Abstract
STriatal-Enriched Phosphatase (STEP) is a brain-specific protein tyrosine phosphatase that plays a role in synaptic plasticity and has recently been implicated in neurodegenerative disease. STEP opposes the development of synaptic strengthening by dephosphorylating and inactivating key signaling proteins that include the MAP kinases ERK1/2 and p38, as well as the tyrosine kinase Fyn. STEP also dephosphorylates the GluR2 subunit of the AMPAR and the NR2B subunit of the NMDAR, which leads to internalization of the NR1/NR2B and GluR1/GluR2 receptors. STEP levels and activity are regulated through phosphorylation, local translation, ubiquitination and degradation and proteolytic cleavage. Here we review recent progress in understanding the normal regulation of STEP and how this regulation is disrupted in Alzheimer's disease, in which abnormally increased STEP levels and activity contribute to the cognitive deficits.
Key words: tyrosine phosphatase, STEP, Alzheimer's disease, glutamate receptors, proteasome, ubiquitination
The ability to learn and react suitably to a changing environment depends on neuronal plasticity. Plasticity refers to the extent to which neurons can modify the structure and strength of their connections in response to synaptic activity (reviewed in ref. 1). While considerable research has focused on the mechanisms by which activity strengthens synaptic connections, less has concentrated on how synaptic connections are weakened. Recent work shows that STriatal-Enriched protein tyrosine Phosphatase (STEP) plays an important role in opposing synaptic strengthening.2 STEP's molecular properties, regulation of its substrates ERK1/2, Fyn and the NMDAR complex, and its interactions with PKA and calcineurin have been reviewed in ref. 2 and are briefly mentioned here. Recent work has augmented our understanding of STEP: STEP activity also leads to the internalization of AMPARs,3 and is regulated by proteolytic cleavage,4 ubiquitin-proteasome degradation,4,5 and local translation.3 We discuss these developments in light of their implications for plasticity and neurodegenerative disease.
Expression and Functional Domains
STEP is expressed exclusively in the central nervous system where it is alternatively spliced into two major isoforms, STEP46 and STEP61. STEP46 is a cytosolic variant, whereas STEP61 is targeted to the post-synaptic density and the endoplasmic reticulum by an additional 172 amino acids at its N-terminus.6 This spatial localization is thought to be important to STEP function, especially as it relates to NMDAR and AMPAR trafficking.
STEP is enriched in medium spiny neurons of the striatum, where both STEP46 and STEP61 are expressed. STEP61 is also found throughout the neocortex, hippocampus, amygdala and developing spinal cord.7 Both STEP46 and STEP61 contain a catalytic protein tyrosine phosphatase (PTP) consensus sequence [I/V]HCxAGxxR[S/T]G and a kinase-interacting motif (KIM) necessary for interaction of STEP with its substrates. Unique to STEP61 are two polyproline rich regions, as discussed below.
STEP Substrates
MAPKs: ERK1/2 and p38.
Several studies, reviewed elsewhere,2 implicate STEP in the regulation of the MAPKs. Extracellular regulated kinase 1 and 2 (ERK1/2) activity induces and sustains synaptic strengthening through parallel processes. ERK1/2 promotes local protein translation8 and gene transcription, and is involved in regulating spine stabilization and back-propagating action potentials (reviewed in ref. 9). ERK1/2 inhibition disrupts these processes. STEP binds ERK1/2 and p38 through its KIM domain and inactivates them by dephosphorylating the regulatory tyrosine in their activation loops.
ERK1/2 activity is significantly increased in the absence of STEP.10 pERK1/2 is elevated in the striatum, area CA2 of the hippocampus, and the lateral and central amygdala in the brains of STEP knock-out (KO) mice. Moreover, STEP KO cultured neurons exhibit exaggerated pERK induction after pharmacological stimulation with DHPG, an agonist of the type I metabotropic glutamate receptor (mGluR) that leads to moderate ERK activation followed by synaptic depression. STEP's ability to bind to its substrates has been disrupted in an attempt to interfere with the consolidation of new memories. Infusion of a substrate-trapping, membrane permeable STEP46 into the lateral amygdala blocked Pavlovian fear learning by preventing ERK1/2 translocation to the nucleus.11
The ability of STEP to regulate both ERK1/2 and p38 initially seemed paradoxical. Whereas ERK1/2 activation promotes cell survival and plasticity, p38 activation leads to cell death. A recent study clarified the mechanism by which STEP regulates both pro-survival and pro-cell-death pathways.4 Extrasynaptic NMDAR stimulation, which leads to cell death, triggers local calpain-mediated cleavage of STEP61 in its KIM domain. Cleavage prevents STEP from binding its substrates and causes selective activation of extrasynaptically concentrated p38.4 In contrast, synaptic NMDAR stimulation leads to the ubiquitination and degradation of STEP61 and predominantly ERK1/2 activation. Cell death is mitigated by preventing STEP cleavage, which suggests STEP plays a neuroprotective role.4
Tyrosine kinase Fyn and NMDARs.
STEP regulates NMDAR trafficking through two parallel pathways. STEP acts as a brake on NMDAR exocytosis by dephosphorylating and inactivating Fyn (Y420). Fyn phosphorylates NR2B at tyr1472, leading to exocytosis of NMDARs to neuronal surfaces.12,13 STEP61 in particular seems important in this process as the presence of its polyproline region increases its affinity for Fyn 10-fold over STEP46. STEP also leads directly to clathrin-dependent endocytosis of the receptor complex by dephosphorylating NR2B (Y1472).14
AMPARs.
STEP also contributes to the regulation of GluR1/GluR2 AMPARs surface expression.3 Pharmacological stimulation of group I mGluRs with DHPG normally promotes endocytosis of AMPARs and long-term depression (mGluR-LTD).15 AMPAR endocytosis in NMDAR-LTD requires the dephosphorylation of a serine residue, whereas AMPAR endocytosis in mGluR-LTD requires the dephosphorylation of a tyrosine16 and is completely blocked by inhibiting PTPs, suggesting that an unknown PTP is essential in mGluR-LTD.17 We have recently shown that AMPAR surface expression is increased in STEP KO mice, whereas mGluR stimulation with DHPG no longer induces GluR1/2 endocytosis.18 The introduction of a membrane-permeable WT STEP rescued the ability of DHPG to induce GluR1/2 internalization in the KO cultures.3 These results implicate STEP as the PTP important in mGluR-dependent AMPAR trafficking. The identification of the specific Y residue on GluR2 that STEP dephosphorylates is subject of current investigation.
STEP Regulation
Phosphorylation.
PKA phosphorylation of S221 in STEP's KIM domain prevents STEP interaction with its substrates. In striatal medium spiny neurons, D1 receptors couple positively to cAMP via Gs to lead to the activation of PKA and consequent phosphorylation of STEP. Phosphorylation at S221 can be reversed by Ca2+ influx (via NMDAR or α7nAChR activation) and activation of the phosphatases, calcineurin and PP1. In this way, STEP acts as a coincidence detector for dopaminergic and glutamatergic or cholinergic signaling. STEP's integration of these systems may be co-opted in psychostimulant addiction19 and Alzheimer's disease.20 Elevated Aβ levels (1 µM) activate α7nAChRs, which leads to calcineurin/PP1 activation, STEP dephosphorylation and activation, Fyn inactivation and NMDAR endocytosis. Disruption of the NMDAR system is thought to contribute to cognitive deficits. A substrate-trapping, catalytically inactive STEP blocked this Aβ-induced NMDAR endocytosis in neuronal cultures and tissue slices.20
Local translation.
The local translation of proteins in dendrites and spines is now known to be integral to the development of synaptic plasticity and its dysregulation is thought to be involved in the cognitive deficits present in Fragile X Syndrome.21 STEP is rapidly translated in the amygdala within minutes after Pavlovian fear conditioning training, which suggested it might be translated locally.11 We recently confirmed that STEP mRNA is present and locally translated upon stimulation of mGluR5 but not mGluR1 in synaptosomes, a preparation in which the presynaptic and postsynaptic compartments are isolated as a functional unit.3 Moreover, translation was necessary for STEP-mediated endocytosis of AMPARs and required both ERK1/2 and PI3K pathways.
A STEP Overboard: Dysregulation in Neurodegeneration
Alzheimer's disease (AD) is characterized by the presence of Aβ plaques and hyperphosphorylated tau tangles. Emerging research suggests that soluble forms of Aβ interfere with synapse function and may contribute to the pathophysiology of the disease.22
Our recent work further implicates STEP in the etiology of AD.5 STEP level and activity are increased in human prefrontal cortex of patients with Alzheimer's disease as well as in cortex of an AD mouse model (Tg2576 mice). Moreover, 12-month but not 3-month old Tg2576 mice exhibit decreased pNR2B levels. Incubation with Aβ-enriched medium (7PA2-CM) is sufficient to increase STEP levels in cultures and slices. Moreover, the Aβ-enriched medium decreases the surface expression of NR1 and NR2B in WT cultured cortical neurons and cortical slices but has no effect in STEP KO cultures, which suggests that STEP is integral to the Aβ-mediated NMDAR endocytosis. Interestingly, a follow-up experiment we performed after the publication of this paper shows that although the Aβ-induced NMDAR internalization does not occur in STEP KO neuronal cultures, it is restored if wild-type STEP protein is added to the cultures prior to Aβ stimulation.23
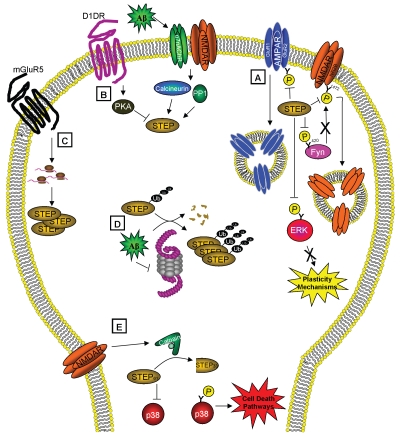
The mechanism by which Aβ increases STEP61 protein levels involves inhibition of the ubiquitin proteasome system.5 Ubiquitinated STEP61 accumulates in cortical cultures and slices treated with Aβ-enriched medium and in the cortex of 12-month (but not 3-month) old Tg2576 mice. Pharmacological proteasome inhibition leads to the increase in STEP61, whereas neither translation nor transcription inhibitors block the increase. Overall, the study supports a model in which STEP61 is normally degraded by the ubiquitin proteasome, but accumulates due to Aβ blockade of the proteasome. STEP accumulation triggers the internalization of glutamate receptors, the inactivation of Fyn and ERK1/2 and thereby contributes to the cognitive deficits that arise in this devastating illness. For a schematic summary of STEP function and regulation, see Figure 1.
Figure 1.
STEP functions and regulation. (A) STEP activity opposes the development of synaptic plasticity by dephosphorylating and inactivating ERK and Fyn and leading to the endocytosis of AMPA and NMDA receptors. STEP is itself regulated by (B) phosphorylation, (C) local translation, (D) ubiquitination and degradation and (E) proteolytic cleavage. (B) STEP is inactivated by PKA-phosphorylation after dopamine stimulation and activated by calcineurin/PP1 dephosphorylation of this residue after α7nAChR and NMDAR stimulation. (C) Stimulation of mGluR5 leads to local translation of STEP. (D) STEP is normally degraded by the ubiquitin proteasome system (UPS), but in the presence of Aβ, the UPS is impaired, causing the accumulation of polyubiquitinated STEP. (E) Activation of extrasynaptic NMDARs triggers calpain to cleave STEP61 into STEP33, which prevents STEP from regulating its substrates and leads to the release of p38 from inhibition and activation of cell death pathways. These regulatory pathways may contribute to the pathophysiology of Alzheimer's if disrupted, and also implicate STEP as an excellent target for drug discovery.
Concluding Remarks
STEP regulates the activity of MAPK, Fyn and the trafficking of NMDARs and AMPARs. Perhaps because of the importance of its substrates to normal neuronal function, regulation of STEP is itself proving enormously complex. Disruption at several levels—phosphorylation, translation, proteolysis, ubiquitination—may trigger a spiral into disease and pharmacological correction of STEP activity may lead to a novel class of drugs for the treatment of Alzheimer's disease. Further research into these mechanisms promises to take us a step forward in our understanding of the biology of STEP and may suggest rational treatments of CNS disorders.
Acknowledgements
We thank the members of our laboratory and Marilee Ogren for helpful discussions and critical reading of the manuscript. The work was funded by The American Health Assistance Foundation, the Institute for the Study of Aging, and NIH grants MH01527 and MH52711 to P.J.L.
Abbreviations
- AD
alzheimer's disease
- AMPAR
α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate receptor
- MAPK
mitogen activated protein kinase
- NMDAR
N-methyl-D-aspartate receptor
- PKA
protein kinase A
- STEP
striatal-enriched protein tyrosine phosphatase
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/12692
References
- 1.Feldman DE. Synaptic mechanisms for plasticity in neocortex. Annu Rev Neurosci. 2009;32:33–55. doi: 10.1146/annurev.neuro.051508.135516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Braithwaite SP, Paul S, Nairn AC, Lombroso PJ. Synaptic plasticity: One STEP at a time. Trends Neurosci. 2006;29:452–458. doi: 10.1016/j.tins.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Y, Venkitaramani DV, Gladding CM, Zhang Y, Kurup P, Molnar E, et al. The tyrosine phosphatase STEP mediates AMPA receptor endocytosis after metabotropic glutamate receptor stimulation. J Neurosci. 2008;28:10561–10566. doi: 10.1523/JNEUROSCI.2666-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu J, Kurup P, Zhang Y, Goebel-Goody SM, Wu PH, Hawasli AH, et al. Extrasynaptic NMDA receptors couple preferentially to excitotoxicity via calpain-mediated cleavage of STEP. J Neurosci. 2009;29:9330–9343. doi: 10.1523/JNEUROSCI.2212-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kurup P, Zhang Y, Xu J, Venkitaramani DV, Haroutunian V, Greengard P, et al. Aβ-mediated NMDA receptor endocytosis in alzheimer's disease involves ubiquitination of the tyrosine phosphatase STEP61. J Neurosci. 2010;30:5948–5957. doi: 10.1523/JNEUROSCI.0157-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bult A, Zhao F, Dirkx R, Jr, Raghunathan A, Solimena M, Lombroso PJ. STEP: A family of brain-enriched PTPs. Alternative splicing produces transmembrane, cytosolic and truncated isoforms. Eur J Cell Biol. 1997;72:337–344. [PubMed] [Google Scholar]
- 7.Boulanger L, Lombroso P, Raghunathan A, During M, Wahle P, Naegele J. Cellular and molecular characterization of a brain-enriched protein tyrosine phosphatase. J Neurosci. 1995;15:1532–1544. doi: 10.1523/JNEUROSCI.15-02-01532.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Costa-Mattioli M, Sossin WS, Klann E, Sonenberg N. Translational control of long-lasting synaptic plasticity and memory. Neuron. 2009;61:10–26. doi: 10.1016/j.neuron.2008.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sweatt JD. Mitogen-activated protein kinases in synaptic plasticity and memory. Curr Opin Neurobiol. 2004;14:311–317. doi: 10.1016/j.conb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Venkitaramani DV, Paul S, Zhang Y, Kurup P, Ding LI, Tressler L, et al. Knockout of STriatal enriched protein tyrosine phosphatase in mice results in increased ERK1/2 phosphorylation. Synapse. 2009;63:69–81. doi: 10.1002/syn.20608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paul S, Olausson P, Venkitaramani DV, Ruchkina I, Moran TD, Tronson N, et al. The striatal-enriched protein tyrosine phosphatase gates long-term potentiation and fear memory in the lateral amygdala. Biol Psychiatry. 2007;61:1049–1061. doi: 10.1016/j.biopsych.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunah AW, Sirianni AC, Fienberg AA, Bastia E, Schwarzschild MA, Standaert DG. Dopamine D1-dependent trafficking of striatal N-methyl-D-aspartate glutamate receptors requires fyn protein tyrosine kinase but not DARPP-32. Mol Pharmacol. 2004;65:121–129. doi: 10.1124/mol.65.1.121. [DOI] [PubMed] [Google Scholar]
- 13.Hallett PJ, Spoelgen R, Hyman BT, Standaert DG, Dunah AW. Dopamine D1 activation potentiates striatal NMDA receptors by tyrosine phosphorylation-dependent subunit trafficking. J Neurosci. 2006;26:4690–4700. doi: 10.1523/JNEUROSCI.0792-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Braithwaite SP, Adkisson M, Leung J, Nava A, Masterson B, Urfer R, et al. Regulation of NMDA receptor trafficking and function by striatal-enriched tyrosine phosphatase (STEP) Eur J Neurosci. 2006;23:2847–2856. doi: 10.1111/j.1460-9568.2006.04837.x. [DOI] [PubMed] [Google Scholar]
- 15.Snyder EM, Philpot BD, Huber KM, Dong X, Fallon JR, Bear MF. Internalization of ionotropic glutamate receptors in response to mGluR activation. Nat Neurosci. 2001;4:1079–1085. doi: 10.1038/nn746. [DOI] [PubMed] [Google Scholar]
- 16.Gladding CM, Collett VJ, Jia Z, Bashir ZI, Collingridge GL, Molnár E. Tyrosine dephosphorylation regulates AMPAR internalisation in mGluR-LTD. Mol Cell Neurosci. 2009;40:267–279. doi: 10.1016/j.mcn.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 17.Moult PR, Correa SAL, Collingridge GL, Fitzjohn SM, Bashir ZI. Co-activation of p38 mitogen-activated protein kinase and protein tyrosine phosphatase underlies metabotropic glutamate receptor-dependent long-term depression. J Physiol. 2008;586:2499–2510. doi: 10.1113/jphysiol.2008.153122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y, Venkitaramani DV, Gladding CM, Zhang Y, Kurup P, Molnar E, et al. The tyrosine phosphatase STEP mediates AMPA receptor endocytosis after metabotropic glutamate receptor stimulation. J Neurosci. 2008;28:10561–10566. doi: 10.1523/JNEUROSCI.2666-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valjent E, Pascoli V, Svenningsson P, Paul S, Enslen H, Corvol J, et al. Regulation of a protein phosphatase cascade allows convergent dopamine and glutamate signals to activate ERK in the striatum. Proc Natl Acad Sci USA. 2005;102:491–496. doi: 10.1073/pnas.0408305102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY, et al. Regulation of NMDA receptor trafficking by amyloid-β. Nat Neurosci. 2005;8:1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- 21.Bardoni B, Davidovic L, Bensaid M, Khandjian EW. The fragile X syndrome: Exploring its molecular basis and seeking a treatment. Expert Rev Mol Med. 2006;8:1–16. doi: 10.1017/S1462399406010751. [DOI] [PubMed] [Google Scholar]
- 22.Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: Lessons from the alzheimer's amyloid β-peptide. Nat Rev Mol Cell Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 23.Kurup P, Zhang Y, Venkitaramani DV, Xu J, Lombroso PJ. The role of STEP in Alzheimer disease. Channels. 2010;4 doi: 10.4161/chan.4.5.12910. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]