Abstract
We recently reported that NMDA (N-Methyl-D-aspartate) and AMPA (α-Amino-3-hydroxy-5-methylisoxazole-4-propionic acid) induce concentration-dependent paroxysms in planarians (Dugesia dorotocephala). Since the postulated mechanisms of action of the sulfamate-substituted monosaccharide antiepileptic drug topiramate include inhibition of glutamate-activated ion channels, we tested the hypothesis that topiramate would inhibit glutamate-induced paroxysms in our model. We demonstrate that: (1) L-glutamate (1–10 mM), but not D-glutamate, induced dose-related paroxysms, and that (2) topiramate dose-relatedly (0.3–3 mM) inhibited L-glutamate-induced paroxysms. These results provide further evidence of a topiramate-sensitive glutamate receptor-mediated activity in this model.
Keywords: topiramate, glutamate, planarians, seizure, anticonvulsant
1. Introduction
Topiramate is an anticonvulsant drug that is used as mono- or adjunctive therapy and a wide spectrum of other CNS disorders (Maryanoff et al., 1987). Topiramate initially was believed to produce these effects by inhibition of carbonic anhydrase (Shank et al., 1994, 2005, 2006; Dodgson et al., 2000). However, following additional extensive study it is currently believed that several mechanisms contribute to topiramate’s anticonvulsant activity, either individually or in concert, including an action on ionotropic (including glutamate) receptors (reviewed in Shank and Maryanoff, 2008). The sugar subunit in topiramate adopts a ‘twist-boat’ conformation that is conducive to its overall spectrum of pharmacologic activities; the sulfamate moiety (OSO2NH2) is an essential component for its anticonvulsant activity (Maryanoff et al., 1987). An effect of topiramate on glutamate in vivo was demonstrated in a study using double mutant spontaneous epileptic rats. These rats have basal hippocampal extracellular levels of glutamate about 2–3-fold higher than do normal Wistar rats (Kanda et al., 1996). Topiramate (20–40 mg/kg, i.p.) dose-relatedly reduces the extracellular levels of glutamate in these rats, but not normal Wistar rats, over a time course similar to its active suppression of tonic seizures (Nakamura et al., 1994). A recent comprehensive review summarizes topiramate’s clinical use in epilepsy (Lyseng-Williamson and Yang, 2007).
Planarians express mammalian-like neurotransmitter and 2nd-messenger systems and provide a simple model of mammalian pharmacologic effects (reviewed in the recent monograph Raffa and Rawls, 2008). Individual planarians contain glutamate levels of at least 323 ± 44 pmol/mg-animal (Rawls et al., 2006) and express the genes products for at least two types of ionotropic glutamate receptors that share high sequence similarity with mouse and human neural specific genes (Cebria et al., 2002).
In a previous study we demonstrated that seizure-like paroxysms are induced in a concentration-dependent manner by the excitatory ligands NMDA and AMPA, but not the inhibitory amino acid glycine (Rawls et al., 2009). Topiramate attenuated the pSLA induced by NMDA and AMPA. The results of that study suggested an inhibitory action of topiramate on glutamate activity. The present study demonstrates this activity directly.
2. Methods
2.1. Animals and drugs
All planarians (Dugesia dorotocephala) were purchased from Carolina Biological Supply (Burlington, NC, USA). They were acclimated to ambient room temperature (21°C) and they were tested within 2 days of receipt. L- and D-glutamate were purchased from Tocris Biosciences (St Louis, MO) or Sigma Chemical Co (St Louis, MO). Topiramate (catalog no. T540250) was purchased from Toronto Research Chemicals (North York, Ontario).
2.2. Behavioral experiments
Planarians were placed individually into round petri dishes (5.1 cm diameter) containing water or test compound(s) and were observed for the occurrence of presumptive seizure-like activity, defined as paroxysms resulting in disruption of normal spontaneous locomotor activity. The planarians were observed for 5 min for the occurrence of paroxysms (the number of events counted). Separate planarians were photographed to illustrate the behavioral response, but the results were not included in the quantification analyses.
Prior to testing, each planarian was individually pretreated by 15-min exposure to either water or topiramate. The test groups were (pretreatment–test): (1) water → water, (2) water → L-glutamate (1, 3, 10 mM), (3) water → D-glutamate (3 mM), (4) water → topiramate (3 mM), (5) topiramate (3 mM) → water, and (5) water → [glutamate (3 mM) + topiramate (0.3, 1, 3 mM)].
Each planarian was used only one time. N = 8 planarians per treatment or control group. The ED50 for L-glutamate-induction and topiramate-antagonism of paroxysms were determined with InStat® (GraphPad Software, Inc., La Jolla, CA).
3. Results
3.1. L-Glutamate induces paroxysms
Consistent with normal behavior, planarians tested in water displayed a constant-velocity spontaneous locomotor activity and no paroxysms (representative photographs shown in Fig 1). In contrast, planarians exposed to L-glutamate displayed recurrent paroxysms (shown for 10 mM glutamate in Fig 2). The onset of paroxysms following L-glutamate exposure was rapid, beginning within 10 s following exposure. The duration of each individual behavior was approximately 1 s. The effect of L-glutamate was concentration-dependent, as planarians that were exposed to 1, 3, or 10 mM L-glutamate displayed an increasing amount of paroxysms with nearly 100% responding at 10 mM. In previous work we showed that planarians exposed to a variety of classes of other compounds do not display paroxysms (Rawls et al., 2009). Locomotor activity, although not specifically quantified here, was reduced in relation to concentration of L-glutamate. The possibility that low pH of the L-glutamate solutions (~3.5) contributed to induction of paroxysms was investigated. Planarians exposed to D-glutamate did not display paroxysms. The concentration-related L-glutamate-induced paroxysms observed qualitatively in the photographs were quantified in subsequent behavioral assay (by counts) (ED50 = 2.1 mM)) and is shown in Fig 3.
Fig 1.
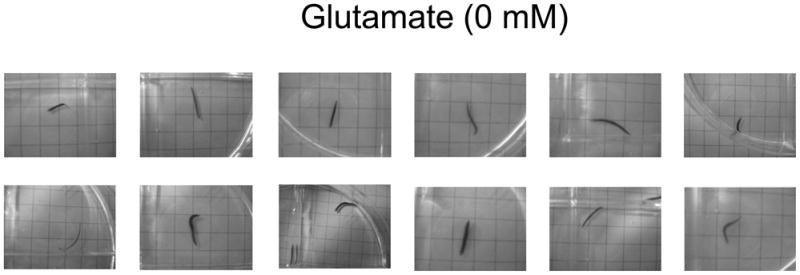
Representative photographs of the spontaneous locomotor activity displayed by planarians tested in water. When quantified as rate of gridlines crossed, locomotor activity is nearly linear for at least 10 minutes (see refs in Raffa and Rawls, 2008).
Fig 2.
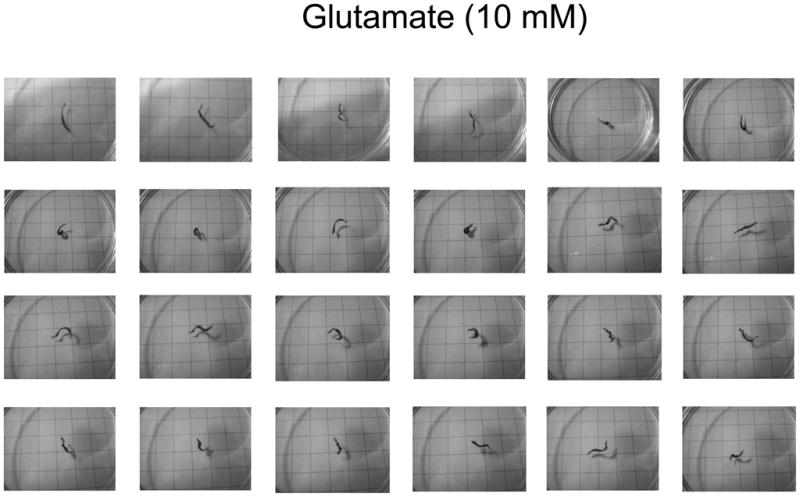
Representative photographs of paroxysms displayed when planarians were tested in L-glutamate. The behavior consisted of spasmodic twisting and contracture in-place, with loss of forward movement.
Fig 3.
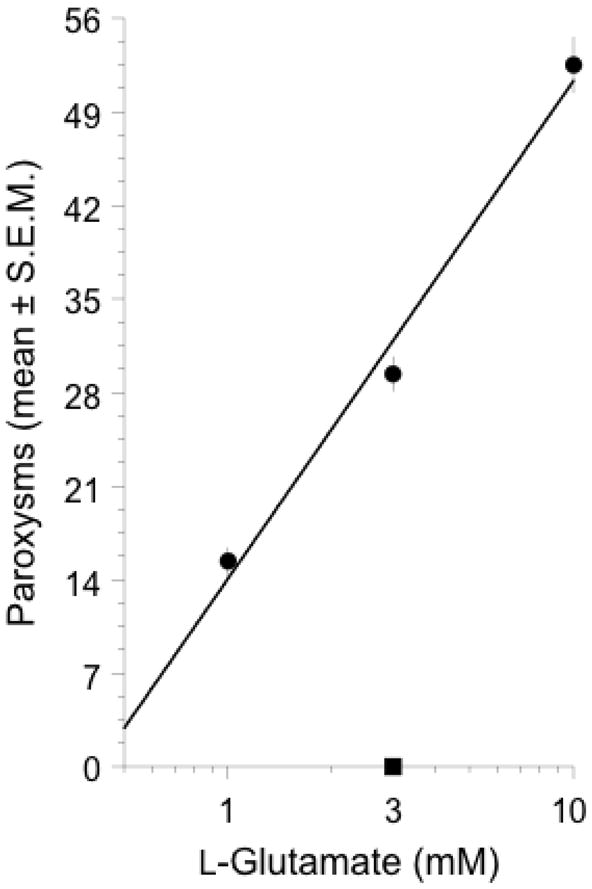
L-glutamate (filled circles), but not D-glutamate (filled square), induced concentration-related paroxysms, expressed as the mean (± S.E.M.) over a 5-min observation period. N = 8 planarians per group.
3.2. Topiramate inhibits L-glutamate-induced paroxysms
Topiramate (3 mM) did not induce paroxysms. However, planarians that were exposed to a combination of L-glutamate (3 mM) and topiramate (0.3, 1, 3 mM) displayed a dose-related reduction in paroxysms induced by L-glutamate (3 mM) (ED50 = 0.3 mM) (Fig. 4).
Fig 4.
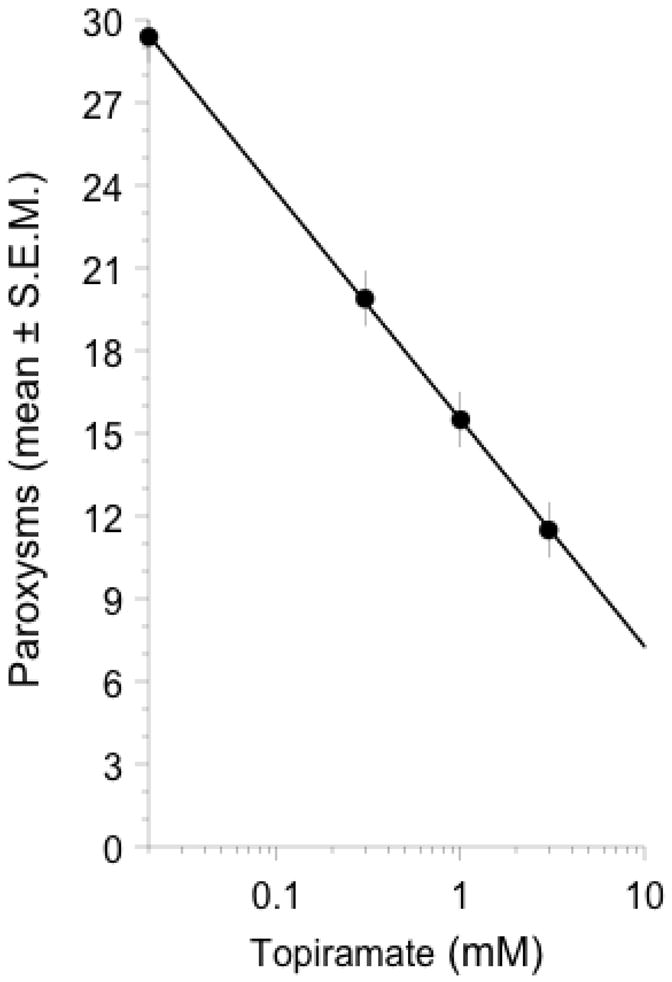
Topiramate (0.3, 1, 3 mM) attenuated the paroxysms induced by a fixed concentration of L-glutamate (3 mM). N = 8 planarians per group.
4. Discussion
Glutamate, the most abundant excitatory amino acid in the mammalian brain, is released in large quantities during seizures and triggers ion fluxes that are mediated or modulated by glutamate receptor subtypes. Fast excitatory glutamate neurotransmission involves ionotropic glutamate receptors (NMDA, AMPA, and kainate), whereas slow responses involve metabatropic G protein-coupled glutamate receptors (mL-GluR1 to mL-GluR8) (see Bazan et al., 2002).
Excitatory glutamate activity has been linked to the etiology, maintenance, or sequelae of epilepsy. Pathophysiology might not require excess glutamate per se, but merely an imbalance between excitatory (glutamate) and inhibitory (γ-aminobutyric acid (GABA)) influences on neurotransmission. As summarized in the comprehensive review by Dalby and Mody (2001): glutamate release increases during kindling epileptogenesis and during and after status epilepticus; chronically elevated glutamate levels caused by a lack of glutamate transporters (GLT-1 and GLAST) renders mice more susceptible to seizures; NMDA receptor levels are increased in epileptic tissue; the gain of NMDA receptor-mediated transmission in temporal lobe epilepsy is also effectively enhanced by post-translational phosphorylation and redox modifications of NMDA receptor subunits; and the enhanced glutamate-induced Ca2+ influx through AMPA receptors in genetically modified mice expressing a Q/R unedited GluR-B subunit render the mice significantly more susceptible than their wild type counterparts to temporal lobe epilepsy.
Planarians express gene products for at least two types of ionotropic glutamate receptors that share a high sequence similarity to neural-specific mouse and human gene products (Cebria et al., 2002). Previous studies that used neurochemical, molecular, and behavioral approaches revealed that planarians have endogenous glutamate and express glutamate-like receptors. We demonstrated the presence of glutamate and aspartate in individual planarians using a fluorescence high-pressure liquid chromatography (HPLC) method after a perchloric acid based extraction and filtration (Rawls et al., 2006).
Topiramate is used as medication for the treatment of a variety of CNS disorders associated with excess glutamatergic activity. In mammals, topiramate exerts an indirect inhibitory effect on glutamate receptor subtypes (e.g., Gibbs et al., 2000; Gryder and Rogawski, 2003; Qian and Noebels, 2003; Angehagen, 2004), possibly via an allosteric modulatory mechanism (Angehagen et al., 2004, 2005; Shank and Maryanoff, 2008).
In our previous study, planarians exposed to NMDA or to AMPA displayed pSLA. The effects of both NMDA and AMPA were antagonized by topiramate (Rawls et al., 2009). In the present study topiramate antagonized pSLA induced by L-Glu. While this inhibitory action of topiramate on the effect of L-Glu is consistent with its documented action in mammals, this is the first study to investigate such a role for topiramate in this model. The mechanism of topiramate’s demonstrated inhibition is not known. Since we showed previously that topiramate inhibits the action of NMDA and AMPA in this model (Rawls et al., 2009), topiramate’s inhibition in the present study might be related to L-Glu action through these ionotropic receptor subtypes. Since topiramate does not have affinity for these receptors, its inhibitory effect on L-Glu-induced pSLA could be due to an effect on the 2nd-messenger transduction of NMDA or AMPA receptors (i.e., ion channels). We cannot currently exclude an interaction at the other proposed mechanisms of action of topiramate summarized in the authoritative review by Shank and Maryanoff (2008): an inhibitory effect on voltage-gated Na+- or Ca2+-channels, or an enhancement of GABA-mediated Cl− fluxes into neurons (Pappalardo et al., 2004; Ziemann, 2003; Nakamura et al., 2000). We are attempting to elucidate the exact mechanism of inhibition.
In summary, the results of the present study demonstrated that planarians exposed to L-glutamate display a concentration-dependent paroxysms and that L-glutamate-induced paroxysms is inhibited by the anticonvulsant drug topiramate. These findings, and this model, might provide novel insight into the mechanism of seizures and the drugs used to treat them.
Acknowledgments
The authors thank Timothy Shickley, Ph.D., for suggesting Planaria as a test model. This work was supported by NIH (NIDA) grants DA15378 (RBR) and DA022694 (SMR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Angehagen M, Ben-Menachem E, Shank R, Ronnback L, Hansson E. Topiramate modulation of kainate-induced calcium currents is inversely related to channel phosphorylation level. J Neurochem. 2004;88:320–325. doi: 10.1046/j.1471-4159.2003.02186.x. [DOI] [PubMed] [Google Scholar]
- Bazan NG, Tu B, Rodriguez de Turco EB. What synaptic lipid signaling tells us about seizure-induced damage and epileptogenesis. Prog Brain Res. 2002;135:175–185. doi: 10.1016/S0079-6123(02)35017-9. [DOI] [PubMed] [Google Scholar]
- Cebrià F, Kudome T, Nakazawa M, Mineta K, Ikeo K, Gojobori T, Agata K. The expression of neural-specific genes reveals the structural and molecular complexity of the planarian central nervous system. Mech Dev. 2002;116:199–204. doi: 10.1016/s0925-4773(02)00134-x. [DOI] [PubMed] [Google Scholar]
- Dalby NO, Mody I. The process of epileptogenesis: a pathophysiological approach. Curr Opin Neurol. 2001;14:187–192. doi: 10.1097/00019052-200104000-00009. [DOI] [PubMed] [Google Scholar]
- Dodgson SJ, Shank RP, Maryanoff BE. Topiramate as aninhibitor of carbonic anhydrase isozymes. Epilepsia. 2000;41(Suppl 1):S35–39. doi: 10.1111/j.1528-1157.2000.tb06047.x. [DOI] [PubMed] [Google Scholar]
- Gibbs JW, III, Sombati S, DeLorenzo RJ, Coulter DA. Cellular actions of topiramate: blockade of kainate-evoked inward currents in cultured hippocampal neurons. Epilepsia. 2000;41(Suppl 1):S10–16. doi: 10.1111/j.1528-1157.2000.tb02164.x. [DOI] [PubMed] [Google Scholar]
- Gryder DS, Rogawski MA. Selective antagonism of L-GluR5 kainate-receptor-mediated synaptic currents by topiramate in rat basolateral amygdala neurons. J Neurosci. 2003;23:7069–7074. doi: 10.1523/JNEUROSCI.23-18-07069.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda T, Kurokawa M, Tamura S, Nakamura J, Ishii A, Kuwana Y, Serikawa T, Yamada J, Ishihara K, Sasa M. Topiramate reduces abnormally high extracellular levels of L-Glutamate and aspartate in the hippocampus of spontaneously epileptic rats (SER) Life Sci. 1996;59:1607–1616. doi: 10.1016/0024-3205(96)00492-4. [DOI] [PubMed] [Google Scholar]
- Lyseng-Williamson KA, Yang LPH. Topiramate: A review of its use in the treatment of epilepsy. Drugs. 2007;67:2231–2256. doi: 10.2165/00003495-200767150-00008. [DOI] [PubMed] [Google Scholar]
- Maryanoff BE, Nortey SO, Gardocki JF, Shank RP, Dodgson SP. Anticonvulsant O-alkyl sulfamates, 23:45-Bis-O-(1-methylethylidene)-β-D-fructopyranose sulfamate and related compounds. J Med Chem. 1987;30:880–887. doi: 10.1021/jm00388a023. [DOI] [PubMed] [Google Scholar]
- Nakamura J, Tamura S, Kanda T, Ishii A, Ishihara K, Serikawa T, Yamada J, Sasa M. Inhibition by topiramate of seizures in spontaneously epileptic rats and DBA/2 mice. Eur J Pharmacol. 1994;254:83–89. doi: 10.1016/0014-2999(94)90373-5. [DOI] [PubMed] [Google Scholar]
- Nakamura J, Kuwana Y, Yukitoshi N. Target pharmacology of topiramate, a new antiepileptic drug. Nippon Yakurigaku Zasshi. 2000;115:53–57. doi: 10.1254/fpj.115.53. [DOI] [PubMed] [Google Scholar]
- Pappalardo A, Liberto A, Patti F, Reggio A. Neuroprotective effects of topiramate. Clin Ter. 2004;155:75–78. [PubMed] [Google Scholar]
- Qian J, Noebels JL. Topiramate alters excitatory synaptic transmission in mouse hippocampus. Epilepsy Res. 2003;55:225–233. doi: 10.1016/s0920-1211(03)00120-7. [DOI] [PubMed] [Google Scholar]
- Raffa RB, Rawls SM. Planaria: A Model for Drug Action and Abuse. Landes Bioscience; Austin TX: 2008. [Google Scholar]
- Rawls SM, Gomez T, Raffa RB. An NMDA antagonist (LY 235959) attenuates abstinence-induced withdrawal of planarians following acute exposure to a cannabinoid agonist (WIN 55212-2) Pharmacol Biochem Behav. 2007;86:499–504. doi: 10.1016/j.pbb.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Rawls SM, Thomas T, Adeola M, Patil T, Raymondi N, Poles A, Loo M, Raffa RB. Topiramate antagonizes NMDA- and AMPA-induced seizure-like activity in planarians. Pharmacol Biochem Behav. 2009;93:363–367. doi: 10.1016/j.pbb.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Shank RP, Maryanoff BE. Molecular pharmacodynamics clinical therapeutics and pharmacokinetics of topiramate. CNS Neurosci Ther. 2008;14:120–142. doi: 10.1111/j.1527-3458.2008.00041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shank RP, Gardocki JF, Vaught JL, Davis CB, Schupsky JJ, Raffa RB, Dodgson SJ, Nortey SO, Maryanoff BE. Topiramate: preclinical evaluation of a structurally novel anticonvulsant. Epilepsia. 1994;35:450–460. doi: 10.1111/j.1528-1157.1994.tb02459.x. [DOI] [PubMed] [Google Scholar]
- Shank RP, Doose DR, Streeter AJ, Bialer M. Plasma and whole blood pharmacokinetics of topiramate: the role of carbonic anhydrase. Epilepsy Res. 2005;63:103–112. doi: 10.1016/j.eplepsyres.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Shank RP, McComsey DF, Smith-Swintosky VL, Maryanoff BE. Examination of two independent kinetic assays for determining the inhibition of carbonic anhydrases I and II: structure-activity comparison of sulfamates and sulfamides. Chem Biol Drug Design. 2006;68:113–119. doi: 10.1111/j.1747-0285.2006.00423.x. [DOI] [PubMed] [Google Scholar]
- Ziemann U. Pharmacology of TMS. Suppl Clin Neurophysiol. 2003;56:226–231. [PubMed] [Google Scholar]


