Abstract
Neurotransmitter release relies on the fusion of synaptic vesicles with the plasma membrane of synaptic boutons, which is followed by the recycling of vesicle components and formation of new vesicles. It is not yet clear whether upon fusion the vesicles persist as multimolecular patches in the plasma membrane, or whether they segregate into individual components. Evidence supporting each of these two models has been suggested in recent years. Using diffraction-unlimited imaging (stimulated emission depletion, or STED) of native synaptic vesicle proteins, we have proposed that vesicle proteins remain in clusters on the neuronal surface. These clusters do not appear to intermix. We discuss here these findings in the context of previous studies on synaptic vesicle fusion, and we propose a recycling model which accounts for most of the recent findings on the post-fusion fate of synaptic vesicle components.
Key words: vesicle, exocytosis, endocytosis, STED, resolution, fusion, recycling
Communication through chemical synapses relies on the fusion of neurotransmitter-loaded synaptic vesicles with the plasma membrane (exocytosis), which results in the release of neurotransmitter onto post-synaptic receptors. The vesicle components are afterwards retrieved (endocytosis) and a new vesicle is generated, completing the so-called synaptic vesicle cycle.1
Vesicles can fuse transiently to the plasma membrane, presumably through small pores (a few nanometers in diameter), a mechanism which has been termed kiss-and-run.2 Alternatively, they may collapse into the plasma membrane (full fusion), to be later endocytosed by clathrin-dependent mechanisms.3 In the kiss-and-run model the identity of the vesicle would be perfectly preserved, with essentially all vesicle proteins (and possibly lipids) persisting within the vesicle structure. It is much less clear whether vesicle identity is preserved in full fusion. As already discussed three decades ago by Ceccarelli and Hurlbut,4 two main models can be envisioned: first, the vesicle components would remain as a patch of molecules on the plasma membrane (essentially like a droplet of oil on the surface of water) and they would be eventually endocytosed as a whole. In the second model, exocytosis may be followed by the wide diffusion of vesicle components, which intermix with other vesicle proteins already present in the plasma membrane. As a result, the subsequent endocytosis returns vesicles composed of both newly exocytosed and membrane-resident proteins.
These alternative models have been discussed for decades, although they have been tested directly only in recent years. One basic aspect is the morphology of the newly fused vesicles—the maintenance of identity would result in vesicle-sized patches on the plasma membrane, while numerous smaller fragments should be observed when vesicle identity is not preserved. However, this could not be tested experimentally for decades, since conventional light microscopy (the optimal tool to investigate such a proposal) is limited by the diffraction of light5 to a spatial resolution of ∼200 nm, much larger than the diameter of vesicles (∼40–50 nm) or even fused vesicles (∼80–90 nm). We have used recently stimulated emission depletion (STED) microscopy to investigate the size of fused vesicles.6 STED is one of the first concepts which breaks the diffraction barrier by combining two light beams, with the first exciting the fluorophores and the second quenching them through stimulated emission.7 The first beam is focused to a conventional diffraction-limited spot and the second beam is modulated to a toroidal (doughnut) shape; by aligning the two beams all molecules in focus are excited, but only the ones in the center of the toroid are allowed to fluoresce—all others are quenched by the second beam. As a result, fluorescence is collected from a smaller area than the one originally excited, providing a substantial enhancement in resolution. Using STED we found that the synaptic vesicle marker synaptotagmin I formed well-defined clusters of ∼80–90 nm in diameter on the plasma membrane. The size and appearance (intensity) of the clusters did not vary with stimulation (with synaptic activity), and the large majority of the clusters appeared to contain several synaptotagmin molecules. The absence of single synaptotagmin molecules on the membrane surface, even after prolonged stimulation, suggested that the diffusion of molecules from fusing vesicles might have been only a rare event.
A different approach to study the same issue was introduced almost simultaneously8,9 by investigating synaptic proteins tagged with a pH-dependent variant of green fluorescent protein (GFP), termed pHluorins.10 pHluorins are normally quenched inside the synaptic vesicle (due to the low pH of ∼5–6). At neutral pH (i.e., upon exocytosis) their fluorescence increases drastically, and allows their visualization. pHluorin-tagged proteins disperse out of synapses upon strong (non-physiological) stimulation,8,9,11,12 suggesting that vesicle proteins diffuse upon exocytosis—either in the form of single copies or as assemblies (patches, clusters). One caveat of these results is the assumption that the overexpressed chimeric pHluorin proteins behave in the same manner as native synaptic proteins; potential problems of expression/protein behavior have been already noted by some authors.13
One characteristic of pHluorin recordings is that the signal increase during exocytosis is perfectly counterbalanced by the decrease caused afterwards through endocytosis and vesicle acidification.14 This aspect was exploited in an elegant approach to distinguish newly exocytosed molecules from their membrane-resident counterparts.8,9 The membrane-resident pHluorin moyeties were either bleached8 or enzymatically removed,9 and synaptic activity was stimulated—surprisingly resulting in the retrieval of only a fraction of all newly exocytosed proteins. Some recently exocytosed molecules were thus left on the plasma membrane, with the cell endocytosing in their place non-fluorescent membrane-resident molecules. Both studies concluded that endocytosis results in the retrieval of a mixture of “old” (membrane-resident) and “new” (newly exocytosed) molecules.8,9
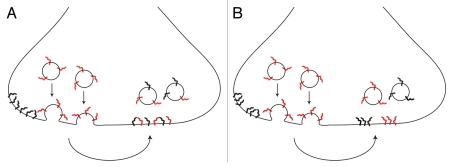
This conclusion was interpreted in the literature as proof for the disintegration of vesicles upon fusion. The components of newly released vesicles dispersed, and they afterwards mixed with vesicle proteins already present on the plasma membrane—with the ensuing endocytosis having no option but to retrieve patchwork vesicles, composed of both membrane-resident and newly exocytosed molecules (see model in Fig. 1A). However, when Wienisch and Klingauf9 used only brief stimuli (which still cause the recycling of several vesicles in each synaptic bouton), the retrieval of exclusively membrane-resident molecules was detected—i.e., no intermixing took place, with one set of vesicles exocytosed and a completely different set endocytosed. This offers a completely different interpretation to the observations of mixed endocytosis: a set of vesicles is released, they persist on the plasma membrane as molecular patches (i.e., they keep their identity), and during the subsequent endocytosis both membrane-resident and newly exocytosed vesicles (rather than molecules, Fig. 1B) are internalized. In this model endocytosis still results in the retrieval of both “old” and “new” molecules, but in different vesicles—thus placing both the pHluorin studies8,9 and our original STED observations6 in perfect agreement.
Figure 1.
Scheme representing two possible cases upon fusion. (A) newly exocytosed vesicle components (red) intermix with components resident on the plasma membrane (black), leading to the endocytosis of intermixed vesicle components. (B) newly exocytosed vesicle components (red) stay clustered and endocytosed vesicles keep their identity, although both newly exocytosed and membrane-resident components are internalized in separate vesicles.
To test this hypothesis, we have recently investigated vesicle recycling by combining STED microscopy with an approach in which native synaptic vesicle proteins, rather than GFP-tagged ones, are investigated.15 The intra-vesicular domain of synaptotagmin I is targeted by antibodies, which can only access it when the vesicle fuses to the plasma membrane. During endocytosis the antibodies are taken up into the vesicle, which recycles normally afterwards.6,16 Using antibodies labeled with fluorescent dyes permits the investigation of particular vesicles by both conventional and STED microscopy.
We first found that the escape of native (antibody-labeled) proteins out of synapses does take place upon strong non-physiological stimulation, confirming the previous pHluorin observations (see above). However, this escape was not detectable when stimulation was restricted to more physiological ranges. Stimulation by 40 action potentials within 2 seconds, which caused the recycling of ∼19% of all vesicles (or about 20–40 vesicles in one synaptic bouton, within only a few seconds; see also17) resulted in no measurable escape of molecules. This correlated well with the finding that not just synaptotagmin, but also two other important synaptic vesicle components, synaptophysin and a vesicular neurotransmitter transporter, were found in small patches on the plasma membrane, with no evidence of single molecules diffusing.15
However, antibody labeling is limited in one respect—the large size of the antibodies does not allow for the identification of all molecules in a cluster, due to steric hindrance. Thus, one could not state whether they were complete vesicles, although the synaptotagmin patches contained on average a sizable 5–6 labeled molecules. The average vesicles may contain ∼15 synaptotagmin molecules,18 so one could not exclude that the patches we observed were actually only vesicle fragments (for example, vesicle halves). To test this directly, we resorted to high-resolution imaging in two color channels. We labeled one set of synaptotagmin molecules by stimulating the preparations in presence of fluorescently labeled antibodies, and, after washing the antibody, we again stimulated the preparations in presence of antibodies labeled with a second dye. The reasoning is that if the vesicles labeled on the first round of labeling do not maintain their identity on the second round of stimulation, an abundance of dual-labeled vesicles would form after the two rounds of stimulation. We employed two types of high-resolution microscopy to investigate the results (two-color STED,19 and conventional/STED imaging of ultrathin fluorescence sections20), and found that essentially no intermixing could be observed, irrespective of the strength of stimulation. The suggestion is thus that the patches of fused vesicle molecules of different ages (membrane-resident or newly exocytosed) do not associate to form new vesicles, which by extension suggests that the patches may represent more or less complete vesicle entities—with only the second model of Figure 1 being accurate.
Finally, one may also wonder whether the model of vesicle molecule intermixing (Fig. 1A) is due to pHluorins functioning different than native proteins. Our interpretation (Fig. 1B) is in perfect agreement with the pHluorin results, with the only caveat that the “intermixing” observed in the pHluorin studies8,9 appears at the level of complete vesicles, rather than single molecules. However, the diffusion of at least some pHluorins into the axonal space upon stimulation does appear to happen even with physiological stimulation (as commented on in the past13), suggesting that they may not form clusters similar to those of the native proteins. A direct investigation of three GFP-tagged synaptic vesicle proteins showed that they indeed seem to diffuse in the plasma membrane, with few protein clusters forming.15
We conclude that synaptic vesicle proteins persist as clusters in the plasma membrane, likely in vesicle-sized units containing most of the vital synaptic components. The high cholesterol contents of the vesicles (∼40%18) may play a role in keeping synaptic vesicle proteins together,21 thus resulting in a patch (or perhaps raft22) of molecules on the plasma membrane. The molecular patch may also persist during other steps in vesicle membrane recycling, such as endosomal sorting1 or may play roles even in vesicle biogenesis, especially as the synaptic vesicle proteins form cholesterol-dependent domains in endosomes.23,24
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/12132
References
- 1.Sudhof TC. The synaptic vesicle cycle. Annu Rev Neurosci. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- 2.Fesce R, Grohovaz F, Valtorta F, Meldolesi J. Neurotransmitter release: fusion or ‘kiss-and-run’? Trends Cell Biol. 1994;4:1–4. doi: 10.1016/0962-8924(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 3.Cremona O, De Camilli P. Synaptic vesicle endocytosis. Curr Opin Neurobiol. 1997;7:323–330. doi: 10.1016/s0959-4388(97)80059-1. [DOI] [PubMed] [Google Scholar]
- 4.Ceccarelli B, Hurlbut WP. Vesicle hypothesis of the release of quanta of acetylcholine. Physiol Rev. 1980;60:396–441. doi: 10.1152/physrev.1980.60.2.396. [DOI] [PubMed] [Google Scholar]
- 5.Abbe E. Beiträge zur Theorie des Mikroskops und der mikroskopischen Wahrnehmung. Arch f Mikr Anat. 1873;9:413–420. (Ger). [Google Scholar]
- 6.Willig KI, Rizzoli SO, Westphal V, Jahn R, Hell SW. STED microscopy reveals that synaptotagmin remains clustered after synaptic vesicle exocytosis. Nature. 2006;440:935–939. doi: 10.1038/nature04592. [DOI] [PubMed] [Google Scholar]
- 7.Hell SW, Wichmann J. Breaking the diffraction resolution limit by stimulated emission: stimulated-emission-depletion fluorescence microscopy. Opt Lett. 1994;19:780–782. doi: 10.1364/ol.19.000780. [DOI] [PubMed] [Google Scholar]
- 8.Fernández-Alfonso T, Kwan R, Ryan TA. Synaptic vesicles interchange their membrane proteins with a large surface reservoir during recycling. Neuron. 2006;51:179–186. doi: 10.1016/j.neuron.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 9.Wienisch M, Klingauf J. Vesicular proteins exocytosed and subsequently retrieved by compensatory endocytosis are nonidentical. Nat Neurosci. 2006;9:1019–1027. doi: 10.1038/nn1739. [DOI] [PubMed] [Google Scholar]
- 10.Miesenböck G, De Angelis DA, Rothman JE. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature. 1998;394:192–195. doi: 10.1038/28190. [DOI] [PubMed] [Google Scholar]
- 11.Fernandez-Alfonso T, Ryan TA. A heterogeneous “resting” pool of synaptic vesicles that is dynamically interchanged across boutons in mammalian CNS synapses. Brain Cell Biol. 2008;36:87–100. doi: 10.1007/s11068-008-9030-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Z, Murthy VN. Visualizing postendocytic traffic of synaptic vesicles at hippocampal synapses. Neuron. 2001;31:593–605. doi: 10.1016/s0896-6273(01)00398-1. [DOI] [PubMed] [Google Scholar]
- 13.Granseth B, Odermatt B, Royle SJ, Lagnado L. Clathrin-mediated endocytosis is the dominant mechanism of vesicle retrieval at hippocampal synapses. Neuron. 2006;51:773–786. doi: 10.1016/j.neuron.2006.08.029. [DOI] [PubMed] [Google Scholar]
- 14.Sankaranarayanan S, Ryan TA. Real-time measurements of vesicle-SNARE recycling in synapses of the central nervous system. Nat Cell Biol. 2000;2:197–204. doi: 10.1038/35008615. [DOI] [PubMed] [Google Scholar]
- 15.Opazo F, Punge A, Bückers J, Hoopmann P, Kastrup L, Hell SW, et al. Limited intermixing of synaptic vesicle components upon vesicle recycling. Traffic. 2010 doi: 10.1111/j.1600-0854.2010.01058.x. In press. [DOI] [PubMed] [Google Scholar]
- 16.Kraszewski K, Daniell L, Mundigl O, De Camilli P. Mobility of synaptic vesicles in nerve endings monitored by recovery from photobleaching of synaptic vesicle-associated fluorescence. J Neurosci. 1996;16:5905–5913. doi: 10.1523/JNEUROSCI.16-19-05905.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schikorski T, Stevens CF. Morphological correlates of functionally defined synaptic vesicle populations. Nat Neurosci. 2001;4:391–395. doi: 10.1038/86042. [DOI] [PubMed] [Google Scholar]
- 18.Takamori S, Holt M, Stenius K, Lemke EA, Grønborg M, Riedel D, et al. Molecular anatomy of a trafficking organelle. Cell. 2006;127:831–846. doi: 10.1016/j.cell.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 19.Donnert G, Keller J, Medda R, Andrei MA, Rizzoli SO, Lührmann R, et al. Macromolecular-scale resolution in biological fluorescence microscopy. Proc Natl Acad Sci USA. 2006;103:11440–11445. doi: 10.1073/pnas.0604965103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Punge A, Rizzoli SO, Jahn R, Wildanger J, Meyer L, Schönle A, et al. 3D reconstruction of high-resolution STED microscope images. Microsc Res Tech. 2008;71:644–750. doi: 10.1002/jemt.20602. [DOI] [PubMed] [Google Scholar]
- 21.Bennett MK, Calakos N, Kreiner T, Scheller RH. Synaptic vesicle membrane proteins interact to form a multimeric complex. J Cell Biol. 1992;116:761–775. doi: 10.1083/jcb.116.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 23.Geumann U, Schäfer C, Riedel D, Jahn R, Rizzoli SO. Synaptic membrane proteins form stable microdomains in early endosomes. Microsc Res Tech. 2009 doi: 10.1002/jemt.20800. In press. [DOI] [PubMed] [Google Scholar]
- 24.Donnert G, Keller J, Wurm CA, Rizzoli SO, Westphal V, Schönle A, et al. Two-color far-field fluorescence nanoscopy. Biophys J. 2007;92:67–69. doi: 10.1529/biophysj.107.104497. [DOI] [PMC free article] [PubMed] [Google Scholar]