Abstract
Objective
To conduct a quasi-experimental comparison of early clinical outcomes between injectable, sustained release, depot naltrexone formulation versus oral naltrexone maintenance therapy.
Method
Early retention and urine results were compared between patients participating in two concurrently run randomized clinical trials of oral (N = 69) and long acting injectable naltrexone maintenance therapy with psychosocial therapy (N = 42). Retention in treatment and opiate use in the first 8-weeks post-detoxification were compared.
Results
Long acting injectable naltrexone produced significantly better outcome than oral naltrexone on days retained in treatment and one measure of opiate use; other measures were not significantly different, but differences were in the same direction. In subanalyses, there were interaction effects between baseline heroin severity and type of treatment. In subanalyses, heroin users with more severe baseline use showed better retention in oral naltrexone maintenance combined with intensive psychotherapy (Behavior Naltrexone Therapy) as compared to retention of severe users treated with long acting naltrexone injections combined with standard cognitive-behavioral psychotherapy; less severe heroin users evidenced better outcomes when treated with long acting injectable naltrexone.
Conclusions
This quasi-experimental analysis provides tentative indications of superior outcomes for heroin dependent patients treated with long acting injectable naltrexone compared to oral naltrexone. The finding that heroin users with more severe baseline use achieved better outcomes with oral naltrexone is most probably attributable to the intensive nature of the psychosocial treatments provided, and points to the opportunity for continued research in augmenting injectable naltrexone with psychosocial strategies to further improve outcome especially in more severe users. The results should be considered exploratory given the quasi-experimental nature of the study.
Keywords: naltrexone, long acting injectable, implants, behavior therapy, heroin
Introduction
The high affinity opioid receptor antagonist naltrexone is a theoretically powerful treatment for opioid dependence, but has had only very limited effectiveness because of poor adherence with the medication in pill form1. It is simply too easy for patients to stop the pills for a few days, after which the blockade wears off and relapse to opioid dependence usually ensues2. Numerous behavioral strategies have been attempted to increase outpatient compliance with oral naltrexone amongst opioid-dependent patients, including contingency management with money and vouchers, behavior therapy, and the inclusion of significant others in conjoint sessions or as medication monitors3-10, although meta-analytic analyses of such studies demonstrate that such strategies have at best a moderate impact on outcome6, 10. Newly developed long acting injectable and implantable naltrexone formulations have the potential to reduce the adherence problem and substantially improve the effectiveness of naltrexone as a treatment alternative for opioid dependence11, 12. However, there are no published randomized controlled trials comparing outcomes for long acting injectable naltrexone versus oral naltrexone for opioid dependence. Three quasi-experimental reports13-15 which compare oral naltrexone outcomes to outcomes from naltrexone implants from treatment providers who offered both forms of treatment plus counseling demonstrate that patients receiving implant treatment demonstrated significantly better opiate abstinence and treatment retention at six- and 12-month follow-up points. A randomized controlled trial comparing injectable naltrexone to oral naltrexone is underway in Russia, and interim results show significantly improved outcomes for patients assigned to injectable naltrexone16.
At the Substance Treatment and Research Service (STARS) at the New York State Psychiatric Institute, we concurrently conducted randomized clinical trials investigating long acting injectable naltrexone in collaboration with investigators from the University of Pennsylvania11, as well as behavioral interventions to improve oral naltrexone compliance6. These recent trials provide an excellent opportunity to examine differences in early retention and urine-confirmed heroin use outcomes between similar groups of heroin-dependent individuals. Despite the given limitations of a quasi-experimental study (non-randomization to groups and potential selection biases), a strong case can be made for this comparison because patients in both trials were recruited concurrently, treated at the same clinic by overlapping staff, with many similarities in background treatment. All participants in both trials completed inpatient, buprenorphene-naltrexone assisted detoxifications and then received either long acting injectable or oral formulations of active naltrexone in separate randomized clinical trials.
In the present analysis, we compared retention and opiate use differences in the first eight weeks of treatment post-detoxification between patients receiving long acting injectable versus patients receiving oral formulations. These two trials were of varying length; the oral naltrexone study6 lasted for 24 weeks, while the injectable naltrexone study11 lasted only 8 weeks. Comparisons to the 8-week injectable naltrexone study11 were drawn from the first 8-weeks of the oral naltrexone study6. This analysis examined primarily length of time retained in treatment and opiate use while in treatment; the variables chosen for analysis (time retained, rate of dropout, and proportion of opiate negative urine samples) were selected because they were primary outcomes used in both of the previous published reports6, 11. We controlled for group differences on baseline patient characteristics. Given the small sample size, we included only one potential moderating variable; our previous work has shown that baseline severity of opiate use can interact with treatment condition, with higher severity patients benefiting from more intensive psychosocial treatment2. We included this severity variable as a potential moderator in all analyses.
In this comparison, approximately half of the patients receiving injectable naltrexone received a less-than therapeutic dose of naltrexone (192 mg/nl), as determined by a previous laboratory study showing that antagonism effects began to diminish by Week 417. In addition, half of the patients receiving oral naltrexone received a much less active psychosocial treatment (compliance enhancement). To estimate the effectiveness of depot naltrexone and oral naltrexone under more optimal conditions as it might be delivered in actual clinical practice (i.e., full dose of injectable naltrexone, integrated evidence-based psychosocial treatment), we conducted a series of sub-analyses comparing outcomes between Behavioral Naltrexone Therapy (BNT), an intensive psychosocial treatment combined with oral naltrexone, to a full-dose long acting injectable naltrexone condition combined with only moderate psychosocial intervention. These exploratory sub-analyses were conducted to assess the degree to which intensive psychosocial strategies may provide comparable results to long acting injectable naltrexone treatment.
Methods
Participants
Recruitment, screening, and eligibility criteria have been described extensively in the published reports6, 11, and are summarized here. Candidates were treatment-seeking heroin users recruited to a research clinic at an urban academic medical center, primarily through newspaper advertisements designating no-cost, confidential treatment for heroin problems in a research setting. Eligibility criteria across the two research protocols varied slightly, but shared in common the following requirements: between the ages of 18 and 59; and if they met DSM-IV criteria for opiate dependence without unstable medical disorders. In both research protocols, participants were excluded if they exhibited any major Axis I disorders such as schizophrenia, or Bipolar Disorder. Both studies permitted participants who exhibited depressive and anxiety symptoms, which are fairly common in opiate dependence but often remit with naltrexone treatment (e.g., diagnoses of Substance Induced Mood Disorder, Major Depression, Anxiety Disorder NOS were acceptable); participants in both studies were assessed using the Hamilton Rating Scale for Depression (HAM-D) to establish baseline level of psychiatric functioning18. In the injectable naltrexone study11, participants could not be dependent on any substances other than opiates, nicotine, and caffeine. Concurrent dependencies on other substances were acceptable in the oral naltrexone study6, as long as opiate dependence was determined to be primary; in addition candidates in the oral naltrexone study6 had to have a non-substance abusing significant other, relative, or sponsor (SO) who was prescreened and willing to participate in treatment.
Measures
This quasi-experimental analysis includes retention and substance use outcome measures analyzed in the original reports6, 11.
Retention
Retention is measured in number of days retained across the first eight weeks of treatment post-detoxification, and measured categorically in terms of whether a patient is retained by eight weeks. Patients were considered dropped from treatment after two weeks absence from the clinic.
Urinalysis
An observed urine sample was collected at all outpatient visits. This analysis employed a comparison of proportions of opiate-negative urine samples across treatment, with missing samples analyzed in two ways: dropped from analysis, and imputed as positive.
Procedures
Patients treated in the conditions in the following comparison received similar clinical care; differences between protocols are summarized here. Detailed descriptions of protocols can be found in the published reports6, 11.
Across conditions, all patients completed an eight-day buprenorphine-assisted detoxification and transition to naltrexone procedure on a locked, inpatient research ward. During the outpatient phase of treatment, patients attended twice-weekly psychosocial treatment. At these visits, patients provided urine-samples, were evaluated by the clinic nursing staff, and saw a clinician for the specified psychosocial treatment. The clinicians (doctoral and pre-doctoral level psychologists) providing the psychosocial treatment were trained in the manualized approaches, and sessions were audiotaped for supervision purposes. Patients were paid nominally for compliance with research visits at various data collection intervals, and were also provided a transportation stipend covering the cost of public transportation to the clinic.
The treatment groups differed in procedures in the following ways:
Oral Naltrexone Maintenance6
Detoxified patients were provided with oral naltrexone (50 mg) and were randomly assigned to Behavior Naltrexone Therapy (Oral/BNT) or Compliance Enhancement Therapy (Oral/CE). Oral/BNT8 strategically integrates several evidence-based behavioral approaches, including elements of cognitive-behavioral relapse prevention therapy19, 20, network therapy21, and voucher incentives (to a maximum of $28 per week, or $224 for the eight-week portion of the study under consideration) for opiate-negative urine samples22. Weekly sessions with the patient’s SO focused on monitoring naltrexone ingestion, and engaging the SO in supporting relapse prevention. Oral/CE is a manual-guided intervention23 delivered by experienced psychiatrists designed as a control condition for medication trials and intended to simulate standard clinic or office-based care, encourage medication compliance, and to control for professional attention. Patients were treated in this study from September of 1999 to May of 2002.
Long acting injectable naltrexone11
Detoxified patients were randomized to receive one of three levels (1. Placebo/CBT, 2. Low Dose/CBT [192 mg] or 3. High Dose/CBT [384 mg]) of an injectable, sustained-release, depot formulation of naltrexone (Depotrex; BIOTEK, Inc, Woburn, Mass). Four weeks later, patients received a second, identical dose of the study medication. Patients received twice-weekly manualized cognitive-behavioral relapse prevention therapy18. Patients were treated in this study from November of 2000 to June of 2003. 21% of this treatment group (N = 9) were recruited, enrolled, and treated at the University of Pennsylvania Treatment Research Center.
Analysis
Differences between groups on demographic and heroin use variables were examined and described, employing chi-square and t-tests to test between groups on categorical and continuous variables. Detected differences between comparison groups were controlled for in outcome analyses. After determining which patient level variables needed to be controlled in each set of analyses, we ran the analyses both with and without the covariates. In all cases, the results, including mean square, F values, and p-values, of the treatment effects were similar.
The analysis was conducted in two stages. First, patients (N = 111) were collapsed across type of naltrexone they received to compare long acting injectable naltrexone (N = 42; patients receiving placebo were excluded) against oral naltrexone (N = 69). Secondly, a set of analyses (N = 58) on a smaller sample was conducted to estimate the effeciveness of full-strength injectable naltrexone (384 mg/nl) when compared to oral naltrexone combined with intensive therapy; we included this set of subanalyses to more closely approximate effectiveness under typical clinical conditions (i.e., full dose of injectable naltrexone, active evidence-based psychosocial treatment). Patients receiving full-strength long acting injectable naltrexone (384 ng/ml) and cognitive-behavioral therapy (High Dose/CBT) were compared to patients receiving oral naltrexone and intensive psychosocial treatment (Oral/BNT). Mean days retained was analyzed with ANCOVA, and time to drop-out was analyzed with Cox regression survival analysis. Opiate use was compared by employing ANCOVA to examine differences across groups in proportion of opiate negative urine samples; a second set of analyses was included with missing urines imputed as positive. Both sets of analyses also included the interaction term between baseline opiate use severity (number of bags of heroin used per day) and the treatment group, as this interaction has been previously demonstrated to moderate the effects of psychosocial treatment when compared against a control oral maintenance condition2.
Days retained was moderately negatively skewed, although within the acceptable range, and proportion of opiate negative urines was more severely positively skewed. We ran a similar set of analyses using non-parametric tests (i.e., Mann-Whitney, Kolmogorov-Smirnoff, etc.) to verify that high skewness values were not inflating our results. In all cases, the results were quite similar, assuring us that issues of skewness were not hampering our analyses.
Results
Patient Characteristics
Combining the patients from each experimental group yielded an N of 111 heroin-dependent patients who completed detoxification and were discharged into outpatient naltrexone maintenance. Descriptive statistics broken down by group are presented in Table 1. Patients were majority male (80.2%), not currently in a cohabiting or marital relationship (75.7%), and were an average age of 37.6 years (SD = 10.0). Patients were majority Caucasian (46.8%), with 24.3% identifying as African-American, 26.1% identifying as Hispanic, and 2.7% of patients identifying that they were of another race.
Table 1.
Descriptive characteristics, retention rates, and substance use outcomes across high and low dose injections from the Comer et al. (3) study of long acting injectable naltrexone, and across behavioral-naltrexone and compliance enhancement conditions of oral maintenancefrom the Nunes et al. (7) study.
| Long Acting Injectable Naltrexone |
Oral Naltrexone |
|||||
|---|---|---|---|---|---|---|
| High Dose/CBT (N = 22) |
Low Dose/CBT (N = 20) |
Combined Injection (N = 42) |
Oral/BNT (N = 36) |
Oral/CE (N = 33) |
Combined Oral (N = 69) |
|
| Gender (% Male) | 86.4% (19) | 75.0% (15) | 81.0% (34) | 77.8% (28) | 81.8% (27) | 79.7% (55) |
| Race | ||||||
| Caucasian | 36.4% (8) | 35.0% (7) | 35.7% (15) | 50.0% (18) | 57.6% (19) | 53.6% (37) |
| African-American | 40.9% (9) | 35.0% (7) | 38.1 % (16) | 19.4% (7) | 12.1% (4) | 15.9% (11) |
| Hispanic | 13.6% (3) | 25.0% (5) | 19.0% (8) | 30.6% (11) | 30.3% (10) | 30.4% (21) |
| Other | 9.1% (2) | 5% (1) | 7.1% (3) | 0% (0) | 0% (0) | 0% (0) |
| Age | 40.6 (10.5) | 42.1 (10.5) | 41.3 (10.4) | 36.3 (10.2) | 34.3 7 (7.7) | 35.3 (9.1) |
|
In cohabiting
relationship |
||||||
| Yes | 27.3% (6) | 40.0% (8) | 33.3% (14) | 19.4% (7) | 18.2% (6) | 18.8% (13) |
| No | 72.7% (16) | 60.0% (12) | 66.7% (28) | 80.6% (29) | 81.8% (27) | 81.2% (56) |
|
21-Item Hamilton
Depression Rating |
11.8 (6.4) | 11.8 (5.5) | 11.8 (5.9) | 13.4 (6.8) | 12.5 (7.0) | 13.0 (6.9) |
| Years Heroin Use | 12.2 (12.3) | 13.6 (12.2) | 12.9 (11.2) | 11.0 (9.4) | 6.3 (5.0) | 8.7 (7.9) |
|
Average Bags
Heroin Per Day |
6.4 (4.0) | 5.4 (4.2) | 5.9 (4.1) | 6.5 (3.4) | 6.1 (3.7) | 6.3 (3.6) |
| Route Heroin Use | ||||||
| IN | 36.4% (8) | 60% (12) | 47.6% (20) | 61.1% (22) | 60.6% (20) | 60.9% (42) |
| IV | 45.5% (10) | 20% (4) | 33.3% (14) | 36.1% (13) | 39.4% (13) | 37.7% (26) |
| SC | ----- | 5% (1) | 2.4% (1) | ----- | ----- | ----- |
| Smoked | 4.5% (1) | 5% (1) | 4.8% (2) | 2.8% (1) | ----- | 1.4% (1) |
|
Percent Completed
8 Treatment Weeks (N) |
68.2% (15) | 45.0% (9)* | 57.1% (24) | 47.2% (17) | 30.3% (10) | 39.0% (27) |
|
Mean Days
Retained (SD) |
47.7 (15.8) | 36.5 (19.3) | 42.33 (18.2) | 36.6 (21.4) | 26.8 (22.7) | 31.9 (22.4) |
|
Percent Missing
Urine Samples |
34.1% | 46.8% | 40.2% | 47.7% | 63.6% | 55.3% |
|
Percent of Patients
Testing Positive For Opiates (N) |
50.0% (11) | 50.0% (10) | 50.0% (21) | 58.3% (21) | 51.5% (17) | 56.5% (38) |
|
Mean Proportion
Opiate-Negative Urine Sam ples (SD) |
0.78 (0.33) | 0.74 (0.35) | 0.76 (0.34) | 0.66 (0.37) | 0.75 (0.33) | 0.70 (0.35) |
|
Mean Proportion
Opiate-Negative Urine Samples (missing imputed as positive) (SD) |
0.57 (0.31) | 0.46 (0.37) | 0.52 (0.34) | 0.40 (0.35) | 0.34 (0.30) | 0.37 (0.33) |
Percent retained in Low Injection/CBT differs from that previously reported in the Comer et al. paper (3) for the 192 mg, as the current report adopted a more rigorous threshold for classifying patients as dropped from treatment. 3 patients considered completers in the Comer paper were reclassified as dropouts due to greater than two weeks absence from the clinic during treatment.
On average, patients reported using 6.1 bags of heroin per day (SD = 3.7), and half (52.4%; N = 77) reported using heroin intra-nasally. 37.4% of patients reported IV injection as their main route of heroin use. The remainder of patients either smoked (5.4%) or injected heroin subcutaneously (1.4%), with 3.4% unknown. Patients reported using heroin regularly an average of 10.2 years (SD = 9.7). Baseline 21-item Hamilton Depression Rating Scores averaged 12.54 (SD = 6.50) and did not differ significantly between groups.
As specified in the exclusion criteria, patients in the long-acting injectable study11 could not meet criteria for dependence on any illicit substances other than opiates. Patients in the oral naltrexone study could meet criteria for dependence on other substances. However, rates of other substance dependencies in the oral naltrexone study were quite low: cocaine (7%), marijuana (3%), alcohol (7%), and sedatives (1%).
The majority of long-acting injectable patients were treated at STARS in New York City, also the site of the oral naltrexone study; four patients from the Low Injection/CBT group and five patients from the High Dose/CBT group were treated at the University of Pennsylvania in Philadelphia. These patients did not differ from the STARS patients in the injectable naltrexone treatment groups on any retention or opiate use variable, and did not differ significantly from the STARS patients on any demographic variable except for age; patients treated at the University of Pennsylvania were on average ten years older than patients treated at STARS (t (40) = 2.79, p = 0.008). However, since age was not correlated with any outcome variable in these groups, this difference was ignored and the University of Pennsylvania patients were included in the analysis. Percentages of patients completing eight weeks of treatment, mean days retained in treatment, and mean proportion of heroin-negative urine samples across the four treatment groups are also presented in Table 1.
Collapsed Conditions: Long Acting Injectable Versus Oral Naltrexone Treatment
High and Low Injection conditions and BNT and CE conditions were combined. There were significant demographic differences found between the Injection and Oral groups. Patients receiving long acting injections tended to be older (Injection M = 41.3, Oral M = 35.3; t (109) = 3.18, p = 0.002), had a longer history of regular heroin use (Injection M = 12.9 years, Oral M = 8.7 years; t (109) = 2.30, p = 0.024), and were more likely to be African-American than patients receiving oral naltrexone (38% in the Injection group, 16% in the Oral group; Chi-square (3) = 13.28, p = 0.004). All analyses exploring differences between Oral and Injection conditions controlled for these three variables.
Fifty-seven percent of Injection patients and 42% of Oral patients completed 8 weeks of treatment. Sixty-seven percent (28 of 42) injection patients consented to receive the second dose of injectable naltrexone after four weeks; patients not receiving the second dose often dropped from treatment at or just before the four week point. Injection patients remained in treatment for an average of 42.3 days (SD = 18.2), while Oral patients remained in treatment for an average of 31.9 days (SD = 22.42); when controlling for differences between groups, this difference was statistically significant (F (1, 106) = 6.49, p = 0.012); see Table 2 for the full model. Cox survival analysis showed no difference between conditions on rate of drop-out (Exp(B) = 0.75, p = 0.20). The interaction between treatment condition and severity of baseline heroin use was included in these two analyses, but was not found to be significant and so was dropped from the models.
Table 2.
Adjusted means, standard deviations, and ANCOVA models1 comparing oral naltrexone versus depot naltrexone on days retained, proportion of heroin negative urines, and proportion of heroin negative urines with missing imputed as positive.
| Depot Naltrexone |
Oral Naltrexone |
|||||
|---|---|---|---|---|---|---|
| ANCOVA |
||||||
| Outcome Variable | M | SD | M | SD | F (1, 111) | Adj. R2 |
| Days Retained in Treatment | 42.5 | 3.3 | 31.8 | 2.5 | 6.49* | 0.086 |
| Proportion of Heroin Negative Urine Samples |
0.77 | 0.06 | 0.70 | 0.05 | 1.00 | 0.024 |
| Proportion of Heroin Negative Urine Samples (Missing Imputed as Positive) |
0.52 | 0.05 | 0.37 | 0.04 | 5.26* | 0.048 |
Models include participant race, age, and years of regular heroin use as covariates.
p < 0.05
Injection patients furnished 62.5% of requested urine samples (M = 9.48, SD = 4.98); Oral patients furnished only 44.7% of requested urine samples (M = 7.67, SD = 5.18). Typically, patients across both conditions provided at least one urine sample per week until they dropped from treatment, and then all remaining urine samples were missing. The differences between conditions in number of provided urine samples did not reach statistical significance (t (109) = 1.81, p = 0.071). Fifty percent (N = 21) of Injection patients tested positive for opioids during the first eight weeks in treatment; 90% (N = 19) of those who tested positive had their first heroin positive urine within the first two weeks of outpatient treatment. Fifty-five percent (N = 38) of Oral patients tested positive for opioids during the first eight weeks in treatment; 87% (N = 33) of those who tested positive had their first positive urine sample with the first two weeks of treatment. We also examined the rate of provision of any positive urine sample in the last two weeks of treatment (Weeks 7 and 8); eight percent (2 of 26) of Injection patients tested positive for opioids during the last two weeks of treatment, and 21% (6 of 29) of Oral patients tested positive in the last two weeks. There were no significant differences between Injection and Oral conditions on proportion of heroin negative urines provided during treatment (F (1, 106) = 1.00, p = 0.32). However, when missing urines were imputed as positive, Injection patients demonstrated a higher proportion of heroin negative urine samples (F (1, 106) = 5.26, p = 0.024). Again, the interaction between treatment condition and severity of baseline heroin use was included in these two analyses, but was not found to be significant and so was dropped from the models.
It is noteworthy that patients receiving injectable naltrexone continued to use opiates at approximately the same frequency and speed as patients receiving oral naltrexone. We looked more closely at the individual opiate use patterns of patients who used opiates early in treatment (the first two weeks), noting whether those patients went on to complete a full 8-week course of treatment. Forty-seven percent (N = 9) of Injection patients who used in the first 8 weeks of care went on to complete treatment, while only 27% (N = 8) of early using Oral patients completed 8 weeks of care.
Intensive Psychosocial Oral Maintenance vs. High Dose Injection Maintenance (N = 58)
A second set of analyses comparing Oral/BNT (N = 36) to High Dose Injection/CBT (N = 22) was undertaken, which also included the interaction between treatment condition and severity of baseline heroin use. These two conditions differed from each other on race (41% in the High Dose Injection/CBT group, 19% in the Oral/BNT group; Chi-square (3) = 7.74, p = 0.052); again, patients in the High Dose Injection/CBT condition were more likely to be African-American.
Sixty-eight percent of High Dose Injection/CBT patients and 53% of Oral/BNT patients completed 8 weeks of treatment. Eighty-two percent (18 of 22) High Dose Injection/CBT patients consented to receive the second dose of injectable naltrexone after four weeks; patients not receiving the second dose often dropped from treatment at or just before the four week point. High Dose Injection/CBT patients remained in treatment for an average of 47.7 days (SD = 15.8), while Oral/BNT patients remained in treatment for an average of 36.6 days (SD = 21.4); when controlling the impact of race on outcome, this difference was statistically significant (F (1, 58) = 5.60, p = 0.021). When the interaction term was added to this equation, it was also significant ((F (1, 58) = 6.51, p = 0.012); see Table 3 for the full model. High severity users remained in treatment longer in Oral/BNT, whereas lower severity users completed more days of treatment in High Dose Injection/CBT.
Table 3.
Full ANCOVA models comparing Oral Behavior Naltrexone Therapy (Oral/BNT) versus full-dose depot naltrexone (Hi-Depot/CBT) on days retained, proportion of heroin negative urines, and proportion of heroin negative urines with missing imputed as positive.
| Outcome Variable | df | Mean Square | F | p-value |
|---|---|---|---|---|
| Days Retained in Treatment | ||||
| Treatment Group | 1 | 3830.85 | 11.84 | 0.001 |
| Race | 1 | 1803.82 | 5.58 | 0.022 |
| Baseline Heroin Use | 1 | 10.80 | 0.03 | 0.856 |
| Treatment Group x Baseline Heroin Use |
1 | 2106.99 | 6.51 | 0.014 |
| Error | 53 | 323.56 | ||
| Adjusted R2 = 0.197 | ||||
|
| ||||
|
Proportion of Heroin
Negative Urine Samples |
||||
| Treatment Group | 1 | 0.867 | 7.32 | 0.009 |
| Race | 1 | 0.094 | 0.71 | 0.379 |
| Baseline Heroin Use | 1 | 0.000 | 0.00 | 0.997 |
| Treatment Group x Baseline Heroin Use |
1 | 0.693 | 5.85 | 0.019 |
| Error | 48 | 0.118 | ||
| Adjusted R2 = 0.077 | ||||
|
| ||||
|
Proportion of Heroin
Negative Urine Samples (Missing Imputed as Positive) |
||||
| Treatment Group | 1 | 1.321 | 13.51 | 0.001 |
| Race | 1 | 0.128 | 1.31 | 0.257 |
| Baseline Heroin Use | 1 | 0.046 | 0.47 | 0.497 |
| Treatment Group x Baseline Heroin Use |
1 | 0.876 | 8.96 | 0.004 |
| Error | 53 | 0.098 | ||
| Adjusted R2 = 0.173 | ||||
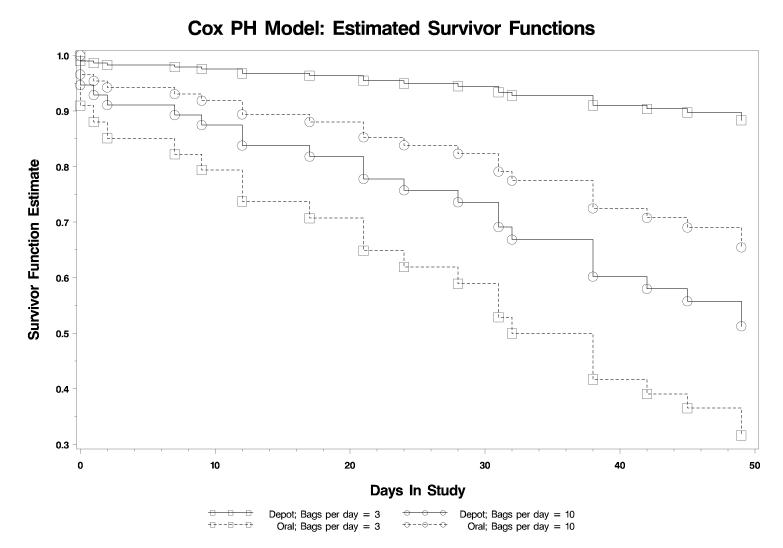
Cox survival analysis models found a significant interaction between treatment groups and baseline severity on time to drop out from treatment (Likelihood ratio Chi-square(1) = 9.31, p = 0.002). In the Oral/BNT group, an increase of one bag per day in baseline use was associated with a 14% decrease in the hazard of dropout (Hazard Ratio = 0.86, 95% CI = 0.73-1.05), while in the High Dose Injection/CBT group, a one bag per day increase in baseline use was associated with a 50% increase (Hazard Ratio = 1.50, 95% CI = 1.13-2.01) in the hazard for dropout. See Figure 1 for a graphic representation of the (Cox model) estimate of the survival function based on severity of baseline heroin use, using 3 bags per day and 10 bags per day as representative levels of baseline use (plus or minus 1 standard deviation from the mean of bags per day).
Figure 1. Survival function over eight weeks in treatment demonstrating the interaction between treatment condition and baseline opioid severity for patients treated in High Dose Injection / CBT versus patients treated with Oral Maintenance and BNT.
Figure displays survival functions for patients treated with 1) 384ng/ml injections of depot naltrexone plus twice weekly CBT or 2) oral naltrexone plus intensive behavioral treatment (BNT) as a function of baseline opioid dependence severity. Designations for high (10 bags/day) and low (3 bags/day) severity are plus or minus one standard deviation (3.6) from the mean (6.4), rounded to the nearest whole unit.
High Dose Injection/CBT patients furnished 65.9% of requested urine samples (M = 10.36, SD = 4.44); Oral/BNT patients provided 52.3% of requested urine samples (M = 8.70, SD = 5.20). This difference was not significant (t (56) = 1.25, p = 0.216). Fifty percent of High Dose Injection/CBT (N = 11) patients tested positive for opioids during the first eight weeks in treatment; all but one of those patients who tested positive had their first heroin positive urine within the first two weeks of outpatient treatment. Fifty-eight percent (N = 20) of Oral/BNT patients tested positive for opioids during the first eight weeks in treatment; 90% (N = 18) of those who tested positive had their first positive urine sample within the first two weeks of treatment. We also examined the rate of provision of any positive urine sample in the last two weeks of treatment (Weeks 7 and 8); six percent (1 of 16) of Injection patients tested positive for opioids during the last two weeks of treatment, and 17% (3 of 18) of Oral patients tested positive in the last two weeks. There were no significant differences between High Dose Injection/CBT and Oral/BNT conditions on proportion of heroin negative urines provided during treatment (F (1, 53) = 1.37, p = 0.25). When the interaction term between treatment condition and baseline severity was entered, it was statistically significant (F (1, 53) = 5.85, p = 0.019); see Table 3 for the full model. High severity users were more likely to exhibit less heroin use in Oral/BNT, whereas lower severity users were more likely to use less heroin in High Dose Injection/CBT. The same comparison was also analyzed when missing urine samples were imputed as negative. When the interaction term between treatment condition and baseline severity was entered, it was statistically significant (F (1, 53) = 8.96, p = 0.004); see Table 3 for the full model. Again, high severity users were more likely to exhibit less heroin use in Oral/BNT, whereas lower severity users were more likely to use less heroin in High Dose Injection/CBT.
Again, we looked more closely at the individual opiate use patterns of patients who used opiates early in treatment (the first two weeks), noting whether those patients went on to complete a full 8-week course of treatment. Seventy percent (N = 7) of High Dose Injection/CBT patients who used in the first 8 weeks of care went on to complete treatment, while only 33% (N = 6) of early using Oral/BNT patients completed 8 weeks of care.
Adverse Events
The use of naltrexone as an antagonist provokes some concern about safety issues, such as concerns that patients will attempt to override the antagonist-blockade, or risk of overdose. Across all 111 patients reported here, we saw no indication that any patient attempted to override the naltrexone-blockade. One patient in the compliance enhancement condition (oral naltrexone combined with minimal psychosocial treatment) did die of an accidental heroin overdose after having discontinued naltrexone and resumed baseline heroin use. This patient presented in week 20 with urine positive for opioids and nonflorescent for riboflavin, indicating discontinuation of naltrexone, and subsequently overdosed.
Discussion
This quasi-experimental comparison of relatively similar heroin-dependent patients participating in varied conditions of outpatient naltrexone-maintenance treatment suggests overall that a long acting, sustained-release, depot formulation of naltrexone may be superior to oral naltrexone for the antagonist treatment of heroin dependence, although it also raises some questions about the robustness of the effect of long-acting naltrexone. When long acting injectable naltrexone conditions from the Comer et al.11 study were combined and compared to the early combined results from the Nunes et al.6 oral naltrexone maintenance study, patients receiving long acting injectable naltrexone were retained for more days in treatment. The difference in dropout rate evaluated with survival analysis was in the same direction but did not reach signficance. When missing urines were imputed as positive, the proportion of opioid negative urines was greater on injectable than oral naltrexone. The difference was not significant when only observed urines were counted, but the imputation of missing urines as positive is reasonable, because most patients who dropout can be assumed to have relapsed.
A second series of analyses compared the group that received an intensive psychosocial treatment (behavior naltrexone therapy), combining oral naltrexone with numerous and powerful psychosocial strategies, against a moderate psychosocial intervention with a full dose of long acting injectable naltrexone; this set of analyses was included because it represented the two most likely manners in which naltrexone might be used in actual clinical practice. In this comparison with relatively small numbers, injectable naltrexone with moderate treatment retained patients significantly longer than the intensive strategy intended to support oral maintenance. But, when the interaction of baseline heroin severity with treatment condition was included in the model, it was significant, indicating that patients with high baseline heroin use demonstrated better retention on oral naltrexone plus intensive psychosocial intervention, while lower severity heroin users were more successful in the condition with moderate psychosocial treatment and long acting injectable naltrexone. Differences in heroin use demonstrated a similar pattern.
The finding of an interaction effect between condition and baseline severity of use should be approached with caution. It is possible that the intensive psychosocial approaches employed in BNT are most needed amongst heavy opioid users, and that while the majority of users might be served by moderate psychosocial treatment and implants or injections, patients with high levels of opioid dependence may require more intensive psychosocial treatment. Of course, given the quasi-experimental nature of this research, these findings should be viewed cautiously and be considered as hypotheses to guide further study. Future research examining long-acting injectable naltrexone in combination with an intensive behavioral regimen similar to BNT in a randomized trial would more definitively measure the value of high-intensity psychosocial treatments.
One of the most interesting findings of this comparison was the relatively similar level of early opiate use by patients across all conditions; approximately 50% of patients used heroin in the first two weeks of treatment, regardless of the route of naltrexone treatment. In this respect, High Dose Injection (384 ng/ml) showed its promise, as 70% of early opiate users recovered from this use to go on to complete eight weeks of treatment.
It is also noteworthy that across these 111 patients, we were aware of no patient attempts to override the naltrexone blockade. This is a common concern about naltrexone, but seems to be rare and our experience bears this out. The main concern with naltrexone is overdose risk after the blockade has worn off. Injectable naltrexone may be safer in this respect because the blockade wears off more slowly.
In comparison to other quasi-experimental studies of oral and implant formulations of naltrexone13-15, this modestly-powered study is the most rigorous comparison to date. The data were collected during the course of randomized clinical trials; consequently, numerous urine samples were collected from patients engaged in treatment, and opiate use results are available over the course of the full eight weeks of treatment, rather than having to rely on self-report or collateral report. Some groups differed significantly from each other on some key variables, such as age and number of years of regular heroin use; these variables were controlled for during analysis.
All advantages to this comparison noted, the study suffers from some significant limitations. Patients were not randomized to conditions, and significant selection biases are possible. The selection criteria for patients and background treatment patients received in both trials were very similar, although patients treated at the STARS clinic had some latitude in choosing which treatment study to participate in. (If anything, the bias in patient selection favors the oral naltrexone study and against injectable naltrexone for two reasons: patients in the oral naltrexone study were required to have a significant other willing to participate in treatment [indicating a certain level of social support and functioning], and half of the injection patients were on a lower, less powerful dose of medication.) Furthermore, these treatments were not originally intended to be compared to one another, so there may be differences that cannot be accounted for. We do not have follow-up data on patients who dropped from treatment early on, and so can only presume relapse to heroin use (the most typical course when patients have been recently detoxed and are not maintained on medication). Finally, while other opiate users were not excluded, we have only heroin users in these samples because this is how the recruitment advertising was worded; further studies with prescription opiate users are needed to assess the effectiveness of these approaches with other opioids of abuse.
Given these limitations, these results should be interpreted with caution, and viewed as exploratory, for the purposes of informing the hypotheses of future research studies, as well as to help manage practitioners expectations when working with oral and injectable naltrexone. However, despite these limitations, the differences between high-dose long acting injectable formulations and other oral maintenance strategies are intriguing. This quasi-experimental study of early outcomes in naltrexone maintenance strategies demonstrates some benefit to long acting injectable formulations, but also shows that heroin use behavior during maintenance treatment is relatively similar despite route of naltrexone administration; this is not surprising, as retention in treatment is the more important outcome in maintenance treatment. If opioid dependent patients are able to stay on naltrexone, opioid taking behavior is likely to extinguish due to the powerful blockade of opioid reinforcing effects. Furthermore, contingent on future replication, the findings of this study also indicate that despite the development of new formulations of naltrexone, the value of intensive psychosocial approaches may continue to be demonstrated for severe users. It is possible that combining monthly injections with some of the strategies which have maximized oral compliance (high-value vouchers, significant-other involvement) could further increase the effectiveness of long acting injectable naltrexone maintenance, and position this treatment as a viable alternative to agonist maintenance (buprenorphine or methadone) for some opioid dependent patients.. There is a need for continued studies that would determine the optimal strategies for combining psychosocial approaches with the new long acting injectable formulations of naltrexone to yield maximal effectiveness.
Acknowledgments
The authors gratefully acknowledge the support of the National Institute of Drug Abuse; support for original data collection drawn from center grants P50 DA09236 and P60 DA05186, as well as RO1 DA-10746 and K02 00288 (Dr. Nunes).
Footnotes
Disclosures The majority of authors of this manuscript (Drs. Brooks, Comer, Sullivan, Bisaga, Raby, Carpenter, Yu, and O’Brien) have no conflicts to disclose that could affect the reporting of this study.
Dr. Nunes has one conflict to report. Dr. Nunes served on the board of Alkermes-Cephalon until he resigned from that position in November of 2007. Alkermes-Cephalon makes a comparable product to Depotrex, the product used in this study.
References
- 1.Rounsaville BJ. Can psychotherapy rescue naltrexone treatment of opioid addiction? In: Onken LS, Blaine JD, Boren JJ, editors. NIDA Research Monograph Series 150: Integrating Behavioral Therapies with Medications in the Treatment of Drug Depedence. U.S. DHHS; 1995. pp. 37–52. [PubMed] [Google Scholar]
- 2.Carpenter KM, Jiang H, Sullivan MA, Bisaga A, Comer SD, Raby WN, Brooks AC, Nunes EV. Betting on change: Modeling transitional probabilities to guide therapy development for opioid dependence. Psych Addict Behav. doi: 10.1037/a0013049. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grabowski J, O’Brien CP, Greenstein RA. Effects of contingent payment on compliance with a naltrexone regimen. Am Jrnl Drug Alc Abuse. 1979;6:355–365. doi: 10.3109/00952997909001724. [DOI] [PubMed] [Google Scholar]
- 4.Carroll KM, Ball SA, Nich C, O’Connor PG, Eagan EA, Frankforter TL, Triffleman EG, Shi J, Rounsaville BJ. Targeting behavioral therapies to enhance naltrexone treatment of opioid dependence: Efficacy of contingency management and significant other involvement. Arch Gen Psych. 2001;58(8):755–761. doi: 10.1001/archpsyc.58.8.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Preston KL, Silverman K, Umbricht A, DeJesus A, Montoya ID, Schuster CR. Improvement in naltrexone treatment compliance with contingency management. Drug Alc Depend. 1999;54(2):127–135. doi: 10.1016/s0376-8716(98)00152-5. [DOI] [PubMed] [Google Scholar]
- 6.Nunes EV, Rothenberg JL, Sullivan MA, Carpenter KM, Kleber HD. Behavioral therapy to augment oral naltrexone for opioid dependence: A ceiling on effectiveness? Am Jrnl Drug Alc Abuse. 2006;32(4):503–517. doi: 10.1080/00952990600918973. [DOI] [PubMed] [Google Scholar]
- 7.Fals-Stewart W, O’Farrell TJ. Behavioral family counseling and naltrexone for male opioid-dependent patients. Journal Consult Clin Psych. 2003;71(3):432–442. doi: 10.1037/0022-006x.71.3.432. [DOI] [PubMed] [Google Scholar]
- 8.Rothenberg JL, Sullivan MA, Church SH, Seracini A, Collins E, Kleber HD, Nunes EV. Behavioral naltrexone therapy: and integrated treatment for opioid dependence. J Subst Abuse Treat. 2002;23(4):351–360. doi: 10.1016/s0740-5472(02)00301-x. [DOI] [PubMed] [Google Scholar]
- 9.Hulse GK, Basso MR. The association between naltrexone compliance and daily supervision. Drug and Alcohol Review. 2000;19(1):41–48. [Google Scholar]
- 10.Johansson BA, Berglund M, Lindgren A. Efficacy of maintenance treatment with naltrexone for opioid dependence: A meta-analytical review. Addiction. 2006;101(4):491–503. doi: 10.1111/j.1360-0443.2006.01369.x. [DOI] [PubMed] [Google Scholar]
- 11.Comer SD, Sullivan MA, Yu E, Rothenberg JL, Kleber HD, Kampman K, Dackis C, O’Brien CP. Injectable, sustained-release naltrexone for the treatment of opioid dependence: A randomized, placebo-controlled trial. Arch Gen Psych. 2006;63(2):210–218. doi: 10.1001/archpsyc.63.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foster J, Brewer C, Steele T. Naltrexone implants can completely prevent early (1-month) relapse after opiate detoxification: A pilot study of two cohorts totaling 101 patients with a note on naltrexone blood levels. Addiction Biology. 2003;8(2):211–217. doi: 10.1080/1355621031000117446. [DOI] [PubMed] [Google Scholar]
- 13.Colquhoun R, Tan DYK, Hull S. A comparison of oral and implant naltrexone outcomes at 12 months. Journal of Opioid Management. 2005;1(5):249–256. doi: 10.5055/jom.2005.0054. [DOI] [PubMed] [Google Scholar]
- 14.Carreno JE, Alvarez CE, Narciso GI San, Bascaran MT, Diaz M, Bobes J. Maintenance treatment with depot opioid antagonists in subcutaneous implants: An alternative in the treatment of opioid dependence. Addiction Biology. 2003;8(4):429–438. doi: 10.1080/13556210310001646402. [DOI] [PubMed] [Google Scholar]
- 15.Reece AS. Psychosocial and treatment correlates of opiate free success in a clinical review of a naltrexone implant program. Substance Abuse Treatment, Prevention, and Policy. 2007;2(35) doi: 10.1186/1747-597X-2-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krupitsky EM, Zvartau E, Egorova V, Masalov D, Burakov A, Tsoy M, Bushara N, Romanova T, Verbitskaya E, O’Brien CP, Woody GE. Double blind randomized placebo controlled study of effectiveness of implantable naltrexone (Prodetoxone) for treatment of heroin addiction: Interim analysis. Presented at 70th Annual Meeting of College on Problems of Drug Dependence; San Juan, Puerto Rico. 2008. [Google Scholar]
- 17.Comer SD, Collins ED, Kleber HD, Nuwayser ES, Kerrigan JH, Fischman MW. Depot naltrexone: Long-lasting antagonism of the effects of heroin in humans. Psychopharmacology. 2002;159:351–360. doi: 10.1007/s002130100909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams JBW. A structured interview guide for the Hamilton Depression Rating Scale. Arch Gen Psych. 45(8):742–747. doi: 10.1001/archpsyc.1988.01800320058007. [DOI] [PubMed] [Google Scholar]
- 19.Marlatt GA, Gordon JR. Relapse prevention and maintenance strategies in the treatment of addictive behaviors. Guilford Press; New York: 1985. [Google Scholar]
- 20.Carroll KM. A cognitive-behavioral approach: Treating cocaine addiction. NIDA Therapy Manuals for Drug Addiction. 1998 [Google Scholar]
- 21.Galanter M. Network therapy for addiction: A model for office practice. Am J Psychiatry. 1993;150(1):28–36. doi: 10.1176/ajp.150.1.28. [DOI] [PubMed] [Google Scholar]
- 22.Higgins ST, Delaney DD, Budney AJ, Bickel WK, Hughes JR, Foerg F, Fenwick JW. A behavioral approach to achieving initial cocaine abstinence. Am J Psychiatry. 1991;148(9):1218–1224. doi: 10.1176/ajp.148.9.1218. [DOI] [PubMed] [Google Scholar]
- 23.Carroll KM, Nuro KR, O’Malley SS. Compliance enhancement: A manual for the clinical management of drug-dependent patients. Yale University School of Medicine, Psychotherapy Development Center; New Haven, CT: 1999. [Google Scholar]