Abstract
Rab proteins are key-regulators of intracellular membrane trafficking. Rab7b is a recently identified Rab protein that may downregulate TLR4 and TLR9-mediated inflammatory responses. Rab7b, believed to have similar function as Rab7, controls however vesicular trafficking from endosomes to the TGN. It is localized to late endosomes/lysosomes as well as the TGN. Rab7b interferes with enzymes delivery to lysosomes and with the retrograde Shiga toxin transport to the Golgi. Furthermore, Rab7b depletion alters CI-MPR and TGN46 trafficking. In conclusion, Rab7b, by regulating the transport from late endosomes to the TGN, is fundamental for trafficking of several receptors, opening for a revised scenario for its influence on signaling of Toll-like Receptors (TLRs) and other receptors.
Key words: membrane traffic, rab proteins, Rab7b, Rab7, toll like receptor, epidermal growth factor receptor, cation independent-mannose-6-phosphate receptor
Eukaryotic cells are organized complexes of subcellular membranous compartments that, in order to function properly, needs to be tightly controlled by specific transport systems. Some proteins, such as Rab proteins, are the responsible for regulating the trafficking between the different compartments. Rab proteins (Ras-related proteins in brain) are small GTPases, members of the Ras superfamily.1 Although more than 60 human Rab proteins have been identified to date, only about half of them has been functionally characterized,2,3 Rab proteins cycle between a GTP-bound active and a GDP-bound inactive form that are connected with different localizations in the cell to carry out their functions.3,4 Each Rab protein interacts with several downstream effectors: motor proteins that transport vesicles along actin or tubulin cytoskeleton,5 tethering factors, as well as SNARES, that help to target the vesicular carrier to the appropriate membrane promoting membrane fusion,6,7 adaptors, lipid kinases and phosphatases to control budding and fusion of vesicles.8,9 Furthermore Rab proteins are also essential for proper signaling and control of cell proliferation and differentiation.10,11
Rab7 regulates the transport to late endosomes and lysosomes (Fig. 1), and is important for lysosomal and phagosomal biogenesis, and for maturation of late autophagic vacuoles.12–14 The active form of Rab7, recruits RILP onto Rab7-positive compartments, and RILP recruits dynein-dynactin motor complexes.13,15 Recruitment of motors causes the movement of Rab7-containing vesicles toward the microtubule-organizing center.
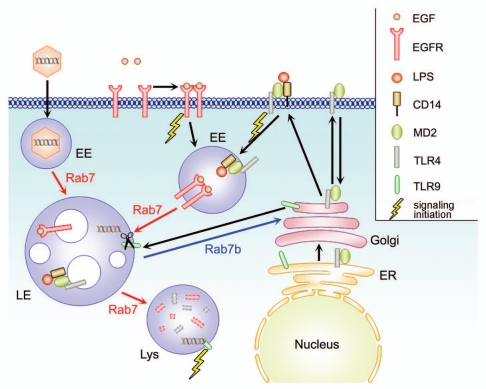
Figure 1.
Simplified overview of Rab 7, Rab7b function and receptor trafficking. Rab7b is localized to late endosomes (LE) and lysosomes (Lys), and to TGN/Golgi, directing the transport between these compartments. EGF binds its receptor EGFR on the plasma membrane, this leads to dimerization which initiate a signaling cascade, and internalization of the complex to early endosomes where it is still able to signal. EGFR is then taken into the the lumen of MVBs of late endosomes and lysosomes for degradation. Rab7, (but not Rab7b) regulates these steps of transport from early endosomes to late endosomes and lysosomes. TLR4 associated with MD2 at the endoplasmic reticulum (ER), and it is transported through the Golgi to the plasma membrane. When it is activated by the LPS/CD14, it starts the signaling and it is internalized in early endosomes and degraded in late endosomes and lysosomes. TLR9 is normally resident in the ER. After cell activation, it is transported to the endolysosomal compartment, passing through the Golgi. In these compartments, the receptor is proteolytically cleaved, allowing the activation of the signaling cascade after binding the endocytosed ligand (CpG DNA).
The localization and function of a new Rab7 like protein called Rab7b has recently been investigated.16–19 In this review we will discuss the role of Rab7b in membrane traffic and we will highlight the implications of its functions in receptor trafficking and signaling.
Role of Rab7b in Membrane Traffic
Rab7b is a small GTPase first identified by Cao and coworkers in 2004.16 It shares approximately 68% similarity with Rab7 and it is highly expressed in monocytes (CD14+ cells), monocyte-derived immature dendritic cells (DCs), and promyeloid or monocytic leukemia cell lines.16 Rab7b expression is downregulated in DCs after stimulation with lipopolysaccharide (LPS), while it is upregulated when monocytes are activated by LPS treatments or when acute promyelocytic leukemia (APL) cell lines are differentiated with phorbol myristate acetate (PMA).16
Cao and coworkers demonstrated that Rab7b is localized on late endosomes and lysosomes, and suggested that, similarly to Rab7, Rab7b could control transport to late endosomes and lysosomes in the endocytic pathway.16 When we investigated the intracellular localization of Rab7b, we confirmed that Rab7b, similarly to Rab7 is localized to late endosomes and lysosomes. However, we also found that, at variance with Rab7, Rab7b is also localized to the Golgi apparatus and to the TGN19 (Fig. 1). In particular, the constitutively active mutant Rab7b Q67L almost completely co-localizes with Golgi and TGN markers, at variance with Rab7 Q67L that is present solely on the late endosomal and lysosomal compartment.19
EGFR, after binding its ligand (EGF) at the plasma membrane, is activated and dimerizes; dimerization induces autophosphorilation of the receptor, followed by ubiquitination and internalization.20 On early endosomes, the ubiquitinated receptor is directed by the Endosomal Sorting Complexes Required for Transport (ESCRTs) into the intralumenal vesicles of multivesicular bodies (MVBs) for lysosomal degradation.21 Degradation of EGF and EGFR is regulated by Rab7, while it is not affected by silencing or overexpression of Rab7b wt and mutant proteins, again arguing for a different role of Rab7b compared to Rab7.19,22,23 Moreover, data form our laboratories demonstrate that Rab7b does not interact with RILP, a Rab7 effector protein (unpublished data), again pointing out to profound differences between Rab7 and Rab7b.
Rab7b depletion increases the secretion of lysosomal enzymes, inhibits Cathepsin D maturation, and increases levels of late endosomal markers (i.e., CI-MPR and Cathepsin D).19 These evidences are hallmarks of alteration in the transport of lysosomal enzymes to their final destination, and this often happens when CI-MPR trafficking is altered.24,25 In agreement, CI-MPR distribution, synthesis and turnover rate are altered in Rab7b-depleted cells. Interestingly, not only CI-MPR but also TGN46 distribution is affected by Rab7b depletion: from clustered in perinuclear position, to scattered in the whole cell.19 Our hypothesis is that these proteins, after leaving the TGN, in the absence of Rab7b are not able to cycle back. Furthermore, internalized Shiga toxin is not able to reach the Golgi in Rab7b-silenced cells,19 demonstrating that Rab7b controls trafficking from endosomes to the TGN.
Role of Rab7b in Signal Transduction
High level expression of Rab7b downregulates the TLR4 and TLR9-mediated inflammatory response whereas depletion leads to upregulation, and suggests that Rab7b function was essential for receptor degradation.17,18 This hypothesis was based on the assumption that Rab7b regulated transport to degradative compartments along the endocytic pathway similarly as Rab7.17,18 However, considering that Rab7b controls transport from endosomes to the TGN, data on TLRs should be reinterpreted.
TLRs are type I transmembrane receptors that initiate the immune responses following the binding with microbial molecules.26 TLR4 is present on the plasma membrane and binds molecules that are on the surface of pathogens (LPS), whereas TLR9 is expressed intracellularly in acidic compartment where recognizes microbial nucleic acids.27 Although there is a good knowledge about the TLRs-triggered signaling cascade, very little is known about their intracellular trafficking.
LPS, after binding CD14 on the cell surface, is presented to TLR4 and initiates the intracellular signaling cascade, resulting in the release of proinflammatory cytokines. The LPS/TLR4/MD-2 complex is internalized by endocytosis in early endosomes, but it is then delivered to different destinations, as recycling endosomes, TGN and Golgi for recycling, and lysosomes for degradation (Fig. 1).28–31 Notably, the receptor complex has been detected in Rab7b-positive compartments.18 In Rab7b-silenced cells, after LPS treatment, it was observed a prolonged persistence of the receptor in both early and late endocytic compartments.18 Since Rab7b regulates the transport from endosomes to the TGN, when Rab7b is depleted TLR4 cannot recycle to the TGN efficiently and therefore it is forced to stay in the early endocytic route longer and this could increase its signaling.
It has been also observed that Rab7b overexpression inhibits unselectively both the MyD88-dependent (occurring at the plasma membrane and terminating with TLR4 endocytosis) and TRIF-dependent (occurring in the endosomes and terminating with the receptor degradation) signaling cascade.18,32,33 However, if Rab7b was controlling early endosomes to late endosomes and lysosomes transport one would expect that only TRIF-dependent signaling would be affected. In addition, in cells overexpressing Rab7b there is a low protein level of the receptor even in unstimulated conditions. Therefore the inhibition of TLR4-mediated signaling in presence of Rab7b could be caused by decrease of the receptor synthesis or, assuming that the receptor complex is not able to signal when localized to the TGN, by increased transport to this compartement from endosomes.
TLR9 localizes to endoplasmic reticulum in unstimulated cells; after activation, it traffics to endolysosomes passing through the Golgi (Fig. 1).34 The ectodomains of TLR9 is cleaved in the endolysosome by cathepsins, and it interacts with endocytosed CpG DNA at acidic pH.35 After DNA binding, TLR9 activates immune response against microbial pathogens, leading to the induction of proinflammatory cytokines.27 Although both full-length and processed forms of the receptor can bind ligand, only the cleaved form of TLR9 is competent for signal transduction.34,35 Rab7b colocalizes with TLR9 on late endosomes/lysosomes after CpG stimulation.
Overexpression of Rab7b inhibits TLR9-mediated activation and expression of proinflammatory cytokines and type I IFN in macrophages, while Rab7b silencing has the opposite effect.17 Interestingly, Rab7b overexpression inhibits TLR9 expression in macrophages even when the macrophages are unstimulated.17 Considering that Rab7b controls trafficking between endosomes and the TGN, it is tempting to speculate that overexpression of Rab7b cause re-routing of the receptor to the TGN thus causing the suppression of TLR9-triggered production of proinflammatory cytokines.17 In addition, it is known that TLR9 is degraded by proteasomes after ubiquitination by the Triad3A ubiquitin ligase, thus ending the signaling.36
Emerging evidence demonstrates the functional significance of compartimentalized signaling by cell surface receptors. Therefore, the endocytic Rab proteins (and in particular of Rab7b) are essential for regulating the kinetics and dynamics of membrane traffic and thereby receptor signaling. Indeed, modulation of endocytic Rab proteins functions could help to regulate signal transduction enabling control of proliferation and differentiation, with important implications for the development of cancer therapies and stem cell-based therapy for genetic and acquired diseases.
Acknowledgements
Work done in the author's laboratories was supported by AIRC (Associazione Italiana per la Ricerca sul Cancro, Grant N. 4496 to C.B.) and NRC (Norwegian Research Council to C.P. and O.B.) and DNK (Norwegian Cancer Society to O.B.).
Abbreviations
- CI-MPR
cation indipendent-mannose phospate receptor
- DCs
dendritic cells
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- LPS
lipopolysaccharide
- MVBs
multivesicular bodies
- TGN
trans golgi network
- RILP
rab interacting lysosomal protein
- TLR
toll like receptor
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/12341
References
- 1.Schwartz SL, Cao C, Pylypenko O, Rak A, Wandinger-Ness A. Rab GTPases at a glance. J Cell Sci. 2007;120:3905–3910. doi: 10.1242/jcs.015909. [DOI] [PubMed] [Google Scholar]
- 2.Colicelli J. Human RAS superfamily proteins and related GTPases. Sci STKE. 2004;2004:13. doi: 10.1126/stke.2502004re13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10:513–525. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- 4.Stein MP, Dong J, Wandinger-Ness A. Rab proteins and endocytic trafficking: potential targets for therapeutic intervention. Adv Drug Deliv Rev. 2003;55:1421–1437. doi: 10.1016/j.addr.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 5.Jordens I, Marsman M, Kuijl C, Neefjes J. Rab proteins, connecting transport and vesicle fusion. Traffic. 2005;6:1070–1077. doi: 10.1111/j.1600-0854.2005.00336.x. [DOI] [PubMed] [Google Scholar]
- 6.Grosshans BL, Ortiz D, Novick P. Rabs and their effectors: achieving specificity in membrane traffic. Proc Natl Acad Sci USA. 2006;103:11821–11827. doi: 10.1073/pnas.0601617103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Markgraf DF, Peplowska K, Ungermann C. Rab cascades and tethering factors in the endomembrane system. FEBS Lett. 2007;581:2125–2130. doi: 10.1016/j.febslet.2007.01.090. [DOI] [PubMed] [Google Scholar]
- 8.Shin HW, Hayashi M, Christoforidis S, Lacas-Gervais S, Hoepfner S, Wenk MR, et al. An enzymatic cascade of Rab5 effectors regulates phosphoinositide turnover in the endocytic pathway. J Cell Biol. 2005;170:607–618. doi: 10.1083/jcb.200505128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao C, Laporte J, Backer JM, Wandinger-Ness A, Stein MP. Myotubularin lipid phosphatase binds the hVPS15/hVPS34 lipid kinase complex on endosomes. Traffic. 2007;8:1052–1067. doi: 10.1111/j.1600-0854.2007.00586.x. [DOI] [PubMed] [Google Scholar]
- 10.Bucci C, Chiariello M. Signal transduction gRABs attention. Cell Signal. 2006;18:1–8. doi: 10.1016/j.cellsig.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Yu X, Prekeris R, Gould GW. Role of endosomal Rab GTPases in cytokinesis. Eur J Cell Biol. 2007;86:25–35. doi: 10.1016/j.ejcb.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Bucci C, Thomsen P, Nicoziani P, McCarthy J, van Deurs B. Rab7: a key to lysosome biogenesis. Mol Biol Cell. 2000;11:467–480. doi: 10.1091/mbc.11.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harrison R, Bucci C, Vieira O, Schroer T, Grinstein S. Phagosomes fuse with late endosomes and/or lysosomes by extension of membrane protrusions along microtubules: role of Rab7 and RILP. Mol Cell Biol. 2003;23:6494–6506. doi: 10.1128/MCB.23.18.6494-6506.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jager S, Bucci C, Tanida I, Ueno T, Kominami E, Saftig P, et al. Role for Rab7 in maturation of late autophagic vacuoles. J Cell Sci. 2004;117:4837–4848. doi: 10.1242/jcs.01370. [DOI] [PubMed] [Google Scholar]
- 15.Johansson M, Rocha N, Zwart W, Jordens I, Janssen L, Kuijl C, et al. Activation of endosomal dynein motors by stepwise assembly of Rab7-RILP-p150Glued, ORP1L, and the receptor betalll spectrin. J Cell Biol. 2007;176:459–471. doi: 10.1083/jcb.200606077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang M, Chen T, Han C, Li N, Wan T, Cao X. Rab7b, a novel lysosome-associated small GTPase, is involved in monocytic differentiation of human acute promyelocytic leukemia cells. Biochem Biophys Res Commun. 2004;318:792–799. doi: 10.1016/j.bbrc.2004.04.115. [DOI] [PubMed] [Google Scholar]
- 17.Yao M, Liu X, Li D, Chen T, Cai Z, Cao X. Late endosome/lysosome-localized Rab7b suppresses TLR9-initiated proinflammatory cytokine and type I IFN production in macrophages. J Immunol. 2009;183:1751–1758. doi: 10.4049/jimmunol.0900249. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Chen T, Han C, He D, Liu H, An H, et al. Lysosome-associated small Rab GTPase Rab7b negatively regulates TLR4 signaling in macrophages by promoting lysosomal degradation of TLR4. Blood. 2007;110:962–971. doi: 10.1182/blood-2007-01-066027. [DOI] [PubMed] [Google Scholar]
- 19.Progida C, Cogli L, Piro F, De Luca A, Bakke O, Bucci C. Rab7b controls trafficking from endosomes to the TGN. J Cell Sci. 2010;123:1480–1491. doi: 10.1242/jcs.051474. [DOI] [PubMed] [Google Scholar]
- 20.Le Roy C, Wrana JL. Clathrin- and non-clathrin-mediated endocytic regulation of cell signalling. Nat Rev Mol Cell Biol. 2005;6:112–126. doi: 10.1038/nrm1571. [DOI] [PubMed] [Google Scholar]
- 21.Raiborg C, Rusten TE, Stenmark H. Protein sorting into multivesicular endosomes. Curr Opin Cell Biol. 2003;15:446–455. doi: 10.1016/s0955-0674(03)00080-2. [DOI] [PubMed] [Google Scholar]
- 22.Vitelli R, Santillo M, Lattero D, Chiariello M, Bifulco M, Bruni C, et al. Role of the small GTPase Rab7 in the late endocytic pathway. J Biol Chem. 1997;272:4391–4397. doi: 10.1074/jbc.272.7.4391. [DOI] [PubMed] [Google Scholar]
- 23.Ceresa BP, Bahr SJ. Rab7 activity affects epidermal growth factor:epidermal growth factor receptor degradation by regulating endocytic trafficking from the late endosome. J Biol Chem. 2006;281:1099–1106. doi: 10.1074/jbc.M504175200. [DOI] [PubMed] [Google Scholar]
- 24.Ganley IG, Carroll K, Bittova L, Pfeffer S. Rab9 GTPase regulates late endosome size and requires effector interaction for its stability. Mol Biol Cell. 2004;15:5420–5430. doi: 10.1091/mbc.E04-08-0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riederer MA, Soldati T, Shapiro AD, Lin J, Pfeffer SR. Lysosome biogenesis requires Rab9 function and receptor recycling from endosomes to the trans-Golgi network. J Cell Biol. 1994;125:573–582. doi: 10.1083/jcb.125.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saitoh S, Miyake K. Regulatory molecules required for nucleotide-sensing Toll-like receptors. Immunol Rev. 2009;227:32–43. doi: 10.1111/j.1600-065X.2008.00729.x. [DOI] [PubMed] [Google Scholar]
- 27.Halaas O, Husebye H, Espevik T. The journey of Toll-like receptors in the cell. Adv Exp Med Biol. 2007;598:35–48. doi: 10.1007/978-0-387-71767-8_4. [DOI] [PubMed] [Google Scholar]
- 28.Thieblemont N, Wright SD. Transport of bacterial lipopolysaccharide to the golgi apparatus. J Exp Med. 1999;190:523–534. doi: 10.1084/jem.190.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Latz E, Visintin A, Lien E, Fitzgerald KA, Monks BG, Kurt-Jones EA, et al. Lipopolysaccharide rapidly traffics to and from the Golgi apparatus with the toll-like receptor 4-MD-2-CD14 complex in a process that is distinct from the initiation of signal transduction. J Biol Chem. 2002;277:47834–47843. doi: 10.1074/jbc.M207873200. [DOI] [PubMed] [Google Scholar]
- 30.Hornef MW, Frisan T, Vandewalle A, Normark S, Richter-Dahlfors A. Toll-like receptor 4 resides in the Golgi apparatus and colocalizes with internalized lipopolysaccharide in intestinal epithelial cells. J Exp Med. 2002;195:559–570. doi: 10.1084/jem.20011788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Husebye H, Halaas Ø, Stenmark H, Tunheim G, Sandanger Ø, Bogen B, et al. Endocytic pathways regulate Toll-like receptor 4 signaling and link innate and adaptive immunity. EMBO J. 2006;25:683–692. doi: 10.1038/sj.emboj.7600991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kagan JC, Su T, Horng T, Chow A, Akira S, Medzhitov R. TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-beta. Nat Immunol. 2008;9:361–368. doi: 10.1038/ni1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Watts C. Location, location, location: identifying the neighborhoods of LPS signaling. Nat Immunol. 2008;9:343–345. doi: 10.1038/ni0408-343. [DOI] [PubMed] [Google Scholar]
- 34.Chockalingam A, Brooks JC, Cameron JL, Blum LK, Leifer CA. TLR9 traffics through the Golgi complex to localize to endolysosomes and respond to CpG DNA. Immunol Cell Biol. 2009;87:209–217. doi: 10.1038/icb.2008.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ewald SE, Lee BL, Lau L, Wickliffe KE, Shi GP, Chapman HA, Barton GM. The ectodomain of Toll-like receptor 9 is cleaved to generate a functional receptor. Nature. 2008;456:658–662. doi: 10.1038/nature07405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chuang TH, Ulevitch RJ. Triad3A, an E3 ubiquitin-protein ligase regulating Toll-like receptors. Nat Immunol. 2004;5:495–502. doi: 10.1038/ni1066. [DOI] [PubMed] [Google Scholar]