing cells in the hippocampus of soman treated rats also gives a clear indication of their identity as neurons.
Fig 6.
Soman causes gliosis in hippocampus. Rats were treated with soman and brains were processed for immunohistochemistry 48 hr after treatment. Astrocytes were labeled in control (A) or soman-treated rats (B) using antibodies against GFAP. Activated microglia were labeled in control (C) or soman-treated rats (D) using ILB4 staining.
Fig 7.
Soman increases COX-2 expression solely in neurons. Rats were treated with soman and brains were processed after 48 hr for double labeling fluorescence immunohistochemistry as described in Materials and Methods. COX-2-positive cells were labeled with Alexa 488-conjugated antibodies (green fluorescence, panels A, D, G). Neurons were identified using antibodies against NeuN and Alexa-555-conjugated secondary antibodies (orange fluorescence, panel B). Activated microglial were identified using Alexa-568-conjugated ILB4 (red fluorescence, panel E). Astrocytes were identified using antibodies against GFAP and Alexa-647-conjugated secondary antibodies (red fluorescence, panel H). Merged images in panels C, F and I indicate that COX-2 labeling overlapped with neurons and not with microglia or astrocytes.
4. Discussion
The present results establish that soman leads to large increases in expression of COX-2 protein. The greatest increase in COX-2 was observed within subregions of the hippocampus (i.e., dentate gyrus, CA1 and CA3). Significant increases in COX-2 were also observed in the basolateral amygdala, piriform cortex and ventral thalamus. COX-1 expression was not altered by soman. The soman-induced increase in COX-2 was time-dependent and developed at a relatively slow rate by comparison to the very rapid onset of hyper-cholinergic (0-5 min) and hyper-glutamatergic (5-40 min) conditions known to occur after soman exposure (Shih and McDonough, 1997). Changes in COX-2 levels were very slight for 12 hr after soman treatment and increased to maximal levels over the course of 24-48 hours. COX-2 levels declined somewhat from 48 hours to 7 days, the last time-point sampled presently, but remained significantly elevated nonetheless. Immunoblotting studies confirmed soman-induced increases in COX-2 with increases as large as 15-fold by comparison to controls. The lack of effect of soman on COX-1 levels was also confirmed by immunoblotting.
These results are significant for several important reasons. First, COX-2 is the initial enzyme in the biosynthesis of PGEs. The PGEs participate in inflammatory processes throughout the body (Kuehl and Egan, 1980). In brain, PGEs have excitatory effects on neurons (Bezzi et al., 1998; Nishihara et al., 1995; Sekiyama et al., 1995) and they have been implicated in numerous seizure conditions (Cole-Edwards and Bazan, 2005) to include roles for both initiation and propagation (Takemiya et al., 2006). Second, soman-induced increases in COX-2 occur in brain regions known to be damaged by NAs (Baille et al., 2005; Collombet et al., 2005; Collombet et al., 2008; McDonough et al., 1989; McDonough et al., 1999; Shih and McDonough, 1999). Prior results linking soman-induced neuropathology to seizure intensity and duration (Chapman et al., 2006) make it all the more interesting that increases in COX-2 protein are positively correlated with seizure intensity after soman exposure. This result also confirms that seizure activity per se triggers COX-2 induction and not soman (i.e., some soman-treated rats do not develop seizures and show very low levels of COX-2). Third, increases in COX-2 develop rather slowly after soman, a compound that causes physiological distress with an extremely rapid onset and which can lead to death within minutes. The identification of increased COX-2 as a late-occurring effect of soman exposure has important implications for the treatment of NA poisoning (see below).
The present results are consistent with prior studies implicating inflammatory processes in the action of NAs. Recent microarray analyses showed increases in COX-2 mRNA that reached a maximum of about 7-fold between 9-12 hours after soman administration (Dillman et al., 2009). Our studies also recapitulate others showing that NAs increase brain PGEs to their highest levels 24-48 hours post-exposure (Chapman et al., 2006). The present results expand these findings substantially by establishing that soman effects on COX-2 gene expression extend to increased translation of COX-2 protein, and they provide the biochemical basis for increased PGE production. Our results are also unique in that they identify the cell-type in which COX-2 is increased by soman. Double-labeling immunohistochemical studies showed clearly that COX-2-positive cells in soman treated rats were co-labeled with NeuN, establishing their identity as neurons. COX-2 immunoreactivity was not observed in activated microglia or in astrocytes. Considering only the hippocampus, the morphology of COX-2 containing cells in soman treated rats suggests their identity as pyramidal and granular neurons. Although gliosis is increased by NAs (Baille et al., 2005; Collombet et al., 2005; Collombet et al., 2008; Grauer et al., 2008; Zimmer et al., 1997), glial activation does not include elevated COX-2 expression and it appears that microglial and astrocyte participation in NA-induced neuropathology involves inflammatory and excitatory mechanisms that could act to enhance COX-2 mediated effects that occur only in neurons.
Significant strides have been made recently in developing therapies for NA intoxication. Current treatment involves a four-pronged approach (i.e., carbamate cholinesterase inhibitor, atropine, 2-PAM Cl and diazepam) aimed at counteracting the immediate effects of NA (Berry and Davies, 1970; Dirnhuber et al., 1979; Moore et al., 1995). This therapy is very effective in increasing survival after NA exposure but it does not always prevent seizures and neuronal damage. A number of modifications in this treatment strategy have been made to optimize seizure control (McDonough et al., 2009; Shih et al., 2007) because the neuropathology associated with NA exposure is linked to seizure intensity and duration (Chapman et al., 2006). It is evident that several different pathological systems are recruited after NA exposure. The early cholinergic overload leads to seizures that can be controlled by anti-muscarinic drugs. A second, later phase of increased excitatory (glutamate/NMDA) transmission follows and seizures occurring in this phase are responsive to benzodiazepines. However, limits in the duration of their anti-seizure activity or delays in their application after NA exposure can lead to the re-emergence of seizures and the risk of additional brain damage.
It has become clear that the development of neuronal damage after exposure to NA can be quite protracted. In animal models of NA poisoning, a progressive neuronal degeneration in hippocampus (Collombet et al., 2006) and amygdala (Collombet et al., 2008) continues for 60-90 days after exposure. Behavioral deficits reflective of the brain region damaged are also seen chronically to include altered cognition in the case of hippocampal damage (Filliat et al., 2007; Grauer et al., 2008) and increased emotional behavior (e.g., conditioned and unconditioned fear) in the case of damage to the amygdala (Coubard et al., 2008). In humans exposed to NA (victims of the Tokyo sarin attack (Ohbu et al., 1997)) emotional and cognitive changes have been seen up to 7 years post-exposure (Miyaki et al., 2005) and may be related to persistent structural changes in brain caused by a single exposure to sarin (Yamasue et al., 2007). In light of these findings, the present results suggest the possibility that COX-2 expression is triggered as a late-occurring response to soman-induced seizures, and the associated increase in the production of PGEs could play a role in neuronal degeneration well after early cholinergic and glutamatergic overloads. Seizures arising in response to a PGE overload will not likely respond to anticholinergics and benzodiazepines. Therefore, some consideration should be given to the use of COX-2 selective inhibitors (NSAIDs) to prevent or minimize neuronal damage that occurs through non-cholinergic and nonglutamatergic mechanisms.
Acknowledgements
This work was supported by grants from the Defense Threat Reduction Agency, NIH and the Department of Veterans Affairs. Its contents are solely the responsibility of the authors and do not necessarily reflect the official views of the federal government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
None declared.
Disclaimer
This research was conducted in compliance with the Animal Welfare Act and other Federal statutes and regulations relating to animals and experiments involving animals and adhered to the principles stated in The Guide for the Care and Use of Laboratory Animals, National Research Council, 1996. The facility where this research was conducted is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. The opinions or assertions contained herein are the private views of the authors and are not to be construed as reflecting the views of the Department of the Army or the Department of Defense.
References
- Baille V, Clarke PG, Brochier G, Dorandeu F, Verna JM, Four E, Lallement G, Carpentier P. Soman-induced convulsions: the neuropathology revisited. Toxicology. 2005;215:1–24. doi: 10.1016/j.tox.2005.05.028. [DOI] [PubMed] [Google Scholar]
- Berry WK, Davies DR. The use of carbamates and atropine in the protection of animals against poisoning by 1,2,2-trimethylpropyl methylphosphonofluoridate. Biochem Pharmacol. 1970;19:927–34. doi: 10.1016/0006-2952(70)90256-x. [DOI] [PubMed] [Google Scholar]
- Bezzi P, Carmignoto G, Pasti L, Vesce S, Rossi D, Rizzini BL, Pozzan T, Volterra A. Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature. 1998;391:281–5. doi: 10.1038/34651. [DOI] [PubMed] [Google Scholar]
- Chapman S, Kadar T, Gilat E. Seizure duration following sarin exposure affects neuro-inflammatory markers in the rat brain. Neurotoxicology. 2006;27:277–83. doi: 10.1016/j.neuro.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Cole-Edwards KK, Bazan NG. Lipid signaling in experimental epilepsy. Neurochem Res. 2005;30:847–53. doi: 10.1007/s11064-005-6878-4. [DOI] [PubMed] [Google Scholar]
- Collombet JM, Carpentier P, Baille V, Four E, Bernabe D, Burckhart MF, Masqueliez C, Baubichon D, Lallement G. Neuronal regeneration partially compensates the delayed neuronal cell death observed in the hippocampal CA1 field of soman-poisoned mice. Neurotoxicology. 2006;27:201–9. doi: 10.1016/j.neuro.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Collombet JM, Four E, Bernabe D, Masqueliez C, Burckhart MF, Baille V, Baubichon D, Lallement G. Soman poisoning increases neural progenitor proliferation and induces long-term glial activation in mouse brain. Toxicology. 2005;208:319–34. doi: 10.1016/j.tox.2004.11.036. [DOI] [PubMed] [Google Scholar]
- Collombet JM, Pierard C, Beracochea D, Coubard S, Burckhart MF, Four E, Masqueliez C, Baubichon D, Lallement G. Long-term consequences of soman poisoning in mice Part 1. Neuropathology and neuronal regeneration in the amygdala. Behav Brain Res. 2008;191:88–94. doi: 10.1016/j.bbr.2008.02.043. [DOI] [PubMed] [Google Scholar]
- Coubard S, Beracochea D, Collombet JM, Philippin JN, Krazem A, Liscia P, Lallement G, Pierard C. Long-term consequences of soman poisoning in mice: part 2. Emotional behavior. Behav Brain Res. 2008;191:95–103. doi: 10.1016/j.bbr.2008.03.027. [DOI] [PubMed] [Google Scholar]
- Dhote F, Peinnequin A, Carpentier P, Baille V, Delacour C, Foquin A, Lallement G, Dorandeu F. Prolonged inflammatory gene response following soman-induced seizures in mice. Toxicology. 2007;238:166–76. doi: 10.1016/j.tox.2007.05.032. [DOI] [PubMed] [Google Scholar]
- Dillman JF, 3rd, Phillips CS, Kniffin DM, Tompkins CP, Hamilton TA, Kan RK. Gene expression profiling of rat hippocampus following exposure to the acetylcholinesterase inhibitor soman. Chem Res Toxicol. 2009;22:633–8. doi: 10.1021/tx800466v. [DOI] [PubMed] [Google Scholar]
- Dirnhuber P, French MC, Green DM, Leadbeater L, Stratton JA. The protection of primates against soman poisoning by pretreatment with pyridostigmine. J Pharm Pharmacol. 1979;31:295–9. doi: 10.1111/j.2042-7158.1979.tb13504.x. [DOI] [PubMed] [Google Scholar]
- Filliat P, Coubard S, Pierard C, Liscia P, Beracochea D, Four E, Baubichon D, Masqueliez C, Lallement G, Collombet JM. Long-term behavioral consequences of soman poisoning in mice. Neurotoxicology. 2007;28:508–19. doi: 10.1016/j.neuro.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Grauer E, Chapman S, Rabinovitz I, Raveh L, Weissman BA, Kadar T, Allon N. Single whole-body exposure to sarin vapor in rats: long-term neuronal and behavioral deficits. Toxicol Appl Pharmacol. 2008;227:265–74. doi: 10.1016/j.taap.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Kadar T, Shapira S, Cohen G, Sahar R, Alkalay D, Raveh L. Sarin-induced neuropathology in rats. Hum Exp Toxicol. 1995;14:252–9. doi: 10.1177/096032719501400304. [DOI] [PubMed] [Google Scholar]
- Kuehl FA, Jr., Egan RW. Prostaglandins, arachidonic acid, and inflammation. Science. 1980;210:978–84. doi: 10.1126/science.6254151. [DOI] [PubMed] [Google Scholar]
- Lee EC. Clinical manifestations of sarin nerve gas exposure. JAMA. 2003;290:659–62. doi: 10.1001/jama.290.5.659. [DOI] [PubMed] [Google Scholar]
- McDonough JH, Jr., Dochterman LW, Smith CD, Shih TM. Protection against nerve agent-induced neuropathology, but not cardiac pathology, is associated with the anticonvulsant action of drug treatment. Neurotoxicology. 1995;16:123–32. [PubMed] [Google Scholar]
- McDonough JH, Jr., Jaax NK, Crowley RA, Mays MZ, Modrow HE. Atropine and/or diazepam therapy protects against soman-induced neural and cardiac pathology. Fundam Appl Toxicol. 1989;13:256–76. doi: 10.1016/0272-0590(89)90262-5. [DOI] [PubMed] [Google Scholar]
- McDonough JH, Jr., McMonagle J, Copeland T, Zoeffel D, Shih TM. Comparative evaluation of benzodiazepines for control of soman-induced seizures. Arch Toxicol. 1999;73:473–8. doi: 10.1007/s002040050637. [DOI] [PubMed] [Google Scholar]
- McDonough JH, Jr., Shih TM. Neuropharmacological mechanisms of nerve agent-induced seizure and neuropathology. Neurosci Biobehav Rev. 1997;21:559–79. doi: 10.1016/s0149-7634(96)00050-4. [DOI] [PubMed] [Google Scholar]
- McDonough JH, Jr., Zoeffel LD, McMonagle J, Copeland TL, Smith CD, Shih TM. Anticonvulsant treatment of nerve agent seizures: anticholinergics versus diazepam in soman-intoxicated guinea pigs. Epilepsy Res. 2000;38:1–14. doi: 10.1016/s0920-1211(99)00060-1. [DOI] [PubMed] [Google Scholar]
- McDonough JH, Van Shura KE, LaMont JC, McMonagle JD, Shih TM. Comparison of the intramuscular, intranasal or sublingual routes of midazolam administration for the control of soman-induced seizures. Basic Clin Pharmacol Toxicol. 2009;104:27–34. doi: 10.1111/j.1742-7843.2008.00326.x. [DOI] [PubMed] [Google Scholar]
- Minghetti L, Levi G. Microglia as effector cells in brain damage and repair: focus on prostanoids and nitric oxide. Prog Neurobiol. 1998;54:99–125. doi: 10.1016/s0301-0082(97)00052-x. [DOI] [PubMed] [Google Scholar]
- Miyaki K, Nishiwaki Y, Maekawa K, Ogawa Y, Asukai N, Yoshimura K, Etoh N, Matsumoto Y, Kikuchi Y, Kumagai N, Omae K. Effects of sarin on the nervous system of subway workers seven years after the Tokyo subway sarin attack. J Occup Health. 2005;47:299–304. doi: 10.1539/joh.47.299. [DOI] [PubMed] [Google Scholar]
- Nishihara I, Minami T, Watanabe Y, Ito S, Hayaishi O. Prostaglandin E2 stimulates glutamate release from synaptosomes of rat spinal cord. Neurosci Lett. 1995;196:57–60. doi: 10.1016/0304-3940(95)11839-o. [DOI] [PubMed] [Google Scholar]
- Ohbu S, Yamashina A, Takasu N, Yamaguchi T, Murai T, Nakano K, Matsui Y, Mikami R, Sakurai K, Hinohara S. Sarin poisoning on Tokyo subway. South Med J. 1997;90:587–93. doi: 10.1097/00007611-199706000-00002. [DOI] [PubMed] [Google Scholar]
- Sekiyama N, Mizuta S, Hori A, Kobayashi S. Prostaglandin E2 facilitates excitatory synaptic transmission in the nucleus tractus solitarii of rats. Neurosci Lett. 1995;188:101–4. doi: 10.1016/0304-3940(95)11407-n. [DOI] [PubMed] [Google Scholar]
- Shih T, McDonough JH, Jr., Koplovitz I. Anticonvulsants for soman-induced seizure activity. J Biomed Sci. 1999;6:86–96. doi: 10.1007/BF02256439. [DOI] [PubMed] [Google Scholar]
- Shih TM, Duniho SM, McDonough JH. Control of nerve agent-induced seizures is critical for neuroprotection and survival. Toxicol Appl Pharmacol. 2003;188:69–80. doi: 10.1016/s0041-008x(03)00019-x. [DOI] [PubMed] [Google Scholar]
- Shih TM, McDonough JH., Jr. Neurochemical mechanisms in soman-induced seizures. J Appl Toxicol. 1997;17:255–64. doi: 10.1002/(sici)1099-1263(199707)17:4<255::aid-jat441>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Shih TM, McDonough JH., Jr. Organophosphorus nerve agents-induced seizures and efficacy of atropine sulfate as anticonvulsant treatment. Pharmacol Biochem Behav. 1999;64:147–53. doi: 10.1016/s0091-3057(99)00114-8. [DOI] [PubMed] [Google Scholar]
- Shih TM, Rowland TC, McDonough JH. Anticonvulsants for nerve agent-induced seizures: The influence of the therapeutic dose of atropine. J Pharmacol Exp Ther. 2007;320:154–61. doi: 10.1124/jpet.106.111252. [DOI] [PubMed] [Google Scholar]
- Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem. 2000;69:145–82. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- Takemiya T, Maehara M, Matsumura K, Yasuda S, Sugiura H, Yamagata K. Prostaglandin E2 produced by late induced COX-2 stimulates hippocampal neuron loss after seizure in the CA3 region. Neurosci Res. 2006;56:103–10. doi: 10.1016/j.neures.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Takemiya T, Suzuki K, Sugiura H, Yasuda S, Yamagata K, Kawakami Y, Maru E. Inducible brain COX-2 facilitates the recurrence of hippocampal seizures in mouse rapid kindling. Prostaglandins Other Lipid Mediat. 2003;71:205–16. doi: 10.1016/s1098-8823(03)00040-6. [DOI] [PubMed] [Google Scholar]
- Thomas DM, Francescutti-Verbeem DM, Kuhn DM. Methamphetamine-induced neurotoxicity and microglial activation are not mediated by fractalkine receptor signaling. J Neurochem. 2008;106:696–705. doi: 10.1111/j.1471-4159.2008.05421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DM, Kuhn DM. Cyclooxygenase-2 is an obligatory factor in methamphetamine-induced neurotoxicity. J Pharmacol Exp Ther. 2005;313:870–6. doi: 10.1124/jpet.104.080242. [DOI] [PubMed] [Google Scholar]
- Williams AJ, Berti R, Yao C, Price RA, Velarde LC, Koplovitz I, Schultz SM, Tortella FC, Dave JR. Central neuro-inflammatory gene response following soman exposure in the rat. Neurosci Lett. 2003;349:147–50. doi: 10.1016/s0304-3940(03)00818-8. [DOI] [PubMed] [Google Scholar]
- Yamasue H, Abe O, Kasai K, Suga M, Iwanami A, Yamada H, Tochigi M, Ohtani T, Rogers MA, Sasaki T, Aoki S, Kato T, Kato N. Human brain structural change related to acute single exposure to sarin. Ann Neurol. 2007;61:37–46. doi: 10.1002/ana.21024. [DOI] [PubMed] [Google Scholar]
- Zimmer LA, Ennis M, Shipley MT. Soman-induced seizures rapidly activate astrocytes and microglia in discrete brain regions. J Comp Neurol. 1997;378:482–92. doi: 10.1002/(sici)1096-9861(19970224)378:4<482::aid-cne4>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]