Abstract
Background
Previous studies have demonstrated that the urinary excretion of angiotensinogen is significantly increased in ANG II-infused hypertensive rats, which is associated with an augmentation of intrarenal ANG II levels. These findings suggest that urinary angiotensinogen excretion rates provide an index of intrarenal ANG II levels in ANG II-dependent hypertensive states. However, little information is available regarding the urinary excretion of angiotensinogen in ANG II-dependent malignant hypertension.
Methods
The present study was performed to determine if urinary angiotensinogen excretion is increased in Cyp1a1-Ren2 transgenic rats [strain name: TGR(Cyp1aRen2)] with inducible ANG II-dependent malignant hypertension. Adult male Cyp1a1-Ren2 rats (n=6) were fed a normal diet containing 0.3% indole-3-carbinol (I3C) for 10 days to induce ANG II-dependent malignant hypertension.
Results
Rats induced with I3C exhibited pronounced increases in systolic blood pressure (SBP) (208±7 vs. 127±3 mmHg, P<0.001), marked proteinuria (29.4±3.6 vs. 5.9±0.3 mg/day, P<0.001), and augmented urinary angiotensinogen excretion (996±186 vs. 241±31 ng/day, P<0.01). Chronic administration of the AT1 receptor antagonist, candesartan (25 mg/L in drinking water, n=6), prevented the I3C-induced increases in SBP (125±5, P<0.001), proteinuria (7.3±1.0 mg/day, p<0.001) and urinary angiotensinogen excretion (488±51 ng/day, P<0.01).
Conclusions
These data demonstrate that the urinary excretion of angiotensinogen is markedly augmented in ANG II-dependent malignant hypertension. Such increased urinary angiotensinogen excretion may contribute to augmented intrarenal ANG II levels and, thereby, to the increased blood pressure in Cyp1a1-Ren2 transgenic rats with inducible ANG II-dependent malignant hypertension.
Keywords: kidney, renin-angiotensin system, malignant hypertension, proteinuria, blood pressure, angiotensinogen
Introduction
Previous studies have demonstrated that kidney ANG II contents of 2K1C Goldblatt hypertensive rats, ANG II-infused hypertensive rats, TGR(mRen2)27 transgenic rats, and hypertensive Cyp1a1-Ren2 transgenic rats are markedly higher than can be explained on the basis of circulating ANG II concentrations even though the kidneys are exposed to markedly elevated arterial blood pressures.1–5 This augmentation of total kidney ANG II content may occur secondary to AT1 receptor-mediated uptake of circulating ANG II and/or AT1 receptor-mediated stimulation of intrarenal angiotensinogen and ANG II generation.6–8 Indeed, it has been demonstrated that ANG II-infusion in normal rats results in paradoxical increases in renal expression of angiotensinogen mRNA and protein.8–10 In addition, the urinary excretion of angiotensinogen is significantly increased in ANG II-infused hypertensive rats, which is associated with an augmentation of intrarenal ANG II levels.8,11,12 These findings suggest that urinary angiotensinogen excretion rates provide an index of intrarenal ANG II levels in ANG II-dependent hypertensive states. However, little information is available regarding the urinary excretion of angiotensinogen in ANG II-dependent malignant hypertension.
Malignant hypertension is a severe form of hypertension characterized by rapidly increasing blood pressure, pressure diuresis and natriuresis, severe renal vasoconstriction and ischemia, activation of the renin-angiotensin system, microangiopathy, hemolytic anemia, and development of retinopathy.5,13–16 The vascular lesions of malignant hypertension in the kidney consist of myointimal proliferation and fibrinoid necrosis.13–16 A transgenic rat line [strain name TGR(Cyp1a1Ren2)] was created that allows the induction of various degrees of ANG II-dependent hypertension.16 This transgenic rat line was generated by inserting the mouse Ren2 renin gene, fused to an 11.5-kb fragment of the cytochrome P-450 1a1 (Cyp1a1) promoter, into the genome of the Fischer 344 rat.16 Cyp1a1, which catalyzes the oxidation of a wide range of endogenous lipophilic compounds and xenobiotics17–19, is not constitutively expressed. However, Cyp1a1 is highly inducible on exposure to various aryl hydrocarbons such as indole-3-carbinol (I3C).17–23 Induction of Cyp1a1 is mediated by the aryl hydrocarbon receptor, which is a basic helix-loop-helix transcription factor that binds to specific DNA elements in the Cyp1a1 promoter.17,19,24 Rats transgenic for the Cyp1a1-Ren2 construct do not constitutively express the Ren2 renin gene. The Ren2 gene is expressed, primarily in the liver, only after induction of the Cyp1a1 promoter by aryl hydrocarbons such as I3C.16 Essentially, induction of the Cyp1a1 promoter by I3C is used to drive hepatic expression of the Ren2 renin gene. In this transgenic rat model, induction of the Cyp1a1 promoter by dietary administration of I3C results in a fixed level of expression of the Ren2 renin gene and in the development of ANG II-dependent hypertension.5,16,25 At a dose of 0.3% (wt/wt), dietary I3C induces malignant hypertension, characterized by loss of body weight, polyuria, polydipsia, lethargy, and piloerection. Therefore, this model allows the induction of ANG II-dependent malignant hypertension using a benign and naturally occurring dietary supplement without the need for surgical intervention, dietary salt manipulation, or the administration of steroids.
The present study was performed to determine if urinary angiotensinogen excretion is increased in Cyp1a1-Ren2 transgenic rats with inducible ANG II-dependent malignant hypertension. In light of the evidence that increased urinary angiotensinogen excretion in ANG II-dependent hypertensive states occurs in part as a consequence of AT1 receptor mediated stimulation of proximal tubular angiotensinogen production8, an additional objective was to determine the effects of chronic AT1 receptor blockade on the urinary excretion of angiotensinogen in Cyp1a1-Ren2 rats with malignant hypertension.
Methods
The experimental procedures in this study conform to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of Tulane University Health Sciences Center. Experiments were performed on adult male transgenic rats [TGR(Cyp1a1Ren2)] with inducible expression of the mouse Ren2 renin gene.16 All transgenic rats used in the present study were bred at Tulane University School of Medicine from stock animals supplied by Harlan UK Limited, Bicester, UK. The experimental animals were divided into three groups. Group 1 (Non-induced; n=6) Cyp1a1-Ren2 rats were maintained on a normal rat diet (diet TD 99414, Harlan-Teklad, Madison, WI). Group 2 (0.3% I3C; n=6) Cyp1a1-Ren2 rats were fed a normal diet containing a I3C at a dose of 0.3% (wt/wt; diet TD 05381, Harlan-Teklad) for 10 days to induce ANG II-dependent malignant hypertension, as described previously.5,26–29 Group 3 (0.3% I3C+Cand; n=6) rats were fed 0.3% I3C and treated chronically with the AT1 receptor blocker candesartan (AstraZeneca UK Ltd., Macclesfield, Cheshire, UK) for 10 days. Candesartan was added to the drinking water at a concentration of 25 mg/L. We have previously demonstrated that this dose of candesartan prevents the development of malignant hypertension in Cyp1a1-Ren2 transgenic rats.5
Measurement of systolic blood pressure was obtained in conscious rats using computerized tail-cuff plethysmography (Model 6R22931, IITC Instruments; Woodland Hills, CA). All rats were trained for two weeks prior to the beginning of the experiment in order to habituate them to this procedure. Blood pressures were measured every day throughout the duration of the study. Body weight was similarly measured every day throughout the course of the study. Rats were placed in metabolic cages and twenty-four hour urine collections were obtained on days −6, −3, 2, 4, and 10 relative to initiating dietary I3C administration for determination of 24-hr urinary angiotensinogen and protein excretion. Urine samples were centrifuged and the supernatant separated and stored at −20°C until assayed for protein or stored at −80°C until assayed for angiotensinogen concentrations. Urine volume was determined gravimetrically. Urine protein concentrations were measured by a colorimetric assay using a commercially available kit (Bio-Rad). Urinary angiotensinogen concentrations were determined using a rodent angiotensinogen sandwich ELISA as described previously.30
Statistical analyses were performed using one-way repeated measures ANOVA followed by Student -Newman-Keuls test for within group analyses, and one-way and two-way repeated measures ANOVA followed by Student-Newman-Keuls test for between group analyses. All statistical analyses were performed using SigmaPlot for Windows (version 11, Systat Software Inc., San Jose, CA). Statistical significance was defined as P < 0.05. All data are expressed as mean ± SE.
Results
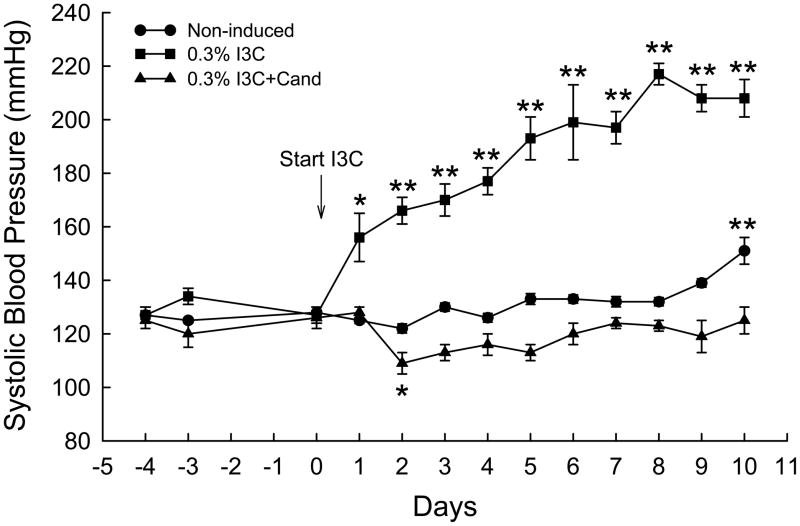
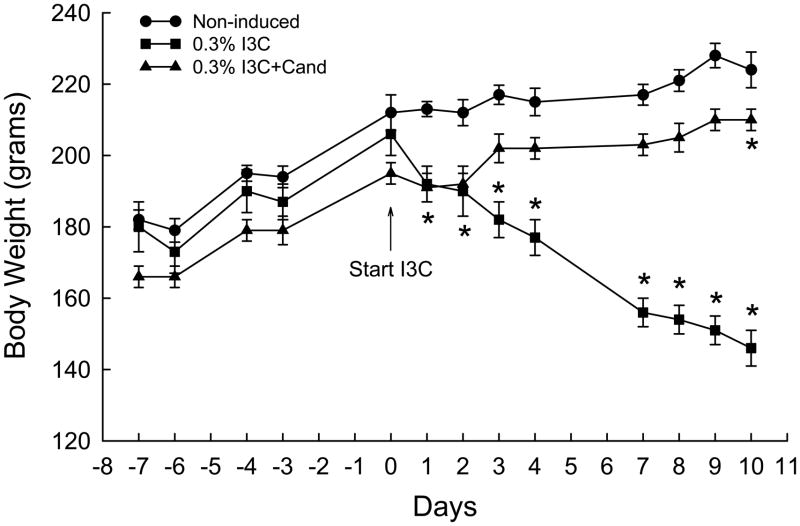
The effects of dietary administration of I3C and I3C+Cand on conscious systolic blood pressure of Cyp1a1-Ren2 transgenic rats are summarized in Fig. 1. Chronic administration of 0.3% I3C for 10 days resulted in the development of severe hypertension, with blood pressure increasing from 127±3 to 208±7 mmHg (P<0.001) over the 10 day period of I3C induction. As shown in Fig. 1, chronic administration of candesartan completely prevented the I3C-induced increase in SBP (126±4 vs. 125±5 mmHg) confirming our previous observations that the I3C-induced increase in SBP is mediated via activation of AT1 receptors by ANG II generated as a consequence of expression of the Cyp1a1-Ren2 transgene. SBP in the non-induced control rats similarly remained unaltered from day 0 to day 8, but increased by day 10 (151±5 vs. 128±2 mmHg, P<0.001). As shown in Fig. 2, the development of hypertension was associated with a pronounced reduction in body weight from 206±6 to 146±5 g (P<0.001). In addition, the hypertensive rats demonstrated severe lethargy, assumption of a hunched posture, and piloerection, which are manifestations of malignant hypertension in the rat.5,13–16,26–29 In contrast, the rats induced with I3C+Cand exhibited a small but significant increase in body weight over the 10 day induction period (195±3 to 210±3 g, P<0.001) (Fig. 2). Body weight in the non-induced rats remained unaltered (212±5 vs. 224±11 g) over the 10 day measurement period.
FIG. 1.
Conscious systolic blood pressures of non-induced Cyp1a1-Ren2 rats (Non-induced); Cyp1a1-Ren2 rats fed a normal-salt diet containing 0.3% I3C for 10 days (0.3% I3C); and Cyp1a1-Ren2 rats induced for 10 days with 0.3% I3C and treated with the AT1 receptor antagonist, candesartan (Cand; 25 mg/L in drinking water) (0.3% I3C+Cand). *P<0.05, **P<0.001 vs. day 0.
Fig. 2.
Body weights of non-induced Cyp1a1-Ren2 rats (Non-induced); Cyp1a1-Ren2 rats fed a normal-salt diet containing 0.3% I3C for 10 days (0.3% I3C); and Cyp1a1-Ren2 rats induced for 10 days with 0.3% I3C and treated with the AT1 receptor antagonist, candesartan (Cand; 25 mg/L in drinking water) (0.3% I3C+Cand). *P<0.001 vs. day 0.
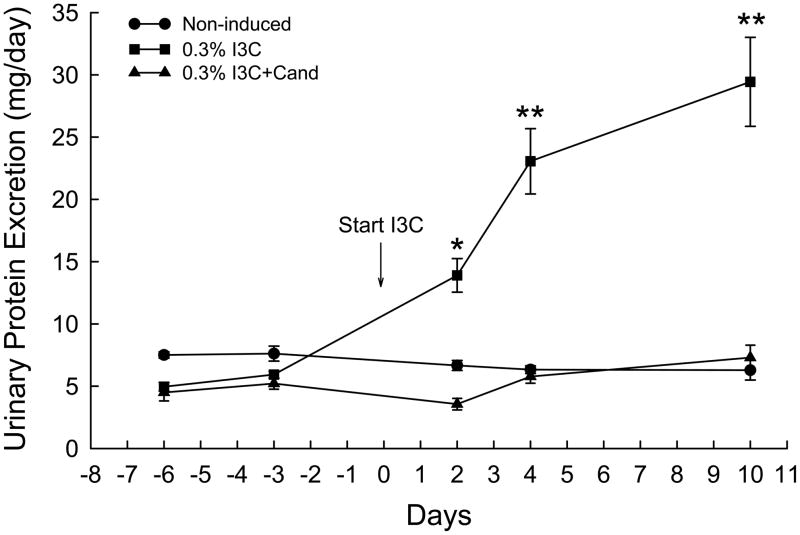
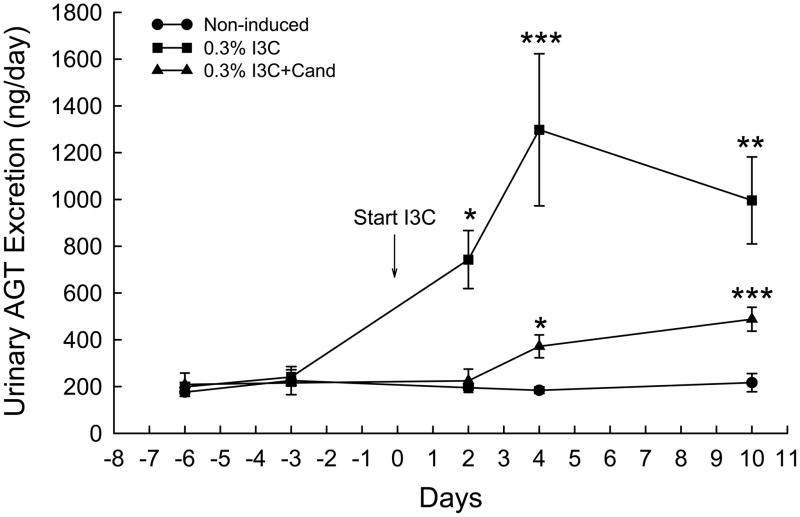
The effects of I3C and I3C+Cand on urinary protein excretion in Cyp1a1-Ren2 rats are shown in Fig. 3. Dietary administration of I3C increased protein excretion from 5.9±0.3 to 29.4±3.6 mg/day (P<0.001) over the 10 days of I3C administration. As shown in Fig. 3, chronic administration of candesartan prevented the I3C-induced increase in urinary protein excretion (5.2±0.5 vs. 7.3±1.0 mg/day). Protein excretion in the non-induced rats remained unaltered (7.6±0.6 vs. 6.3±0.8 mg/day) throughout the 10 day measurement period. Fig. 4. shows the effects of I3C and I3C+Cand on urinary angiotensinogen excretion in Cyp1a1-Ren2 rats. Urinary angiotensinogen excretion was significantly elevated on day two of I3C induction (743±124 vs. 241±31 ng/day, P<0.05) and increased further to 1298±325 ng/day (P<0.001) by day 4. Urinary angiotensinogen remained substantially elevated on day 10 (996±186 ng/day, P<0.01). As shown in Fig. 4, chronic administration of candesartan markedly attenuated the I3C-induced increase in urinary angiotensinogen excretion with urinary angiotensinogen excretion increasing from 216±14 to 488±51 ng/day (P<0.001) in the rats induced with I3C and treated chronically with candesartan. Urinary angiotensinogen excretion was markedly lower in rats induced with I3C and treated with candesartan at all time points (P<0.001) compared with corresponding values in rats induced with I3C alone (Fig. 4.). Urinary angiotensinogen excretion in the non-induced control rats remained unaltered (225±60 vs. 217±39 ng/day) throughout the course of the experiment.
FIG. 3.
Effects of I3C, and I3C+Cand on urinary protein excretion in Cyp1a1-Ren2 transgenic rats. *P<0.01, **P<0.001 vs. day -3.
FIG. 4.
Effects of I3C, and I3C+Cand on urinary angiotensinogen excretion in Cyp1a1-Ren2 transgenic rats. *P<0.05, **P<0.01, ***P<0.001 vs. day -3.
Discussion
The present study was performed to determine if urinary angiotensinogen excretion is increased in Cyp1a1-Ren2 transgenic rats with inducible ANG II-dependent malignant hypertension. Malignant hypertension is a form of severe hypertension characterized by fibrinoid necrosis of arterioles and vascular damage in many tissues, including the kidney.13–16,28 The Cyp1a1-Ren2 transgenic line allows the induction of ANG II-dependent malignant hypertension.5,16,25–29 This transgenic line was generated by inserting a mouse Ren2 renin gene into the genome of the Fischer 344 rat.16 Extrarenal Ren2 renin gene expression is induced by the administration of the aryl hydrocarbon I3C, resulting in the development of ANG II-dependent malignant hypertension.5,16,25–29 Such induction of Ren2 renin gene expression using a benign and naturally occurring dietary supplement leads to the development of ANG II-dependent hypertension as a result of increased renin gene expression and plasma renin levels, which are not subject to normal physiological feedback control mechanisms. In the present study, induction of the Ren2 renin gene by dietary administration of 0.3% I3C for 10 days resulted in the development of severe hypertension. As described previously5,16,26–29, the hypertension was associated with a marked decrease in body weight, and the rats exhibited extreme lethargy, assumption of a hunched posture, and piloerection, which are clinical manifestations of malignant hypertension in the rat.5,13–16 The development of malignant hypertension was associated with pronounced increases in the urinary excretion of angiotensinogen.
The development of malignant hypertension in Cyp1a1-Ren2 rats was also associated with increased urinary protein excretion. Thus, it is possible that the increased urinary angiotensinogen excretion occurred as a consequence of the nonspecific effects of the hypertension or of the enhanced urinary excretion of protein. In essence, it is possible that glomerular filtration of angiotensinogen into the tubular fluid, driven by high intraglomerular pressure and glomerular damage, contributed to the observed increase in urinary angiotensinogen excretion. However, previous studies performed in DOCA-salt hypertensive rats demonstrated urinary protein excretion in DOCA-salt hypertensive rats was significantly higher than in ANG II-infused hypertensive rats.12 In contrast, urinary excretion of angiotensinogen was significantly lower in DOCA-salt hypertensive rats than in ANG II-infused hypertensive rats indicating dissociation between urinary protein excretion and the urinary angiotensinogen excretion rate.12 However, we have demonstrated that renal cortical angiotensinogen mRNA expression in Cyp1a1-Ren2 transgenic rats with malignant hypertension is not different from that observed in normotensive control rats.31 These findings suggest that increased glomerular filtration of angiotensinogen contributed substantially to the observed increase in urinary angiotensinogen excretion in Cyp1a1-Ren2 rats with malignant hypertension. Nevertheless, one cannot rule out the possibility that ANG II-induced proximal tubular secretion of angiotensinogen contributed, in part, to the observed increase in urinary angiotensinogen excretion in Cyp1a1-Ren2 rats with malignant hypertension. Further studies are required to address this issue.
The observation that urinary protein excretion, a parameter of renal injury, was markedly increased in the hypertensive rats is consistent with our previous observations that the kidneys of Cyp1a1-Ren2 rats with malignant hypertension exhibit glomerulosclerosis, myointimal hyperplasia and tubular dilation, and tubulointerstitial inflammation and proliferation, particularly in the perivascular areas.28 Collectively, these findings indicate that Cyp1a1-Ren2 rats with malignant hypertension exhibit pronounced renal injury. In the present study, chronic blockade of AT1 receptors with candesartan completely prevented the I3C-induced increase in proteinuria indicating that AT1 receptor activation by ANG II is responsible for the development of proteinuria and the associated renal injury in Cyp1a1-Ren2 rats with malignant hypertension. However, the present data do not allow determination of the degree to which the prevention of the proteinuria by candesartan occurred as a consequence of blockade of the direct blood pressure-independent renal actions of AT1 receptor activation by ANG II or was due to the associated prevention of the hypertension. Additional studies are required to address this issue.
It has been demonstrated that kidney ANG II contents of 2K1C Goldblatt hypertensive rats, ANG II-infused hypertensive rats, TGR(mRen2)27 transgenic rats, and hypertensive Cyp1a1-Ren2 transgenic rats are substantially higher than the prevailing circulating ANG II concentrations even though the kidneys are exposed to markedly elevated arterial blood pressures.1–5 In the present study, the rats with malignant hypertension exhibited substantially elevated urinary angiotensinogen excretion rates compared with non-induced controls. This observation is consistent with previous observations that the urinary excretion of angiotensinogen is significantly increased in ANG II-infused hypertensive rats, which is associated with an augmentation of intrarenal ANG II levels.8,11,12 The present finding that urinary angiotensinogen excretion is increased in Cyp1a1-Ren2 rats with malignant hypertension together with our previous observations that the pathogenesis of ANG II-dependent malignant hypertension in Cyp1a1-Ren2 transgenic rats involves augmentation of plasma and total kidney ANG II levels5,27 indicates that Cyp1a1-Ren2 rats with malignant hypertension exhibit a paradoxical increase in the activity of the intrarenal renin-angiotensin system. Chronic blockade of AT1 receptors with candesartan markedly attenuated the I3C-mediated augmentation of urinary angiotensinogen excretion indicating that the elevated urinary angiotensinogen excretion in the rats with malignant hypertension was dependent on activation of AT1 receptors. In this regard, it is possible that the augmentation of urinary angiotensinogen excretion in the rats with malignant hypertension may have occurred, in part, to AT1 receptor-mediated stimulation of angiotensinogen secretion by the proximal tubules, such as occurs in ANG II-infused hypertensive rats.8 It is also possible that other mechanisms such as oxidative stress may have contributed to the increased urinary angiotensinogen excretion in the hypertensive Cyp1a1-Ren2 rats. Indeed, we have previously demonstrated that Cyp1a1-Ren2 transgenic rats with malignant hypertension exhibit elevated basal rates of urinary excretion of 8-isoprostane indicating oxidative stress in this model of ANG II-dependent malignant hypertension.29 In addition, administration of the AT1 receptor antagonist, candesartan, significantly decreased the urinary excretion of 8-isoprostane, indicating that ANG II stimulates the production of superoxide anion in Cyp1a1-Ren2 rats with malignant hypertension.29 Such ANG II-mediated increase in superoxide anion levels may contribute to the increase in urinary angiotensinogen excretion in hypertensive Cyp1a1-Ren2 rats.
The increased urinary angiotensinogen excretion indicates that greater amounts of angiotensinogen, either filtered at the glomerulus or secreted into the tubular fluid by the proximal tubule cells, traverse the distal nephron segments and may result in increased generation of ANG II in distal tubular fluid as well as proximal tubular fluid.8 In this regard, we have recently demonstrated that renin immunoreactivity in the JG cells is not suppressed and that collecting duct renin immunoreactivity is markedly increased in kidneys of Cyp1a1-Ren2 transgenic rats with malignant hypertension.32 Similarly, kidney cortex renin content in hypertensive Cyp1a1-Ren2 rats is significantly elevated compared with normotensive control rats.31 These recent findings are in contrast to the previous observation that rat renin and mRen2 renin expression are absent in the kidneys of Cyp1a1-Ren2 rats with malignant hypertension.16 The reason for this apparent discrepancy is not clear; however, it has been demonstrated that collecting duct renin is enhanced in the non-clipped kidneys of 2K1C Goldblatt hypertensive rats and in the kidneys of ANG II infused hypertensive rats despite the fact that these kidneys are exposed to markedly elevated arterial blood pressures.33 The mechanisms responsible for the increased collecting duct renin in ANG II-dependent hypertensive states remain unclear. However, it is possible that a local amplification mechanism exists whereby intrarenal ANG II stimulates collecting duct renin synthesis and secretion into the cortical and medullary collecting duct fluid. Such increased cortical and medullary collecting duct renin levels together with elevated intratubular angiotensinogen levels may result in increased intratubular ANG II generation and, thereby, allow moderate increases in intrarenal ANG II levels to further augment intratubular ANG II levels.33 Regardless of the specific mechanisms involved, our previous observation that renin immunoreactivity in the JG cells is not suppressed and that collecting duct renin immunoreactivity is markedly increased in kidneys of hypertensive Cyp1a1-Ren2 transgenic rats indicates that the kidneys of Cyp1a1-Ren2 rats with malignant hypertension are not renin-depleted. The maintained JG cell and augmented collecting duct renin together with the enhanced tubular fluid angiotensinogen levels may contribute to augmented intratubular ANG II levels. Such increased tubular fluid ANG II generation may account, at least in part, for the elevated intrarenal ANG II levels observed in Cyp1a1-Ren2 transgenic rats with ANG II-dependent malignant hypertension.5 It is also possible that, in addition to increased intratubular generation of ANG II, the elevated intrarenal ANG II levels observed in hypertensive Cyp1a1-Ren2 rats occurred, in part, as a consequence of uptake of the peptide via AT1 receptor sequestration. Thus, increased kidney ANG II content may result from the combination of uptake of circulating ANG II and local formation of ANG II from enhanced intraluminal angiotensinogen levels. Whatever the mechanism, to the extent that AT1 receptors are located on both the luminal and basolateral membranes of the proximal tubule as well as in the distal nephron segments and collecting ducts34 it is likely that increases in intrarenal ANG II levels would act to increase proximal and distal nephron sodium chloride and fluid reabsorption rate. Such renal tubular actions of the inappropriately elevated intrarenal ANG II levels would contribute to an impaired ability of the kidney to maintain normal rates of sodium excretion except at hypertensive arterial pressures, and impair the pressure natriuretic response to the ANG II-mediated increases in arterial blood pressure.5,35 In this manner, by maintaining an inappropriately high reabsorptive status and an impaired pressure natriuresis relation, the inappropriately augmented intrarenal ANG II content would contribute to the pathogenesis of malignant hypertension in Cyp1a1-Ren2 transgenic rats.
In summary, the present findings confirm that AT1 receptor activation contributes to the increase in SBP and proteinuria in Cyp1a1-Ren2 transgenic rats with ANG II-dependent malignant hypertension. These data also demonstrate that the urinary excretion of angiotensinogen is markedly augmented in ANG II-dependent malignant hypertension. Such increased urinary angiotensinogen excretion likely reflects an AT1 receptor-mediated augmentation of intraluminal angiotensinogen levels which may contribute to augmented intrarenal ANG II levels and, thereby, to the increased blood pressure in Cyp1a1-Ren2 transgenic rats with inducible ANG II-dependent malignant hypertension.
Acknowledgments
This study was supported by Tulane COBRE in Hypertension and Renal Biology (NCRR 2P20RR017659-06), NIDDK grant DK072408, and NHLBI grant HL26371.
The authors would like to thank Porcha D. Davis, Dale M. Seth, Weijian Shao, and Jessica L. Mucci for excellent technical assistance. We also thank Dr. Barb Mickelson, Harlan-Teklad, for help with the design and production of the I3C-containing rat diet. This study was supported by The Tulane COBRE in Hypertension and Renal Biology (NCRR 2P20RR017659-06), NIDDK grant DK072408, and NHLBI grant HL26371.
References
- 1.Guan S, Fox J, Mitchell KD, et al. Angiotensin and angiotensin converting enzyme tissue levels in two-kidney, one clip hypertensive rats. Hypertension. 1992;20:763–767. doi: 10.1161/01.hyp.20.6.763. [DOI] [PubMed] [Google Scholar]
- 2.Von Thun AM, Vari RC, El-Dahr SS, et al. Augmentation of intrarenal angiotensin II levels by chronic angiotensin II infusion. Am J Physiol Renal Fluid Electrolyte Physiol. 1994;266:F120–F128. doi: 10.1152/ajprenal.1994.266.1.F120. [DOI] [PubMed] [Google Scholar]
- 3.Zou L, Hymel A, Imig JD, et al. Renal accumulation of circulating angiotensin II in angiotensin II-infused rats. Hypertension. 1996;27:658–662. doi: 10.1161/01.hyp.27.3.658. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell KD, Jacinto SM, Mullins JJ. Proximal tubular fluid, kidney, and plasma levels of angiotensin II in hypertensive ren-2 transgenic rats. Am J Physiol Renal Physiol. 1997;273:F246–253. doi: 10.1152/ajprenal.1997.273.2.F246. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell KD, Bagatell SJ, Miller CS, et al. Genetic clamping of renin gene expression induces hypertension and elevation of intrarenal Ang II levels of graded severity in Cyp1a1-Ren2 transgenic rats. Journal of the Renin Angiotensin Aldosterone System. 2006;7(2):74–86. doi: 10.3317/jraas.2006.013. [DOI] [PubMed] [Google Scholar]
- 6.Zou L, Imig JD, Von Thun AM, et al. Receptor-mediated intrarenal ANG II augmentation in ANG II-infused hypertensive rats. Hypertension. 1996;28:669–677. doi: 10.1161/01.hyp.28.4.669. [DOI] [PubMed] [Google Scholar]
- 7.Zou L, Imig JD, Hymel A, et al. Renal uptake of circulating angiotensin II in Val5- angiotensin II infused rats is mediated by AT1 receptor. Am J Hypertens. 1998;11:570–578. doi: 10.1016/s0895-7061(97)00410-x. [DOI] [PubMed] [Google Scholar]
- 8.Kobori H, Prieto-Carrasquero MC, Ozawa Y, et al. AT1 receptor mediated augmentation of intrarenal angiotensinogen in angiotensin II-dependent hypertension. Hypertension. 2004;43:1126–1132. doi: 10.1161/01.HYP.0000122875.91100.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kobori H, Harrison-Bernard LM, Navar LG. Expression of angiotensinogen mRNA and protein in angiotensin II-dependent hypertension. J Am Soc Nephrol. 2001;12:431–439. doi: 10.1681/asn.v123431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kobori H, Harrison-Bernard LM, Navar LG. Enhancement of angiotensinogen expression in angiotensin II-dependent hypertension. Hypertension. 2001;37:1329–1335. doi: 10.1161/01.hyp.37.5.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobori H, Harrison-Bernard LM, Navar LG. Urinary excretion of angiotensinogen reflects intrarenal angiotensinogen production. Kidney Int. 2002;61:579–585. doi: 10.1046/j.1523-1755.2002.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kobori H, Nishiyama A, Harrison-Bernard LM, et al. Urinary angiotensinogen as an indicator of intrarenal angiotensin status in hypertension. Hypertension. 2003;41:42–49. doi: 10.1161/01.hyp.0000050102.90932.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whitworth CE, Fleming S, Cumming AD, et al. Spontaneous development of malignant phase hypertension in transgenic ren-2 rats. Kidney Int. 1994;46:1528–1532. doi: 10.1038/ki.1994.437. [DOI] [PubMed] [Google Scholar]
- 14.Whitworth CE, Fleming S, Kotelevtsev Y, et al. A genetic model of malignant phase hypertension in rats. Kidney Int. 1995;47:529–535. doi: 10.1038/ki.1995.66. [DOI] [PubMed] [Google Scholar]
- 15.Kantachuvesiri S, Haley CS, Fleming S, et al. Genetic mapping of modifier loci affecting malignant hypertension in TGRmRen2 rats. Kidney Int. 1999;56:414–420. doi: 10.1046/j.1523-1755.1999.00571.x. [DOI] [PubMed] [Google Scholar]
- 16.Kantachuvesiri S, Fleming S, Peters J, et al. Controlled hypertension, a transgenic toggle switch reveals differential mechanisms underlying vascular disease. J Biol Chem. 2001;276:36727–36733. doi: 10.1074/jbc.M103296200. [DOI] [PubMed] [Google Scholar]
- 17.Campbell SJ, Carlotti F, Hall PA, et al. Regulation of the CYP1A1 promoter in transgenic mice: an exquisitely sensitive on-off system for cell specific gene regulation. J Cell Sci. 1996;109:2619–2625. doi: 10.1242/jcs.109.11.2619. [DOI] [PubMed] [Google Scholar]
- 18.Forrester LM, Henderson CJ, Glancey MJ, et al. Relative expression of cytochrome P450 isoenzymes in human liver and association with the metabolism of drugs and xenobiotics. Biochem J. 1992;281:359–368. doi: 10.1042/bj2810359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith JD, Wong E, Ginsberg M. Cytochrome P450 1A1 promoter as a genetic switch for the regulatable and physiological expression of a plasma protein in transgenic mice. Proc Natl Acad Sci USA. 1995;92:1926–11930. doi: 10.1073/pnas.92.25.11926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gelboin HV. Benzo[alpha]pyrene metabolism, activation and carcinogenesis: role and regulation of mixed-function oxidases and related enzymes. Physiol Rev. 1980;60:1107–1166. doi: 10.1152/physrev.1980.60.4.1107. [DOI] [PubMed] [Google Scholar]
- 21.Jellinck PH, Forkert PG, Riddick DS, et al. Ah receptor binding properties of indole carbinols and induction of hepatic estradiol hydroxylation. Biochem Pharmacol. 1993;45:1129–1136. doi: 10.1016/0006-2952(93)90258-x. [DOI] [PubMed] [Google Scholar]
- 22.Loub WD, Wattenberg LW, Davis DW. Aryl hydrocarbon hydroxylase induction in rat tissues by naturally occurring indoles of cruciferous plants. J Natl Cancer Inst. 1975;54:985–988. [PubMed] [Google Scholar]
- 23.Pelkonen O, Nebert DW. Metabolism of polycyclic aromatic hydrocarbons: etiologic role in carcinogenesis. Pharmacol Rev. 1982;34:189–222. [PubMed] [Google Scholar]
- 24.Fujii-Kuriyama Y, Masatsuga E, Junsei M, et al. Polymorphic forms of the Ah receptor and induction of the CYP1A1 gene. Pharmacogenetics. 1995;5:149–153. doi: 10.1097/00008571-199512001-00018. [DOI] [PubMed] [Google Scholar]
- 25.Vanourkova Z, Kramer HJ, Huskova Z, et al. AT1 receptor blockade is superior to conventional triple therapy in protecting against end-organ damage in Cyp1a1-Ren-2 transgenic rats with inducible hypertension. J Hypertens. 2006;24:2465–2472. doi: 10.1097/01.hjh.0000251909.00923.22. [DOI] [PubMed] [Google Scholar]
- 26.Opay AL, Mouton CR, Mullins JJ, et al. Cyclooxygenase-2 inhibition normalizes arterial blood pressure in CYP1A1-REN2 transgenic rats with inducible ANG-dependent malignant hypertension. Am J Physiol Renal Physiol. 2006;291:F612–F618. doi: 10.1152/ajprenal.00032.2006. [DOI] [PubMed] [Google Scholar]
- 27.Ortiz RM, Graciano ML, Mullins JJ, et al. Aldosterone receptor antagonism alleviates proteinuria, but not malignant hypertension in Cyp1a1-Ren2 transgenic rats. Am J Physiol Renal Physiol. 2007;293:F1584–F1591. doi: 10.1152/ajprenal.00124.2007. [DOI] [PubMed] [Google Scholar]
- 28.Graciano ML, Mouton CR, Patterson ME, et al. Renal vascular and tubulointerstitial inflammation and proliferation in Cyp1a1-Ren2 transgenic rats with inducible ANG II-dependent malignant hypertension. Am J Physiol Renal Physiol. 2007;292:F1858–F1866. doi: 10.1152/ajprenal.00469.2006. [DOI] [PubMed] [Google Scholar]
- 29.Patterson ME, Mullins JJ, Mitchell KD. Interactive effects of superoxide anion and nitric oxide on blood pressure and renal hemodynamics in transgenic rats with inducible malignant hypertension. Am J Physiol Renal Physiol. 2005;289:F754–F759. doi: 10.1152/ajprenal.00419.2004. [DOI] [PubMed] [Google Scholar]
- 30.Kobori H, Katsurada A, Miyata K, et al. Determination of plasma and urinary angiotensinogen levels in rodents by newly developed ELISA. Am J Physiol Renal Physiol. 2008;294:F1257–F1263. doi: 10.1152/ajprenal.00588.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams DE, Kavanagh K, Davis PD, et al. Cyp1a1-Ren2 transgenic rats with ANG II-dependent malignant hypertension exhibit maintained kidney angiotensinogen mRNA expression and elevated prorenin receptor levels. J Am Soc Nephrol. 2009;20:226A. [Google Scholar]
- 32.Prieto MC, Botros FT, Martin VL, et al. Increased collecting duct renin protein expression in Cyp1a1-Ren2 transgenic rats with inducible ANG II-dependent malignant hypertension. FASEB J. 2009;23:1016.3. [Google Scholar]
- 33.Prieto-Carrasquero MC, Botros FT, Kobori H, et al. Collecting duct renin: a major player in angiotensin II-dependent hypertension. J Am Soc Hypertens. 2009;3(2):96–104. doi: 10.1016/j.jash.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harrison-Bernard LM, Navar LG, Ho MM, et al. Immunohistochemical localization of ANG II AT1 receptor in adult rat kidney using a monoclonal antibody. Am J Physiol Renal Physiol. 1997;273:F170–F177. doi: 10.1152/ajprenal.1997.273.1.F170. [DOI] [PubMed] [Google Scholar]
- 35.Mitchell KD, Navar LG. Intrarenal actions of angiotensin II in the pathogenesis of experimental hypertension. In: Laragh JH, Brenner BM, editors. Hypertension: Pathophysiology, Diagnosis, and Maintenance. 2. New York, NY: Raven; 1995. pp. 1437–1450. [Google Scholar]