Abstract
The Golgi apparatus (GA) is an intracellular organelle that plays a central role in lipid and protein posttranslational modification and sorting. In addition, the GA has been also shown to be involved in Ca2+ signalling, as: (i) it accumulates Ca2+ within its lumen in an ATP-dependent process catalyzed by two enzymes, the sarco-endoplasmic reticulum Ca2+ ATPase (SERCA) and the secretory pathway Ca2+ ATPase1 (SPCA1), and (ii) it releases Ca2+ during cell stimulation in response to inositol 1,4,5-trisphosphate (IP3) receptor activation. Therefore, on this aspect, the GA appears to behave similarly to the major intracellular Ca2+ store, the endoplasmic reticulum (ER). By using a new FRET-based Ca2+ probe, specifically targeted to the trans-compartment of the GA, we demonstrate that the organelle is heterogeneous in terms of Ca2+ handling, the trans-Golgi being insensitive to IP3 and capable of accumulating Ca2+ solely through the activity of SPCA1. The SERCA and the IP3 receptor appear to be restricted to the cis- and intermediate GA compartments. Moreover, selective reduction of Ca2+ concentration within the trans-Golgi, obtained by reducing the level of SPCA1 by RNAi, results in major alterations of protein trafficking within the secretory pathway and induces the collapse of the entire GA morphology.
Key words: Golgi apparatus, calcium, FRET, SPCA
The Golgi apparatus (GA) is a specialized membranous organelle involved in lipids and proteins modification during transport from their site of synthesis in the endoplasmic reticulum (ER) to other subcellular compartments, such as lysosomes, secretory vesicles and plasma membrane.1 Morphologically it is quite heterogeneous and, by EM analysis, it is possible to distinguish stacks of flat cysternae (cis- and medial Golgi), tubular-reticular networks and vesicles (trans-Golgi).2–4 These morphological differences parallel a distinct functionality: for example, glycosyl-transferase enzymes, acting on newly synthesized proteins, have distinct distribution and complementary role in the various GA compartments: mannosidase I is primarily located and active in the cis- and medial Golgi, while sialyl-transferase, fucosyl-transferase or sulphatases are found within the trans-Golgi cisternae and its more distal tubular reticular membrane network (the trans-Golgi network, TGN).5
In the last decade, it became clear that the GA also plays a key role as intracellular Ca2+ store: using the aequorin Ca2+ probe targeted to the organelle, it has been demonstrated that the compartment behaves similarly to the main intracellular Ca2+ store of non-excitable cells, the ER. It is indeed endowed, for Ca2+ uptake, with the sarcoplasmic-endoplasmic reticulum Ca2+ ATPase, SERCA (together with the secretory pathway Ca2+ ATPase1, SPCA1,6) and with inositol-trisphosphate receptors, IP3Rs, as Ca2+ release channels.7,8 The GA, therefore, has been considered as another important dynamic Ca2+ store that participates in determining the spatio-temporal complexity of the Ca2+ signal within the cell (reviewed in ref. 9). A number of indirect evidence suggests that the lumenal Ca2+ within the GA is fundamental in controlling some key processes occurring in the organelle (post-translational modifications, protein sorting and trafficking, etc.;10–12), and thus dynamic variations of the [Ca2+] within the Golgi could affect cell functions in a variety of ways.
Whether the GA is homogeneous in term of Ca2+ handling or whether it can be divided in different sub-compartments, as its morphology and functionality suggest, remained obscure,13 due to lack of tools to directly investigate this question. In a very recent paper, we have addressed this problem by developing a new, genetically encoded fluorescent Ca2+ indicator specifically targeted to the trans-Golgi that allows the quantitative and dynamic measurement of luminal [Ca2+] in this compartment at the single cell level. This probe has unexpectedly revealed that the trans-Golgi compartment behaves differently from the overall GA: it takes up Ca2+ almost exclusively via SPCA1 (and not by SERCA); it does not release Ca2+ in response to IP3 generation (but rather accumulates the cation as a consequence of the cytoplasmic Ca2+ rises); it is endowed (in some cells) with functional ryanodine receptors, RyRs, thus representing a potential store responding to local Ca2+-induced Ca2+ release or to second messengers such as cADPR and NAADP that activate the RyRs.14
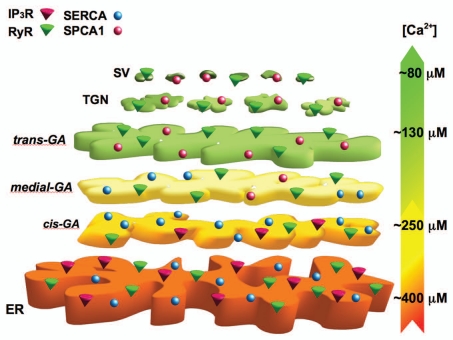
The Ca2+ concentration within the trans-Golgi (∼130 µM)14 appears to be significantly different than that measured in overall GA (∼2–300 µM)7 and ER (∼3–400 µM)15,16 of the same cells or in secretory granules (∼80 µM) as measured in other cell types.17 Taken together, the data from different laboratories, cells and probes suggest that there is a decrease in the lumenal Ca2+ concentration down the secretory pathway, ER > cis-Golgi > trans-Golgi > secretory vesicles (Fig. 1). Worth noting, this decrease in the free Ca2+ within the lumena of these compartments is not paralleled by a reduction in total Ca2+ content, rather the opposite, indicating that the Ca2+ buffering capacity increases drastically from the ER to the secretory compartment.
Figure 1.
Ca2+ concentration and molecular tool-kit gradient through the secretory pathway. The endoplasmic reticulum (ER) is endowed with SERCA, IP3Rs and, in some cells, RyRs and its luminal [Ca2+] is estimated to be around 400 µM. The Golgi apparatus (GA) can be divided in three distinct sub-compartments: the cis-Golgi, with a luminal [Ca2+] around 250 µM and expressing mainly SERCA and IP3Rs; the medial-Golgi with SERCA and SPCA1, but not with IP3Rs; the trans-Golgi with SPCA1 and RyRs (but not IP3Rs) and a luminal [Ca2+] of ∼130 µM. Finally, secretory vesicles (SV) are endowed with SPCA1 and RyRs and show a [Ca2+] around 80 µM. TGN, trans-Golgi network. This model is based on quantitative data for the ER and GA obtained in HeLa cells,7,14,15 while the data on the secretory vesicles have been extrapolated from experiments carried out in insulin secreting cells.17
In addition, using brefeldin A to block the forward, but not the backward, flow of vesicles in the GA18 and so inducing the back flow of most trans-Golgi membrane and luminal content (including the Ca2+ probe) into the medial- and cis-Golgi and eventually into the ER, we obtained indications for the presence of a Ca2+ toolkit protein gradient within the GA: the SERCA and IP3Rs are excluded from the trans-Golgi; the sensitivity to SERCA inhibitors appears in a compartment still devoid of IP3 sensitivity (medial-Golgi?); eventually, a compartment (presumably the cis-Golgi), can be revealed where both IP3Rs and SERCA are highly expressed (Fig. 1).
Since the new trans-Golgi Ca2+ probe utilized for this study14 has been constructed by including the trans-Golgi targeting sequence of the resident enzyme sialyl-transferase (the same used by Pinton et al. to targeted the aequorin Ca2+ sensor to the GA, Go-Aeq)7 at the N-terminus of a low Ca2+ affinity, FRET based indicator (Go-D1cpv),19 the question that arises is why Go-Aeq is retained in a different GA sub-compartment (not only in the trans-Golgi, but also in the cis/medial-GA) and why the signal of Go-Aeq is so dramatically biased towards reporting the Ca2+ changes from the compartment with the high sensitivity to IP3. As to the first question, the simplest explanation is that, because Go-Aeq is expressed at much higher levels than Go-D1cpv, its targeting is less accurate than that of the novel probe and, therefore, Go-Aeq is easily mis-targeted to the whole GA. Indeed, we found that the distribution of Go-Aeq in the Golgi overlaps not only with that of canonical trans-Golgi markers, but also with proteins typically located in the cis-GA compartment.14 In addition we often found cells with strong expression in which Go-Aeq was substantially retained also in the ER, while no mis-targeting of the Go-D1cpv in this compartment was ever observed. As to the second question, not only the signal of Go-Aeq is the mean of thousands of cells and of the different Golgi compartments, but, given the non-linear dependence of luminescence on the [Ca2+], the overall signal of this probe is intrinsically dominated by the compartments with highest Ca2+ concentration.20 A simple numerical example may explain this concept. Let's assume for simplicity that the Golgi is composed of two compartments, each trapping the same amount of aequorin, one with a [Ca2+] of 450 µM and the other of 150 µM. The normalized rates of photon emission (counts/s, cps) from the two compartments would be ∼l00 cps from the first compartment and ∼15 cps from the second. The mean luminescent signal would thus be dominated by the first compartment (average 55 cps). Most important, if only the first compartment is sensitive to IP3, the average response would be again biased towards reporting this event and not the small increase of the second compartment (the first would drop from 100 to ∼10 cps and the second would rise from ∼10 to ∼20 cps, on average a mean drop from 55 to 15 cps).
As to the importance of Ca2+ within the trans-Golgi, where the only Ca2+ uptake mechanism is based on SPCA1 activity, several authors showed that SPCA1 downregulation affects a number of cellular and Golgi specific functions.21,22 A SPCA1 knockout mouse is also available;23 in homozygote animals, the loss of the pump causes Golgi stress, expansion of the apparatus, increased apoptosis and embryonic lethality. Moreover, SPCA1 haploinsufficiency causes a genetic predisposition to cancer.23 The best example of SPCA1-dependent cellular defect comes from keratinocytes of patients with Hailey Hailey disease (with mutations in one allele for SPCA1).24,25 These cells have been thoroughly investigated for their defects in protein sorting and other specific cell functions.26–28 On this aspect, we found that reduction of SPCA1 protein level, by impairing trans-Golgi Ca2+ homeostasis, resulted in disturbed trafficking of different classes of proteins as well as in marked morphological alterations of the entire Golgi structure.14 Thus, maintaining the correct luminal [Ca2+] within the trans-Golgi compartment is essential not only for its specific functions, but also for the entire GA architecture.
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/12473
References
- 1.Keller P, Simons K. Post-Golgi biosynthetic trafficking. J Cell Sci. 1997;110:3001–3009. doi: 10.1242/jcs.110.24.3001. [DOI] [PubMed] [Google Scholar]
- 2.Mellman I, Simons K. The Golgi complex: in vitro veritas? Cell. 1992;68:829–840. doi: 10.1016/0092-8674(92)90027-A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polishchuk RS, Mironov AA. Structural aspects of Golgi function. Cell Mol Life Sci. 2004;61:146–158. doi: 10.1007/s00018-003-3353-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rambourg A, Clermont Y. Three-dimensional electron microscopy: structure of the Golgi apparatus. Eur J Cell Biol. 1990;51:189–200. [PubMed] [Google Scholar]
- 5.Breton C, Mucha J, Jeanneau C. Structural and functional features of glycosyltransferases. Biochimie. 2001;83:13–18. doi: 10.1016/s0300-9084(01)01298-6. [DOI] [PubMed] [Google Scholar]
- 6.Van Baelen K, Dode L, Vanoevelen J, Callewaert G, De Smedt H, Missiaen L, et al. The Ca2+/Mn2+ pumps in the Golgi apparatus. Biochim Biophys Acta. 2004;1742:103–112. doi: 10.1016/j.bbamcr.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 7.Pinton P, Pozzan T, Rizzuto R. The Golgi apparatus is an inositol 1,4,5-trisphosphate-sensitive Ca2+ store, with functional properties distinct from those of the endoplasmic reticulum. EMBO J. 1998;17:5298–5308. doi: 10.1093/emboj/17.18.5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Missiaen L, Van Acker K, Van Baelen K, Raeymaekers L, Wuytack F, Parys JB, et al. Calcium release from the Golgi apparatus and the endoplasmic reticulum in HeLa cells stably expressing targeted aequorin to these compartments. Cell Calcium. 2004;36:479–487. doi: 10.1016/j.ceca.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 9.Rizzuto R, Pozzan T. Microdomains of intracellular Ca2+: molecular determinants and functional consequences. Physiol Rev. 2006;86:369–408. doi: 10.1152/physrev.00004.2005. [DOI] [PubMed] [Google Scholar]
- 10.Carnell L, Moore HP. Transport via the regulated secretory pathway in semi-intact PC12 cells: role of intra-cisternal calcium and pH in the transport and sorting of secretogranin II. J Cell Biol. 1994;127:693–705. doi: 10.1083/jcb.127.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Austin CD, Shields D. Prosomatostatin processing in permeabilized cells. Calcium is required for prohormone cleavage but not formation of nascent secretory vesicles. J Biol Chem. 1996;271:1194–1199. doi: 10.1074/jbc.271.2.1194. [DOI] [PubMed] [Google Scholar]
- 12.Duncan JS, Burgoyne RD. Characterization of the effects of Ca2+ depletion on the synthesis, phosphorylation and secretion of caseins in lactating mammary epithelial cells. Biochem J. 1996;317:487–493. doi: 10.1042/bj3170487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vanoevelen J, Raeymaekers L, Parys JB, De Smedt H, Van Baelen K, Callewaert G, et al. Inositol trisphosphate producing agonists do not mobilize the thapsigargin-insensitive part of the endoplasmicreticulum and Golgi Ca2+ store. Cell Calcium. 2004;35:115–121. doi: 10.1016/j.ceca.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Lissandron V, Podini P, Pizzo P, Pozzan T. Unique characteristics of Ca2+ homeostasis of the trans-Golgi compartment. Proc Natl Acad Sci USA. 2010;107:9198–9203. doi: 10.1073/pnas.1004702107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Montero M, Brini M, Marsault R, Alvarez J, Sitia R, Pozzan T, et al. Monitoring dynamic changes in free Ca2+ concentration in the endoplasmic reticulum of intact cells. EMBO J. 1995;14:5467–5475. doi: 10.1002/j.1460-2075.1995.tb00233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Montero M, Alvarez J, Scheenen WJJ, Rizzuto R, Meldolesi J, Pozzan T. Ca2+ homeostasis in the endoplasmic reticulum: coexistence of high and low [Ca2+] subcompartments in intact HeLa cells. J Cell Biol. 1997;139:601–611. doi: 10.1083/jcb.139.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitchell KJ, Pinton P, Varadi A, Tacchetti C, Ainscow EK, Pozzan T, et al. Dense core secretory vesicles revealed as a dynamic Ca2+ store in neuroendocrine cells with a vesicle-associated membrane protein aequorin chimaera. J Cell Biol. 2001;155:41–51. doi: 10.1083/jcb.200103145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doms RW, Russ G, Yewdell JW. Brefeldin A redistributes resident and itinerant Golgi proteins to the endoplasmic reticulum. J Cell Biol. 1989;109:61–72. doi: 10.1083/jcb.109.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palmer AE, Giacomello M, Kortemme T, Hires SA, Lev-Ram V, Baker D, et al. Ca2+ indicators based on computationally redesigned calmodulin-peptide pairs. Chem Biol. 2006;13:521–530. doi: 10.1016/j.chembiol.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 20.Montero M, Barrero MJ, Alvarez J. [Ca2+] microdomains control agonist-induced Ca2+ release in intact HeLa cells. FASEB J. 1997;11:881–885. doi: 10.1096/fasebj.11.11.9285486. [DOI] [PubMed] [Google Scholar]
- 21.Durr G, Strayle J, Plemper R, Elbs S, Klee SK, Catty P, et al. The medial-Golgi ion pump Pmr1 supplies the yeast secretory pathway with Ca2+ and Mn2+ required for glycosylation, sorting and endoplasmic reticulum-associated protein degradation. Mol Biol Cell. 1998;9:1149–1162. doi: 10.1091/mbc.9.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramos-Castaneda J, Park YN, Liu M, Hauser K, Rudolph H, Shull GE, et al. Deficiency of ATP2C1, a Golgi ion pump, induces secretory pathway defects in endoplasmic reticulum (ER)-associated degradation and sensitivity to ER stress. J Biol Chem. 2005;280:9467–9473. doi: 10.1074/jbc.M413243200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okunade GW, Miller ML, Azhar M, Andringa A, Sanford LP, Doetschman T, et al. Loss of the Atp2c1 secretory pathway Ca(2+)-ATPase (SPCA1) in mice causes Golgi stress, apoptosis and midgestational death in homozygous embryos and squamous cell tumors in adult heterozygotes. J Biol Chem. 2007;282:26517–26527. doi: 10.1074/jbc.M703029200. [DOI] [PubMed] [Google Scholar]
- 24.Hu Z, Bonifas JM, Beech J, Bench G, Shigihara T, Ogawa H, et al. Mutations in ATP2C1, encoding a calcium pump, cause Hailey-Hailey disease. Nat Genet. 2000;24:61–65. doi: 10.1038/71701. [DOI] [PubMed] [Google Scholar]
- 25.Behne MJ, Tu CL, Aronchik I, Epstein E, Bench G, Bikle DD, et al. Human keratinocyte ATP2C1 localizes to the Golgi and controls Golgi Ca2+ stores. J Invest Dermatol. 2003;121:688–694. doi: 10.1046/j.1523-1747.2003.12528.x. [DOI] [PubMed] [Google Scholar]
- 26.Foggia L, Hovnanian A. Calcium pump disorders of the skin. Am J Med Genet C Semin Med Genet. 2004;131:20–31. doi: 10.1002/ajmg.c.30031. [DOI] [PubMed] [Google Scholar]
- 27.Missiaen L, Dode L, Vanoevelen J, Raeymaekers L, Wuytack F. Calcium in the Golgi apparatus. Cell Calcium. 2007;41:405–416. doi: 10.1016/j.ceca.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 28.Missiaen L, Raeymaekers L, Dode L, Vanoevelen J, Van Baelen K, Parys JB, et al. SPCA1 pumps and Hailey-Hailey disease. Biochem Biophys Res Commun. 2004;322:1204–1213. doi: 10.1016/j.bbrc.2004.07.128. [DOI] [PubMed] [Google Scholar]