Abstract
Pulmonary bacterial infections are a leading cause of death. Since the introduction of antibiotics, multidrug-resistant Klebsiella pneumoniae (Kp) became an escalating threat. Therefore, development of methods to augment antibacterial defense is warranted. Neutrophil recruitment is critical to clear bacteria and neutrophil migration in the lung requires the production of ELR+ CXC chemokines. Although lung specific CXCL1/KC transgene expression causes neutrophil-mediated clearance of Kp, the mechanisms underlying KC-mediated host defense against Kp have not been explored. Here we delineated the host defense functions of KC during pulmonary Kp infection using KC-/- mice. Our findings demonstrate that KC is important for expression of CXCL2/MIP-2 and CXCL5/LIX and activation of NF-κB, and MAPKs in the lung. Furthermore, KC-derived from both hematopoietic and resident cells contributes to host defense against Kp. Neutrophil depletion in mice prior to Kp infection reveals no differences in the production of MIP-2 and LIX or activation of NF-κB and MAPKs in the lung. Using murine bone marrow-derived (BMMs) and alveolar macrophages, we confirmed KC-mediated upregulation of MIP-2 and activation of NF-κB and MAPKs upon Kp infection. Moreover, neutralizing KC in BMMs prior to Kp challenge decreases bacteria-induced production of KC, MIP-2 and activation of NF-κB and MAPKs. These findings reveal the importance of KC produced by hematopoietic and resident cells in regulating pulmonary host defense against a bacterial pathogen via the activation of transcription factors and MAPKs as well as the expression of cell adhesion molecules and other neutrophil chemoattractants.
INTRODUCTION
Despite the development of newer generation antibiotics, the morbidity and mortality associated with bacterial pneumonia is still extensive (1-3). In addition, clinical management of patients infected with antibiotic-resistant bacterial strains, including Klebsiella pneumoniae (Kp), has been difficult. Innate host defense mediated through neutrophils is pivotal to controlling bacterial replication in the host. However, neutrophil influx acts as a double-edged sword: an insufficient neutrophil recruitment can lead to life-threatening infection whereas an extreme accumulation of neutrophils can result in excessive lung injury, such as ALI/ARDS (4-6). Therefore, the ideal therapeutic approach to target neutrophils would be to attenuate their destructive potential while maintaining their antibacterial defense function. However, understanding the mechanisms contributing to neutrophil influx is a prerequisite to designing improved treatment and/or prevention strategies to attenuate excessive neutrophil-mediated lung parenchymal damage.
Bacterial lung infection is characterized by excessive capillary leakage, activation of transcription factors, upregulation of cell-adhesion molecules, expression of proinflammatory mediators, and accumulation of neutrophils in the alveolar spaces with diffuse damage of alveolar epithelial and endothelial cells (7-9). The production of chemokines is a critical step leading to the recruitment of neutrophils to organs during microbial insult (7-9). These chemokines are classified into four groups based on the arrangement of a cysteine motif positioned near the N terminus: C, CC, CXC, and CX3C. These groups are further characterized by the position of the ELR motif (glutamic acid-leucine-arginine) prior to the CXC sequence. These ELR+CXC chemokines are powerful neutrophil chemoattractants and seven of this group have been identified in humans: interleukin (IL)-8; neutrophil-activating peptide 2 (NAP-2); growth-related oncogenes (GRO)-α, β, and γ; epithelium-derived neutrophil-activating peptide 78 (ENA-78); and granulocyte chemotactic protein 2 (GCP-2). Of these, IL-8 is the most potent neutrophil chemoattractant in humans. Although a homolog of human IL-8 has not been identified, CXCL1/KC (10, 11), CXCL2/ MIP-2 (12, 13) and CXCL5/LIX (14, 15) have been found to be potent neutrophil chemoattractants in mice.
KC has been shown to be essential in inducing neutrophil recruitment to the lungs because 1) inhibition of KC using a blocking Ab resulted in attenuation of neutrophil migration to the airspaces following E. coli LPS challenge in a rat model (10, 11) ; and 2) transgenic KC mice, which constitutively expressed KC within the lungs, have more neutrophil influx and less bacterial burden in the organs following challenge with Kp (16). However, the signaling cascades associated with neutrophil-mediated bacterial clearance in the lungs have yet to be explored. With the advent of gene deletion technology in mice, it is possible to determine the mechanisms of these chemokines in pulmonary host defense. In this study, we sought to determine the mechanisms underlying KC-mediated pulmonary defense using mice with a targeted disruption in KC. Our results reveal that KC derived from both hematopoietic and resident cells regulate host defense in the lung via expression of other neutrophil chemokines and activation of NF-κB and MAPKs. Moreover, our findings suggest that KC mediates pulmonary host defense in an autocrine and paracrine manner.
MATERIALS AND METHODS
Animals
KC gene-deficient mice (KC-/-) (17) were backcrossed 10 times with C57Bl/6 and therefore, C57Bl/6 mice were used as controls (KC+/+). All animal experiments were conducted using a protocol approved by the Louisiana State University Animal Welfare Committee. Female mice 8- 10-wk-old, ranging from 20 to 25 g in weight, were used in our experiments. Mice were maintained under specific pathogen-free conditions and kept on a 12:12-h light: dark cycle with free access to food and water.
Kp inoculation
We used serotype 2 of K. pneumoniae strain (Kp: ATCC 43816) because this strain induces substantial inflammation in mice at 103 CFU/animal (18). Briefly, the bacteria were grown for 6 h to mid-logarithmic phase at 37°C in tryptic soy broth while shaking at 225 rpm, harvested by centrifugation, and washed twice in sterile isotonic saline. The bacteria were resuspended in saline at a concentration of 103 CFU/50 μl/mouse for in vivo experiments. The mice were anesthetized with ketamine (100 mg/kg) /xylazine (10 mg/kg) mixture and a mid-ventral incision was performed. After isolation of muscles, the trachea was exposed and inoculated with 103 CFU of Kp. A 50 μl aliquot of a serially diluted suspension of the initial inoculums was plated onto a Tryptic Soy Agar (TSA) and a MacConkey plates for validation of CFUs.
Survival studies
Mice were inoculated i.t. with 103 CFU of Kp in 50 μl of 0.9% saline and survival was examined up to 15 days.
Determination of lung and spleen bacterial CFUs
The lungs and spleens of mice were weighed and homogenized in 1 ml of 0.9% saline using a tissue homogenizer. The solid tissue was allowed to sediment for 10 min at room temperature and the supernatants were serially diluted. Twenty μl aliquots of each sample were plated on tryptic soy agar (TSA) and MacConkey agar plates and bacterial colonies were enumerated after incubation at 37°C.
BAL fluid (BALF) collection
At the designated time points, the animals were euthanized and exsanguinated by cardiac puncture. The trachea was cannulated with a 20-gauge catheter and BALF was collected as described earlier (18-21). A total of 3.0 ml BALF was retrieved from each mouse, and 140 μl of BALF was centrifuged and placed on glass cytospin slides, which were then stained by diff-quick reagents (Fisher, Chicago, IL) to enumerate leukocyte subtypes. Two ml of the undiluted BALF was centrifuged, passed via a 0.22-μm filter and used immediately or stored at -80°C for future use.
Myeloperoxidase (MPO) assay
Neutrophil migration to the lung can be assessed by MPO activity in the lungs which was determined as previously described (18-21). Briefly, the lungs were weighed, homogenized, centrifuged, and the pellet was resuspended in 50 mM potassium phosphate buffer, pH 6.0 (supplemented with 0.5% hexadecyltrimethylammonium bromide). Samples were then sonicated, incubated at 60°C for 2 h, and assayed for MPO activity in a hydrogen peroxide/O-dianisidine buffer at 460 nm. The MPO activity was calculated between 0 sec and 90 sec. The lung samples were used for MPO activity within 3 weeks post harvest.
Cytokine and chemokine determination
Cytokine and chemokine levels were measured in BALF, lung homogenates, culture supernatants obtained from bone marrow-derived macrophages (BMMs) and alveolar macrophages (AMs) using a specific sandwich ELISA as described in earlier publications. The minimum detection limit is 8 pg/ml of cytokine protein (18-21).
Western blotting of lung homogenates
The harvested lungs were homogenized in 1 ml of PBS buffer containing 0.1% Triton X-100, and complete protease and phosphatase inhibitor cocktail (Roche Co, Indianapolis, IN) using a TissueLyser II (Qiagen) for 30 s in chilled conditions and centrifuged at maximum speed in a microcentrifuge at 4°C for 20 min to remove debris. The resulting supernatant fluids were used for Western blotting. To ensure equal loading of protein onto the gel, a Bradford protein assay was performed (Bio-Rad, Herculus, CA). The lung homogenates were analyzed on 8-15% Tris-glycine gels. The resolved proteins were transferred on to a PVDF membrane using standard protocols. The specific antibodies to phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204), phospho-p38 MAPK (Thr180/Tyr182), phospho-SAPK/JNK (Thr183/Tyr185), phospho-IKKα/β (Ser 176/180), phospho-NF-κB/p65 (Ser 536), phospho-Iκ-Bα (Ser 32), IKKβ, NF-κB/p65, IκBα, VCAM-1 and ICAM-1 were added at a 1:1000 dilution, whereas, Abs to total p38 and GAPDH were added to the concentration of 1:5000. Immunostaining was performed using an appropriate secondary Ab at a dilution of 1: 2,000 and developed with ECL plus western blot detection system (Thermo-Fisher, Piscataway, NJ).
NF-κB activation
An ELISA-based NF-κB DNA binding assay was performed in the lungs according to the manufacturer's protocol (Active Motif, Carlsbad, CA) in order to detect the activation of the p65 subunit of NF-κB as described earlier (18). Nuclear extract was prepared using the Nuclear Extraction Kit from Active Motiff. Briefly, a total of 7.5 μg nuclear extract from each lung samples post-Kp challenge was added to the NF-κB specific oligonucleotide coated 96-well plate and incubated for 1 h at room temperature. After washing the plate 3 times, a primary Ab specific for NF-κB/p65 was added and incubated for 1 h at room temperature. After 3 washings to remove excess primary Ab, an anti-HRP conjugate was added to the plate and the color development was monitored at the OD of 450 nm.
Generation of KC chimeras
Donor and recipient mice between 6-8 wk old were used to generate chimeras, as described in our earlier publication (19). Recipient mice were γ irradiated in two 525-rad doses between 3 hours. Bone marrow cells (8 × 106/mouse) were injected into the tail vein of the irradiated recipients and mice were maintained on 0.2% neomycin sulfate for the first 3 weeks. The reconstituted mice were used for experiments 6 weeks after the transplantation. We found that greater than 84% of blood leukocytes were derived from donor mice at the time the mice were used for experiments (6-8 wk after transplantation). Irradiated mice that were not transplanted with donor cells died between days 18 and 22 after transplantation (data not shown).
Neutrophil depletion
The protocol used to deplete neutrophils (GR1+) in mice has been described in our earlier reports (21) with modifications. A total of 50 μg anti-GR1+ mAb (sodium azide-free and low LPS content; RB6-8C5 from BD Pharmingen) in 50 μl was administrated i.p. at 12 and 2 h before bacterial infection. In control experiments, 50 μg of non specific isotype-matched control mAb in equal volumes was administrated at the same time points before bacterial infection. To validate the efficiency of neutrophil depletion by the Ab, differential WBC counts in blood were obtained every 12 h up to 3 days, and ≤2% neutrophils were found up to 3 days after depletion.
BMM culture
BMMs were differentiated using DMEM containing 10% FBS and MCSF. BM cells were differentiated in culture dishes by supplementing DMEM with MCSF on days 3 and 5 for 7 days. Cells were then stimulated for the indicated times with Kp (MOI of 1), and processed in urea/chaps buffer before being separated on SDS polyacrylamide gels. Proteins were transferred onto PVDF membranes and were detected using the indicated Ab specific for activated/phosphorylated NF-κB or total MAPKs, such as JNK, ERK, and p38. The Western blots were quantitated against GAPDH and expressed as fold increase in response to Kp after background (unstimulated) subtraction. In addition, culture media obtained from macrophages at 18 h following Kp infection were used for cytokine/chemokine determination by ELISA.
AM culture
AMs were isolated as described in earlier publications (22, 23). Positive selection by CD11b MicroBeads (Stem Cell technologies) was applied to isolate AMs from BAL cells. Pooled BAL cells were resuspended in RoboSep Buffer and CD11b PE labeling reagent was mixed with cell suspension followed by incubation at room temperature for 15 mins. EasySep PE selection cocktail was thereafter added to the cell suspension and the suspension was further incubated for 15 mins at room temperature. EasySep Magnetic nanoparticles were lastly mixed with the cell suspension followed by magnetic separation of CD11b positive cells. Cells were washed with PBS, centrifuged at 1,200 rpm for 10 min and resuspended in DMEM containing 5% FBS. Cells were infected with Kp (MOI of 1) and harvested at designated time points using urea/chaps/tris buffer. Samples were centrifuged and supernatants were used for Western blotting. In addition, culture media was collected at 18 h post-infection and was used for cytokine/chemokines determination. Finally, the total number of live cells and the percentage of purified AMs were determined by utilizing trypan blue exclusion and cytospin preparations stained with Wright-Giemsa respectively. Percent recovery of AMs was calculated as the ratio of AMs to the total macrophages isolated from the BALF. Using this technique, we obtained about 85-90 % AMs.
Statistical analysis
Data are expressed as mean ± SE. The intensity of immunoreactive bands was determined using a Gel Digitizing Software (UN-SCAN-IT gel™) from Silk Scientific, Inc, Utah. Data were analyzed by one way ANOVA followed by Bonferroni's post-hoc analysis for multiple comparisons. All statistical calculations were performed using InStat software and GraphPad Prism 4.0. Differences were considered statistically significant at *P<0.05 when compared with control. Survival curves were compared by Wilcoxon rank sign test.
RESULTS
KC-/- mice are highly susceptible to K. pneumoniae infection
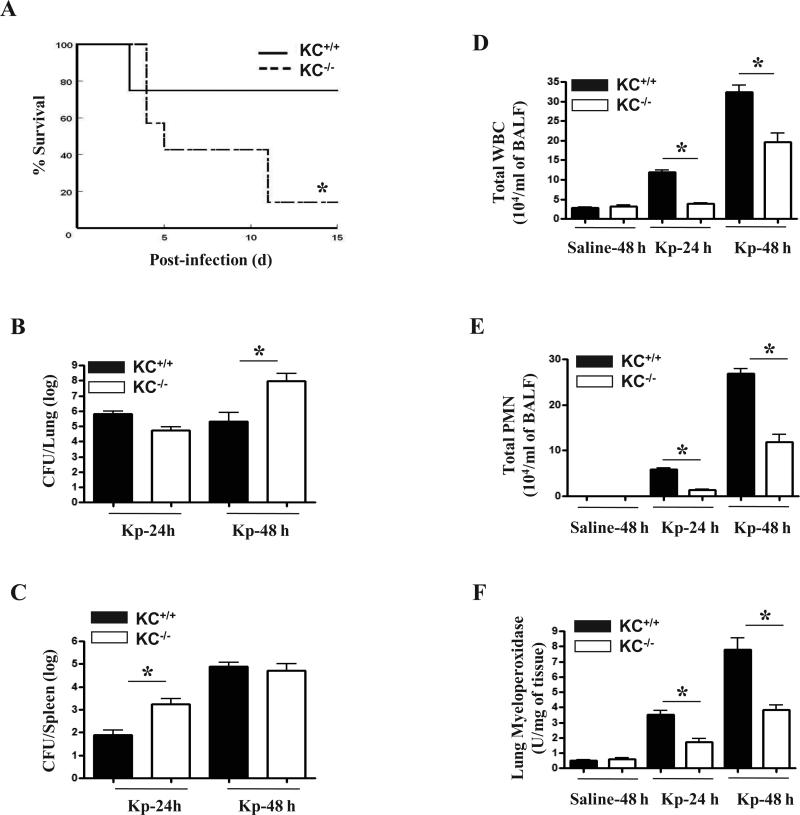
To assess the requirement of KC in host defense against Kp, we infected KC-/- and KC+/+ animals with Kp (103 CFU/mouse) through the i.t. route and survival was monitored for up to 15 days. We observed decreased survival in the KC–/– group and only 14.3% of mice survived to day 15 (Fig. 1A). On the other hand, 75% of Kp-infected KC+/+ mice survived up to 15 days following Kp infection (Fig. 1A).
Figure 1. Mortality in KC-/- mice infected with Kp.
(A) Enhanced mortality in CXCL1/KC-/- mice following i.t. Kp challenge. Mice were i.t. infected with 103 CFU Kp per mouse and survival was assessed up to 15 days. Data are from two separate experiments (n=10 mice/group). Significance between groups was examined by Wilcoxon rank sign test and asterisk indicates the difference between KC-/- and KC+/+ mice (p < 0.05). (B-C) Impaired bacterial clearance in the lungs and spleens in KC-/- mice challenged i.t. with Kp. The CFUs were examined in homogenates obtained from the lungs and spleens at 24 and 48 h post Kp challenge (n=5/group from 2 independent experiments; p< 0.05; significant differences between KC–/– and KC+/+ mice. (D-E) KC-/- mice have reduced cellular/neutrophil accumulation in the airspaces (BALF) after Kp (103 CFU/mouse) inoculation. (F) Attenuated neutrophil influx in the lung parenchyma, as measured by MPO activity, in KC-/- mice following Kp infection. (n=6/group from 2 separate experiments for D-F; p < 0.05; significant differences between KC–/– and KC+/+ mice.
KC–/– mice have impaired clearance of Kp
To determine whether the enhanced mortality in KC-/- mice to Kp was due to an impaired ability to limit bacterial growth in the lungs and/or bacterial dissemination to the bloodstream, we infected KC-/- and their control mice (KC+/+) with 103 CFU of Kp and assessed bacterial numbers in the lung and spleen at 24 and 48 h. As compared with KC+/+ (C57Bl/6) mice, KC–/– animals had a higher bacterial burden in the lungs at 48 h and more bacterial dissemination at 24 h (Fig. 1B and C).
KC deficiency impairs inflammatory cell recruitment to the lung following Kp challenge
To assess the myeloid cells recruitment to the lungs of KC-/- and KC+/+ mice post Kp infection, we i.t. infected both groups of mice with Kp and then enumerated the recruited cells. In KC–/– mice, pulmonary total white blood cell and neutrophil recruitment was attenuated both at 24 and 48 h following Kp challenge. In control (saline-challenged) groups, however no neutrophil influx in the lungs was observed in both KC-/- and KC+/+ mice (Figs. 1D-E). We also measured MPO activity in the lung parenchyma of infected KC-/- and KC+/+ mice and observed decreased activity in the lungs of KC-/- mice as compared with their WT counterparts (Fig. 1F), demonstrating attenuated neutrophil recruitment to the lung parenchyma.
KC deficiency results in decreased MIP-2 and LIX production in response to Kp
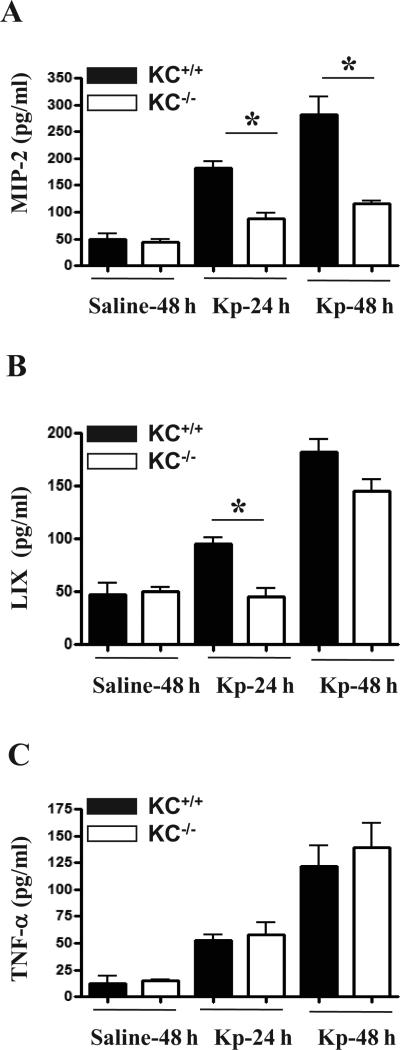
Since the impairment in Kp-induced neutrophil accumulation observed in KC-/- mice could reflect a decrease in the levels of cytokines/chemokines, we quantified the expression of TNF-α, MIP-2, and LIX in BALF at 24 and 48 h after Kp challenge. The levels of MIP-2 were found to be attenuated in KC-/- mice at both 24 and 48 h following Kp infection whereas the levels of LIX were found to be decreased at 24 h post infection (Figs. 2A-B). However, TNF-α levels in BALF were not different between KC-/- and KC+/+ mice (Fig. 2C).
Figure 2. Kp-induced cytokine/chemokine production in KC-/- mice.
(A-C) KC-dependent signaling regulates the expression of MIP-2 and LIX in the lungs post Kp inoculation. Mice were infected with Kp, BALF was collected and MIP-2, LIX and TNF-α concentrations were measured at 24 and 48 h post infection. A total of 4-6 animals were used at each time point in each group. Significant differences between KC-/- and control (KC+/+) mice are indicated by asterisks; *, p < 0.05.
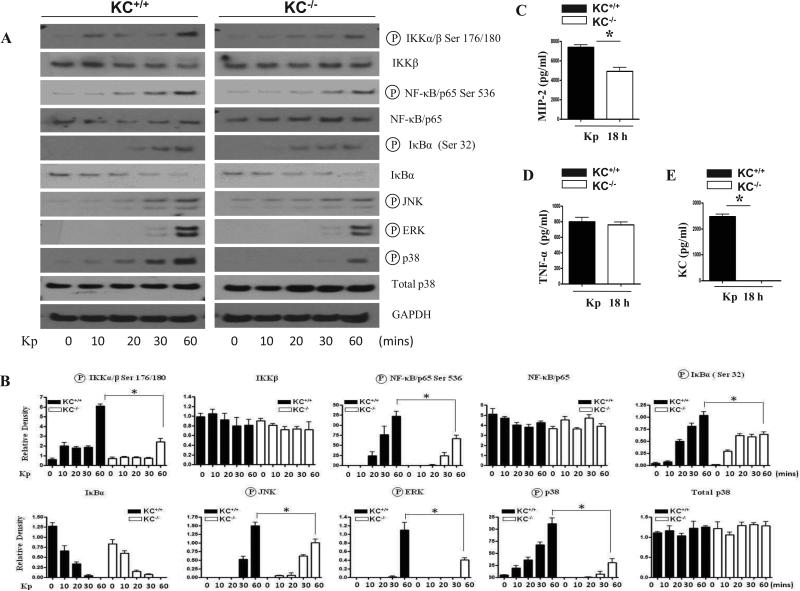
Kp-induced KC regulates NF-κB activation in the lung
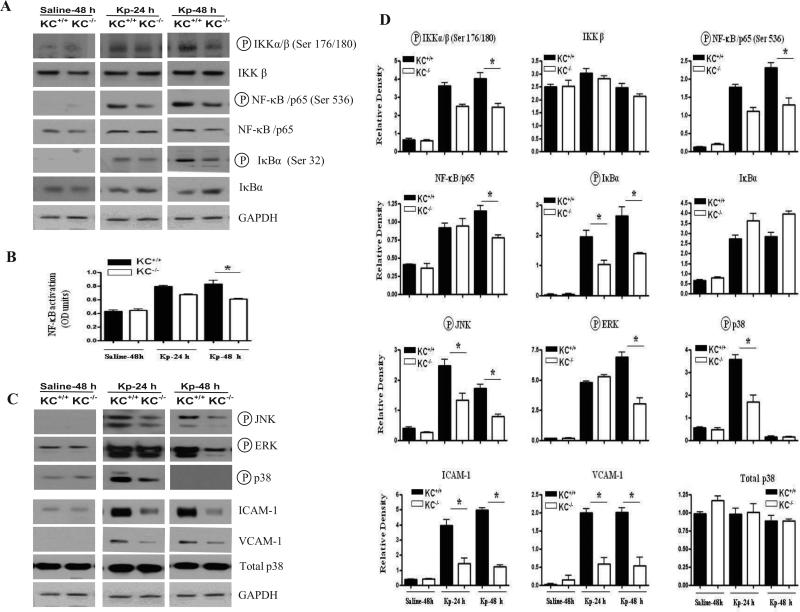
Previous reports have demonstrated that NF-κB is a major regulator of cytokines and chemokines, we next examined whether KC modulates NF-κB activation post Kp challenge. In order to elucidate NF-κB activation, we first determined the effect of Kp-induced KC on the phosphorylation of IKK, the upstream kinase of NF-κB and Iκ-Bα. Our data revealed activation of IKKα/β by KC. In a similar manner, we observed an increase in NF-κB /p65 phosphorylation at Ser536 at 48 h post infection. Although we also observed a modest decrease in the expression of IKKβ and NF-κB /p65 proteins in KC-/- mice after Kp infection, these changes were not significant as compared to the post-translational modifications (phosphorylation) of NF-κB (Figs 3A-D). In addition, we repeatedly could not detect phosphorylated IKKα/β or NF-κB/p65 in the control (saline-challenged) KC-/- and KC+/+ animals. Moreover, our time course experiments revealed a decrease in the phosphorylation of IκBα (Ser 32) in the lungs of KC-/- mice starting from 24 h post-treatment and persisted up to 48 h following Kp infection. Concomitantly, we also observed an increased accumulation of IκBα protein in KC-/- mice at 48 h post Kp infection (Figs. 3A-D). To confirm decreased activation of NF-κB /p65 in KC-/- mice following Kp infection, we determined p65 DNA binding using the nuclear extracts from the lungs. Our findings revealed a decrease in NF-κB/p65 DNA binding in KC-/- mice as compared to their wild type counterparts although this decrease was only significant at a later time point (48 h) (Figs. 3B, 3D).
Figure 3. Activation of Kp-mediated NF-κB and MAPKs and expression of ICAM-1 and VCAM-1 in lung tissues of KC-/- mice.
(A) KC mediated regulation of IKK (an upstream kinase of NF-κB) and NF-κB/p65 and IκBα in the lungs of KC-/- mice post Kp infection. The blots are a representative of 3 independent experiments with similar results. (B) Reduced activation of the NF-κB/p65 subunit of NF-κB as detected by p65 DNA binding in the nuclear extracts of murine lungs at 24 and 48 h following Kp infection (n = 3–4 mice per group at a time point.). (C) Attenuated upregulation of MAPKs and cell adhesion molecules (VCAM-1 and ICAM-1) in the lungs of KC-/- mice following Kp inoculation. Results are representative of three separate experiments with identical results. Values that are significantly different between KC–/– and KC+/+ are indicated by asterisks (p < 0.05). (D) Densitometric analysis of Western blots from three independent experiments to quantify the protein levels of IKK, NF-κB, and phospho-IKK, -NF-κB, -IκBα and -MAPKs as well as ICAM-1, VCAM-1, total p38 following Kp infection. The results obtained were normalized against GAPDH and expressed as mean ± SE. The difference between the KC-/- and KC+/+ animals was determined and the difference was expressed as p < 0.05.
Kp-induced MAPK activation is impaired in KC-/- mice
Decreased neutrophil accumulation in the lungs in response to Kp in KC-/- mice could be attributed to reduced activation of MAPKs, which can then regulate other transcription factors besides NF-κB, such as AP-1 and STAT-1 (24, 25). Therefore, we examined the activation of JNK, ERK and p38 kinases in the lungs of KC-/- and KC+/+ mice following Kp infection. Activation of JNK was attenuated both at 24 and 48 h post Kp infection whereas the activation of ERK was reduced only at 48 h (Figs. 3C-D). In addition, activation of p38 was attenuated in the lungs of KC-/- mice at 24 h following infection (Figs. 3C-D).
KC contributes to Kp-induced ICAM-1 and VCAM-1 expression
Successful neutrophil recruitment to the lung in response to infection requires the efficient upregulation of cell adhesion molecules on vascular endothelium and leukocytes. We therefore examined the expression of ICAM-1 and VCAM-1 in lung homogenates following Kp infection. In KC-/- mice, the expression of both ICAM-1 and VCAM-1 was attenuated in the lung at 48 h following Kp infection relative to the KC+/+ mice (Figs. 3C-D).
Bone marrow cell and non-bone marrow cell-derived KC contributes to host defense following pulmonary Kp infection
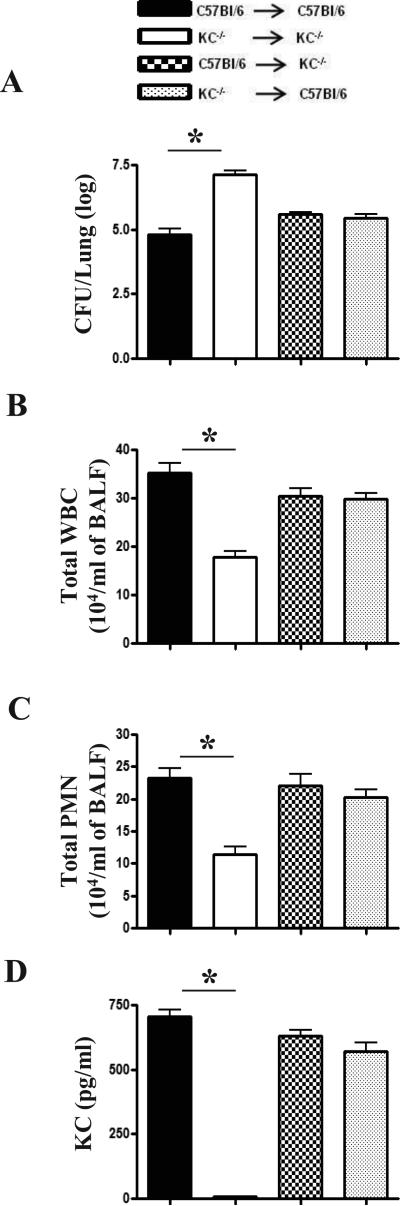
We next studied the relative importance of bone marrow (radiosensitive) vs. resident/non-hematopoietic (radioresistant) cell-derived KC in host defense using bone marrow chimeras. Lung CFUs were in response to Kp infection were increased in irradiated KC-/- reconstituted with KC+/+ bone marrow (Fig. 4A). Kp-induced total leukocyte and neutrophil influx as well as KC production was attenuated in irradiated KC-/- reconstituted with KC-/- bone marrow (Figs. 4B-D). As compared with irradiated KC+/+ mice reconstituted with KC+/+ bone marrow., irradiated KC-/- mice reconstituted with KC+/+ bone marrow or KC+/+ irradiated mice reconstituted with KC-/- bone marrow did not show differences in lung CFU, neutrophil influx or KC levels following Kp infection (Figs. 3A-D) As expected, neutrophil recruitment to the lung or KC production was not observed in reconstituted mice following saline challenge (data not shown).
Figure 4. Role of bone marrow and non-bone marrow cells in host defense against Kp.
KC produced from bone marrow and non-bone marrow/resident cells following Kp infection is essential for host defense. Bone marrow chimeras were generated by lethal irradiation of KC-/- and KC+/+ mice and reconstituted with bone marrow cells via tail injection. (A) Bacterial CFU in the lungs at 48 h post-Kp infection. (B-C) Total WBC and neutrophil counts in BALF at 48 h post Kp infection. (D) KC levels in BALF at 48 h following Kp challenge. A total of 5-7 mice/group were used in 2 independent experiments and statistical significance was expressed as p < 0.05.
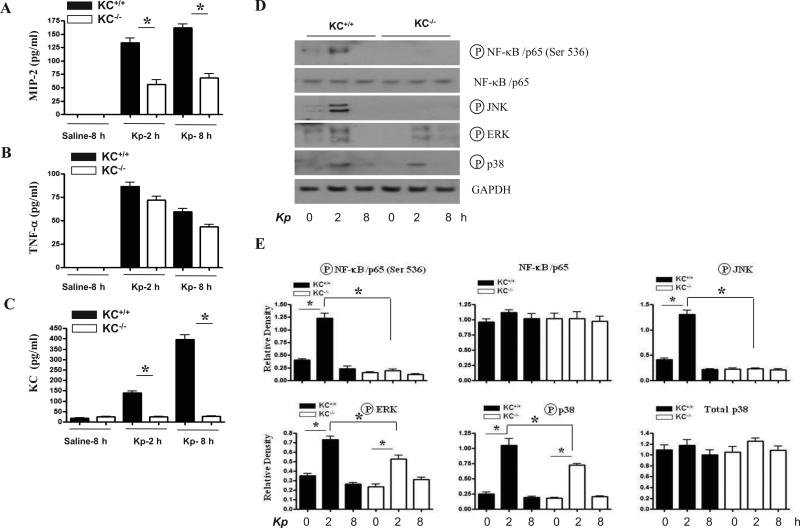
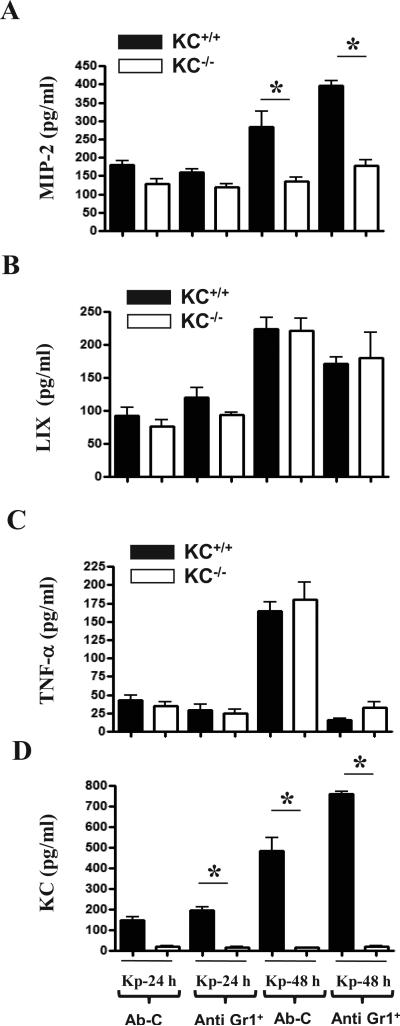
Neutrophils do not contribute to KC production in the lungs following Kp challenge
We observed substantial neutrophil recruitment to the lung at 48 h following Kp infection (Figs. 1C-E). Therefore, we depleted neutrophils from KC+/+ and KC-/- mice to assess their role in the production of cytokines/chemokines as well as activation of NF-κB and MAPKs in the lungs. To deplete neutrophils, mice were treated with anti-Gr-1 (RB6-8C5) Ab at 12 and 2 h prior to Kp challenge whereas isotype matched Ab was used as a control. Neutrophil depletion did not substantially alter the differences in MIP-2, LIX and TNF-α levels between KC+/+ and KC-/- mice following Kp infection (Fig. 6). In a similar manner, activation of NF-κB and MAPKs and expression of VCAM-1 and ICAM-1 was not differed between KC+/+ and KC-/- mice post-infection (Fig. 6).
Figure 6. Activation of Kp-induced NF-κB and MAPKs in lung tissues of neutrophil depleted KC+/+ and KC-/- mice.
(A) Wild-type and KC-/- mice challenged with 103 CFU i.t. were euthanized at 24 and 48 h post infection and lungs were used for western blotting. The membranes were probed with activated NF-κB and MAPK Abs. (B) Densitometric analysis of Western blots from three independently performed experiments to quantify the protein levels of IKK, NF-κB, ICAM-1, VCAM-1 and phospho-IKK, -NF-κB, and -MAPKs following Kp challenge. The data obtained from these experiments were normalized against GAPDH and expressed as mean ± SE from 2 independent experiments). The significance between the KC-/- and KC+/+ mice was indicated as p < 0.05.
BMMs obtained from KC–/– mice have attenuated activation of NF-κB and MAPKs in response to Kp
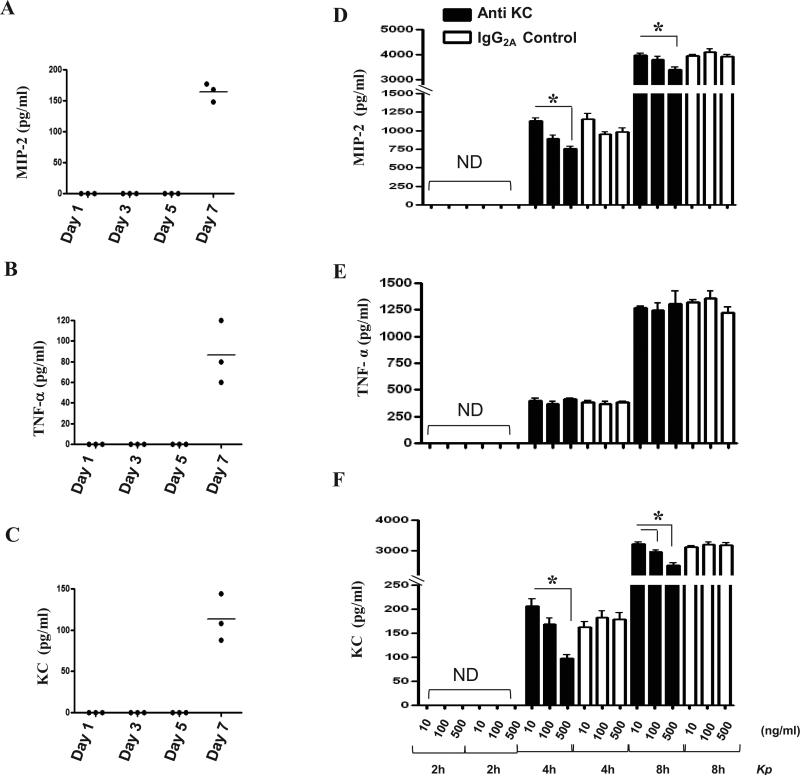
We next assessed whether KC deficiency has any effect on activation of NF-κB and MAPKs in myeloid cells. In particular, we have used macrophages since these cells are known to mediate host defense. When KC–/– macrophages were stimulated with Kp, reduced activation of NF-κB as well as JNK, ERK, and p38 was observed (Fig. 7A-B). We observed attenuated MIP-2 production in macrophages obtained from KC-/- mice, although TNF-α levels between KC-/- and KC+/+ mice were not different following Kp infection (Fig. 7C-E). To examine the basal levels of cytokines/chemokines in BMMs, we measured the protein levels of MIP-2, KC and TNF-α. Our results also show that BMMs release KC, MIP-2 And TNF-α during differentiation on day 7 in the absence of Kp infection although these levels are much less than in the presence of Kp infection (Figs. 8A-C),
Figure 7. Activation of NF-κB and MAPKs in BMMs of KC-/- mice infected with Kp.
(A) Decreased activation of NF-κB and MAPKs in BMMs obtained from KC-/- mice post Kp infection. Activation of IKK, NF-κB and MAPKs was detected at 24 and 48 h following Kp stimulation. The blot is a representative of three independent experiments with identical results. (B) Densitometric analysis of IKK, NF-κB and MAPK activation in BMMs up to 60 min following stimulation with Kp (MOI of 1). Data expressed as mean ± SE of three blots from three mice in each group (p < 0.05). (C) Attenuated production of MIP-2 in BMMs obtained from KC-/- mice post Kp infection. BMMs were stimulated with Kp at an MOI of 1 and supernatants collected at 18 h post infection were used to determine MIP-2, KC and TNF-α release. The data obtained from 3 independent experiments.
Figure 8. Effect of KC neutralization on the production of cytokines/chemokines in BMMs following Kp challenge.
(A-C) BMMs from KC+/+ mice produce KC, MIP-2 and TNF-α during differentiation in the absence of Kp infection on day 7. Supernatants were collected 1, 3, 5 and 7 days culture and cytokine/chemokine levels were assessed by sandwich ELISA. Data are representative of 2 independent experiments using 3 mice/group (p < 0.05). (D-E) Blocking KC using Ab attenuated KC and MIP-2 but not TNF-α production in BMMs. BMMs were pretreated with KC neutralizing Ab 2 h prior to Kp infection (MOI of 1). Supernatants were collected 2, 4 and 8 h after Kp challenge and cytokine/chemokines levels were measured by sandwich ELISA. Data are pooled from 2 independent experiments using 5 mice/group and significant difference was expressed as p < 0.05.
KC mediates expression of cytokine/chemokines and activation of NF-κB and MAPKs in an autocrine and paracine manner
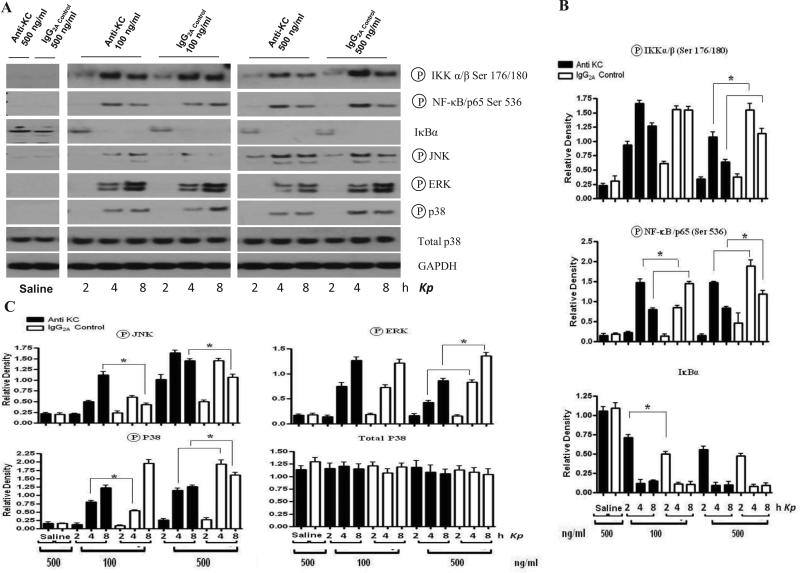
In order to test the hypothesis that KC-induced production of cytokine/chemokines and activation of NF-κB and MAPKs involves autocrine and paracrine responses in BMMs, we used a KC neutralizing Ab. As demonstrated in Figs. 8 and 9, blocking KC reduced release of KC and MIP-2 but not TNF-α (Fig. 8D-E) and activation of NF-κB and MAPKs (Fig. 9). We used murine alveolar macrophages to confirm the findings obtained from BMMs and demonstrated that KC and MIP-2 production and activation of NF-κB and MAPKs was attenuated in AMs obtained from KC-/- mice as compared with cells obtained from their littermate controls (Fig. 10).
Figure 9. Effect of KC blocking on activation of NF-κB and MAPKs in BMMs challenged with Kp.
(A) KC blocking decreased activation of NF-κB and MAPKs in BMMs obtained from KC+/+ (C57Bl/6) mice following pulmonary Kp infection.Activation of NF-κB and MAPKs was detected at various time points after stimulation.The blot is a representative of four blots with identical results. (B) Densitometric analysis of IKK, NF-κB, IκBα and MAPK activation in BMMs at 2, 4 and 8 h post-stimulation with an MOI of 1. Data expressed as mean ± SE of 3 independent experiments in each group (p < 0.05).
Figure 10. Effect of KC disruption on cytokine/chemokine production and activation of NF-κB and MAPKs in AMs following Kp infection.
(A-C) Decreased production of MIP-2 in alveolar macrophages obtained from KC-/- mice to Kp challenge. Alveolar macrophages were stimulated with Kp at an MOI of 1 and supernatants collected at 18 h post infections were used to determine MIP-2, TNF-α, and KC levels. The data obtained from 2 independent experiments using 4 mice in total. (D) Reduced activation of NF-κB and MAPKs in alveolar macrophages obtained from KC-/- mice following Kp infection. Activation of IKK, NF-κB and MAPKs was detected at various time points following Kp stimulation (MOI of 1). The blot is a representative of three experiments with identical results. (E) Densitometric analysis of IKK, NF-κB and MAPK activation in BMMs up to 8 h following Kp stimulation with an MOI of 1. Data expressed as mean ± SE of three blots from three mice in each group from 2 independent experiments (p < 0.05).
DISCUSSION
Community acquired pneumonia (CAP) caused by Kp is a leading cause of death due to extensive lung damage (26). Unlike many other Gram-negative bacterial pathogens, Kp can induce disseminated infection even with a relatively low dose. While initial treatment of CAP is empirical antibiotic coverage, increasing numbers of immunocompromised patients, a growing proportion of elderly individuals, and the emergence of antibiotic resistant Kp makes this treatment difficult. Therefore, modulation of the host immune response itself is an attractive option for clinical management, particularly among patients with immune system impairment or infected with antibiotic resistant bacteria. Development of more effective agents requires a better understanding of the mechanisms by which innate defense against bacteria is regulated.
Successful host defense against bacteria involves the brisk recruitment of neutrophils because they phagocytose and eliminate microbes. Neutrophil migration to the lungs involves the expression of chemokines. Earlier reports have demonstrated that KC, MIP-2 and LIX are important for neutrophil accumulation in the lungs (11, 13-15). These investigations have primarily been performed using blocking Abs. Subsequently, several KC transgenic mouse strains have been used to demonstrate the importance of KC in neutrophil influx in the lungs although the transgene expression was expressed in all tissues, including the lung (27, 28). Therefore, a lung specific KC transgene expressing mouse was later developed to demonstrate the importance of KC in Kp-induced neutrophil recruitment and bacterial clearance. An important challenge that remains is the determination of the KC-mediated signaling mechanisms in the lungs during pulmonary bacterial infection. In this report, we have used a unique (loss-of-function) model using mice deficient in KC to delineate the mechanisms associated with Kp-induced neutrophil-mediated bacterial clearance in the lungs. This model provided a unique opportunity to address the mechanisms related to KC-induced neutrophil-mediated host defense in the lungs against bacterial pathogens, including Kp. Using this murine model, we confirmed the previous observations (27, 28) that KC is essential for survival and the clearance of Kp from the host.
A role for chemokines to amplify the inflammation in the lungs has been documented in an investigation using KC transgenic mice (28). That report provided evidence that KC regulates the expression of MIP-2 in the lungs in KC transgenic mice infected with Kp whereas KC does not regulate TNF-α, IFN-γ, IL-12 and IL-10 (28). Our data show that KC also regulates other chemokines, such as LIX, in response to Kp infection. These observations reveal that KC can augment neutrophil-mediated lung inflammation and host defense against bacteria via the upregulation of MIP-2 and LIX. These findings support that KC not only has a direct role but also has an indirect role via upregulating other pro-inflammatory mediators in the lungs to augment host defense during bacterial infections.
Neutrophil infiltration into mucosal epithelia is a hallmark of bacterial pneumonia and this involves a multistep paradigm. Neutrophils in the bloodstream adhere to the vascular endothelium and migrate across the endothelium to reach sites of inflammation in various organs, including the lungs (29). This process is complex and involves multiple adhesion molecules from different families that are expressed on the surface of endothelial cells and leukocytes in response to different cytokines. These endothelial proteins recognize specific leukocyte glycoproteins and facilitate their reversible adherence to the vessel wall, known as tethering. This is followed by firm attachment of neutrophils to the endothelium and eventual extravasation through diapedesis. In this investigation, we identified that Kp regulates the expression of VCAM-1 and ICAM-1 following Kp infection and this is one of the KC-mediated defense mechanisms involved in neutrophil accumulation and bacterial clearance in the lungs.
A central feature of inflammation is the activation of transcription factors. Among these, NF-κB is well-studied and becomes activated in response to stress, cytokines, free radicals, ultraviolet irradiation, oxidized LDL, and bacterial or viral antigens. NF-κB activation has been reported to contribute to neutrophil accumulation in the lungs by bacteria via multiple mechanisms, including upregulation of cell adhesion molecules (VCAM-1 and ICAM-1). In this regard, studies demonstrate that endogenous NF-κB protects the host by preventing excessive inflammation and injury during E. coli (30) and pneumococcal pneumonia (31). Moreover, NF-κB is shown to be essential for antibacterial host defense (30, 31). Our findings from this study support an important role for KC in late NF-κB activation (48 h) in the lungs following Kp infection. In addition, our current findings also suggest the involvement of other transcription factors, such as AP-1/STAT-1 since MAPKs are known to regulate AP-1 and STAT-1 (32) and our results reveal that KC mediates activation of all three MAPKs at late time point (48 h).
The relative contribution of myeloid cells (macrophages and neutrophils) versus resident tissue cells in neutrophil accumulation into the lung from the bloodstream has not been clear. However, the contribution of specific cell type is dependent on the stimulus, since both of these cell types are exposed to inflammatory components during an insult. In earlier studies, it has been proposed that myeloid cells in the lung produce a battery of neutrophil chemotactic substances such as KC (10, 11) and MIP-2 (12, 13) whereas resident cells, including alveolar epithelial type II (AEII) cells produce other neutrophil chemoattractants, such as LIX (14). An interesting finding of this investigation was the dependence on both hematopoietic/myeloid cell and resident/non-hematopoietic cell-derived KC for host defense during Kp infection. The conclusions obtained from this investigation are consistent with prior reports of the role of hematopoietic and nonhematopoietic cells in pulmonary inflammation. For example, an earlier study has shown that MyD88 derived from hematopoietic cells is more important for LPS-induced expression of TNF-α and IL-12p40 (33) although both hematopoietic and resident cell–derived MyD88 are important for LPS-induced neutrophil influx (31)(34, 35). We have shown earlier that MD-2 in both cell types is important for neutrophil-mediated inflammation and the expression of MIP-2, TNF-a and IL-6 is mediated by both cell types in the lungs following LPS challenge (19).
A key finding of our investigation is that depletion of neutrophils resulted in no substantial change in the production of KC in the lungs. These results are consistent with the fact that several other cell types are involved in the initiation of KC production during pulmonary infectious challenge. Our observations suggest that neutrophils may be the key source for TNF-α, a cytokine which is not regulated by KC-dependent signaling cascade(s). We used macrophages in our in vitro system since this cell type is a vital mediator of host defense in the lungs and found that macrophages modulate lung inflammation upon Kp infection via KC-mediated activation of NF-κB and MAPKs. Our data also show that KC regulates MIP-2 in macrophages upon Kp infection. Since KC controls the production of MIP-2 from macrophages and LIX from AEII cells in the lung, it is likely that KC-mediates both autocrine and paracrine effects upon Kp infection. In this regard, we have observed expression of KC and its receptor (CXCR2) in large and small airway cells, alveolar type II cells, macrophages and neutrophils (data not shown).
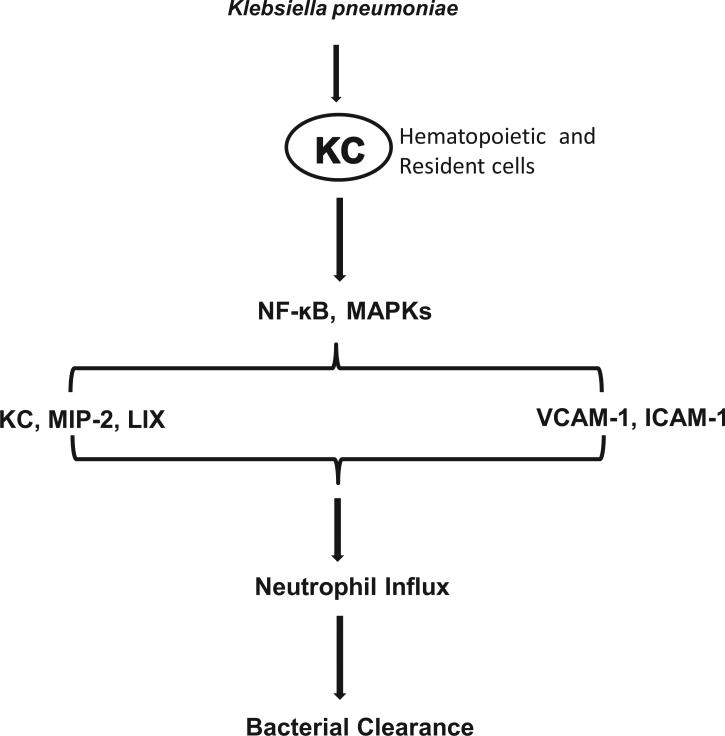
KC-/- mice have been used in a few prior studies: 1) KC/low density lipoprotein receptor (LDLR)-/- mice exhibit protective effects against the development of atherosclerosis (36) and 2) KC-/- mice also show protection against dextran sulphate sodium-induced colitis (17). We took advantage of the gene-deficient mice to demonstrate the host defense mechanisms induced by KC following Kp infection. The findings from this study unveil that multiple signaling cascades involving transcription factors, kinases and the expression of proinflammatory mediators are altered in Gram-negative pneumonia via KC (Fig 11, proposed model). This investigation offers the first step into the biology of KC in mediating pulmonary antibacterial defense upon bacterial infection.
Figure 11. Schematic depicting the functions of KC leading to neutrophil accumulation following Kp infection.
KC activates of NF-κB and MAPK causes upregulation of chemokines, such as KC, MIP-2 and LIX and adhesion molecules, including VCAM- and ICAM-1. In turn, these events ultimately cause neutrophil recruitment to clear the bacterial infection from the lungs.
Figure 5. Kp-induced cytokine/chemokine production in lungs of neutrophil depleted KC+/+ and KC-/- mice.
(A-D) KC derived from neutrophils does not contribute to MIP-2 and LIX production. Mice were pretreated with anti-Gr1 or control mAb at 12 and 2 h prior to infection with 103 CFU of Kp. Cytokine/chemokines levels were determined at 24 and 48 h post Kp infection (n=6-8 animals per group; * p < 0.05).
ACKNOWLEDGMENTS
The authors thank Nades Palaniyar at the University of Toronto for helpful discussions. We thank Rachel Zemans and Ken Malcolm at National Jewish Health, Dan Chisenhall and Pete Mottram at LSU and Lung Biology lab members, Gayathriy Balamayooran and Himanshu Raje, for critical reading of the manuscript.
Supported by a Scientist Award from the Flight Attendant Medical Research Institute (YCSA-062466); and grants from the NIH (R01 HL-091958 and R01 HL-091958S1 via ARRA) to SJ
LIST OF ABBREVIATIONS
- ICAM-1
Intracellular cell-adhesion molecule 1
- VCAM-1
Vascular cell-adhesion molecule 1
- LIX
Lipopolysaccharide-induced CXC chemokine
- KC
Keratinocyte cell-derived chemokine
- MIP-2
Macrophage inflammatory protein-2
- i.t.
Intratracheal
REFERENCES
- 1.Mizgerd JP. Lung infection--a public health priority. PLoS Med. 2006;3:e76. doi: 10.1371/journal.pmed.0030076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fein AM. Pneumonia in the elderly: overview of diagnostic and therapeutic approaches. Clin Infect Dis. 1999;28:726–729. doi: 10.1086/515218. [DOI] [PubMed] [Google Scholar]
- 3.Lynch JP, 3rd, Martinez FJ. Community-acquired pneumonia. Curr Opin Pulm Med. 1998;4:162–172. [PubMed] [Google Scholar]
- 4.Craig A, Mai J, Cai S, Jeyaseelan S. Neutrophil recruitment to the lungs during bacterial pneumonia. Infect Immun. 2009;77:568–575. doi: 10.1128/IAI.00832-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mizgerd JP. Acute lower respiratory tract infection. N Engl J Med. 2008;358:716–727. doi: 10.1056/NEJMra074111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abraham E. Neutrophils and acute lung injury. Crit Care Med. 2003;31:S195–199. doi: 10.1097/01.CCM.0000057843.47705.E8. [DOI] [PubMed] [Google Scholar]
- 7.Strieter RM, Kunkel SL. Acute lung injury: the role of cytokines in the elicitation of neutrophils. J Investig Med. 1994;42:640–651. [PubMed] [Google Scholar]
- 8.Goodman RB, Strieter RM, Martin DP, Steinberg KP, Milberg JA, Maunder RJ, Kunkel SL, Walz A, Hudson LD, Martin TR. Inflammatory cytokines in patients with persistence of the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1996;154:602–611. doi: 10.1164/ajrccm.154.3.8810593. [DOI] [PubMed] [Google Scholar]
- 9.Zhang P, Summer WR, Bagby GJ, Nelson S. Innate immunity and pulmonary host defense. Immunol Rev. 2000;173:39–51. doi: 10.1034/j.1600-065x.2000.917306.x. [DOI] [PubMed] [Google Scholar]
- 10.Bozic CR, Kolakowski LF, Jr., Gerard NP, Garcia-Rodriguez C, von Uexkull-Guldenband C, Conklyn MJ, Breslow R, Showell HJ, Gerard C. Expression and biologic characterization of the murine chemokine KC. J Immunol. 1995;154:6048–6057. [PubMed] [Google Scholar]
- 11.Frevert CW, Huang S, Danaee H, Paulauskis JD, Kobzik L. Functional characterization of the rat chemokine KC and its importance in neutrophil recruitment in a rat model of pulmonary inflammation. J Immunol. 1995;154:335–344. [PubMed] [Google Scholar]
- 12.Wolpe SD, Sherry B, Juers D, Davatelis G, Yurt RW, Cerami A. Identification and characterization of macrophage inflammatory protein 2. Proc Natl Acad Sci U S A. 1989;86:612–616. doi: 10.1073/pnas.86.2.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Driscoll KE, Hassenbein DG, Howard BW, Isfort RJ, Cody D, Tindal MH, Suchanek M, Carter JM. Cloning, expression, and functional characterization of rat MIP-2: a neutrophil chemoattractant and epithelial cell mitogen. J Leukoc Biol. 1995;58:359–364. doi: 10.1002/jlb.58.3.359. [DOI] [PubMed] [Google Scholar]
- 14.Jeyaseelan S, Manzer R, Young SK, Yamamoto M, Akira S, Mason RJ, Worthen GS. Induction of CXCL5 during inflammation in the rodent lung involves activation of alveolar epithelium. Am J Respir Cell Mol Biol. 2005;32:531–539. doi: 10.1165/rcmb.2005-0063OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jeyaseelan S, Chu HW, Young SK, Worthen GS. Transcriptional profiling of lipopolysaccharide-induced acute lung injury. Infect Immun. 2004;72:7247–7256. doi: 10.1128/IAI.72.12.7247-7256.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsai WC, Strieter RM, Wilkowski JM, Bucknell KA, Burdick MD, Lira SA, Standiford TJ. Lung-specific transgenic expression of KC enhances resistance to Klebsiella pneumoniae in mice. J Immunol. 1998;161:2435–2440. [PubMed] [Google Scholar]
- 17.Shea-Donohue T, Thomas K, Cody MJ, Aiping Z, Detolla LJ, Kopydlowski KM, Fukata M, Lira SA, Vogel SN. Mice deficient in the CXCR2 ligand, CXCL1 (KC/GRO-alpha), exhibit increased susceptibility to dextran sodium sulfate (DSS)-induced colitis. Innate Immun. 2008;14:117–124. doi: 10.1177/1753425908088724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cai S, Batra S, Shen L, Wakamatsu N, Jeyaseelan S. Both TRIF and MyD88-dependent signaling contribute to host defense against pulmonary Klebsiella infection. J Immunol. 2009;183:6629–6638. doi: 10.4049/jimmunol.0901033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cai S, Zemans RL, Young SK, Worthen GS, Jeyaseelan S. Myeloid differentiation protein-2-dependent and -independent neutrophil accumulation during Escherichia coli pneumonia. Am J Respir Cell Mol Biol. 2009;40:701–709. doi: 10.1165/rcmb.2008-0152OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeyaseelan S, Young SK, Fessler MB, Liu Y, Malcolm KC, Yamamoto M, Akira S, Worthen GS. Toll/IL-1 receptor domain-containing adaptor inducing IFN-beta (TRIF)-mediated signaling contributes to innate immune responses in the lung during Escherichia coli pneumonia. J Immunol. 2007;178:3153–3160. doi: 10.4049/jimmunol.178.5.3153. [DOI] [PubMed] [Google Scholar]
- 21.Jeyaseelan S, Young SK, Yamamoto M, Arndt PG, Akira S, Kolls JK, Worthen GS. Toll/IL-1R domain-containing adaptor protein (TIRAP) is a critical mediator of antibacterial defense in the lung against Klebsiella pneumoniae but not Pseudomonas aeruginosa. J Immunol. 2006;177:538–547. doi: 10.4049/jimmunol.177.1.538. [DOI] [PubMed] [Google Scholar]
- 22.Xu J, Xu F, Barrett E. Metalloelastase in lungs and alveolar macrophages is modulated by extracellular substance P in mice. Am J Physiol Lung Cell Mol Physiol. 2008;295:L162–170. doi: 10.1152/ajplung.00282.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagatani K, Dohi M, To Y, Tanaka R, Okunishi K, Nakagome K, Sagawa K, Tanno Y, Komagata Y, Yamamoto K. Splenic dendritic cells induced by oral antigen administration are important for the transfer of oral tolerance in an experimental model of asthma. J Immunol. 2006;176:1481–1489. doi: 10.4049/jimmunol.176.3.1481. [DOI] [PubMed] [Google Scholar]
- 24.Kwon D, Fuller AC, Palma JP, Choi IH, Kim BS. Induction of chemokines in human astrocytes by picornavirus infection requires activation of both AP-1 and NF-kappa B. Glia. 2004;45:287–296. doi: 10.1002/glia.10331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soriano SF, Serrano A, Hernanz-Falcon P, Martin de Ana A, Monterrubio M, Martinez C, Rodriguez-Frade JM, Mellado M. Chemokines integrate JAK/STAT and G-protein pathways during chemotaxis and calcium flux responses. Eur J Immunol. 2003;33:1328–1333. doi: 10.1002/eji.200323897. [DOI] [PubMed] [Google Scholar]
- 26.Renckens R, Roelofs JJ, Stegenga ME, Florquin S, Levi M, Carmeliet P, Van't Veer C, van der Poll T. Transgenic tissue-type plasminogen activator expression improves host defense during Klebsiella pneumonia. J Thromb Haemost. 2008;6:660–668. doi: 10.1111/j.1538-7836.2008.02892.x. [DOI] [PubMed] [Google Scholar]
- 27.Lira SA, Zalamea P, Heinrich JN, Fuentes ME, Carrasco D, Lewin AC, Barton DS, Durham S, Bravo R. Expression of the chemokine N51/KC in the thymus and epidermis of transgenic mice results in marked infiltration of a single class of inflammatory cells. J Exp Med. 1994;180:2039–2048. doi: 10.1084/jem.180.6.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lira SA, Fuentes ME, Strieter RM, Durham SK. Transgenic methods to study chemokine function in lung and central nervous system. Methods Enzymol. 1997;287:304–318. doi: 10.1016/s0076-6879(97)87022-x. [DOI] [PubMed] [Google Scholar]
- 29.Balamayooran G, Batra S, Fessler MB, Happel KI, Jeyaseelan S. Mechanisms of neutrophil accumulation in the lungs against bacteria. Am J Respir Cell Mol Biol. 2010;43:5–16. doi: 10.1165/rcmb.2009-0047TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mizgerd JP, Lupa MM, Kogan MS, Warren HB, Kobzik L, Topulos GP. Nuclear factor-kappaB p50 limits inflammation and prevents lung injury during Escherichia coli pneumonia. Am J Respir Crit Care Med. 2003;168:810–817. doi: 10.1164/rccm.200303-412OC. [DOI] [PubMed] [Google Scholar]
- 31.Quinton LJ, Jones MR, Simms BT, Kogan MS, Robson BE, Skerrett SJ, Mizgerd JP. Functions and regulation of NF-kappaB RelA during pneumococcal pneumonia. J Immunol. 2007;178:1896–1903. doi: 10.4049/jimmunol.178.3.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin WN, Luo SF, Lin CC, Hsiao LD, Yang CM. Differential involvement of PKC-dependent MAPKs activation in lipopolysaccharide-induced AP-1 expression in human tracheal smooth muscle cells. Cell Signal. 2009;21:1385–1395. doi: 10.1016/j.cellsig.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 33.Noulin N, Quesniaux VF, Schnyder-Candrian S, Schnyder B, Maillet I, Robert T, Vargaftig BB, Ryffel B, Couillin I. Both hemopoietic and resident cells are required for MyD88-dependent pulmonary inflammatory response to inhaled endotoxin. J Immunol. 2005;175:6861–6869. doi: 10.4049/jimmunol.175.10.6861. [DOI] [PubMed] [Google Scholar]
- 34.Skerrett SJ, Liggitt HD, Hajjar AM, Ernst RK, Miller SI, Wilson CB. Respiratory epithelial cells regulate lung inflammation in response to inhaled endotoxin. Am J Physiol Lung Cell Mol Physiol. 2004;287:L143–152. doi: 10.1152/ajplung.00030.2004. [DOI] [PubMed] [Google Scholar]
- 35.Quinton LJ, Jones MR, Robson BE, Simms BT, Whitsett JA, Mizgerd JP. Alveolar epithelial STAT3, IL-6 family cytokines, and host defense during Escherichia coli pneumonia. Am J Respir Cell Mol Biol. 2008;38:699–706. doi: 10.1165/rcmb.2007-0365OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boisvert WA, Rose DM, Johnson KA, Fuentes ME, Lira SA, Curtiss LK, Terkeltaub RA. Up-regulated expression of the CXCR2 ligand KC/GRO-alpha in atherosclerotic lesions plays a central role in macrophage accumulation and lesion progression. Am J Pathol. 2006;168:1385–1395. doi: 10.2353/ajpath.2006.040748. [DOI] [PMC free article] [PubMed] [Google Scholar]