Abstract
Background. The Centers for Disease Control and Prevention (CDC) and others reported that methicillinresistant S. aureus (MRSA) are significant causes of serious human infections, including pulmonary illnesses. We investigated the role played by superantigens in lung-associated lethal illness in rabbits.
Methods. A rabbit model was established to investigate the potential role played by superantigens, staphylococcal enterotoxin B (SEB), staphylococcal enterotoxin C , and toxic chock syndrome toxin-1 (TSST-1). Rabbits received intrabronchial community-associated (CA) MRSA strains USA200 (TSST-1+), MW2 (SEC+), c99-529 (SEB+), Or Purified Superantigens. Some Rabbits Were Preimmunized Against Superantigens Or Treated With Soluble High-Affinity T Cell Receptors (Vβ-TCR) to Neutralize SEB and then challenged intrabronchially with CA-MRSA or superantigens.
Results. Rabbits challenged with CA-MRSA or superantigens developed fatal, pulmonary illnesses. Animals preimmunized against purified superantigens, or treated passively with Vβ-TCRs and then challenged with CAMRSA or superantigens, survived. Lung histological analysis indicated that nonimmune animals developed lesions consistent with necrotizing pneumonia after challenge with CA-MRSA or purified superantigens. Superantigenimmune animals or animals treated with soluble Vb-TCRs did not develop pulmonary lesions.
Conclusions. Superantigens contribute to lethal pulmonary illnesses due to CA-MRSA; preexisting immunity to superantigens prevents lethality. Administration of high-affinity Vβ-TCR with specificity for SEB to nonimmune animals protects from lethal pulmonary illness resulting from SEB+ CA-MRSA and SEB.
Staphylococcus aureus is a significant human pathogen that causes multiple illnesses [1]. In recent years, there has been a rapid emergence of severe soft tissue and pulmonary infections caused by community-associated methicillin-resistant S. aureus (CA-MRSA) [2, 3]. These potentially fatal infections, including toxic shock syndrome (TSS), purpura fulminans, and necrotizing pneumonia occur in individuals lacking predisposing risk factors, although the majority may have had prior upper respiratory viral infections [2–4].
Staphylococcal superantigens are exotoxins that stimulate massive cytokine production by both T lymphocytes and macrophages [5, 6]. These cytokines include tumor necrosis factor α and β, interleukin 1β, interleukin 2, and interferon g [7] and cause many of the clinical features of TSS. Superantigens bind to and crosslink variable regions of certain β-chains of T cell receptors (Vβb-TCRs) and either one or both of the α-or β-chains of major histocompatibility complex (MHC) II molecules on macrophages [8, 9].
Superantigens, such as TSS toxin-1 (TSST-1) made by CA-MRSA USA200 strains (CDC designation based on pulsed-field gel electrophoresis) and staphylococcal enterotoxins B and C (SEB and SEC, respectively) made by CA-MRSA USA400 strains [4], are associated with TSS and other serious illnesses in humans [5, 6]. TSST1 is associated with nearly all cases of menstrual TSS, and 50% of nonmenstrual cases. SEB and SEC are associated with most of the remaining cases of nonmenstrual TSS [10, 11].
In the present study, we investigated the role played by these 3 superantigens produced by CA-MRSA in rabbit models of lethal pulmonary illness.
Materials and Methods
CA-MRSA strains. USA200 strains included MNPA (menstrual TSS isolate), MN1021, and MN128. These organisms appear to be highly related, with MN1021 and MN128 coming from the same outbreak; data presented are accumulated from the use of all 3 organisms. These isolates produce elevated amounts of TSST-1 compared with USA200 Methicillin-sensitive S. aureus (MSSA) but do not produce α, β, γ, or Panton-Valentine leukocidin (PVL) cytotoxins, and the organisms are nonpigmented [12]. The strains have mutations in the α-and γ-toxin genes as determined by nucleotide sequencing and lack PVL genes as determined by polymerase chain reaction [13, 14]. USA400 strains were MW2 and c99–529, isolated from children who died of necrotizing pneumonia [4]. MW2 produces SEC, whereas CA-MRSA c99–529 produces SEB. Both of the USA400 strains also produce α-and γ-toxins and PVL but not β-toxin.
Rabbits. Dutch belted rabbits (1.5–2 kg) were used in accordance with guidelines established by the University of Minnesota Institutional Animal Care and Use Committee (IACUC).
Superantigens. All reagents used for preparation of purified superantigens were maintained lipopolysaccharide-free. TSST-1 was purified to homogeneity from S. aureus clone RN4220 (pCE107); this strain does not produce other super-antigens. SEB was purified from S. aureus MNHo and SEC from MW2. Superantigens were purified after growth of organisms in dialyzable beef heart media [15]. Superantigens were precipitated from culture fluids with 4 volumes of absolute ethanol, resolubilized in distilled water, and purified by thin-layer isoelectric focusing [15, 16]. Initial isoelectric focusing pH gradients were 3.5–10, followed by second gradients of pH 6- 8 for TSST-1 and pH 7–9 for SEB and SEC; the isoelectric point for TSST-1 is 7.2 and for SEB and SEC is 8.3 [17, 18]. Purified superantigens were quantified using the BioRad Protein assay (BioRad), with SEB as the protein standard. Purity was confirmed by superantigen migration as single bands when subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis [19] with silver staining and reversed phase high-performance liquid chromatography [20] (also to confirm lack of contaminating lipoteichoic acid), Limulus assay to confirm lack of detectable lipopolysaccharide, and bioassays to confirm absence of detectable cytolysins, lipase, and protease [21]. Purified superantigens were also free of detectable peptidoglycan and lipopolysaccharide as demonstrated by lack of pyrogenicity with 2-h and 3-h fever peaks [22].
Pulmonary illness model. Rabbits were administered CAMRSA or purified superantigens through intrabronchial inoculation (2 X 109colony-forming units in 200 μL of dialyzable beef heart medium and 100–200 μg of purified superantigens in 200 μL of phosphate-buffered saline). Rabbits were anesthetized by subcutaneous injections of ketamine (25 mg/kg) and xylazine (25 mg/kg) (Phoenix Pharmaceuticals) [23]. Once under anesthesia, their necks were shaved, and small incisions were made along the tracheas. Incisions (3 mm) were made in the tracheas, and polyethylene catheters (1-mm diameter; Fisher Scientific) were inserted and threaded into the left bronchi. Bacteria or purified superantigens were administered through the catheters. Once exposed to CA-MRSA or superantigens, rabbits were monitored for up to 7 days for development of respiratory distress and lethal illness (this point is defined as death or, in agreement with the University of Minnesota IACUC and 28 years research experience, rabbit failure to exhibit both escape behavior and ability to right themselves). At this point, the animals were killed by intravenous administration of 1 mL/kg Beuthanasia D (Schering-Plough Animal Health Corp). Rabbits that did not develop respiratory distress and lethal illness were killed after 7 days.
Immunizations. Dutch belted rabbits were hyperimmunized against either purified TSST-1 or SEC before receiving intrabronchial CA-MRSA or purified superantigens. Superantigens (in phosphate-buffered saline, 0.005 mol/L NaPO4,pH 7.2, 0.15 mol/L NaCl) were mixed with equal volumes of incomplete Freund adjuvant (Difco Laboratories). Final concentrations of 50 μg/mL of superantigens were used for injections, with each rabbit receiving 1.0 mL, injected subcutaneously into 4 sites on the nape of the neck. Animals received initial injections, followed by booster injections every 2 weeks until antibody titers were >10,000, as determined by enzyme-linked immunosorbent assay (ELISA); antibody titers of >10,000 were considered hyperimmune (humans who do not develop menstrual TSS typically have immunoglobulin G [IgG] titers of 160). For ELISA, wells of flat-bottom 96-well plates were coated with 1.0 μg/well of purified TSST-1 or SEC [24] and washed. Rabbit serum samples were serially diluted 2-fold in the wells beginning with 1/10 dilutions; the plates were incubated for 2 h at room temperature, and then wells were washed. Horseradish peroxidase-conjugated anti-rabbit IgG (Sigma-Aldrich) were added to wells, the plates were incubated for 2 h at room temperature, and the wells were again washed. The relative levels of IgG were determined by the addition of color substrate containing o-phenylenediamine and H2O2 (100 μL/well). Reactions were stopped by addition of 12.5% sulfuric acid (50 μL), and then absorbances at 490 nm wavelength were measured spectophotometrically.
Soluble high-affinity Vβ-T cell receptor (G5–8). Some rabbits received soluble high-affinity Vβ-TCR (100 μg administered intravenously daily) G5–8 in addition to CA-MRSA or superantigens. Soluble high-affinity Vβ-TCR G5–8 with 48 pmol/L specificity for SEB was generated by Vβ-TCR mutagenesis and selection by flow cytometry [25]. This 12,000 molecular weight molecule is highly specific for reactivity to SEB, but not SEC and TSST-1; it effectively neutralizes SEB super-antigenicity and thus neutralizes lethal activity through competition with Vβ-TCR.
Histology. Rabbit lung tissue samples were excised immediately upon death of animals or at the termination of experimentation (7 days), fixed in 10% formalin, and embedded in paraffin wax. Thick tissue sections (10 mm) were obtained using a microtome (Leica RM2235, Wetzlar). Sections were stained with hematoxylin (Fisher Scientific) and eosin (Sigma-Aldrich) (&) following standard protocols at the University of Minnesota Veterinary Animal Pathology Laboratory.
Statistical analyses. Data were analyzed by Fisher exact test. P values of ⩽.05 were considered significant. The Reed and Muench method was used to calculate the median lethal dose endpoint (LD50) for pulmonary exposure to purified SEC [26].
Results
CA-MRSA rabbit pulmonary model. Rabbits were exposed intrabronchially to CA-MRSA and monitored for signs of respiratory distress and lethal illness. To initiate pulmonary infections, bacteria were administered into rabbit bronchi. CAMRSA strains tested were USA200 MNPA, MN1021 or MN128 (TSST-1+; these strains do not produce the cytotoxins α-toxin, β-toxin, γ-toxin, and PVL), and USA400 MW2 (SEC4+, α-toxin+, β-toxin-, γ-toxin+, and PVL+) [4] and USA400 c99- 529 (SEB +, a-toxin +, b-toxin -, g-toxin +, and PVL +) [4]. The animals were monitored for signs of illness for up to 7 days. Typically, rabbits exposed to CA-MRSA died 2–3 days after infection and had high fevers on days 1 and 2 after infection. Rabbits exposed to both USA200 and USA400 CA-MRSA organisms developed illness associated with respiratory distress and lethal illness, and the animals died (Figure 1); only 1 of 8 animals exposed to the TSST-1+ USA200 organisms survived (Figure 1 A), and no animals survived when challenged intrabronchially with SEC+ USA400 MW2 organisms (Figure 1 B).
Figure 1.
Immunization against superantigens or administration of soluble high-affinity variable β-Chains of T Cell receptors (Vβ-TCR) G5-8 protects rabbits from lethal community-associated methicillin-resistant S. Aureus (CA-MRSA) pulmonary illness. A , Nonimmunized and toxic Shock syndrome toxin-1 (TSST-1)-Immunized rabbits alive after challenge with TSST-1+ USA200 strain. B , Nonimmunized and staphylococcal enterotoxin C - Immunized rabbits alive after challenge with SEC+ USA400 strain. C , Rabbits treated with phosphate-buffered saline and high affinity Vβ-TCR (G5-8) alive after challenge with SEB+ USA400 strain
Excised lungs from rabbits exposed to CA-MRSA USA200 bacteria and USA400 MW2 were severely hemorrhagic (Figure 2 A -2 B and 3 A -3 B), compared with rabbits challenged with phosphate-buffered saline (Figure 2 E -2 F and 3 I -3 J). Histological analysis of representative lung sections confirmed the presence of hemorrhagic tissue (Figure 4 A -4 B and 5 A -5 B), compared with rabbits challenged with phosphate-buffered saline (Figure 4 E -4 F and 5 I -5 J).
We attempted to construct an allelic replacement sec (gene encoding SEC) knockout to test the role of SEC in lethality in this rabbit model. Although we were successful in constructing knockouts, making isogenic strains, the strains were not isophenotypic. Thus, the constructed knockouts lacked production of both SEC and α-toxin (only proteins tested), despite having similar growth kinetics. Therefore, to assess the role played by TSST-1 and SEC in lethal illness in rabbits, groups of rabbits were hyperimmunized against highly purified TSST1 or SEC (ELISA IgG titers >10,000) [15, 16] and then challenged intrabronchially with corresponding CA-MRSA isolates that produce TSST-1 or SEC. Rabbits preimmunized against TSST-1 or SEC did not develop respiratory distress or lethal illness when challenged (P<.001 and P< .005, respectively) (Figure 1 A -1 B) and, other than fever, did not develop overt clinical signs.
Excised lungs removed from nonimmune animals challenged with CA-MRSA USA200 or USA400 MW2 showed hemorrhagic tissue (Figure 2 A -2 B and 3 A -3 B). Lungs from TSST-1 or SEC hyperimmunized rabbits challenged with USA200 bacteria or USA400 MW2 did not have visible hemorrhagic lesions (Figure 2 C -2 D and 3 C -3 D), although the lungs appeared somewhat congested, consistent with staphylococcal infections. Lungs from rabbits treated with phosphate-buffered saline did not show hemorrhagic tissue and were not congested (Figure 2 E -2 F and 3 I -3 J).
Histological analysis showed hemorrhagic lung tissue in non-immune rabbits (Figure 4 A -4 B and 5 A -5 B) challenged with CA-MRSA USA200 bacteria or MW2, normal lung tissue in TSST-1 and SEC-hyperimmunized rabbits challenged with USA200 and MW2 organisms (Figure 4 C -4 D and 5 C -5 D) or rabbits challenged with phosphate-buffered saline (Figure 4 E -4 F and 5 I -5 J).
Additional experiments investigated the ability of soluble high affinity Vβ-TCR G5–8, specific for neutralization of SEB lethality in rabbits, through inhibition of superantigenicity [25], to provide passive protection from CA-MRSA or purified SEB intrabronchial challenge. Rabbits that received high-affinity Vβ-TCR G5–8 intravenously at the same time as the intrabronchial SEB+ CA-MRSA c99–529 did not develop respiratory distress and lethal TSS (Figure 1 C), whereas animals that did not receive G5–8 died.
Pulmonary exposure to purified SEB or SEC. Rabbits were administered purified SEC (0, 50, 100, or 200 μg) intrabronchially in phosphate-buffered saline. The LD50 of SEC was 75 μg by this route. Postmortem examination of lungs from SEC-treated rabbits revealed hemorrhagic tissue (Figure 3 E -3 F), compared with normal tissues from animals treated with phosphate-buffered saline (Figure 3 I -3 J).
Rabbits were also hyperimmunized against purified SEC and then administered 200 μg of SEC intrabronchially in phosphate-buffered saline. SEC hyperimmunized rabbits did not develop respiratory distress and lethal illness (data not shown) over the 7-day test period. In contrast, nonimmunized rabbits exposed to 200 μg of purified SEC showed respiratory distress and died within 24 h (data not shown). Excised lungs from nonimmunized rabbits revealed the presence of hemorrhagic lesions (Figure 3 E -3 F). Lung samples from SEC hyperimmunized rabbits did not show hemorrhagic lesions (Figure 3 G -3 H); they resembled lungs from animals treated with phosphate-buffered saline (Figure 3 I -3 J).
Histological analysis confirmed that excised lung sections from nonimmunized rabbits administered 200 μg of SEC contained hemorrhagic lesions (Figure 5 E -5 F). Tissue from SEC hyperimmunized rabbits administered 200 μg of SEC (Figure 5 G -5 H) and rabbits that received phosphate-buffered saline (Figure 5 I -5 J) showed normal lung tissue. Intravenous administration of high-affinity G5–8 with intrabronchial SEB also protected rabbits from respiratory distress and lethal TSS (Figure 6).
Figure 6.
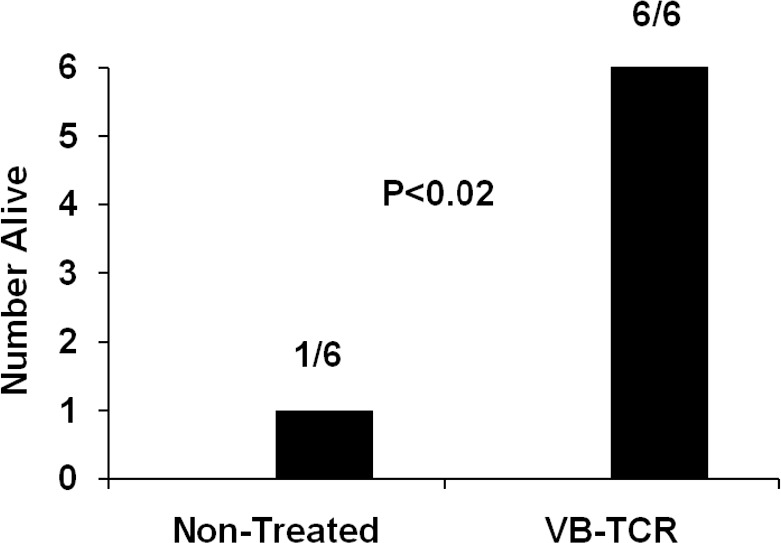
Soluble high affinity variable β-chains of T cell receptors (Vβ-TCR) (G5-8) protects rabbits from respiratory distress and lethal illness due to intrabronchial purified staphylococcal enterotoxin B (SEB) (100 or 200 μg). Phosphate-buffered saline or high affinity Vβ-TCR (G5-8)treated rabbits alive after intrabronchial administration of 100 (3 rabbits) or 200 μg of SEB (3 rabbits) Note that all rabbits that received Vβb-TCR G5-8 were administered 200 μg of purified SEB.
Discussion
We evaluated the role played by staphylococcal superantigens in serious pulmonary CA-MRSA infections and intoxications. Through TSST-1 and SEC immunization studies and use of soluble high-affinity Vβ-TCR G5–8 to neutralize SEB, we showed that these 3 superantigens are critical for development of serious pulmonary illness caused by both CA-MRSA and highly purified superantigens. We used rabbits as models because these animals are more similar to humans in susceptibility to superantigens [6, 27–31]; rabbits are also highly susceptible to cytotoxins [32]. Previous studies of CA-MRSA pulmonary infections used mice as the animal model [13, 33, 34] and have generated conflicting results regarding the roles played by staphylococcal exotoxins; one group suggested that PVL is critical to necrotizing pneumonia [34], whereas other groups suggested that α-toxin and phenol-soluble modulins, but not PVL, are critical [13, 33]. However, none of these studies assessed the role played by superantigens in disease because mice are at least 1011 more resistant to superantigens on a weight basis than are humans [35, 36]. Indeed, the presence of superantigens increases the resistance of mice to infections [37]. In contrast, young adult rabbits are only 102-103 more resistant than humans to superantigens, and rabbits ⩾8 months of age are as equally susceptible as humans to superantigens.
Previous hyperimmunization against TSST-1 protected rabbits from the lethality associated with intrabronchial challenge with CA-MRSA USA200. Interestingly, these CA-MRSA strains do not produce α, β, γ, or PVL cytotoxins, yet they cause fatal pulmonary illness. The studies suggest that cytotoxins are not required for the fatal outcomes. Our hyperimmunization of rabbits against PVL followed by challenge with USA400 MW2 (SEC+, α-toxin+, β-toxin-, γ-toxin+, and PVL+) resulted in lethal pulmonary illnesses, suggesting that PVL is not critical for lethality (unpublished data). It appears that these redundantly expressed cytotoxins, including α-toxin, β-toxin, γ-toxin, and PVL, when produced, contribute significantly to serious lung diseases, either through direct toxicity or induction of inflammation, but are not required for lethality in rabbits.
Presently, MRSA and their MSSA counterparts are highly significant causes of infectious disease deaths in the United States, including fatal pulmonary infections [3]. Our data suggest that superantigens are important contributors to those fatal infections. The initial report of CA-MRSA USA400 strains associated with deaths of 4 young children in the Upper Midwest due to necrotizing pneumonia demonstrated that 2 isolates produced SEB and the other 2 produced SEC, including MW2 and c99–529 [4]. Nonimmunized rabbits used in our studies that received intrabronchial MW2 and c99–529 developed illness that resembled necrotizing pneumonia, including by histologic examination of lung tissue. A subsequent larger study of CA-MRSA USA400 strains indicated that the vast majority produce either SEB or SEC [38]. It is also our experience that some regions of the United States are experiencing emergences of CA-MRSA USA200 S. aureus that produce TSST-1. As shown in the present studies, TSST-1 is critical in fatal pulmonary illness associated with these strains.
Of great significance, our studies show that administration of soluble high-affinity Vβ-TCR G5–8 or prior hyperimmunization to raise neutralizing antibodies against superantigens dramatically increases rabbit survival. There are anecdotal studies of patients with severe S. aureus pulmonary infections being treated successfully with intravenous immunoglobulin (IVIG) [39, 40]. IVIG is highly capable of neutralizing superantigens [41]. Additionally, a study has shown that IVIG reduces the case: fatality rate of streptococcal TSS in humans. However, IVIG is costly, requires large amounts of immunoglobulin to be administered, and has side effects. Our previous studies have shown that the soluble high-affinity Vβ-TCR G5–8 requires 2200 times less than IVIG for comparable ability to neutralize SEB in rabbits. G5–8 is easy to prepare from bacterial clones and requires only equimolar amounts to neutralize SEB, such small amounts for SEB neutralization that it may be possible to nebulize into the lungs, as well as administered intravenously.
Figure 2.

Rabbit lungs after exposure to community-associated methicillin-resistant S. aureus (CA-MRSA) USA200 (TSST-1+, α-toxin-, β-toxin-, γ-toxin-, and PVL-). A-B , Left and right lungs removed from a nonimmunized rabbit that received CA-MRSA USA200. C-D , Left and right lungs from a rabbit immunized against purified toxic shock syndrome toxin-1 (TSST-1) prior to administration of CA-MRSA USA200. E-F , Left and right lungs removed from a rabbit exposed only to phosphate-buffered saline.
Figure 3.
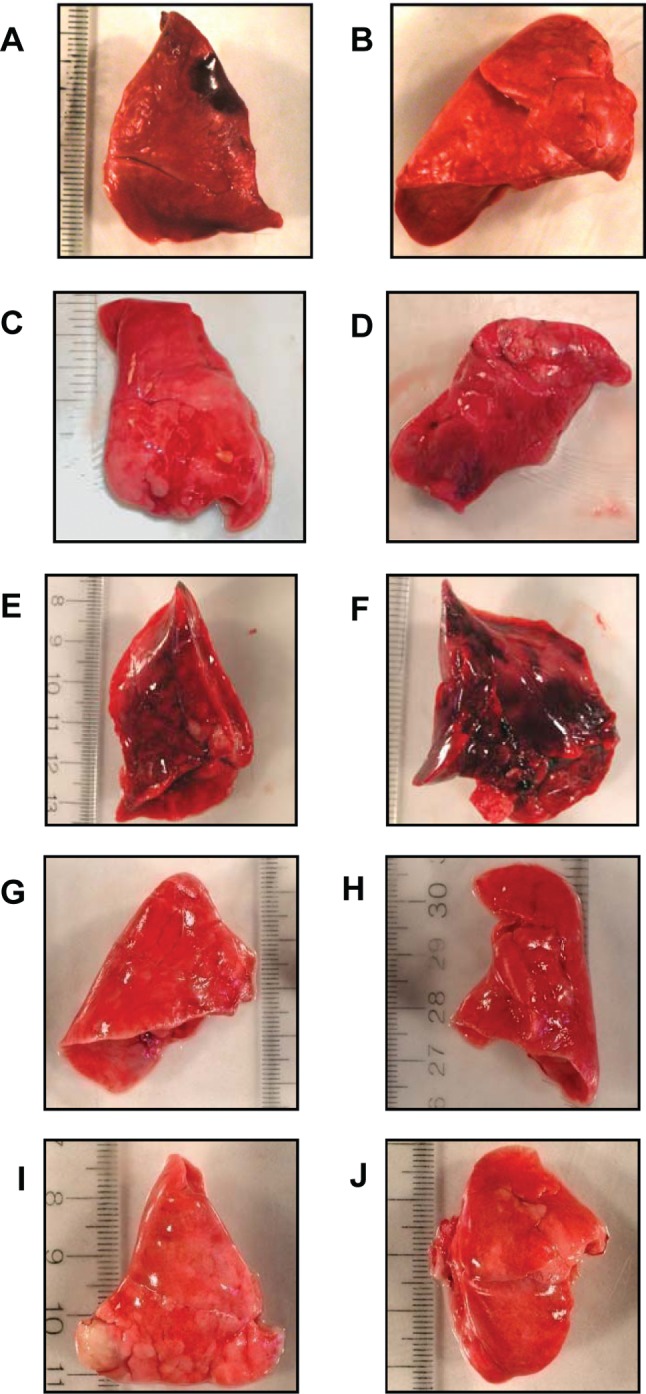
Rabbit lungs after exposure to community-associated methicillin-resistant S. aureus (CA-MRSA) USA400 MW2 (SEC+, α-toxin+, β-toxin-, γ-toxin+, and PVL+) or purified staphylococcal enterotoxin C (SEC). A-B , Left and right lungs from a nonimmune rabbit that received MW2 CA-MRSA. C-D , Left and right lungs from a rabbit immunized against purified SEC prior to administration of MW2 CA-MRSA. E-F , Left and right lungs from a nonimmune rabbit after intrabronchial administration of 200 μg purified SEC. G-H , Left and right lungs from a rabbit immunized against purified SEC prior to administration of 200 μg purified SEC. I-J , Left and right lungs from a rabbit administered phosphate-buffered saline.
Figure 4.

Histology of lung sections from rabbits challenged with community-associated methicillin-resistant S. aureus (CA-MRSA) USA200 MNPA (TSST-1+, α-toxin-, β-toxin-, γ-toxin-, and PVL-). A-B , & staining of sections from a rabbit (lung image shown in Figure 2A -2B) that received MNPA CA-MRSA. C-D , & staining of sections from a rabbit (lung image shown in Figure 2C -2D) hyperimmunized against purified toxic shock syndrome toxin-1 (TSST-1) prior to administration of MNPA CA-MRSA. E-F , & staining of sections from a rabbit (lung image shown in Figure 2E -2F) challenged with only phosphate-buffered saline. Images in A , C , and E : 100X magnification; images in B , D , and F :200X magnification.
Figure 5.

Histology of lung sections from rabbits challenged with community-associated methicillin-resistant S. aureus (CA-MRSA) MW2 (SEC+, α-toxin+, β-toxin-, γ-toxin+, and PVL+) or purified staphylococcal enterotoxin C (SEC). A-B , & staining of sections from a nonimmune rabbit (lung images shown in Figure 3A -3B) that received CA-MRSA MW2. C- D , & staining of sections from a rabbit (lung images shown in Figure 3C -3D) immunized against purified SEC prior to administration of CAMRSA MW2. E-F , & staining of sections from a rabbit (lung images shown in Figure 3E -3F) that received 200 μg of purified SEC. G-H , & staining of sections from a rabbit (lung images shown in Figure 3G -3H) hyperimmunized against purified SEC prior to administration of 200 μg of purified SEC. I-J , & staining of sections from a rabbit (lung images shown in Figure 3I -3J) administered only phosphate-buffered saline. Image in A :40X magnification; images in B , C , E , G , and I :100X magnification; images in D , F , H ,and J :200X magnification.
Footnotes
Potential conflicts of interest: none reported.
Presented in part: American Society for Microbiology 108th General Meeting; June 2008, Boston, Massachusetts.
Financial support: US Public Health Service (research grants AI057153, AI074283, and AI064611) from the National Institute of Allergy and Infectious Diseases. P.M.S. and D.M.K. acknowledge membership in and support from the Region V “ Great Lakes” Regional Center of Excellence in Biodefense and Emerging Infectious Diseases Consortium.
References
- 1.Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998;339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 2.Daum RS, Ito T, Hiramatsu K, et al. A novel methicillin-resistance cassette in community-acquired methicillin-resistant Staphylococcus aureus isolates of diverse genetic backgrounds. J Infect Dis. 2002;186:1344–1347. doi: 10.1086/344326. [DOI] [PubMed] [Google Scholar]
- 3.Klevens RM, Morrison MA, Nadle J, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763–1771. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention Four pediatric deaths from community-acquired methicillin-resistant Staphylococcus aureus -Minnesota and North Dakota, 1997–1999. JAMA. 1999;282:1123–1125. [PubMed] [Google Scholar]
- 5.Marrack P, Kappler J. The staphylococcal enterotoxins and their relatives. Science. 1990;248:705–711. doi: 10.1126/science.2185544. [DOI] [PubMed] [Google Scholar]
- 6.McCormick JK, Yarwood JM, Schlievert PM. Toxic shock syndrome and bacterial superantigens: an update. Annu Rev Microbiol. 2001;55:77–104. doi: 10.1146/annurev.micro.55.1.77. [DOI] [PubMed] [Google Scholar]
- 7.Li H, Llera A, Malchiodi EL, Mariuzza RA. The structural basis of T cell activation by superantigens. Annu Rev Immunol. 1999;17:435–466. doi: 10.1146/annurev.immunol.17.1.435. [DOI] [PubMed] [Google Scholar]
- 8.Wang L, Zhao Y, Li Z, et al. Crystal structure of a complete ternary complex of TCR, superantigen and peptide-MHC. Nat Struct Mol Biol. 2007;14:169–171. doi: 10.1038/nsmb1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marrack P, Kappler J. The staphylococcal enterotoxins and their relatives. Science. 1990;248:1066. [PubMed] [Google Scholar]
- 10.Schlievert PM. Staphylococcal enterotoxin B and toxic-shock syndrome toxin-1 are significantly associated with non-menstrual TSS. Lancet. 1986;1:1149–1150. doi: 10.1016/s0140-6736(86)91859-3. [DOI] [PubMed] [Google Scholar]
- 11.Schlievert PM, Tripp TJ, Peterson ML. Reemergence of staphylococcal toxic shock syndrome in Minneapolis-St. Paul, Minnesota, during the 2000–2003 surveillance period. J Clin Microbiol. 2004;42:2875–2876. doi: 10.1128/JCM.42.6.2875-2876.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schlievert PM, Strandberg KL, Lin YC, Peterson ML, Leung DY. Secreted virulence factor comparison between methicillin-resistant and methicillin-sensitive Staphylococcus aureus , and its relevance to atopic dermatitis. J Allergy Clin Immunol. 2010;125:39–49. doi: 10.1016/j.jaci.2009.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Voyich JM, Otto M, Mathema B, et al. Is Panton-Valentine leukocidin the major virulence determinant in community-associated methicillinresistant Staphylococcus aureus disease? J Infect Dis. 2006;194:1761–1770. doi: 10.1086/509506. [DOI] [PubMed] [Google Scholar]
- 14.O'Reilly M, Kreiswirth B, Foster TJ. Cryptic alpha-toxin gene in toxic shock syndrome and septicaemia strains of Staphylococcus aureus. Mol Microbiol. 1990;4:1947–1955. doi: 10.1111/j.1365-2958.1990.tb02044.x. [DOI] [PubMed] [Google Scholar]
- 15.Blomster-Hautamaa DA, Schlievert PM. Preparation of toxic shock syndrome toxin-1. Methods Enzymol. 1988;165:37–43. doi: 10.1016/s0076-6879(88)65009-9. [DOI] [PubMed] [Google Scholar]
- 16.Blomster-Hautamaa DA, Kreiswirth BN, Novick RP, Schlievert PM. Resolution of highly purified toxic-shock syndrome toxin 1 into two distinct proteins by isoelectric focusing. Biochemistry. 1986;25:54–59. doi: 10.1021/bi00349a009. [DOI] [PubMed] [Google Scholar]
- 17.Schlievert PM, Shands KN, Dan BB, Schmid GP, Nishimura RD. Identification and characterization of an exotoxin from Staphylococcus aureus associated with toxic-shock syndrome. J Infect Dis. 1981;143:509–516. doi: 10.1093/infdis/143.4.509. [DOI] [PubMed] [Google Scholar]
- 18.Bohach GA, Fast DJ, Nelson RD, Schlievert PM. Staphylococcal and streptococcal pyrogenic toxins involved in toxic shock syndrome and related illnesses. Crit Rev Microbiol. 1990;17:251–272. doi: 10.3109/10408419009105728. [DOI] [PubMed] [Google Scholar]
- 19.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 20.Schlievert PM, Case LC, Nemeth KA, et al. Alpha and beta chains of hemoglobin inhibit production of Staphylococcus aureus exotoxins. Biochemistry. 2007;46:14349–14358. doi: 10.1021/bi701202w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schlievert PM, Osterholm MT, Kelly JA, Nishimura RD. Toxin and enzyme characterization of Staphylococcus aureus isolates from patients with and without toxic shock syndrome. Ann Intern Med. 1982;96:937–940. doi: 10.7326/0003-4819-96-6-937. [DOI] [PubMed] [Google Scholar]
- 22.Schlievert PM, Bettin KM, Watson DW. Effect of antipyretics on group A streptococcal pyrogenic exotoxin fever production and ability to enhance lethal endotoxin shock. Proc Soc Exp Biol Med. 1978;157:472–475. doi: 10.3181/00379727-157-40079. [DOI] [PubMed] [Google Scholar]
- 23.Schlievert PM, Gahr PJ, Assimacopoulos AP, et al. Aggregation and binding substances enhance pathogenicity in rabbit models of Enterococcus faecalis endocarditis. Infect Immun. 1998;66:218–223. doi: 10.1128/iai.66.1.218-223.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hay FC, Olwyn MR. Oxford: Blackwell; 1980. Practical Immunology. [Google Scholar]
- 25.Buonpane RA, Churchill HR, Moza B, et al. Neutralization of staphylococcal enterotoxin B by soluble, high-affinity receptor antagonists. Nat Med. 2007;13:725–729. doi: 10.1038/nm1584. [DOI] [PubMed] [Google Scholar]
- 26.Reed LJ, Muench H. A simple method of estimating 50 per cent endpoints. Am J Hyg. 1938;27 [Google Scholar]
- 27.Dinges MM, Orwin PM, Schlievert PM. Exotoxins of Staphylococcus aureus. Clin Microbiol Rev. 2000;13:16–34. doi: 10.1128/cmr.13.1.16-34.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.riskandan S, Moyes D, Buttery LK, et al. Streptococcal pyrogenic exotoxin A release, distribution, and role in a murine model of fasciitis and multiorgan failure due to Streptococcus pyogenes. J Infect Dis. 1996;173:1399–1407. doi: 10.1093/infdis/173.6.1399. [DOI] [PubMed] [Google Scholar]
- 29.Giantonio BJ, Alpaugh RK, Schultz J, et al. Superantigen-based immunotherapy: a phase I trial of PNU-214565, a monoclonal antibody-staphylococcal enterotoxin A recombinant fusion protein, in advanced pancreatic and colorectal cancer. J Clin Oncol. 1997;15:1994–2007. doi: 10.1200/JCO.1997.15.5.1994. [DOI] [PubMed] [Google Scholar]
- 30.Dinges MM, Gregerson DS, Tripp TJ, McCormick JK, Schlievert PM. Effects of total body irradiation and cyclosporin A on the lethality of toxic shock syndrome toxin-1 in a rabbit model of toxic shock syndrome. J Infect Dis. 2003;188:1142–1145. doi: 10.1086/378514. [DOI] [PubMed] [Google Scholar]
- 31.Dinges MM, Schlievert PM. Comparative analysis of lipopolysaccharide-induced tumor necrosis factor alpha activity in serum and lethality in mice and rabbits pretreated with the staphylococcal superantigen toxic shock syndrome toxin 1. Infect Immun. 2001;69:7169–172. doi: 10.1128/IAI.69.11.7169-7172.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dinges MM, Orwin PM, Schlievert PM. Exotoxins of Staphylococcus aureus. Clin Microbiol Rev. 2000;13:16–34. doi: 10.1128/cmr.13.1.16-34.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bubeck Wardenburg J, Bae T, Otto M, Deleo FR, Schneewind O. Poring over pores: alpha-hemolysin and Panton-Valentine leukocidin in Staphylococcus aureus pneumonia. Nat Med. 2007;13:1405–1406. doi: 10.1038/nm1207-1405. [DOI] [PubMed] [Google Scholar]
- 34.Labandeira-Rey M, Couzon F, Boisset S, et al. Staphylococcus aureus Panton-Valentine leukocidin causes necrotizing pneumonia. Science. 2007;315:1130–1133. doi: 10.1126/science.1137165. [DOI] [PubMed] [Google Scholar]
- 35.Dinges MM, essurun J, Schlievert PM. Comparisons of mouse and rabbit models of toxic shock syndrome. International Congress and Symposium Series. 1998;229:167–168. [Google Scholar]
- 36.Dinges MM, Schlievert PM. Comparative analysis of lipopolysaccharide-induced tumor necrosis factor alpha activity in serum and lethality in mice and rabbits pretreated with the staphylococcal superantigen toxic shock syndrome toxin 1. Infect Immun. 2001;69:7169–7172. doi: 10.1128/IAI.69.11.7169-7172.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.riskandan S, Unnikrishnan M, Krausz T, Cohen J. Molecular analysis of the role of streptococcal pyrogenic exotoxin A (SPEA) in invasive soft-tissue infection resulting from Streptococcus pyogenes. Mol Microbiol. 1999;33:778–790. doi: 10.1046/j.1365-2958.1999.01525.x. [DOI] [PubMed] [Google Scholar]
- 38.Fey PD, Said-Salim B, Rupp ME, et al. Comparative molecular analysis of community-or hospital-acquired methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2003;47:196–203. doi: 10.1128/AAC.47.1.196-203.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schlievert PM. Use of intravenous immunoglobulin in the treatment of staphylococcal and streptococcal toxic shock syndromes and related illnesses. J Allergy Clin Immunol. 2001;108:107–110. doi: 10.1067/mai.2001.117820. [DOI] [PubMed] [Google Scholar]
- 40.Barry W, Hudgins L, Donta ST, Pesanti EL. Intravenous immunoglobulin therapy for toxic shock syndrome. JAMA. 1992;267:3315–3316. [PubMed] [Google Scholar]
- 41.Holm SE, Kohler W, Kaplan EL, et al. Streptococcal toxic shock syndrome (STSS). An update: a roundtable presentation. Adv Exp Med Biol. 1997;418:193–199. doi: 10.1007/978-1-4899-1825-3_47. [DOI] [PubMed] [Google Scholar]



