Abstract
BACKGROUND
To investigate the pathophysiology of anemia and responses to RBC transfusions and erythropoietin, repeated measurement of the circulating red cell volume (RCV) would be useful. Ovine erythropoiesis is similar to human erythropoiesis. Accordingly, a method for measuring RCV using either human or sheep RBCs labeled at different biotin densities has been previously validated in vitro. Here preclinical studies validating this method for in vivo measurement of circulating RCV in sheep are reported.
STUDY DESIGN AND METHODS
For each sheep, autologous RBCs were biotinylated were at four discrete densities (12, 24, 48, and 96 µg biotinylation reagent per mL RBC). The densities were mixed and infused intravenously. Blood was sampled five times over one hour beginning at 4 minutes. RCV values were determined based on dilution of each population of biotinylated RBCs and by the 14C-cyanate method.
RESULTS
There was no difference among RCVs measured at all densities through 16 minutes; however, by 60 minutes, RBCs biotinylated at the highest density overestimated RCV by 7.6%. RCV values increased 41% over the hour, consistent with equilibration with a pool of RBCs sequestered in the spleen. RCV by the 14C-cyanate method paralleled results by the biotin method but averaged 8% greater.
CONCLUSION
These studies provide evidence that all four densities of biotinylated RBCs can be used in sheep to simultaneously and independently determine RCV. We speculate that the multidensity biotinylation method will be useful both for multiple simultaneous measurements and for repeated measurement of circulating RCV and blood volume in humans.
Keywords: circulating red cell volume, blood volume, biotin, multiple densities, sheep, spleen
INTRODUCTION
Studies of circulating RBC volume (RCV), which is sometimes termed the red cell mass, in very low birth weight infants and other vulnerable populations are hampered by two problems. First, application of the standard methods employed in adults that utilize radioisotopes is impeded by concerns about radiation exposure in fetuses, infants, and children. Second, methods that do not expose the patient to radiation have technical limitations including cost, feasibility, and sensitivity. Accordingly, a reliable method for measuring RCV that does not use radioactive labels would be of considerable value to further define the physiology, pathophysiology, and responses to treatment of a variety of conditions in pregnant women, fetuses, infants, and children. To this end, we developed a method to label RBCs at four or more distinct densities of biotin and then validated this method in vitro using sheep and human RBCs.1
Ovine fetal and newborn cardiovascular, pulmonary, and hematologic development and physiology resemble those of humans.2 In particular, ovine erythropoiesis in the fetus, neonate, and adult is similar to the human, and regulation of erythropoiesis and RBC kinetics in the ovine model are currently active areas of study.3–6 Accordingly, lambs and sheep are logical animal models for preclinical testing of a method for measuring RCV.
Successful validation is important because the biotin method does not expose the patient or study subject to radiation and is sufficiently sensitive for use in neonates and adults.7,8 Moreover, the biotin method is practical because 1) the required biotinylation reagents are inexpensive and commercially available, 2) the required instruments (a flow cytometer and sterile hood) are available in most medical center clinical laboratories, and 3) technician time required for analysis is modest.8,9
In this study, we report additional progress made toward the in vivo validation of an RCV method using RBCs labeled at multiple biotin densities. In both sheep and humans, RCV measurements using a single density of biotin-labeled RBCs have been validated in vitro and in vivo.7,10 Because the density of the biotin label on the RBC surface can be reproducibly and predictably increased in incremental steps,1 multiple distinct populations of biotinylated RBCs can be prepared, mixed together, infused, sampled, and enumerated individually. This permits repeated determination of RCV and blood volume over time in the same subject despite the confounding factors of variable erythropoiesis, RBC transfusions, and somatic growth; these events are confounding to models that assume steady state because these factors increase RBC volume, blood volume, or both. We measured RCV in vitro using at least four discrete populations of RBCs that differed in biotinylation densities and documented this method to be accurate within 3% with precision ≤ 6% (expressed as one standard deviation of replicates).1 The objective of the current study in sheep was to measure RCV in vivo simultaneously and independently using four distinct densities of biotinylated RBCs; we tested the hypothesis that RCV values determined using multiple densities would agree.
MATERIALS AND METHODS
Animal studies
All animal studies were approved by the Institutional Animal Care and Use Committees at both the University of Arkansas for Medical Sciences and the University of Iowa. The nine sheep studied were females, approximately 2 years old. None had previous exposure to biotinylated RBCs. Additional demographic information is provided in the Appendix.
Biotinylation of RBCs
The method for biotinylating RBCs to produce distinct biotinylation densities on the cell surface has been described.1 Briefly, individual aliquots of autologous ovine RBCs were incubated with increasing concentrations of sulfo-succinimido-biotin (Pierce Chemical Co., Rockford, IL). The stock biotinylation solution and the serial dilutions were prepared at pH 5.0 to favor the biotinylation reaction over hydrolysis of the reagent. Serial dilutions of the stock solution of the biotinylation reagent were used to produce discrete densities of biotin label on the RBC surface proteins; these reactions were conducted in the wash buffer (pH= 7.4). The wash buffer composition is 11.1 mmol per L glucose, 20 mmol per L sodium bicarbonate, 2.3 mmol per L NaHPO4, 1.14 mmol per L Na2PO4, and 154 mmol per L NaCl (356 mOsm/L). After 30 minutes, RBCs were washed to stop the reaction and to remove the remaining biotinylating reagent as well as any reaction and hydrolysis byproducts.
Infusion and sampling
Approximately 1 week prior to infusion of biotinylated RBCs, a catheter (Intracath 16G x 8 in, Becton Dickinson Infusion Therapy Systems, Inc., Sandy, Utah, USA) was placed percutaneously in the left jugular vein; this catheter was used for both withdrawal of blood and for infusion of biotinylated RBCs. After placement, daily blood samples were collected for determination of hemoglobin concentration and Hct to confirm the steady-state condition of the sheep prior to study (data not shown). On the day of the planned infusion of biotinylated RBCs, sufficient blood was collected into heparin (10 Units/mL) to prepare the 4 densities of biotinylated RBCs as described above. Biotinylated RBCs from each distinct density population were mixed together and infused as a bolus over 3 – 4 minutes; time at the end of the bolus infusion was designated time zero. The number of biotin-labeled RBCs in each population density was determined precisely from red cell count (RCC) per µL and volume of transfused RBC mixture, which was determined gravimetrically. The quantity of each population of biotinylated RBCs transfused was designed to produce a final percentage of approximately 3% of the total RBCs in circulation. After infusion, venous blood was sampled from an indwelling jugular central venous catheter at approximately 4, 8, 16, 32, and 60 minutes. Exact times of infusion and sampling were recorded and used in the data depictions. To further test this interpretation, additional studies were performed in the final three sheep. For these three sheep, a second catheter (Percutaneous Sheath Introducer Set, 8 Fr, Arrow International, Inc., Reading, PA, USA) was placed in the opposite jugular vein. This larger catheter permitted infusion of the entire mixture of biotinylated RBCs within one minute. Four additional blood samples were collected for these three sheep at 1, 2, 90, and 120 minutes post-infusion using the second catheter. The 1 and 2 minute samples were used to assess earlier mixing and equilibration of biotinylated RBCs.
The RCC of the individual unmixed populations of biotinylated RBCs, the mixture to be infused, and the post-infusion venous blood samples were measured by a Sysmex XT-2000iV (Sysmex Corp, Kobe, JP). The total number of infused RBCs was determined by multiplying mass delivered (Wi= mass difference of the infusion syringe) by the RCC divided the specific gravity SGi of the blood using the following equation:
Quantitation of % enrichment of biotinylated RBCs
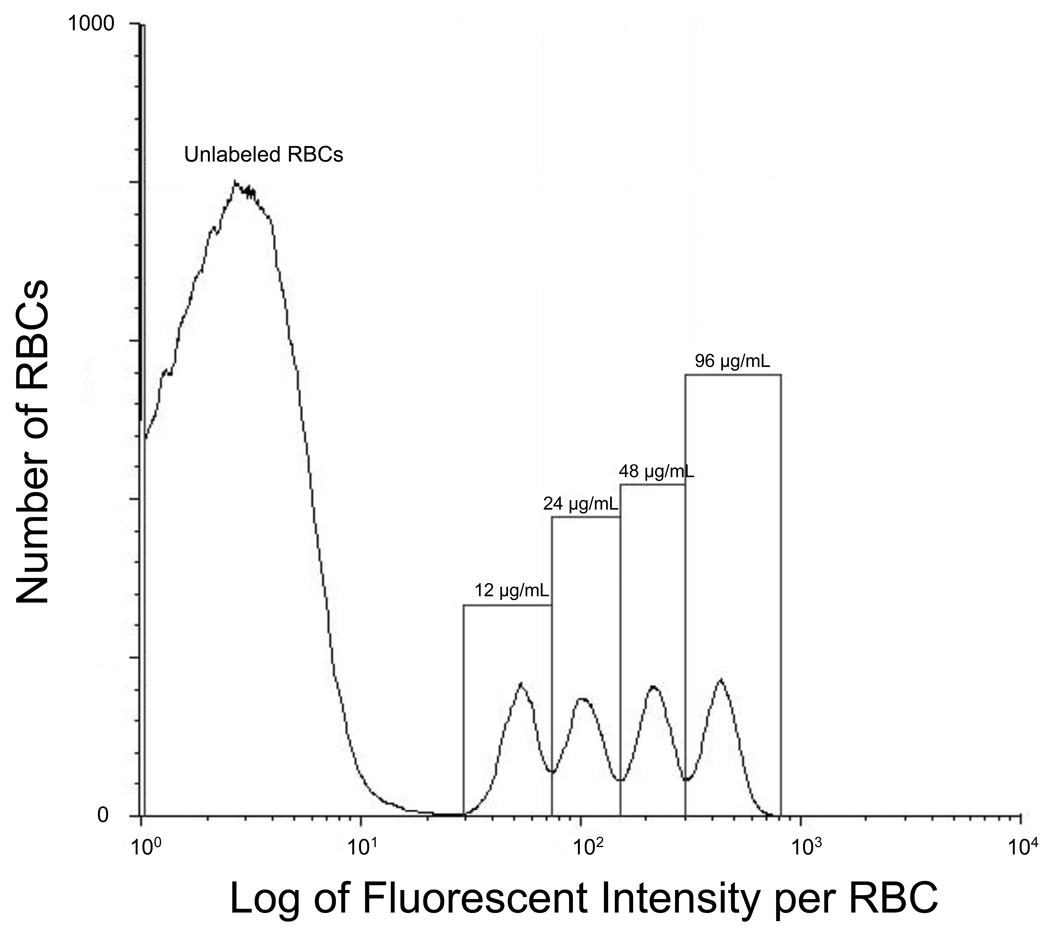
For each sample of venous blood, the concentration of each distinct population of biotinylated RBCs was determined by flow cytometric enumeration as previously described.1 The histogram display of event number versus the log of the fluorescent intensity was divided into discrete populations of biotinylated RBCs using the interval region “markers”; the total number of events, i.e., RBCs, within each region was determined by the flow enumeration with the following minor change. Alexa (Alexa 488-streptavidin, Invitrogen, Carlsbad, CA) replaced fluorescenated avidin as the flow cytometry fluor because Alexa provides both superior separations between the peaks originating from each distinct RBC population and a greater separation of the peak containing unlabeled RBCs (autofluorescence) from the peak containing RBCs with the lowest density of biotin labels. Each population of biotinylated RBCs appeared as a separate peak on the fluorescent intensity histogram obtained from flow cytometry; the number of biotin labels per RBC determines the separation between peaks (Figure 1).
Figure 1.
Flow cytometry histogram relating number of RBCs enumerated to log of fluorescent intensity for four populations of biotinylated sheep RBCs demonstrates separation from unlabeled RBCs but overlap in the biotinylated RBC populations. “12, 24, 48, and 96 µg/mL” denote the populations of biotinylated RBCs produced by successive two-fold increments of the biotinylation reagent.
The number of biotin labels per RBC (i.e., the density of biotin labeling) was determined by the mass of sulfo-succinimido-biotin reagent per mL of RBCs. In this study, we observed the same linear relationship between fluorescent intensity per RBC and mass of biotinylation reagent per mL of RBCs that we reported previously.1 The RBC population with the biotinylation density of 12 µg/mL of RBC (35 nmol/mL of RBC) was chosen as the lowest density because this density still provides complete separation from the unlabeled RBCs (Figure 1). To minimize overlap between peaks while maximizing the number of RBC populations studied, biotinylation reagent concentration was increased in two–fold increments (i.e., 24, 48, and of 96 µg/mL of RBCs; equivalent to 70, 140, 280 nmol/mL of RBCs). In initial pilot studies in sheep (data not shown), we observed that densities greater than about 120 µg/mL exhibit reduced in vivo survival over the first hour, making accurate determination of RCV problematic.
A two-fold increment does produce some overlap of the peaks (i.e., the lowest part of the curve does not reach the horizontal axis between peaks; Figure 1). Our standard approach for peak quantitation was the “drop line” method in which peaks were divided by a vertical line dropped between the minimums of the peaks in the cell count histogram (Figure 1). To assess any inaccuracy introduced by drop line quantitation of the percentage of biotinylated RBCs, a mathematical modeling method was applied to the same quantitation. In this mathematical method, a mixture of distributions from pure samples of each population of biotinylated RBCs was fit to the cell count histogram using ordinary least squares. The model-determined percentage of each biotin-labeled RBC peak (from the estimated mixture proportions) was then used to calculate RCV as usual. This drop line method of peak quantitation produced RCV values that were virtually identical to the more numerically intensive mixture distribution technique (i.e., the absolute relative error was < 2.5%). Because the “drop line” method was the simpler, and the RCV values were virtually identical with the two methods, only the “drop line” results are presented.
Enumeration of the RBCs within each peak provided the percentage of biotinylated RBCs in each distinct population relative to the total number of RBCs circulating. The percentage of biotinylated RBCs was corrected against a standard curve prepared as previously described.1,7,11,12 The slope of the standard curve was typically within 10% of unity, suggesting that there is currently little error in the absolute enumeration of the populations of biotinylated RBCs.
Calculation of RCV and blood volume
RCV at each sample time (4, 8, 16, 32, and 60 minutes post-infusion) was calculated by applying a formula RCVi = RBCi x MCV/ %BUi described previously1 where RCVi is the RCV determined by biotin density i, RBCi is the number of RBCs infused for the ith density as calclulated above, MCV is the mean corpuscular volume in fL/cell, and % BUi is the % of biotinylated RBC of ith density. However, in this study, we used the following minor modification. The MCV term for the recipient’s sample was replaced by the ratio of the Hct to the RCC using the following identity:
This substitution was made because the RCC is particularly stable in blood samples and because the Hct can be independently verified by the manual capillary tube method, if needed.
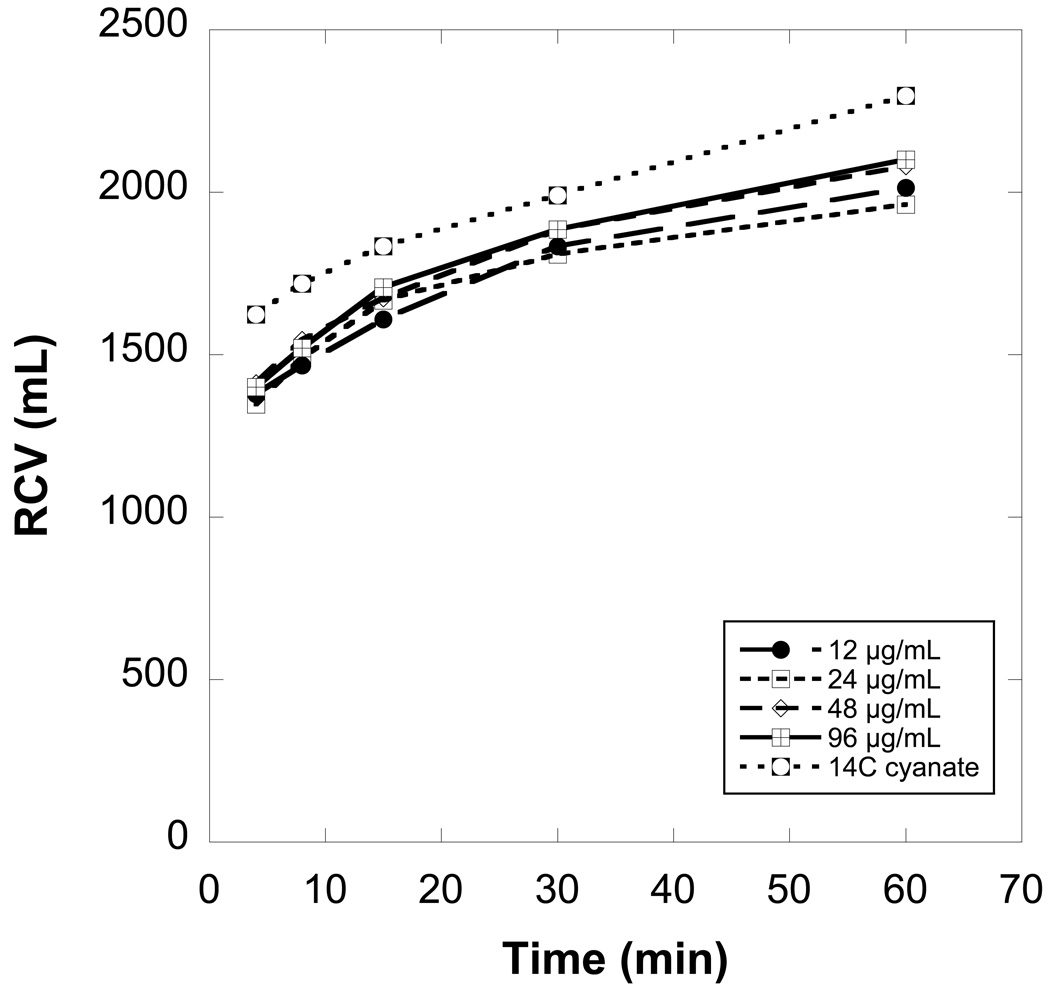
In addition to the RCV values calculated at each blood sampling time (i.e., 4 to 60 minutes), RCV was determined by extrapolation to zero time of the linear regression of the first three points from an RCV versus time plot (See Figure 2 for data from a representative sheep). On average, the RCV extrapolated to zero time was smaller than the RCV from the 4 minute sample by an average of 6.8% (range 2.9 to 10.9%). For simplicity of presentation, the 4 minute RCV is used hereafter as an estimate of the volume of RBCs immediately available for mixing in circulation (i.e., the “circulating RCV”). Circulating blood volume was calculated from the ratio of the circulating RCV to the Hct. No correction for central whole body vs. peripheral venous Hct was used. RCV determined at 60 minutes (after the slower equilibration of circulating RBCs with splenic RBCs) is denoted as “total RCV”; total blood volume was calculated as total RCV / Hct.
Figure 2.
Red cell volumes (RCVs) determined independently and simultaneously from the four biotinylation densities agree well with each other and moderately well with 14C-cyanate values, but all RCV values increase importantly over the first hour after infusion of the mixture of biotinylated cells. These data from a representative sheep depict the time dependence of the calculated RCV for the four biotin densities 12, 24, 48, and 96 µg of biotinylation reagent per mL of autologous RBCs.
Measurement of RCV by 14C-cyanate
In sheep, we previously validated quantitation of RCV using RBCs labeled at a single low density of biotin against both the 51Cr and 14C-cyanate reference methods.10 To further validate that RCV determined from the RBCs biotinylated at the lowest density agrees with RCV determined by another reference method, additional aliquots of RBCs from each of the first six sheep were labeled with 14C-cyanate (as previously described13,14), mixed with the biotinylated RBCs, and infused. Blood volume was determined from liquid scintillation counting of the 14C-cyanate-labeled hemoglobin,14 and RCV was calculated using the venous Hct as previously described.14
Statistical methods
To assess changes in RCV determined by the 14C-cyanate method over time, linear mixed model analysis for repeated measures was used to test data from 4 minutes to 60 minutes. To further assess agreement of RCV determined by biotinylated RBCs with RCV determined by the 14C-cyanate method, linear mixed model analysis for repeated measures was used to estimate the mean relative difference between RCV determined using 14C-cyanate and RCV determined from the lowest density of biotinylated RBCs. Linear mixed model analysis for repeated measures was also used to assess whether this difference changed with time following infusion.
For assessing effects of time that involved multiple tests (e.g., the mixing and equilibration comparing RCVs at 1 and 2 minutes to those at 4 minutes or the stability of equilibration after 60 minutes), the p-values were adjusted using Bonferroni’s method by multiplying the unadjusted p-value by the number of tests performed, with a Bonferroni adjusted p-value<0.05 considered as statistically significant.
In the primary comparison of the study, we assessed the accuracy of RCV determined simultaneously and independently from RBCs biotinylated at higher densities against RCVs determined from the lowest density of biotinylated RBCs (12 µg/mL); thus, the RCV determined from the lowest density was the reference value. Two statistical approaches were used:
1. RCV values from the RBCs labeled at the higher biotin densities 24, 48, and 96 µg/mL were plotted against the RCV values determined from the reference 12 µg/mL density. Linear mixed model analysis was used to compute intercept and slope estimates for each density, and test for differences in the slope among the densities. The fitted line for each of the three densities was examined for statistical difference from the line of identity (adjusted for multiple testing) by assessing whether the 98.3% [(1−.05/3) × 100%] confidence interval included 1.0 for the slope and 0 for the intercept.
2. Because in practice, the comparison of RCV is usually performed on relative scales, an analysis based on the ratio of RCV values relative to the 12 µg/mL density was also performed. The linear mixed model analysis for repeated measures was used to test for differences among the mean RCV ratios, with density, time, and density x time interaction as the fixed effects. In addition to estimating the fixed effects in the mixed model, this method of analysis allows selection of the covariance structure that best fits the relationship of the RCVs for the different densities and duration that were measured in the same sheep (i.e., repeated measures). The best covariance structure is then used in the statistical tests. From the mean and standard deviation estimates of the fitted linear mixed model, the 95% limits of agreement for the mean ratio (mean + 1.96 SD) was computed.15 This interval defines the range within which most of the individual RCV ratios with the higher biotin density of 24 µg/mL, 48 µg/mL, and 96 µg/mL relative to the reference density of 12 µg/mL will lie. Bland-Altman plots were constructed to show the distribution of the RCV ratios and the 95% limits of agreement.16
RESULTS
A table with the complete RCV values for each of the nine sheep, as determined at all time points for all four biotin densities, is provided in the Supplement. Figure 2 presents data from a single representative sheep; the RCV values calculated from the four biotin densities at any single time tend to agree (e.g., at 4 minutes, mean RCV = 1384 mL and range = 1346 to 1412 mL). However, the apparent RCV increased with time; at 60 minutes, mean RCV = 2039 mL and range = 1962 to 2101 mL. For the 12 µg/mL reference biotin density, mean RCV (± 1 SD) for the nine sheep increased by 37 ± 12%. If data for all densities are combined, the mean increase in RCV was 41 ± 14%. Presumably, the infused biotinylated RBCs equilibrated over time with a pool of RBCs sequestered in the spleen;17 the equilibration process is nearly complete within 32 minutes of infusing the biotin-labeled RBCs (Figure 2). As further evidence that the equilibration process occurred within the first 60 minutes, the percentage of all the labels remaining 24 hours after transfusion relative to 60 minutes was 95% ± 4% (n=9). Recovery at 24 hours (PTR24) for each individual density was 95% ± 4% for 12 µg/mL, 96% ± 3% for 24 µg/mL, 94% ± 4% for 48 µg/mL, and 94% ± 4% for 96 µg/mL.
The observation that RCVs measured using RBCs labeled at different biotin densities increased in parallel provides evidence that the spleen interacts similarly with each population of biotinylated RBCs. To further test this interpretation, additional studies were performed in the final three sheep. For these three sheep, a second catheter was placed in the opposite jugular vein. This larger catheter permitted infusion of the entire mixture of biotinylated RBCs within one minute. In addition to the usual five samples between 4 and 60 minutes post-infusion, four additional blood samples were collected for these three sheep at 1, 2, 90, and 120 minutes post-infusion using the second catheter. The 1 and 2 minute samples were used to assess earlier mixing and equilibration of biotinylated RBCs. Compared to RCVs from the 1 minute samples, RCVs from 2 minute samples were 3.3% larger (p<0.002 by paired t test with Bonferroni correction), and RCVs from four minute samples were 6.7% larger (p<0.001 by paired t test with Bonferroni correction). These increases in RCV, present in blood from the non-infusion jugular, provide evidence that the decrease in enrichment (and consequent increase in RCV) over the first 32 minutes is most likely due to splenic equilibration17 rather than blood mixing.
The 90 and 120 minute samples were used to assess whether 60 minutes was sufficient for complete equilibration of the infused biotinylated RBCs with the splenic pool of sequestered RBCs. Of the 24 RCV values calculated from the 90 and 120 minute samples (3 sheep x 4 densities x 2 time points), 23 RCV values agreed with the RCVs calculated from the 60 minute samples within 5% - the analytical accuracy of the biotinylated RBC method. Overall mean difference was 0.2 ± 2.5 % for 90 minute RCVs versus 60 minute RCVs, and mean difference was 2.8 ± 2.2 % for 120 minute RCVs versus 60 minute RCVs. These observations provide evidence that 60 minutes was sufficient time for equilibration of infused biotinylated RBCs with splenic RBCs.
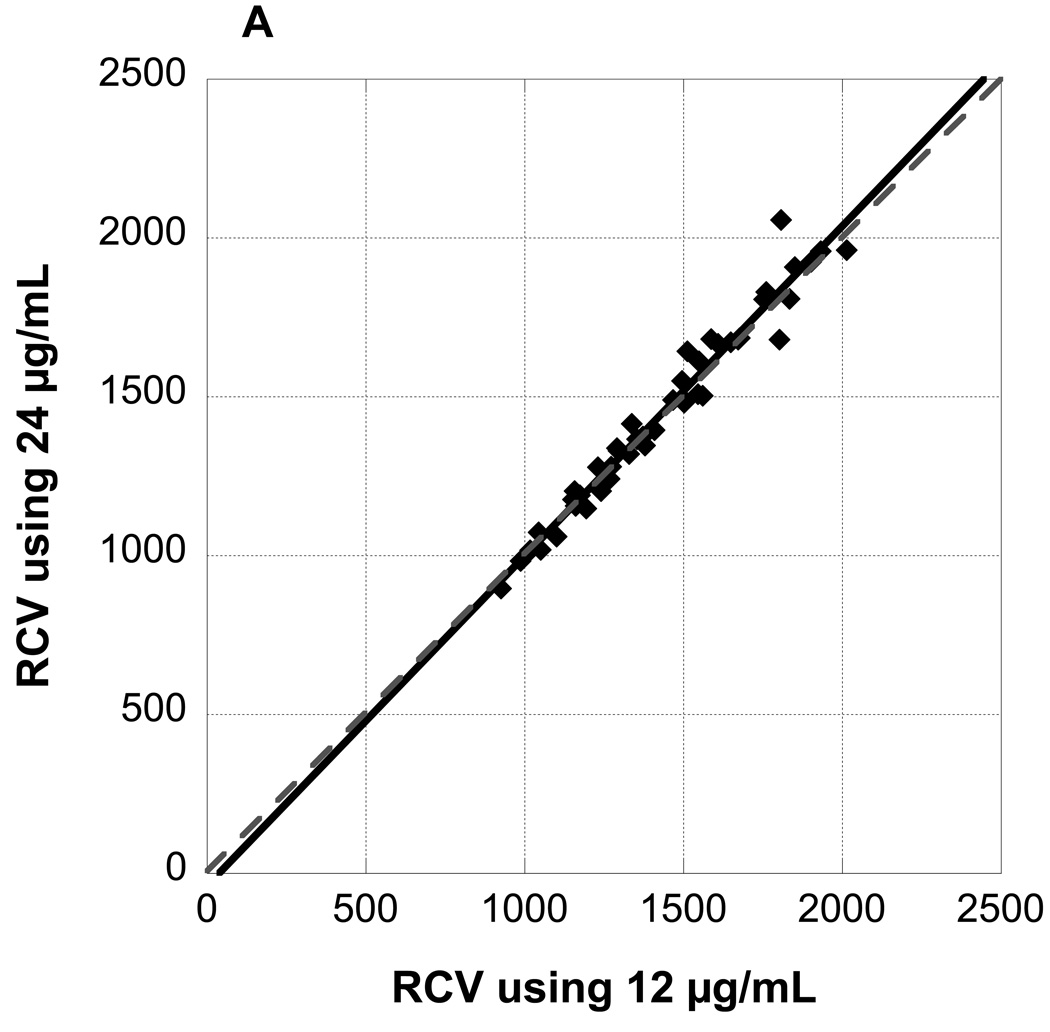
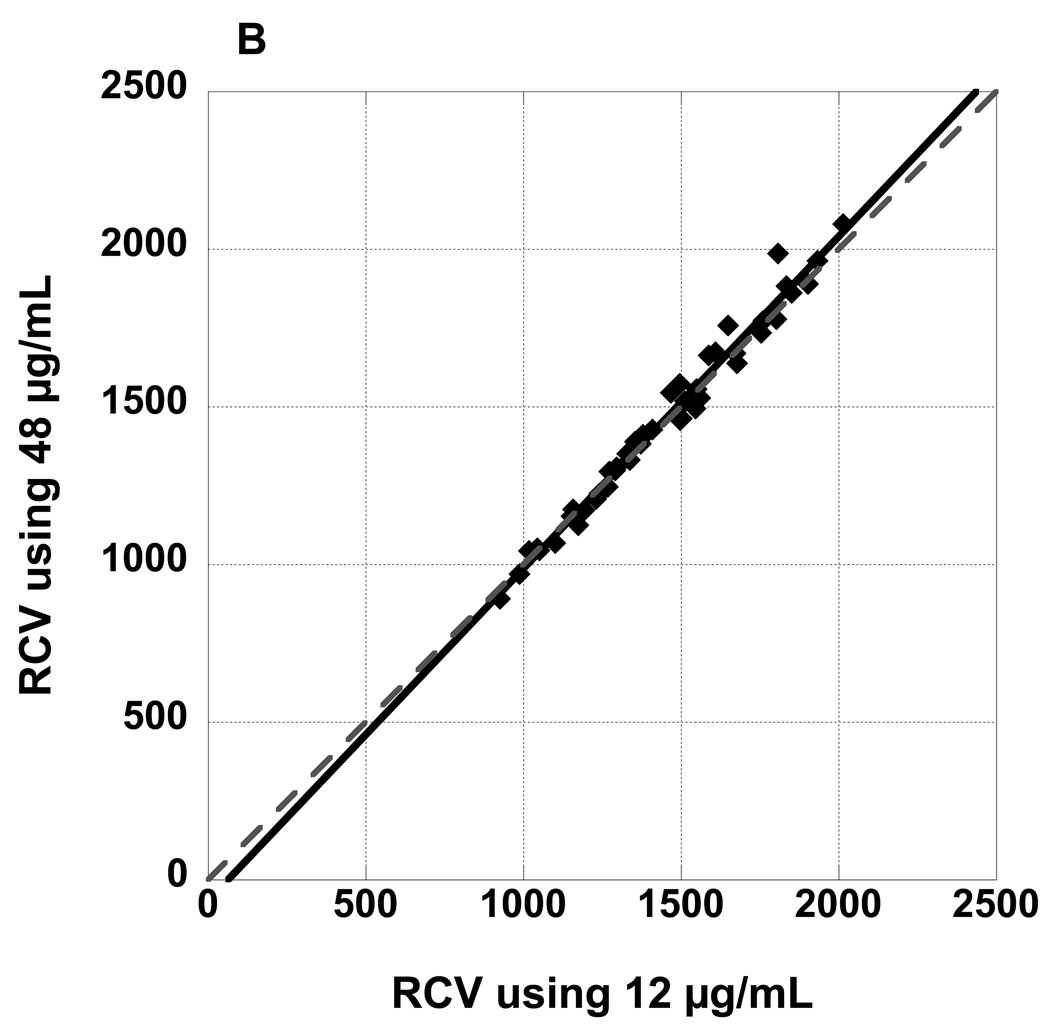
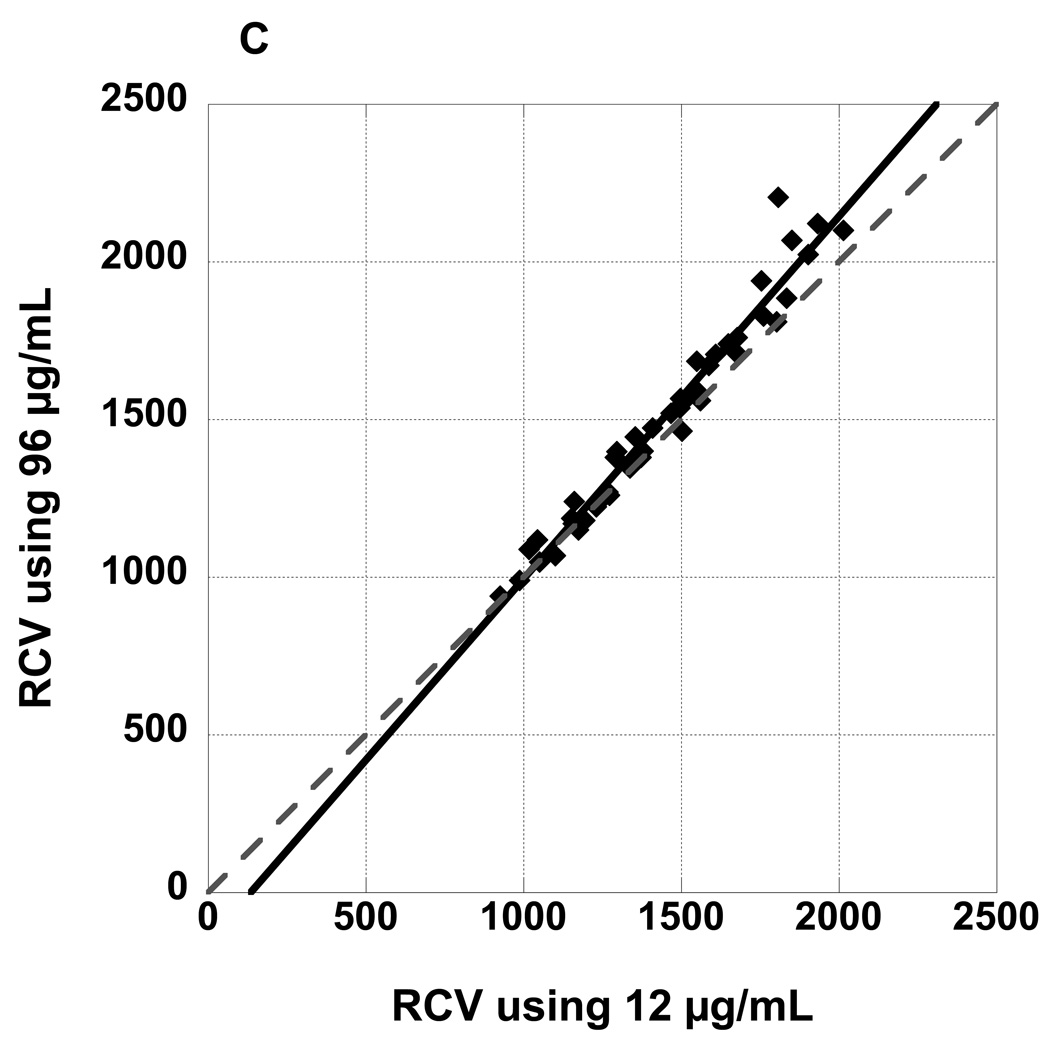
Figure 3 panel A depicts a plot of RCV values observed at 4, 8, 16, 32, and 60 minutes post-infusion for RBCs labeled at biotin density of 24 µg/mL versus RCV values determined from the reference biotin density of 12 µg/mL. The slope of the fitted line from the linear mixed model was not significantly different from the line of identity; the confidence interval (CI) for the slope included 1, and the intercept included 0 (Table 1). Figure 3 panels B and C depict plots of RCV values for RBCs labeled at biotin density of 48 and 96 µg/mL respectively versus RCV values determined from 12 µg/mL. The slopes of the fitted lines were both significantly steeper than a line of identity (Table 1); moreover, the slopes of the three fitted lines differed significantly (p=0.016) and the slope of the fitted line for 96 µg/mL was significantly steeper than both the slope of the fitted line for 24 (Bonferroni adjusted p=0.038) and 48 (Bonferroni adjusted p=0.041).
Figure 3.
RCV values determined from three populations of biotinylated RBCs (A: 24 µg/mL, B: 48 µg/mL, and C: 96 µg/mL) plotted against RCV determined from the reference biotin density of 12 µg/mL demonstrate excellent agreement. All time points from 4 minutes to 60 minutes are depicted. Regression lines are not significantly different from the line of identity.
Table 1.
Regressions of RCVs for 24, 48, and 96 against 12 (Figures 3 A, B, and C) at all time points. Regression for 24 is not significantly different from a line of identity but regressions for 48 and 96 are.
| Biotinylation density |
Slope | 98.3%* CI of the slope |
Intercept | 98.3%* CI of the intercept |
R2 | ||
|---|---|---|---|---|---|---|---|
| 24 µg/mL | 1.068 | 0.948 | 1.189 | −84 | −249 | 81 | 0.96 |
| 48 µg/mL | 1.074 | 1.003 | 1.144 | −96 | −192 | 1 | 0.98 |
| 96 µg/mL | 1.156 | 1.050 | 1.261 | −169 | −313 | −26 | 0.96 |
(1−.05/3) × 100% was used to account for the 3 comparisons performed.
To assess whether there was a detectable effect of biotin density on RCV when all data are considered, we performed a linear mixed model analysis for repeated measures on the ratios of the RCV values from biotinylated RBCs labeled at the higher biotin densities (24, 48, and 96 µg/mL) versus RCV values for the reference 12 µg/mL. Table 2 gives the mean RCV ratios of densities 24, 48, and 96 to density 12 µg/mL at each time post-infusion. There was a significant density x time interaction (p=0.017). Specifically, the mean RCV ratio to density 12 µg/mL did not differ significantly among the densities 24, 48, and 96 at 4 minutes (p>0.99), 8 minutes (p=0.76), or 16 minutes (p=0.39), indicating that a significant artifact was not introduced by heavier biotinylation for RCV measurements made during the first 16 minutes. In contrast, at 60 minutes, the difference in mean RCV ratio density 96 to density 12 µg/mL was significantly larger than the ratio of densities 24 and 48 to density 12 (1.076 for density 96 vs. 1.023 for density 24 and 1.016 for density 48; p<0.0001).
Table 2.
Mean RCV ratios to biotinylation density 12 µg/mL are not different for biotin densities 24 µg/mL and 48 µg/mL but are different for 96 µg/mL
| Biotinylation density | Time (min) |
Mean ratio | 95 % CI of the mean ratio |
|
|---|---|---|---|---|
| 24 µg/mL * | 4 | 0.990 | 0.957 | 1.024 |
| 8 | 1.007 | 0.973 | 1.040 | |
| 16 | 1.015 | 0.982 | 1.048 | |
| 32 | 1.015 | 0.982 | 1.048 | |
| 60 | 1.023 | 0.989 | 1.056 | |
| Mean | 1.010 | 0.974 | 1.046 | |
| 48 µg/mL * | 4 | 0.992 | 0.971 | 1.012 |
| 8 | 1.001 | 0.981 | 1.022 | |
| 16 | 1.009 | 0.989 | 1.030 | |
| 32 | 1.014 | 0.994 | 1.035 | |
| 60 | 1.016 | 0.995 | 1.036 | |
| Mean | 1.006 | 0.985 | 1.028 | |
| 96 µg/mL † | 4 | 1.010 | 0.976 | 1.044 |
| 8 | 1.024 | 0.990 | 1.058 | |
| 16 | 1.036 | 1.002 | 1.070 | |
| 32 | 1.046 | 1.012 | 1.080 | |
| 60 | 1.076 | 1.042 | 1.109 | |
| Mean | 1.038 | 1.002 | 1.074 | |
The mean RCV ratios did not differ significantly among the 5 time points for biotin densities 24 µg /mL (p=0.81) and 48 µg /mL (p=0.23).
The mean RCV ratios differed significantly over the 5 time points for biotin density 96 µg/mL (p=0.005). The 60 minute RCV ratio at the 96 µg/mL was significantly greater compared to the mean ratio at 24 µg/mL and 48 µg/mL (p<0.0001).
Considering the effect of time alone, there was not a significant effect of time on mean RCV ratios of either density 24 or density 48 to density 12. However, for density 96, the mean RCV ratio increased with time (p=0.005); the mean RCV ratio at 4 minutes was 1.010, at 32 minutes was 1.046 minutes, and at 60 minutes was 1.076. Thus, using RBCs biotinylated at 96 µg/mL, RCV was over-estimated by 4.6% at 32 minutes and 7.6% at 60 minutes. Although detected by the statistical analysis, this error is similar to the overall error of the method; the average accuracy using sheep RBCs is −3% and the average precision (expressed as ±1SD) is ±6%.1
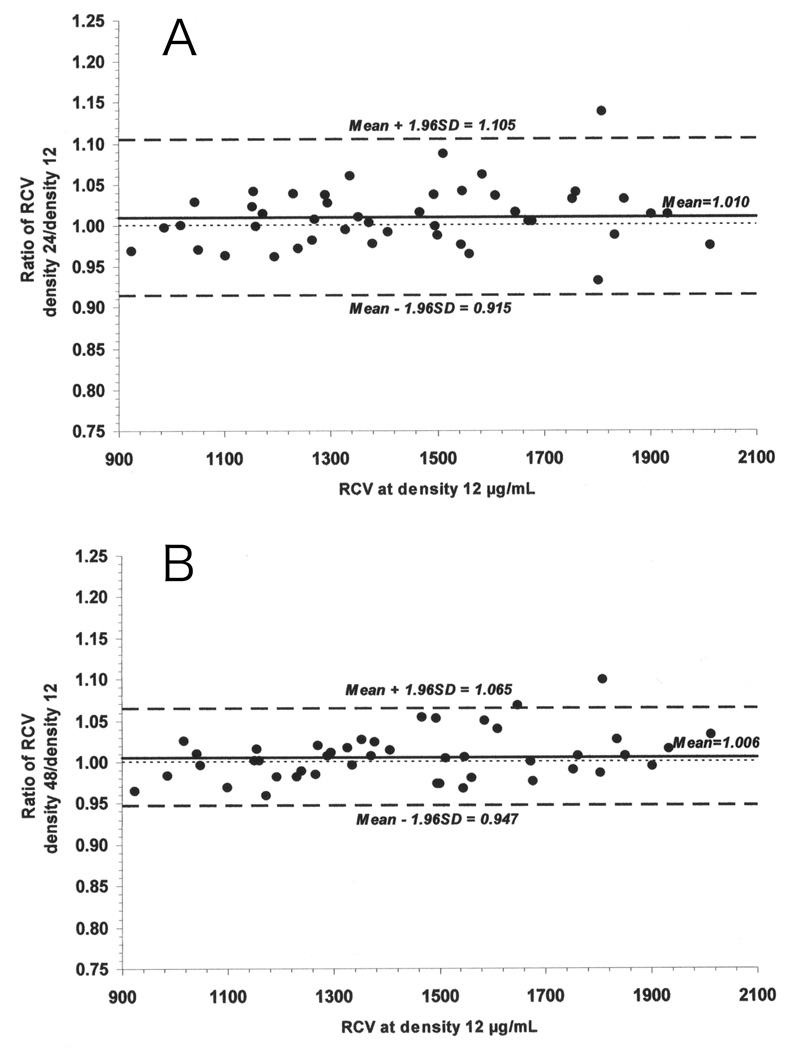
Using mean and standard deviation estimates from the fitted linear mixed model, the 95% limits of agreement for individual sheep RCV ratios were computed for each density. For densities 24 and 48, the 95% limits of agreement were based on pooled estimates from all 5 time points because there was no significant difference in mean RCV across time for these densities. The Bland-Altman plots showing the individual RCV ratios for densities 24 and 48 to density 12 µg/mL with the mean RCV ratio and the 95% limits of agreement are presented in Figure 4 panels A and B. For density 96, limits of agreement were calculated separately for the combined data from 4, 8, and 16 minutes (Figure 5 panel A) and for the combined data from 32 and 60 minutes (Figure 5 panel B) in order to accommodate the significant time effect discussed earlier. Based on this analysis, 95% of the RCVs determined using RBCs biotinylated at biotin density 24 µg/mL are predicted to be within −8.5% to +10.5% of the RCV measured using the reference biotin density of 12 µg/mL, and 95% of the RCVs determined using RBCs biotinylated at biotin density 24 µg/mL are predicted to be within −5.3% to +6.5%. A similar range of −4.6% to +9.6% is predicted for RCVs measured at 4, 8, and 16 minutes using RBCs biotinylated at 96 µg/mL. In contrast, for RCV at 32 and 60 minutes with biotin density 96 µg/mL, the net overestimate of RCV is predicted be 6.1% with variability of ±11.6%; thus overestimates of as great as +17.7% are possible.
Figure 4.
Individual RCV values from RBCs biotinylated at 24 µg/mL (Panel A) and 48 µg/mL (Panel B) agree well with the lowest biotin density (12 µg/mL). Depicted are ratios to the corresponding RCV value from 12 µg/mL for individual RCV values for 24 µg/mL (Panel A) and 48 µg/mL (Panel B). Values for all of the five timed blood samples and all the nine sheep are depicted. Mean ratio (solid line) and the 95% limits of agreement (dashed line) are also depicted.
Figure 5.
Individual RCV values from RBCs biotinylated at 96 µg/mL agree well with the lowest biotin density (12 µg/mL) at 4, 8, and 16 minutes (Panel A), but not at 32 and 60 minutes (Panel B). Depicted are ratios of 96 µg/mL to the corresponding RCV value from 12 µg/mL for individual RCV values at 4, 8, and 16 minutes (Panel A), and 32 and 60 minutes (Panel B). Individual ratios for all of the nine sheep and the 95% limits of agreement (dashed line) are depicted. Mean ratio (solid line) agrees well for at 4, 8, and 16 minutes but differs importantly for 32 and 60 minutes.
As illustrated in Figure 2, RCV values determined using 14C-cyanate consistently paralleled changes in RCV determined using biotinylated RBCs. These 14C-cyanate results are also consistent with equilibration of the 14C-cyanate labeled RBCs into a splenic pool of similar size as that observed using biotinylated RBCs. However, the 14C-cyanate method consistently produced greater RCV values compared to those determined using biotinylated RBCs (Figure 2). The relative mean difference (± SD) using 14C-cyanate compared to biotinylated RBCs at the reference concentration of 12 µg/mL, averaged over the all time points, was 8.3% (± 7.8%); p=0.029. The explanation for this 8.3% overestimate of RCV by 14C-cyanate relative to the biotin method is not known and contrasts with the previously reported closer agreement between the two methods.10
DISCUSSION
A method to accurately measure circulating RCV (often referred to as red cell mass) without exposure of the recipient to radioactivity is highly desirable – particularly for studies of fetuses, infants, children, and pregnant women. The method utilizing RBCs labeled with biotin is such a method. The method using a single density of biotin has been validated against 51Cr-labeled RBCs (as the established standard method) and employed in studies of animals and humans included developing and validation appropriate measures for administration of biologics to human subjects, monitoring for safety (toxicity of chemicals, in vivo hemolysis of biotinylated RBCs, and formation of antibodies to biotinylated RBCs), and obtaining FDA permission for an Investigational New Drug.1,7,8,10,12,18,19 To further enhance the utility of the method, we developed a technique for labeling four separate populations of RBCs with increasing quantities of biotin per RBC; each subpopulation of RBCs can be quantified independently by flow cytometry.1 Thus, the distinct RBC subpopulations labeled with different densities of biotin can potentially be used to measure RCV simultaneously or repetitively in the same subject. Because the densities are independently enumerated, no base line correction8 is needed. Such serial measurements would assist in quantitative estimates of erythropoiesis despite the intervening events such as hemmorhage, transfusion, and somatic growth that typically confounds attempts to quantify erythropoiesis in clinical situations.
In this report, we document that four distinct populations of RBCs, labeled with increasing quantities of biotin, agree reasonably well with one another when used to measure RCV in sheep. Separate cohorts of RBCs were labeled with either 12 µg of biotinylation reagent per mL of RBCs (reference concentration) or 24, 48, or 96 µg/mL. All four populations of autologous RBCs were mixed together and transfused. Post-transfusion blood samples were drawn at 4, 8, 16, 32, and 60 minutes after the infusion, each population of biotinylated RBCs was enumerated in each sample by flow cytometry, and RCV was calculated by the dilution principle.
RCV values, as determined from the four biotin densities, were in close agreement at each time up to 16 minutes. Thereafter, RCVs from the 12, 24, and 48 biotinylated RBCs continued to agree, but RCV values at 60 minutes calculated from 96 µg/mL RBCs overestimated the reference RCV (12 µg/mL) by about 8%. This overestimate was detected by both of our statistical approaches. The mixed model linear regressions found that the 96 versus 12 regression was significantly different from a line of identity; the slope of the line was greater than 1 and the intercept greater than 0. For the 48 versus 12 regression, the CI of the lower limit of the slope (1.003) was slightly greater than1, but the CI for the intercept did incorporate 0. We interpret these findings as evidence that there is a small, but statistically significant artifactual acceleration of removal of RBCs biotinylated at the higher densities, which result in small overestimates of RCV. We speculate that these RBCs are cleared by the RE system (e.g., spleen) rather than hemolyzed in the vascular system; this speculation is consistent with the observation the plasma free hemoglobin and haptoglobin do not increase coincident with clearance of RBCs that are biotinylated at densities even greater than the ones used in this study (unpublished data).
We have no explanation for the 8.3% overestimate of RCV by 14C-cyanate relative to the biotin method. These results contrast with the previously reported closer agreement between the two methods (a smaller overestimate by the biotin method).10 One explanation would be a more rapid removal of 14C-cyanate labeled RBCs, but we speculate that we consistently underestimated the 14C-cyanate concentrations (and hence overestimating the volume measured by 14C-cyanate) and that this artifact likely arose from incomplete correction of the quench caused by residual amounts of bleached hemoglobin and protein in blood.
Several technical points need emphasis. The four-minute RCV was used to represent the immediate circulating RCV; extrapolation back to time zero produced a set of RCV values that was only a minimally smaller. RCV results from all biotin densities increased in parallel over the 60 minutes of measurement. This finding is likely caused by equilibration of RBCs in the spleen with those in the bloodstream.17,20 RCV values from the four populations of biotinylated RBCs followed a similar relative time course, providing evidence that each population of biotinylated RBCs equilibrated with the spleen RBCs at the same rate and into the same size pool of unlabeled splenic RBCs. The mean increase of RCV between four and 60 minutes was 41% (combined data for all biotin densities); most of the equilibration was complete by 32 minutes post-infusion. When additional blood samples were obtained at 90 and 120 minutes post-infusion, RCV values agreed with 60-minute values within the 5% analytical accuracy of the biotinylated RBC method. Thus, blood samples later than 60 minutes post-infusion are not needed to accurately measure total (circulating plus splenic) RCV in adult sheep.
This interpretation regarding circulating RBC equilibration with a splenic pool of more sequestered RBCs in sheep is supported by the findings of others.17,20,21 Using 59Fe enrichment of erythrocytes and sheep in a resting state, Wade observed that the RCV increased 38% with epinephrine injection and calculated that the sheep spleen contains about 27% of the total red cell mass 20. In measuring the increase in the circulating red cell mass that can be induced by intrasplenic injection of epinephrine, Hecker reported an increase of “up to 38%”22. Likely, this approach underestimates the total equilibrated RCV and represents only that which can be immediately squeezed from the sheep’s spleen. Torrington, et al reported 1) that equilibration of 51Cr labeled RBCs intravenously infused in sheep requires a similar equilibration period to that observed in the present study, i.e., about 30 minutes, 2) that the size of the slowly equilibrating pool was about 5–10% of the RCV, and 3) that splenectomy effectively removed the pool.17 These investigators concluded that for sheep the non-circulating RBC pool was located in the spleen.
The equilibration exchange of RBCs between the spleen and the peripheral circulation had important implications in assessing total RCV in sheep (and perhaps other species) in that the timing of blood sampling for the determination of RCV is critical. Measurements made in the first few minutes following transfusion provide information only on the volume of RBCs within the circulation, without including RBCs sequestered in the spleen. For the human, this may have important implications in RCV measurements in human patients with functional splenic abnormalities. One possible example is patients with sickle cell disease. Young patients with sickle cell disease may have spleens that sequester abnormally larger numbers of RBCs; in contrast, older sickle cell patients are likely to have experienced splenic infarcts substantial enough that the spleen RBC pool is insignificant, thus rendering timing of blood sampling for RCV measurement unimportant.
The evidence provided here that RCV can be accurately measured in sheep using RBCs labeled with multiple distinct densities of biotin likely has several important implications. First, this capability adds to the potential utility of the sheep as a model for preclinical testing.2–6 Second, we speculate that extending the studies of RBCs labeled with multiple distinct biotin densities to humans including sequential measurement of RCV and red cell survival (e.g., 24-hour survival post-transfusion recovery and circulating lifespan) will be successful and will offer the opportunity to perform these studies in human fetuses, infants, children, and pregnant women. Third, the strikingly similarity of ovine and human physiology and pathophysiology suggests that the splenic phenomena should be characterized in humans under carefully controlled experimental conditions to determine whether such sequestration may affect the accuracy of estimates of RCV under normal or pathophysiological conditions.
Table A1.
Red cell volumes (RCVs) determined independently and simultaneously from the four biotinylation densities agree well with each other but increase importantly over the first hour after infusion of the mixture of biotinylated cells. RCV values were determined for each of the four biotin densities (12, 24, 48, and 96 µg of biotinylation reagent per mL of autologous RBCs) at each of five time points for all nine sheep.
| Sheep | Body weight (kg) |
Biotinylation density (µg/mL RBC) |
RCV (mL) | ||||
|---|---|---|---|---|---|---|---|
| 4 min | 8 min | 16 min | 32 min | 60 min | |||
| 1 | 72.2 | 12 | 1,156 | 1,240 | 1,353 | 1,586 | 1,803 |
| 24 | 1,204 | 1,204 | 1,367 | 1,682 | 1,681 | ||
| 48 | 1,175 | 1,226 | 1,390 | 1,663 | 1,778 | ||
| 96 | 1,171 | 1,240 | 1,446 | 1,672 | 1,811 | ||
| 2 | 84 | 12 | 925 | 987 | 1,050 | 1,152 | 1,291 |
| 24 | 897 | 984 | 1,018 | 1,178 | 1,338 | ||
| 48 | 892 | 970 | 1,046 | 1,154 | 1,299 | ||
| 96 | 941 | 991 | 1,049 | 1,188 | 1,381 | ||
| 3 | 70.5 | 12 | 1,017 | 1,043 | 1,160 | 1,295 | 1,497 |
| 24 | 1,017 | 1,073 | 1,157 | 1,330 | 1,495 | ||
| 48 | 1,043 | 1,053 | 1,162 | 1,309 | 1,458 | ||
| 96 | 1,088 | 1,119 | 1,240 | 1,398 | 1,567 | ||
| 4 | 74.4 | 12 | 1,271 | 1,373 | 1,495 | 1,648 | 1,808 |
| 24 | 1,280 | 1,376 | 1,550 | 1,672 | 2,058 | ||
| 48 | 1,295 | 1,383 | 1,574 | 1,759 | 1,988 | ||
| 96 | 1,260 | 1,381 | 1,537 | 1,740 | 2,206 | ||
| 5 | 77.8 | 12 | 1,546 | 1,548 | 1,677 | 1,754 | 1,851 |
| 24 | 1,509 | 1,612 | 1,685 | 1,807 | 1,909 | ||
| 48 | 1,496 | 1,557 | 1,638 | 1,735 | 1,863 | ||
| 96 | 1,595 | 1,686 | 1,760 | 1,941 | 2,069 | ||
| 6 | 74 | 12 | 1,379 | 1,467 | 1,609 | 1,834 | 2,014 |
| 24 | 1,346 | 1,490 | 1,667 | 1,809 | 1,962 | ||
| 48 | 1,412 | 1,546 | 1,673 | 1,883 | 2,080 | ||
| 96 | 1,400 | 1,521 | 1,707 | 1,885 | 2,101 | ||
| 7 | 76.8 | 12 | 1,561 | 1,672 | 1,761 | 1,902 | 1,933 |
| 24 | 1,504 | 1,680 | 1,831 | 1,925 | 1,959 | ||
| 48 | 1,529 | 1,671 | 1,774 | 1,891 | 1,964 | ||
| 96 | 1,560 | 1,713 | 1,828 | 2,024 | 2,123 | ||
| 8 | 65.2 | 12 | 1,173 | 1,231 | 1,337 | 1,502 | 1,512 |
| 24 | 1,190 | 1,278 | 1,416 | 1,482 | 1,644 | ||
| 48 | 1,125 | 1,208 | 1,332 | 1,463 | 1,518 | ||
| 96 | 1,150 | 1,224 | 1,346 | 1,463 | 1,563 | ||
| 9 | 68.4 | 12 | 1,101 | 1,194 | 1,267 | 1,328 | 1,408 |
| 24 | 1,061 | 1,149 | 1,242 | 1,321 | 1,396 | ||
| 48 | 1,068 | 1,173 | 1,247 | 1,352 | 1,429 | ||
| 96 | 1,068 | 1,181 | 1,270 | 1,356 | 1,473 | ||
ACKNOWLEDGEMENTS
This work was supported by the NIH Program Project Grant P01 HL046925. We also thank Brandy VanDenBerg, Research Assistant to Dr. Mock, for providing editorial assistance to the authors during preparation of this manuscript. Ms. VanDenBerg is supported by funding from the above grant. The Sysmex XE-2100 automatic hematology analyzer used in this study was provided on an on-loan basis from Sysmex Corporation, Kobe, Japan.
APPENDIX
Characteristics of Sheep In Study
All sheep for this study were females about 2 years of age. The average weight was 73.7 kg (65.2 – 84.0 kg). None had been exposed to biotinylated RBCs before serving in this study. Individual sheep RCV was estimated by using a value of 71.4 mL of blood per kg body weight x Hct. Estimation of enrichment of each individual population of biotinylated RBCs transfused was estimated as a percentage of the total RBCs according to the formula:
Footnotes
COI Statement: None of the authors have declared a Conflict of Interest.
REFERENCES
- 1.Mock D, Matthews N, Strauss R, Burmeister L, Schmidt R, Widness J. Red cell volume can be independently determined in vitro using sheep and human red cells labeled at different densities of biotin. Transfusion. 2009;49:1178–1185. doi: 10.1111/j.1537-2995.2009.02095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lister G, Walter TK, Versmold PR, Dallman PR, Rudolph AM. Oxygen delivery in lambs: cardiovascular and hematologic development. Am J Physiol. 1979:H668–H675. doi: 10.1152/ajpheart.1979.237.6.H668. [DOI] [PubMed] [Google Scholar]
- 3.Blunt MH, Huisman THJ. The Blood of Sheep: Composition and Function. New York: Springer-Verlag; 1975. The hemoglobins of sheep. [Google Scholar]
- 4.Brace RA. Blood volume in the fetus and methods for its measurement. In: Nathanielsz PW, editor. Animal Models in Fetal Medicine. Ithaca: Perinatology Press; 1984. pp. 19–36. [Google Scholar]
- 5.Moritz KM, Lim GB, Wintour EM. Developmental regulation of erythropoietin and erythropoiesis. Am J Physiol Regul Integr Comp Physiol. 1997;273:R1829–R1944. doi: 10.1152/ajpregu.1997.273.6.R1829. [DOI] [PubMed] [Google Scholar]
- 6.Widness JA, Lowe LS, Bell EF, Mock DM, Kistard JA, Bard H. Adaptive responses during anemia and its correction in lambs. J Appl Physiol. 2000;88:1397–1406. doi: 10.1152/jappl.2000.88.4.1397. [DOI] [PubMed] [Google Scholar]
- 7.Mock DM, Lankford GL, Widness JA, Burmeister LF, Kahn D, Strauss RG. Measurement of circulating red blood cell volume using biotin labeled red cells: Validation against 51Cr labeled red cells. Transfusion. 1999;39:149–155. doi: 10.1046/j.1537-2995.1999.39299154728.x. [DOI] [PubMed] [Google Scholar]
- 8.Mock DM, Lankford GL, Widness JA, Burmeister LF, Kahn D, Strauss RG. RBCs labeled at two biotin densities permit simultaneous and repeated measurements of circulating RBC volume. Transfusion. 2004;44:431–437. doi: 10.1111/j.1537-2995.2004.00654.x. [DOI] [PubMed] [Google Scholar]
- 9.Mock DM, Lankford GL, Widness JA, Burmeister LF, Kahn D, Strauss RG. Measurement of red cell survival using biotin labeled red cells: Validation against 51Cr labeled red cells. Transfusion. 1999;39:156–162. doi: 10.1046/j.1537-2995.1999.39299154729.x. [DOI] [PubMed] [Google Scholar]
- 10.Mock DM, Mock NI, Lankford GL, Burmeister LF, Straus RG, Widness JA. Red cell volume can be accurately determined in sheep using a nonradioactive biotin label. Pediatr Res. 2008;64:528–532. doi: 10.1203/PDR.0b013e318183f119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mock DM, Bell EF, Lankford GL, Widness JA. Hematocrit correlates well with circulating red blood cell volume in very low birth weight infants. Pediatr Res. 2001;50:525–531. doi: 10.1203/00006450-200110000-00017. [DOI] [PubMed] [Google Scholar]
- 12.Strauss RG, Mock DM, Johnson K, Mock NI, Cress G, Knosp L, Lobas L, Schmidt RL. Circulating RBC volume, measured with biotinylated RBCs, is superior to the Hct to document the hematologic effects of delayed versus immediate umbilical cord clamping in preterm neonates. Transfusion. 2003;43:1168–1172. doi: 10.1046/j.1537-2995.2003.00454.x. [DOI] [PubMed] [Google Scholar]
- 13.Mock DM, Lankford GL, Burmeister LF, Strauss RG. Circulating red cell volume and red cell survival can be accurately determined in sheep using the [14C]cyanate label. Pediatr Res. 1997;41:916–921. doi: 10.1203/00006450-199706000-00020. [DOI] [PubMed] [Google Scholar]
- 14.Mock DM, Strauss RG, Lankford GL. 14C-cyanate labeling of sheep red cells: Covalent binding to hemoglobin continues in vivo for a day. Pediatr Res. 1997;41:424–429. doi: 10.1203/00006450-199703000-00020. [DOI] [PubMed] [Google Scholar]
- 15.Tolerance intervals for a normal distribution NIST/SEMATECH e-Handbook of Statistical Methods. http://wwwitlnistgov/div898/handbook/prc/section2/prc263htm.
- 16.Bland J, Altman D. Measuring agreement in method comparison studies. Statistical Methods in Medical Research. 1999;8:135–160. doi: 10.1177/096228029900800204. [DOI] [PubMed] [Google Scholar]
- 17.Torrington KG, McNeil JS, Phillips YY, Ripple GR. Blood volume determinations in sheep before and after splenectomy. Laboratory Animal Science. 1989;39:598–602. [PubMed] [Google Scholar]
- 18.Valeri C, MacGregor H, Giorgio A, Srey R, Ragno G. Comparison of radioisotope methods and a nonradioisotope method to measure the RBC volume and RBC survival in the baboon. Transfusion. 2003;43:1366–1373. doi: 10.1046/j.1537-2995.2003.00528.x. [DOI] [PubMed] [Google Scholar]
- 19.Valeri CR, Pivacek LE, Cassidy GP, Ragno G. Volume of RBCs, 24- and 48-hour posttransfusion survivals, and the lifespan of (51)Cr and biotin-X-N-hydroxysuccinimide (NHS)-labeled autologous baboon RBCs: effect of the anticoagulant and blood pH on (51)Cr and biotin-X-NHS elution in vivo. Transfusion. 2002;42:343–348. doi: 10.1046/j.1537-2995.2002.00071.x. [DOI] [PubMed] [Google Scholar]
- 20.Wade L., Jr Splenic sequestration of young erythrocytes in sheep. Am J Physiol. 1973;2:265–267. doi: 10.1152/ajplegacy.1973.224.2.265. [DOI] [PubMed] [Google Scholar]
- 21.Brace RA. Blood volume and its measurement in the chronically catheterized sheep fetus. Am J Physiol Heart Circ Physiol. 1983;244:H487–H494. doi: 10.1152/ajpheart.1983.244.4.H487. [DOI] [PubMed] [Google Scholar]
- 22.Hecker JF. The Sheep as an Experimental Animal. New York: Academic Press; 1983. [Google Scholar]