Abstract
Background. Influenza is an uncontrolled epidemic disease that is vaccine preventable. New recommendations for universal immunization present a challenge to the implementation of vaccine delivery. This field trial examines the effectiveness of school-based clinics for vaccine delivery before an epidemic caused by 3 new influenza virus variants not contained in the vaccine.
Methods. Live attenuated influenza vaccine (LAIV) was offered to eligible children in elementary schools of eastern Bell County, Texas. Age-specific rates of medically attended acute respiratory illness for health plan members at the intervention site were compared with those for members at comparison sites during the epidemic, defined by viral surveillance at all sites.
Results. Almost 48% of children in elementary schools were vaccinated. Significant herd protection attributed to LAIV was detected for all age groups except 12–17-year-old students, who were not offered free vaccine. Approximately 2500 medical encounters were prevented at the intervention site. Inactivated vaccine provided marginal protection against the epidemic viruses.
Conclusions. LAIV delivered to elementary-school children before an epidemic caused by 3 new variant influenza viruses generated significant cross-protection for the recipients and indirect (herd) protection for the community.
Trial registration. ClinicalTrials.gov identifier: NCT00138294.
Even before the emergence of the novel influenza an uncontrolled epidemic disease that exacts a heavy A(H1N1) virus in 2009 [1], influenza was and remains toll in human lives and human suffering [2]. The incidence of serious morbidity attributed to influenza is increasing [3]. The economic burden of seasonal epidemics averages ∼$87 billion per year [2]. The novel H1N1 pandemic has accentuated many of the problems of influenza control, including the timely availability of influenza vaccine [4]. Lacking is public health infrastructure for rapid deployment of vaccines to the susceptible population within the brief period of time between the availability of the vaccines and the onset of the epidemic. The new recommendation by the Advisory Committee for Immunization Practices for universal influenza immunization of all persons ⩾6 months old dictates the development of new vaccine delivery strategies that are efficient and effective [5].
The Central Texas Field Trial was begun in 1998 with an intention to deliver the live attenuated influenza vaccine (LAIV) to as many children in the community as possible [6, 7]. The primary objective has been to determine the proportion of schoolchildren who must be vaccinated to effect indirect protection for the community with the goal of controlling epidemic influenza [7]. A secondary goal has been to assess the direct effectiveness of a single dose of LAIV for eligible children and of the inactivated influenza vaccine (TIV) for children not eligible for LAIV [8–10]. From a public health standpoint, it is very difficult to deliver 11 dose to sufficient numbers of children in a timely manner. Furthermore, the combination of the standing recommendation for vaccination of preschool children and sequential annual vaccination reduces the necessity of a 2-dose schedule for the younger 5–9-year-old schoolchildren.
Since its initiation, the Central Texas Field Trial has evolved from clinic-based delivery of vaccine supplemented by a steadily increasing number of community outreach programs to predominately school-based clinics in 2007–2008. Indirect (herd) protection for the community has been demonstrated by increasing the proportion of immune children, who serve as barriers to the spread of influenza [11, 12]. Implementation of universal vaccination for children [5] requires the determination of an efficient and sustainable system for vaccine delivery. School-based influenza vaccine clinics are a reasonable option to improve vaccine coverage. By 2007, community confidence and trust had been gained by demonstration of the safety of LAIV after administration of 130,000 doses to children in the intervention community during the previous 9 years [11- 14]. The enrollment and consent procedures were simplified, making it unnecessary for the parent or guardian to be present at the time of vaccination of the child. The school-based program is a community collaboration of Scott and White Memorial Hospital and Clinics, independent school districts, the Bell County Health Department, the University of Mary Hardin Baylor Nursing School, and the Texas &cf1 University and Baylor Colleges of Medicine. During the first season, on-site vaccine delivery focused on the 25 elementary schools of the 7 independent school districts of eastern Bell County. This report demonstrates the feasibility of school-based clinics, the direct protection of schoolchildren against influenza-associated illness, and indirect (herd) protection for the community.
Methods
Study design. This community trial investigated nonrandomized, open-label delivery of influenza vaccine targeted to children aged 4–11 years in the elementary schools of 7 independent school districts in eastern Bell County. Temple, Texas, the home of Scott and White Memorial Hospital and Clinics, as well as the adjoining town of Belton plus the communities of Academy, Holland, Rogers, Salado, and Troy make up the population targeted for the intervention. The methods for measuring indirect protection have been described elsewhere and consist of determining the age-specific incidence rates for medically attended acute respiratory illness (MAARI) during the influenza epidemic, to be compared for members of the Scott and White Health Plan (SWHP) at the intervention and comparison sites (located in Waco and Bryan-College Station) [11, 12]. SWHP provides information on MAARI along with demographic information, diagnostic codes, and the census of members by age group from administrative data files for all sites. The trial was approved by the Institutional Review Boards of Scott and White Memorial Hospital and Baylor College of Medicine.
Subjects. Age-eligible healthy children (4 years old and older) received a single dose of the trivalent LAIV (0.2 mL) by intranasal spray. Also included were children with no active asthma treatment or wheezing within 1 year and those receiving nasal steroids. Trivalent TIV (0.5 mL) was given by intramuscular injection to children with active asthma and other underlying conditions and to those with immunocompromised household contacts. Children were excluded from receiving either vaccine if they were allergic to eggs or previous influenza vaccine.
Most of the vaccine was delivered at public elementary schools and parochial schools; some, including a few older children <18 years old, received vaccine during weekend catchup and outreach clinics. Immunization status for all enrolled children and those who were Scott and White patients was ascertained electronically by accessing the systemwide Scott and White immunization registry and administrative database [15]. Safety was monitored by tracking participants for medical encounters at Scott and White clinics, emergency services, and hospitals monthly until 6 weeks after administration of the last dose [13, 14]. Both vaccines contained influenza A/Solomon Islands/3/2006(H1N1), influenza A/Wisconsin/67/2005(H3N2), and influenza B/Ohio/01/2005 (Victoria lineage).
Enrollment. Information about influenza, the vaccines, and the school clinics was delivered to parents through the schools. The parents provided a short medical history to determine eligibility and signed a consent form for each child. Capable children 7 years old and older gave signed assent. The school staff brought the consented children to a schoolroom designated for vaccination. The appropriate vaccine was administered to the students by the Scott and White clinical research team after review of the information and confirmation of parents' consent.
Demographics. Community characteristics have been described in previous reports [11, 12]. The ethnic and racial distributions of the intervention and comparison communities were similar. The membership of SWHP—50,665 persons in the Temple-Belton area and 67,036 persons in the Bryan-College Station and Waco areas—provided a defined population within the intervention and comparisons communities, respectively, for determination of age-specific rates. Approximately 50% of persons in the intervention area (Temple-Belton) and ∼25% of persons in the comparisons communities (Bryan- College Station and Waco) were SWHP members. The age distributions of SWHP members were similar except for the numbers of those >65 years old. The intervention community had more members who were >75 years old—4830 (9.5%) versus 2953 (4.4%) for the comparison sites.
Influenza virus surveillance. Influenza virus surveillance was maintained at all Scott and White clinics in the intervention and comparison areas, as described elsewhere [11, 12]. Patients presenting with a history of febrile acute respiratory illness were candidates for a throat culture for virus isolation. The decision to obtain a culture was made by the clinician seeing the patient without regard for influenza vaccine history. The throat swab specimens were tested by shell vial technique in the viral diagnostic laboratory at Scott and White Memorial Hospital in Temple, Texas. Specimens positive for influenza viruses were reisolated in tissue culture in the laboratory of P.A.P. at Baylor College of Medicine in Houston for subtyping by reverse-transcription polymerase chain reaction and characterization at the Centers for Disease Control and Prevention (CDC) in Atlanta.
Data analysis. Demographic information for enrollees was entered and tracked in the computerized vaccine registry, as described elsewhere [11, 12]. Demographic information and diagnostic categories for MAARI, coded on the basis of the International Classification of Diseases, Ninth Revision , were abstracted from administrative data files for SWHP patients seen during the epidemic year (July 2007 through June 2008). Age-specific rates for MAARI were compared for the influenza epidemic period, determined by virus surveillance. For assessment of the primary outcome, indirect effectiveness, point estimates, and 95% confidence intervals (CIs) for incidence rate ratios (RRs) were calculated. Effectiveness of the influenza vaccine was equal to (1 -RR) X 100% . Age-specific MAARI rates in the prevaccine, vaccine, and postepidemic periods were also calculated to determine the comparability of the sites.
The vaccine status of persons cultured for virus surveillance was determined to allow comparison of influenza virus infection rates for those who received LAIV, TIV, or no vaccine. Rates for appropriate pairs were compared by the x 2test. Poisson regression was used within the subgroup of children who had at least 1 MAARI documented in the administrative database to calculate the average number of MAARIs per week during the epidemic period for age-eligible children by vaccine status.
Medical records for all patients presenting to Scott and White Memorial Hospital or emergency services were retrievable electronically for assessment of safety. All enrollees were tracked for at least 6 weeks after vaccination for adverse events.
Results
Vaccination with LAIV and TIV. Vaccine administration began on 26 October and continued until 19 December 2007. Scott and White clinical research teams supported by Bell County Health Department nurses and student nurses from Mary Hardin Baylor University enrolled students at 25 public elementary schools and 3 parochial schools. A total of 4951 (47.5%) of 10,418 students were vaccinated in the public schools. Catch-up visits to schools, community outreach clinics, and weekend clinics at Scott and White increased the total number to 6191, of whom 5247 (84.8%) received LAIV and 944 (15.2%) received TIV. Vaccine coverage for all Scott and White patients at both the intervention and comparison sites was estimated from the Scott and White immunization registry for children and from administrative records. The proportion of Scott and White patients vaccinated in each age group for the intervention and comparison sites is illustrated in Figure 1. The influenza immunization rates were comparable for all age groups except the 5–11-year-old students (75.8% of Scott and White patients at the intervention site and 24.5% at the comparison sites), who included most of the elementary-school children.
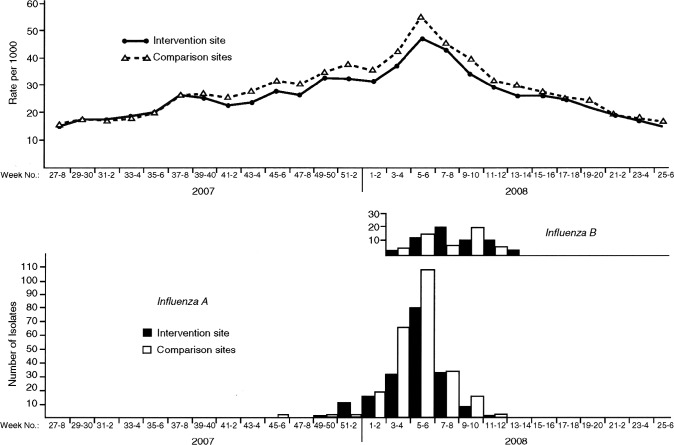
Influenza virus surveillance. Epidemic activity began during week 52 of 2007 at the intervention site and during week 1 of 2008 at the comparison sites and ended on week 11 of 2008 (Figure 2). During that time, 95.5%, 97.4%, and 98.5% of all influenza viruses were detected by surveillance at the Temple-Belton intervention site and at the Bryan-College Station and Waco comparison sites, respectively.
Figure 2.
Biweekly rates of medically attended acute respiratory illness for members of the Scott and White Health Plan (top) and number of Scott and White patients with positive culture results for influenza viruses at intervention and comparison sites (bottom), Central Texas, 2007–2008.
Virus characterization. A sample of 50 influenza viruses recovered throughout the epidemic was characterized at the CDC. The viruses included 22 influenza A(H1N1), 12 influenza A(H3N2), and 16 influenza B viruses. All 50 were classified as new antigenic variants—A/Brisbane/59/2007(H1N1), A/Brisbane/10/2007(H3N2), and B/Florida/04/2005—not included in the 2007–2008 vaccine. For the United States, the CDC reported the new variants for only 24%, 69%, and 95%, respectively, of all viruses characterized. The influenza B/Florida virus was from the Yamagata lineage, whereas the influenza B component of the vaccine was from the distinctly different Victoria lineage. Viruses antigenically similar to the 3 new variants prevalent in Central Texas in 2007–2008 were chosen for the 2008–2009 vaccine.
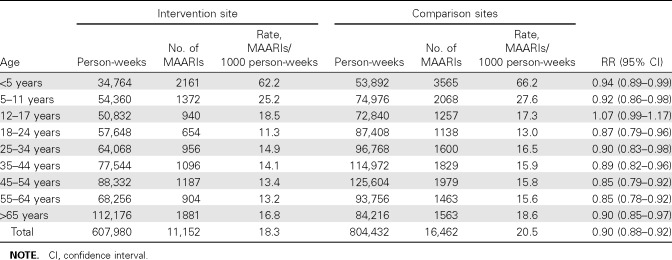
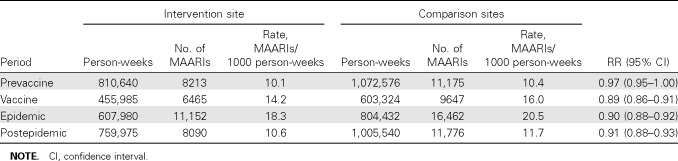
Indirect protection. Biweekly MAARI rates for SWHP members presenting to intervention and comparison site clinics for the 2007–2008 epidemic year are illustrated in Figure 2. Early during the epidemic, influenza A(H1N1) contributed to influenza A activity, as shown in the bar graph, but peak activity consisted mainly of influenza A(H3N2), followed by influenza B activity. The biweekly MAARI rates for intervention and comparison groups were almost identical for the first 15 weeks of the year, as documented for the prevaccine period in Table 1. MAARI rates were lower at the intervention site from week 43 through week 51 when 5247 doses of LAIV were administered mainly to the elementary-school children. The RR was 0.89 (95% CI, 0.86–0.91) for the period during vaccine administration. Indirect protection was evident during the epidemic period and persisted during the early weeks of the postepidemic period; the rates for intervention and comparison sites were comparable for the last 12 weeks of the year (Figure 2). The age-specific MAARI rates for SWHP patients from intervention and comparison sites during the epidemic period are shown in Table 2. Significant indirect protection was detected for all age groups except the 12- 17-year-olds, who were not offered free vaccine in the schools; these students have multiple opportunities for exposure to infectious contacts at school and during other activities.
The expected number of illnesses for the intervention site (12,464) can be estimated by applying the MAARI rate for the comparison sites (20.5 episodes per 1000 person-weeks) to the 607,980 person-weeks of observation of the intervention communities (Table 2). Subtracting the observed number of MAARIs (11,152) from this number suggests that 1312 SWHP medical encounters were prevented by influenza immunization of the elementary-school children. Given that SWHP provides health care for approximately one-half of the people in the intervention area, an estimated 2500 medical encounters were avoided in the Temple-Belton area.
Table 2.
Risk Ratios (RRs) for Age-Specific Rates of Medically Attended Acute Respiratory Illness (MAARI) for Intervention and Comparison Sites during the Influenza Epidemic, 23 December 2007 to 15 March 2008, Central Texas Field Trial
Direct effectiveness. At the intervention site, direct effectiveness was influenced by the indirect benefit of vaccination of a large proportion (47.5%) of elementary-school children, who typically have the highest influenza virus infection rate. Extensive vaccination coverage reduces exposure of unvaccinated persons to infection, resulting in indirect protection (in addition to direct protection for the vaccinated). This was evident from the age distribution and frequency that cultures were obtained for surveillance at the intervention and comparison sites. Both the number of patients of all ages for whom cultures were performed and the proportion positive tended to be lower at the intervention site. Of 482 persons cultured at intervention clinics, 236 (48.5%) yielded an influenza virus; 524 were cultured at the comparison clinics, and 288 (55.0%) were positive for an influenza virus (P = .066). Of patients presenting to clinics with no history of current influenza vaccination, 203 (51.1%) of 397 were culture positive at the intervention clinics, compared with 250 (55.7%) of 449 cultured at the comparison clinics (difference not significant). For recipients of both vaccines combined, the proportion with positive culture results— 31 (37.3%) positive of 83 cultured—was significantly lower, compared with those obtained from unvaccinated patients cultured at intervention clinics (P = .052) but not at comparison sites, where 38 (50.7%) were positive of 75 cultured (P = .370). Influenza B accounted for only 84 (18.5%) of isolates from unvaccinated subjects at all sites and was recovered from 39 (28.1%) of persons who received TIV. Influenza B virus was not isolated from LAIV recipients.
LAIV protection is more evident when the data for the target population (5–11 years old) are examined (Table 3). The culture rate for LAIV recipients was only 2.5 per 1000 persons, significantly lower than for unvaccinated children at the intervention site and children who received TIV or no vaccine at comparison sites (P< .01). At the intervention site, only 1 positive culture result each was found for 11 LAIV and 6 TIV recipients; the numbers were too small to be compared separately. When combined, the proportion positive (11.8%) for vaccinated children was significantly lower than that (46.5%) for unvaccinated children at the intervention site (P = .023). In contrast, at the comparison sites the proportion of positive culture results for vaccinated children was not different than that for unvaccinated children (P = .323). For the comparison sites, the proportion of Scott and White children given LAIV was only 15.2%, compared with 73.8% for those at the intervention site.
Table 3.
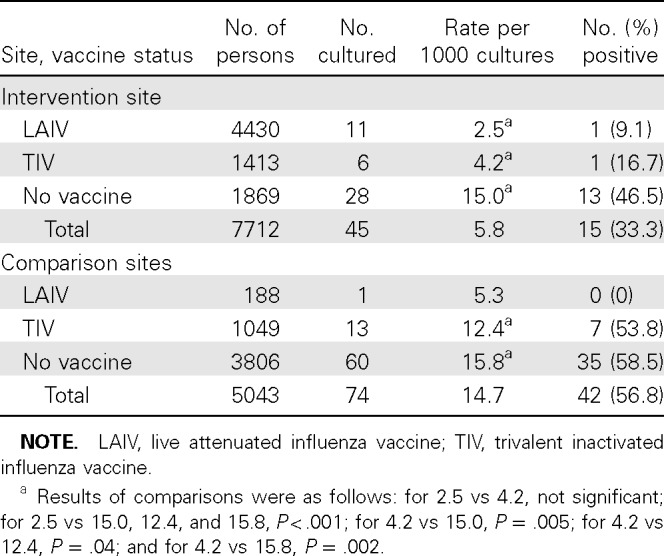
Surveillance of Influenza Virus Infections in a Sample of Age-Eligible Persons, 5–11 Years Old, from Intervention and Comparison Clinics of Scott and White, 2007–2008
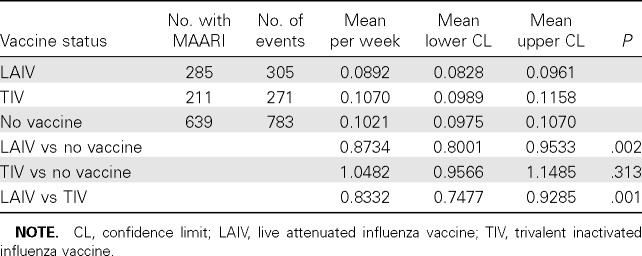
The 3 groups defined by vaccine status and at least 1 MAARI were compared using Poisson regression for the number of visits per week during the epidemic period (Table 4). The subgroup of LAIV recipients had a significantly lower weekly visit rate than the subgroups with no vaccine or TIV recipients. The combined surveillance and MAARI visit data suggest that good protection is provided by a single dose of LAIV and that marginal protection is provided by TIV.
Table 4.
Number of Medically Attended Acute Respiratory Illness (MAARI) Events for Schoolchildren Aged 5–11 Years by Vaccine Status Who Presented for Care at the Intervention Site during the Influenza Epidemic, 23 December 2007 to 15 March 2008, Central Texas Field Trial
Discussion
Significant direct and indirect protection, mainly attributable to the LAIV administered to elementary-school children at the intervention site, was achieved, despite a mismatch between the vaccine strains and the epidemic viruses. Influenza vaccine coverage—mainly TIV—was comparable for Scott and White patients in the intervention and comparison communities except for the 5–11-year-age group, which received most of the LAIV at the intervention site (Figure 1). Only marginal protection was associated with TIV. A single dose of LAIV has been shown to provide better cross-protection than TIV in children exposed to new variants [8–10, 12, 16]. LAIV has several other advantages for children. It is easier to administer and is better accepted. The relative efficacy of LAIV has been shown to be superior to TIV in head-to-head comparisons [17–19]. Furthermore, LAIV provides almost-immediate protection (nonspecific) [12, 13], and the protection (specific) [8, 9, 20, 21] persists through the second season.
Figure 1.
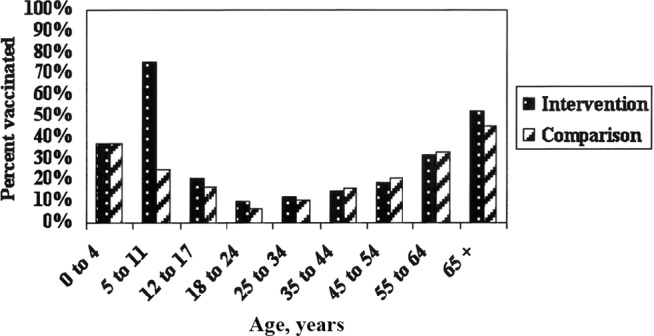
Influenza vaccine coverage for Scott and White clinic patients by age at intervention and comparison sites, Central Texas, 2007–2008.
Indirect protection (herd immunity) is usually more evident in older adults in the community, who generally have a lower risk of exposure to infectious persons [11]. The excess of persons >75 years old in the intervention community would make it more difficult to demonstrate indirect protection. Persons in this age group have serious consequences resulting from influenza virus infection and are less likely to respond to active immunization [22]. Despite the excess of high-risk persons in the intervention community, significant protection was evident for those ⩾65 years old compared with elderly persons at the comparison sites, who had similar TIV immunization rates. Younger persons, such as the 12–17-year-olds in this trial, have many more community contacts and may not have detectable indirect benefits resulting from immunization of 48% of the younger schoolchildren—especially given the circumstances of this trial performed with vaccines containing antigens that did not match those of the prevalent influenza viruses. The absence of influenza vaccine-like viruses in the intervention community is also evidence of protection provided by LAIV and indirect protection of adult populations. Furthermore, influenza B virus was not recovered from LAIV recipients despite the fact that the circulating influenza B virus was of a different lineage, Yamagata, than that in the vaccine (Victoria lineage) [23].
Schoolchildren have the highest influenza attack rate each year and are recognized to be the introducers of influenza into the household and spreaders in the community [6, 7]. LAIV administered to elementary-school children in this trial significantly reduced the number presenting to clinics for culture, the proportion with influenza-positive infections, and the MAARI rates at the intervention site. These observations were made within the structure of an open-label, community-based trial. Therefore, it is important that the concept of indirect (herd) protection was recently reinforced by a randomized controlled trial in small communities of western Canada; vaccine coverage of 83% among children 3–15 years old resulted in a 61% reduction in influenza among unvaccinated subjects in the intervention communities [24].
Reducing the number of susceptible children will limit transmission in the community; however, the risk for serious consequences of influenza virus infection for schoolchildren is generally overlooked. Many laboratory-confirmed deaths are reported annually in healthy schoolchildren [25, 26]. The numbers due to infection with the novel H1N1 virus are alarmingly high. Therefore, vaccine coverage for schoolchildren will provide direct benefit by preventing serious morbidity as well as community benefit if sufficient numbers of children can be vaccinated each year. To maximize effectiveness, it is important to immunize schoolchildren early during the school year. In 2009, the school-based Central Texas Field Trial immunized 13,107 children 4–18 years old (55% of the school population) with seasonal influenza vaccines between August 25 and September 22 (M.J.G. and P.A.P., unpublished data). Early immunization is practical for LAIV because it is effective with a single dose, is easy to administer, and provides almost immediate and long-lasting protection [8, 9, 20, 21].
Immunization of a sizable proportion of schoolchildren will dampen the spread of influenza and provide more time to achieve universal coverage. Universal influenza immunization in Ontario, Canada, is a model for effectiveness of this strategy [27–30]. Reduction of influenza-related mortality and morbidity compared with that in other Canadian provinces has been attributed to the Ontario program.
To facilitate universal immunization in the United States, free vaccine should be delivered to children at school and working adults at the workplace. This, of course, includes health care workers, first responders, and employees of vital community services. Preschool children may continue to receive influenza vaccines at their medical homes or public health clinics. The opportunity to accomplish this will benefit from technological advances in vaccine production. Cell-based substrates for vaccine production should become available soon, allowing a reduction in the time needed to produce vaccine. Cell-based vaccines may also provide broader protection against new variants of influenza virus not included in the vaccine, as occurred in 2007–2008 [31]. The new recommendation for universal immunization should improve influenza control by simplifying the recommendations and stabilize the vaccine supply by increasing demand.
Table 1.
Risk Ratios (RRs) for Medically Attended Acute Respiratory Illness (MAARI) Rates for Scott and White Health Plan Members at Intervention versus Comparison Sites for Prevaccination, Vaccination, Epidemic, and Postepidemic Periods, Central Texas Field Trial, 2007–2008
Acknowledgments
This report is dedicated to the memory of Nadeen Zimmerman, who faithfully extracted Scott and White Health Plan data for the program for 10 years. We are grateful for the contributions of Gayla Herschler, clinical research coordinator, and the Scott and White clinical research team (Charles Fewlass, Scott and White data analyst; Lindsay Newman, school coordinator; Ricky O'Bannon, data acquisition; and Dr Robert L. Fader, director of laboratories at Scott and White Memorial Hospital); the Bell County Health Department under Dr Wayne Farrell (health director) and public health nurses Bonnie Scurzie, Tina Gibson, and Kathy Carlisle, who provided support; and Dr Alexander Klimov (Centers for Disease Control and Prevention), who provided the classification of the sample of influenza surveillance viruses. We also thank Dr Linda Lambert and Sonnie Kim (program officer, National Institute for Allergy and Infectious Diseases), Dr Frank Malinoski (MedImmune), and Dr Ted Tsai (Novartis) for their support. We were privileged to have the full cooperation of the Temple, Belton, Academy, Holland, Rogers, Salado, and Troy Independent School Districts, as well as the parents and children of the districts.
Footnotes
Potential conflicts of interest: The authors acknowledge the investigator-initiated grant from MedImmune for the live attenuated influenza vaccine and funds for surveillance and analysis. (The vaccine-delivery phase was supported by the NIH grant.) Novartis provided the inactivated vaccine. The authors had no other commercial associations that posed a conflict of interest.
Presented in part: Fourth Meeting on Influenza Vaccines That Induce Broad-Spectrum and Long-Lasting Immune Responses, London, United Kingdom, 9–10 November 2009 (Stephenson et al. Vaccine 2010;28:3875–3882).
Financial support: National Institutes of Health (grant UO1-A131050, Control of Epidemic Influenza); MedImmune.
References
- 1.Centers for Disease Control and Prevention Update: novel influenza A(H1N1) virus infection-worldwide, May 6, 2009. MMWR Morb Mortal Wkly Rep. 2009;58:453–458. [PubMed] [Google Scholar]
- 2.Molinari N-AM, Ortega-Sanchez IR, Messonnier ML, et al. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine. 2007;25:5086–5096. doi: 10.1016/j.vaccine.2007.03.046. [DOI] [PubMed] [Google Scholar]
- 3.Thompson WW, Shay DK, Weintraub E, et al. Influenza-associated hospitalizations in the United States. JAMA. 2004;292:1333–1340. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- 4.Jain S, Kamimoto L, Bramley AM, et al. Hospitalized patients with 2009 H1N1 influenza in the United States, April-June 2009. N Engl J Med. 2009;361:1935–1944. doi: 10.1056/NEJMoa0906695. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep. 2010;59(RR-8):1–62. [PubMed] [Google Scholar]
- 6.Glezen WP. Emerging infections: pandemic influenza. Epidemiol Rev. 1996;18:64–76. doi: 10.1093/oxfordjournals.epirev.a017917. [DOI] [PubMed] [Google Scholar]
- 7.Glezen WP. Herd protection against influenza. J Clin Virol. 2006;37:237–243. doi: 10.1016/j.jcv.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 8.Gaglani MJ, Piedra PA, Herschler GB, et al. Direct and total effectiveness of the intranasal, live-attenuated, trivalent cold-adapted influenza virus vaccine against the 2000–2001 influenza A(H1N1) and B epidemic in healthy children. Arch Pediatr Adolesc Med. 2004;158:65–73. doi: 10.1001/archpedi.158.1.65. [DOI] [PubMed] [Google Scholar]
- 9.Halloran ME, Longini IM Jr, Gaglani MJ, et al. Estimating efficacy of trivalent, cold-adapted, influenza virus vaccine (CAIV-T) against influenza A(H1N1) and B using surveillance cultures. Am J Epidemiol. 2003;158:305–311. doi: 10.1093/aje/kwg163. [DOI] [PubMed] [Google Scholar]
- 10.Halloran ME, Piedra PA, Longini IM Jr, et al. Efficacy of trivalent, cold-adapted, influenza virus vaccine against influenza A(Fujian), a drift variant, during 2003–2004. Vaccine. 2007;25:4038–4045. doi: 10.1016/j.vaccine.2007.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piedra PA, Gaglani MJ, Kozinetz CA, et al. Herd immunity in adults against influenza-related illnesses with use of the trivalent-live attenuated influenza vaccine (CAIV-T) in children. Vaccine. 2005;23:1540–1548. doi: 10.1016/j.vaccine.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 12.Piedra PA, Gaglani MJ, Kozinetz CA, et al. Trivalent live attenuated intranasal influenza vaccine administered during the 2003–2004 influenza type A(H3N2) outbreak provided immediate, direct, and indirect protection in children. Pediatrics. 2007;120:553–564. doi: 10.1542/peds.2006-2836. [DOI] [PubMed] [Google Scholar]
- 13.Piedra PA, Gaglani MJ, Riggs M, et al. Live attenuated influenza vaccine, trivalent, is safe in healthy children 18 months to 4 years, 5 to 9 years, and 10 to 18 years of age in a community-based, non-randomized, open-label trial. Pediatrics. 2005;116:397–407. doi: 10.1542/peds.2004-2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaglani MJ, Piedra PA, Riggs M, Herschler G, Fewlass C, Glezen WP. Safety of the intranasal, trivalent, live attenuated influenza vaccine (LAIV) in children with intermittent wheezing in an open-label field trial. Pediatr Infect Dis J. 2008;27:444–452. doi: 10.1097/INF.0b013e3181660c2e. [DOI] [PubMed] [Google Scholar]
- 15.Gaglani MJ, Riggs M, Kamenicky C, Glezen WP. A computerized reminder strategy is effective for annual influenza immunization of children with asthma or reactive airway disease. Pediatr Infect Dis J. 2001;20:1155–1160. doi: 10.1097/00006454-200112000-00012. [DOI] [PubMed] [Google Scholar]
- 16.Belshe RB, Gruber WC, Mendelman PM, et al. Efficacy of vaccination with live attenuated. cold-adapted, trivalent, intranasal influenza virus vaccine against a variant (A/Sydney) not contained in the vaccine. J Pediatr. 2000;136:168–175. doi: 10.1016/s0022-3476(00)70097-7. [DOI] [PubMed] [Google Scholar]
- 17.Belshe RB, Edwards KM, Vesikari T, et al. Live attenuated versus inactivated influenza vaccine in infants and young children. N Engl J Med. 2007;356:685–696. doi: 10.1056/NEJMoa065368. [DOI] [PubMed] [Google Scholar]
- 18.Ashkenazi S, Vertruyen A, Aristegui J, et al. Superior relative efficacy of live attenuated influenza vaccine compared with inactivated influenza vaccine in young children with recurrent respiratory tract infections. Pediatr Infect Dis J. 2006;25:870–879. doi: 10.1097/01.inf.0000237829.66310.85. [DOI] [PubMed] [Google Scholar]
- 19.Fleming DM, Crovari P, Wahn U, et al. Comparison of the efficacy and safety of live attenuated cold-adapted influenza vaccine, trivalent, with trivalent inactivated influenza virus vaccine in children and adolescents with asthma. Pediatr Infect Dis J. 2006;25:860–869. doi: 10.1097/01.inf.0000237797.14283.cf. [DOI] [PubMed] [Google Scholar]
- 20.Piedra PA, Glezen WP. Influenza in children: epidemiology, immunity, and vaccines. Semin Pediatr Infect Dis. 1991;2:140–146. [Google Scholar]
- 21.Ambrose CS, Yi T, Walker RE, Connor EM. Duration of protection provided by live attenuated influenza vaccine in children. Pediatr Infect Dis J. 2008;27(8):744–748. doi: 10.1097/INF.0b013e318174e0f8. [DOI] [PubMed] [Google Scholar]
- 22.Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine. 2006;24:1159–1169. doi: 10.1016/j.vaccine.2005.08.105. [DOI] [PubMed] [Google Scholar]
- 23.Belshe RB, Coelingh K, Ambrose CS, Woo JC, Wu X. 23 . Efficacy of live attenuated influenza vaccine in children against influenza B viruses by lineage and antigenic similarity. Vaccine. 2010;28:2149–2156. doi: 10.1016/j.vaccine.2009.11.068. [DOI] [PubMed] [Google Scholar]
- 24.Loeb M, Russell ML, Moss L, et al. Effect of influenza vaccination of children on infection rates in Hutterite communities. a randomized trial. JAMA. 2010;303:943–950. doi: 10.1001/jama.2010.250. [DOI] [PubMed] [Google Scholar]
- 25.Bhat N, Wright JG, Broder KR, et al. Influenza-associated deaths among children in the United States, 2003–2004. N Engl J Med. 2005;353:2559–2567. doi: 10.1056/NEJMoa051721. [DOI] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention Surveillance for pediatric deaths associated with 2009 pandemic influenza A(H1N1) virus infection-United States, April-August 2009. MMWR Morb Mortal Wkly Rep. 2009;58(34):941–947. [PubMed] [Google Scholar]
- 27.Glezen WP. Benefits of a universal influenza immunization program: more than the reduction in the use of antibiotics. Clin Infect Dis. 2009;49:757–758. doi: 10.1086/605088. [DOI] [PubMed] [Google Scholar]
- 28.Kwong JC, Rosella LC, Johansen H. Trends in influenza vaccination in Canada, 1996/1997 to 2005. Health Rep. 2007;18:9–19. [PubMed] [Google Scholar]
- 29.Kwong JC, Stukel TA, Lim J, et al. The effect of universal immunization on mortality and health care use. PLoS Med. 2008;5:211. doi: 10.1371/journal.pmed.0050211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kwong JC, Maaten S, Upsur REG, Patrick DM, Marra F. The effect of universal immunization on antibiotic prescriptions: an ecological study. Clin Infect Dis. 2009;49:750–756. doi: 10.1086/605087. [DOI] [PubMed] [Google Scholar]
- 31.Minor PD. Vaccines against seasonal and pandemic influenza and the implications of changes in substrates for virus production. Clin Infect Dis. 2010;50:560–565. doi: 10.1086/650171. [DOI] [PubMed] [Google Scholar]