Abstract
Correlation between spike trains or neurons sometimes indicates certain neural coding rules in the visual system. In this paper, the relationship between spike timing correlation and pattern correlation is discussed, and their ability to represent stimulus features is compared to examine their coding strategies not only in individual neurons but also in population. Two kinds of stimuli, natural movies and checkerboard, are used to arouse firing activities in chicken retinal ganglion cells. The spike timing correlation and pattern correlation are calculated by cross-correlation function and Lempel–Ziv distance respectively. According to the correlation values, it is demonstrated that spike trains with similar spike patterns are not necessarily concerted in firing time. Moreover, spike pattern correlation values between individual neurons’ responses reflect the difference of natural movies and checkerboard; neurons cooperate with each other with higher pattern correlation values which represent spatiotemporal correlations during response to natural movies. Spike timing does not reflect stimulus features as obvious as spike patterns, caused by their particular coding properties or physiological foundation. As a result, separating the pattern correlation out of traditional timing correlation concept uncover additional insight in neural coding.
Keywords: Neural coding, Spike pattern correlation, Spike timing correlation, Cross-correlation, Lempel–Ziv distance
Introduction
It has been already known that stimulus information is represented with various rules in neuron spike trains called neural coding. The aim for understanding neural coding is to explore the distinct relationship between stimulus and the individual or ensemble neural responses. Many features in spike trains: firing rate, precise spike time (Butts et al. 2007; Uzzell and Chichilnisky 2004; Berry 1998), spike timescale (Butts et al. 2007), response latency (Gollisch and Meister 2008), special temporal patterns (Gollisch and Meister 2008; Lesica and Stanley 2004; Willmore and Tolhurst 2001; Berry et al. 1997), as well as the relationship among spike trains or neurons: firing reliability (Van Steveninck et al. 1997; Berry et al. 1997), synchronization and correlated temporal firing activity (Shlens et al. 2009; Puchalla et al. 2005; Schnitzer and Meister 2003; Devries 1999; Meister et al. 1995; Singer 2009), have been demonstrated playing a role more or less in neural coding in visual system, although it still remain unclear that whether these elements of neural coding are related to each other and how they cooperate and integrate to carry stimulus information.
In this paper, we focus on two aspects: the temporal coherence of spikes and the firing pattern correlation in both single and population neural responses.
The neurons’ temporal firing coherence is considered as spike timing correlation, emphasizing on whether spikes fire at the same time in spike trains. It has been widely discussed in previous researches. As for individual neurons, timing correlation is analyzed by studying firing reliability and precise temporal coding. In response to multiple stimulus trials, individual neurons can have extremely precise and repeatable responses (down to millisecond variability) (Van Steveninck et al. 1997; Berry et al. 1997). When it goes to neuron population, synchronized firing is a major component of neural activity in retina (DeVries 1999; Meister et al. 1995; Meister 1996). Synchronized activity is supposed to be more reliable and precise in coding information, which enables downstream neurons easier to extract the stimulus information conveyed, and helps neurons to carry more information than independent coding (Pillow et al. 2008; Desbordes et al. 2008; Dan et al. 1998). There are many methods available to quantify the timing correlation between spike trains, such as the cross-correlation analysis (Perkel et al. 1967; De Boer and Jongkees 1968), correlation index (Meister et al. 1995; Schnitzer and Meister 2003), spike trains distance (Victor and Purpura 1996; Van Rossum 2001) and other methods considering temporal characters (Kreuz et al. 2007). Cross-correlation function is used to measure the timing correlation here which gives more useful correlation information such as the peak width or time delay in diagrams.
The spike pattern correlation discussed here means the similarity of firing patterns in spike trains. The difference between the correlation of spike timing and spike patterns lies in whether the correlation is considered in temporal order. Supposing that two spike trains contain similar firing patterns which do not appear in the same time, measurement of timing correlation may give the result of low correlation value between the spike trains. However, it is hard to determine from this result that whether these two spike trains are related in other aspects. The Lempel–Ziv distance (LZ distance) addressed by Christen et al. (2006a) is calculated to evaluate the pattern similarity between two spike trains. LZ distance is calculated from Lempel–Ziv complexity (LZ complexity) which is used to measure the information carried along sequences (Wang et al. 2007; Amigo 2004; Kaspar and Schuster 1987). LZ distance considers spike trains with similar but possibly not temporal concerted firing patterns as related (Christen et al. 2006a). It has been proved effective in measuring neural firing reliability and clustering neuron groups (Christen et al. 2006b).
Using recordings of ganglion cells in five pieces of chicken retinas, we probed measurement of spike timing and pattern correlation in both individual and population responses to natural movies and checkerboard stimuli. By comparing how well timing correlation and pattern correlation reflect the stimulus information, their relationship and coding strategy are discussed respectively.
Methods
Experiment procedure
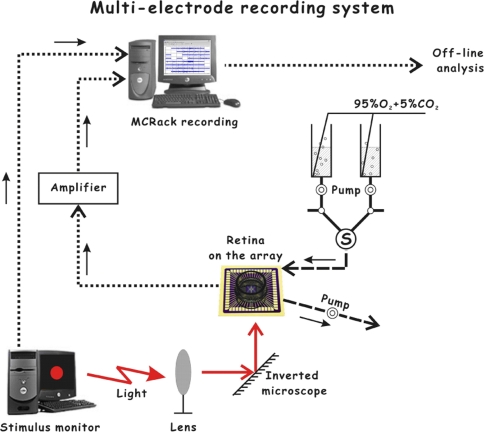
Experimental operations were described as our previous reports (Zhang et al. 2010). Newly-hatched chickens (3–15 days after hatching) were investigated in this study. After decapitation and enucleation of the eye, the eyeball was hemisected with a fine razor blade, and the vitreous body and cornea were removed carefully. A small piece (4 × 4 mm2) of isolated retina was placed on a micro-electrode array (MEA60, MCS GmbH, Germany) with the ganglion-cell-side contacting the electrodes. The micro-electrode array consists of 60 electrodes (10 μm in diameter) arranged in an 8 × 8 matrix (leaving the 4 corners void) with 100 μm tip-to-tip distances (horizontal and vertical). The preparation was perfused in oxygenated Ringer’s solution (containing in mM: 120.0 NaCl, 5.0 KCl, 3.0 MgCl2, 1.8 CaCl2, 25.0 NaHCO3, 1.2 HEPES, 25.0 glucose) with pH value of 7.5 ± 0.2. The tissue and perfusate were kept at 38°C by a temperature control unit (Thermostat HC-X, MCS GmbH, Germany). A small Ag/AgCl pellet with wire was immerged into the bath solution and acted as the reference electrode. The neuronal responses were recorded simultaneously by the micro-electrode array, and the signals were amplified through a 60-channel amplifier (single-ended amplifier, amplification 1,200×, amplifier input impedance >1010 Ω, output impedance 330 Ω). Signals were sampled at a rate of 20 kHz (MC_Rack, MCS GmbH, Germany). Spikes from individual neurons were sorted by principal component analysis (PCA) (Zhang et al. 2004) and K-means clustering by the commercial software MC_Rack and OfflineSorter (Plexon Inc., TX, USA). The whole recording system is shown in Fig. 1.
Fig. 1.
The multi-electrode recording system
Stimulus
The stimulus from a computer monitor was projected onto the retina piece via an optical lens system and covered the whole area of the micro-electrode array. The stimulation protocols are: (1) Three pieces of digitized segments of grayscale video recordings which covered a wide range of natural scenes (street, woods, houses etc.). The natural movies are presented at 10 Hz (the speed as being recorded) with the monitor refresh rate 120 Hz. Each frame contains 128 × 128 pixels and total 1,920 frames last 192 s (downloaded from the website of Hateren’s lab, http://hlab.phys.rug.nl/vidlib/index.html, Van Hateren and Van Schaaf 1998). (2) The checkerboard is made up of 16 × 16 pixels at the frame rate 9.05 Hz lasting 221 s. For each frame of the stimulus, the pixels are either black or white according to a binary pseudorandom m sequence.
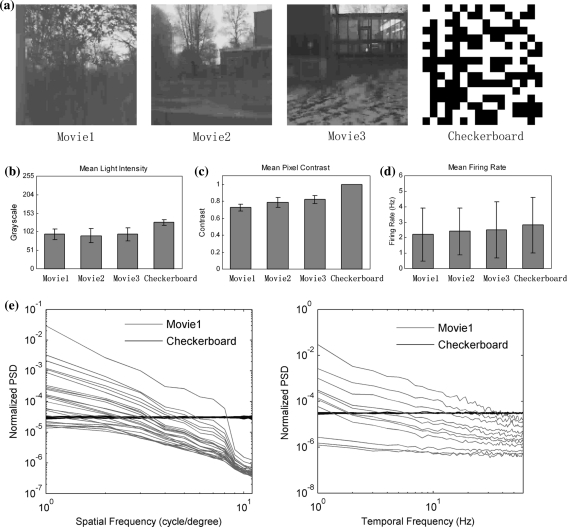
The two kinds of stimuli, natural movies and checkerboard, are quite different in light intensity, contrast, and temporal and spatial correlations. To illustrate their difference, the mean light intensity of stimulus frames, the mean contrast in pixels, and the spatial and temporal power spectral densities of the checkerboard and natural movies are compared in Fig. 2. The statistical characteristics of three natural movies are similar whereas they differ a lot from the checkerboard. The checkerboard has the highest contrast for its pixel switching between absolute white and black, while the natural movies’ contrast vary around lower value due to certain temporal correlations (Fig. 2c). The checkerboard has higher mean light intensity and contrast, leading to slightly higher mean firing rate in overall neural responses (Fig. 2b–d). The power spectral density (PSD) of each stimulus is calculated to characterize the underlying spatial and temporal correlation structure as shown in Fig. 2e. The checkerboard’s PSD lines lie horizontally showing a flat PSD in both temporal and spatial frequency domains, indicating it as the white noise that is random in space and time. Natural scenes have more PSD at low frequencies reflecting higher spatial and temporal correlations and this PSD decreases roughly as a reciprocal power of spatial or temporal spectrum (Dong and Atick 1995; Lesica and Stanley 2004). All these different aspects of two kind stimuli are likely to arouse different styles of neural responses. For example, detecting determinism in firing activities of retinal ganglion cells shows different nonlinear properties of neuron’s response to natural movies and checkerboard (Cai et al. 2008). The stimuli difference may be also reflected by timing correlation or firing pattern correlation between spike trains.
Fig. 2.
Natural movies and checkerboard stimuli. a Sample frames of three natural movies and checkerboard. b The mean light intensity and c the mean contrast of four stimuli. The errorbars stand for the variation of mean light intensity of each frame and the variation of contrast of each pixel respectively. d The overall firing rate averaging across 24 neurons’ response in the first retina to four stimuli. e The spatial and temporal frequency power spectral densities of the checkerboard (black) and natural movie (gray) stimuli. Movie and checkerboard PSD were averaged by each 128 frames over the whole length. The PSD were normalized so that both stimuli had the same total power. The temporal PSD are shown at a range of spatial frequencies and the spatial PSD are shown at a range of temporal frequencies. Each line stands for the spatial PSD in a certain temporal frequency (in the firstfigure) or the temporal PSD in a certain spatial frequency (in the secondfigure). Due to the property of the white noise, all the darkblacklines that stand for checkerboard lie horizontally so that they gather to a boldline. All the other non-horizontal lines are gray belonging to the natural movie
Cross-correlation function
Firstly we get spike trains x and y consisted of ‘0’ and ‘1’, denoting the absence or presence of a spike in the time bin respectively. The time bin is chosen as 1 ms so that there is no more than one spike in each bin. The normalized cross-correlation function for calculating the degree of timing correlation is defined as follows:
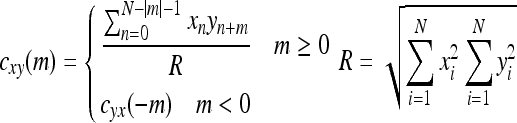 |
where xn denotes the value of spike train x at moment n; yn+m is the value of spike train y at moment n + m; R is the normalizing factor; cxy(m) stands for the spike timing correlation between x and y at the time delay m. The peak value is chosen describing the maximum timing correlation value between two spike trains (ranging from 0 to 1), which is considered significant if it exceeds expected value by 3 standard deviations (Liu et al. 2007). From this function, we can learn that it is quite strict in measuring timing correlation, requiring most spikes in trains to locate at the same time point or appear in a constant time delay to satisfy high timing correlation.
LZ complexity and LZ distance
LZ complexity is applied to measure the generation rate of new patterns along a sequence (Wang et al. 2007; Amigo 2004). Firstly we get spike train Xn of length n consisted of ‘0’ and ‘1’ as described in cross-correlation calculation. Then the spike train is parsed using LZ78 coding which separates the train into no overlapped pattern subsequences (Ziv and Lempel 1978). As an illustration, the string 0100110001001010 is parsed as 0|1|00|11|000|10|01|010. Each substring stands for the new pattern growing along the train. Then the total number of substrings c(Xn) is regarded as the LZ complexity reflecting the amount of information carried by particular patterns in the spike train Xn. The normalized LZ complexity Cn is derived from dividing c(Xn) by n/log2n, the maximum complexity of random sequence with length n→∞ (Kaspar and Schuster 1987). Christen et al. (2006a) developed the Lempel–Ziv coding into LZ distance. The normalized LZ distance is calculated by
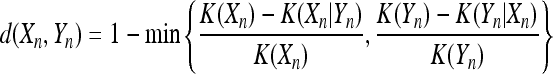 |
where the complexity K(Xn) is given by
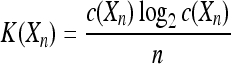 |
and 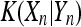 is the mutual information complexity between spike trains Xn and Yn which is calculated using
is the mutual information complexity between spike trains Xn and Yn which is calculated using 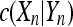 , denoting the number of substrings in Xn but not in Yn.
, denoting the number of substrings in Xn but not in Yn.
A large number of similar patterns appearing in both spike trains will lead to a large overlap of the substrings. Thus, the distance between spike trains with similar patterns is small (tend to zero), whereas the distance between spike trains with different patterns is large (Christen et al. 2006a). Note that firing rate is considered as a part of firing patterns, so that the probability modification by spike number is not involved here. Pattern similarity is compared without temporal order during the process for calculating LZ distance, so pattern correlation considers the overall neural firing form but not the detailed firing time. Overall features like the firing rates, the firing styles such as sparse or dense, or distribution of firing events may affect the result of pattern correlation measured by LZ distance.
Results
Five retinas are stimulated by checkerboard and three natural movies with 24, 20, 50, 55, 44 ganglion cells recorded respectively. The LZ distance and cross-correlation function are calculated using 50 s spike trains under each stimulus.
Single neuron’s response
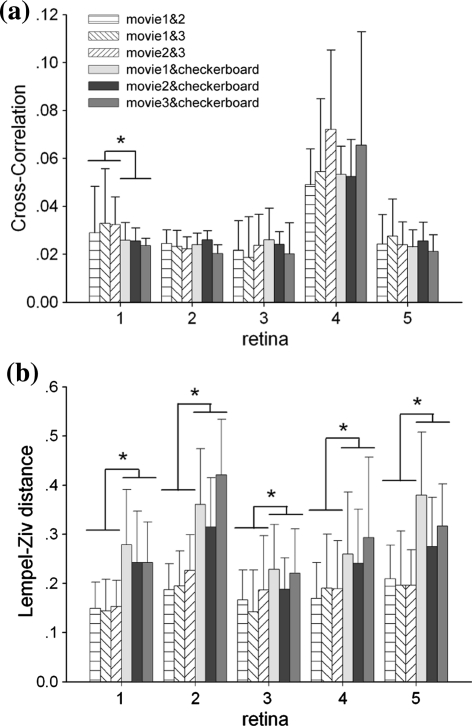
Firstly, the cross-correlation function and LZ distance of each individual neuron during response to each two stimuli of four was calculated, and the results were averaged among all the neurons recorded in each retina. All spike timing correlation measured between different stimulus remains unapparent with the peak cross-correlation value < 0.1. Moreover, no obvious spike timing correlation is observed related to stimulus indicated by cross-correlation value which changes with whatever similar or totally different stimulus (Fig. 3a).
Fig. 3.
The spike timing correlations and pattern correlations were calculated by cross-correlation function (a) and LZ distance (b) between each single neuron’s responses to different stimulus. The result was averaged in each retina. The paired t-test is implemented between the movie-movie correlation values and movie-checkerboard correlation values of each neuron’s response. For the cross-correlation result, p = 0.0028, 0.5746, 0.8148, 0.3466, 0.0512, respectively, in 5 retina. Only the neural responses in the first retina are correlated to similar stimuli. For the LZ distance result, p = 6.5353e-013, 0, 0.0015, 2.2926e-008, 0, respectively, in 5 retinas. Consequently, cross-correlation values under two kind stimuli groups do not make any obvious difference here and Lempel–Ziv distances are significantly smaller in movie-movie pairs than those in movie-checkerboard pairs. But no obvious difference is shown within movie-movie or movie-checkerboard groups in both cross-correlation and LZ distance results (balanced one-way ANOVA, cross-correlation: p = 0.0545, 0.9347, LZ distance: p = 0.3005, 4.6910e-005 for movie-movie and movie-checkerboard respectively)
On the other hand, LZ distance shows that neural firing patterns are always correlated more closely between movie-movie stimuli than the movie-checkerboard group (p < 0.01, paired t-test), and no obvious difference is shown within movie-movie group (p > 0.05, balanced one-way ANOVA), implying that the same kind stimulus arouse similar firing patterns in spike trains (Fig. 3b). As Fig. 3 implies, firing patterns in spike trains may carry information about ensemble stimulus mode, such as the overall contrast and spatiotemporal correlations, despite of failing to tell the further difference in details like precise temporal and spatial features in three natural movies. The reason may lies in that the neural response difference caused by different natural movies is no more obvious than the variation between individual neurons’ response to the same stimulus, so the subtle stimulus-induced difference is covered after averaging the overall neuron activities.
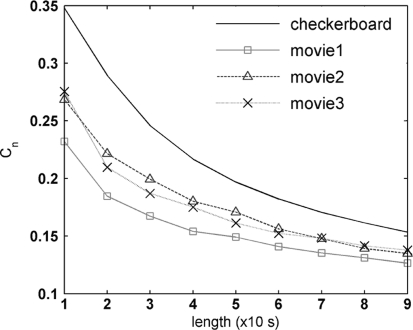
To further study how the firing patterns change with stimulus, we divide spike trains with 10 s increment from the beginning to 10 s, 20 s,…, 90 s respectively and use LZ complexity to calculate the speed of new pattens increase which the spike train carries to code stimulus. The results of all retina pieces reach the similar conclusion and Fig. 4 presents the results in the first retina with 24 neurons recorded for illustration.
Fig. 4.
Normalized LZ complexity Cn of 24 neurons’ responses in the first retina with the spike train length from the beginning to 10 s, 20 s ,…, 90 s, respectively. The overall complexity of response to checkerboard is obviously larger than that of natural movies. The complexity is decreasing with longer spike train, indicating that less new patterns appear and the neural firing is becoming more and more stable and regular
After normalization and averaging across the 24 neurons, LZ complexity trend is shown in Fig. 4. Since the checkerboard presents new random information continuously as white noise, and the natural movies contains rich temporal correlation along the time, so that compared to white noise less new independent information appear in the movies. The overall complexity of response to checkerboard is obviously larger than that of natural movies whereas the firing patterns under natural movies are almost less various. The normalized LZ complexity is decreasing with longer spike train, indicating that less new patterns appear and the neural firing is becoming more and more stable and regular, which may be regarded as the pattern adaptation. As a result, firing patterns appear to be closely related to the information carried by stimulus, and the adaptation seems to be a general mechanism in efficient coding.
Neuron population’s response
While early research suggested that individual neurons are sufficient to represent information of the stimulus (Nirenberg et al. 2001), it is now generally accepted that unambiguous representations are based on population codes. Next, we extend to examine how correlations between neurons organize in population coding. Here we only consider the correlation between two neurons since it has been suggested that pairwise correlation plays dominant role in neurons’ correlation coding (Schneidman et al. 2006; Shlens et al. 2009). For checkerboard and natural movies, respectively, spike trains of each two neurons are calculated by cross-correlation function and LZ distance to assess the concerted firing degree and the pattern similarity. The results of all retina pieces reach the same conclusion and the first retina is presented here as an example.
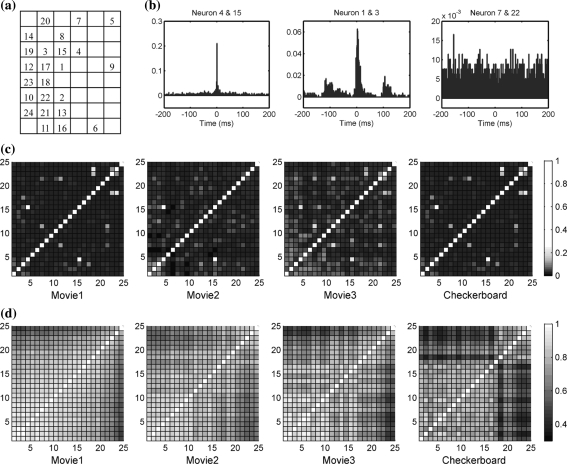
Figure 5a gives recording electrodes’ location of 24 neurons with their numbers. Typical cross-correlation diagrams showing timing correlation with different degrees of highly synchronized, correlated, no obvious correlation respectively are shown in Fig. 5b. Figure 5c plots all the cross-correlation values by grayscales between each two neurons in the retina, showing the neural correlation activities in an all-round manner. Most neurons are poorly correlated in spike timing while only several pairs are highly synchronized, just as the first kind of cross-correlation diagram in Fig. 5b. We also find that most of these highly synchronized neuron pairs remain concerted firing during response to all four stimuli, with their recording electrodes in Fig. 5a as neighbors (for example, neuron 4 and 15). Distant neurons generally correlate poorly in spike timing, as described in previous research that the timing correlation decrease with the distance between two neurons (Smith and Kohn 2008).
Fig. 5.
The correlation distributions in neuron population. a The recording electrodes’ location with their corresponding numbers (in this retina 24 neurons are recorded). b Different cross-correlation results between two neurons under checkerboard, showing three typical timing correlation: highly synchronized, correlated and no obviously timing correlation. c The spike timing correlation between each two neurons in all 24 neurons under four stimuli. Each block’s grayscale stands for the cross-correlation peak value between neurons with their numbers in x and y axis. The brighter the grayscale is, the more synchronized two neurons are. Along the diagonal neurons are calculated auto-correlation reaching the max correlation value 1. d The spike pattern correlation between each two neurons are calculated by LZ distance. The grayscale is set by 1 − dLZ to match the cross- correlation value so that the brighter the grayscale is, the more similar two neurons’ spike patterns are. The timing correlations and pattern correlations are organized differently in neuron population and show no direct relationship with each other
However, measurement of spike pattern similarity shows different organization in neuron’s correlation. As illustrated in Fig. 5d, pattern correlation appear to be independent of neuron locations. Compared with timing correlation, highly synchronized neurons are not necessarily the most similar ones in spike patterns in all neuron pairs relatively, and neurons with similar spike patterns are not necessarily firing temporally concerted.
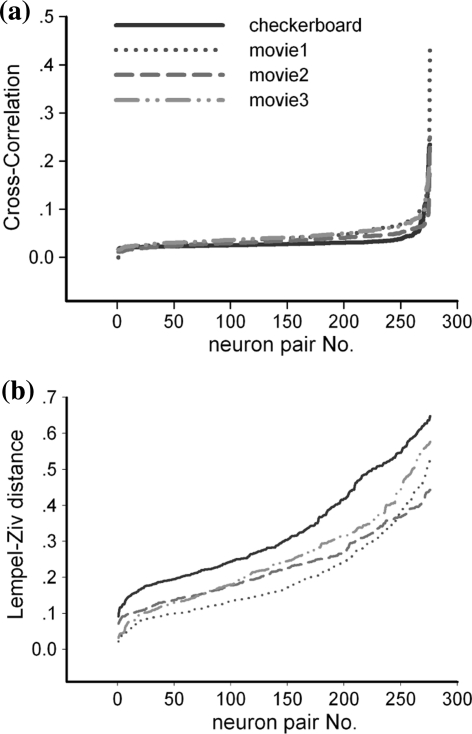
To better characterize the overall correlation distributions, cross-correlation values and LZ distances between the 24 neurons in Fig. 5 are reordered and drawn in Fig. 6. In Fig. 6a, most neuron pairs are not obviously correlated and only a few are highly synchronized, so that the curve of cross-correlation value remains in the low level and rise abruptly at last a few neurons pairs. However, pattern correlations are dispersedly distributed in the distance range as shown in Fig. 6b. The overall LZ distances between neurons in response to checkerboard stimulus are obviously larger than those in response to natural movies. Timing correlations are also observed subtly lower under checkerboard stimuli in Fig. 6a, but not as significant as pattern correlation in Fig. 6b, and the overall correlation degree of neurons in response to three similar natural movies can not be clearly separated in Fig. 6a. The results indicate that stimulus features would be encoded by both the neurons’ timing correlation and pattern correlation, but more remarkable in firing patterns.
Fig. 6.
The total 276 correlation values of neuron pairs are reordered from low to high, expressing overall distribution of timing (a) and pattern correlations (b) respectively. The LZ distances between neurons’ response to natural movies are shorter than those to the checkerboard, demonstrating that natural movies make neurons cooperate closer in firing patterns than checkerboard
Conclusion
In sum, by calculating the spike timing correlation and pattern correlation between spike trains, we find that overall spike patterns can clearly tell the difference of two kind stimuli but the spike timing fails, and neural population also cooperate in firing patterns to encode the spatial and temporal correlations in stimulus. Particularly, the firing pattern shows stronger correlation with stimulus features compared with spike timing, which implies that useful coding information may lose if we only consider the timing correlation with strictly temporal limitations.
Discussion
In this paper, we use two different kinds of stimuli, natural movies and white noise, to test whether overall firing pattern without consideration of strict temporal orders can present any additional information about the stimuli. According to the different light intensity, contrast changes, and temporal or spatial correlations in stimulus, the neural response may vary in overall patterns. For example, neurons may fire sparsely (Willmore and Tolhurst 2001) with long timescales (Butts et al. 2007) under natural stimulus. The latency or distribution of the firing events may be related to certain stimulus patterns (Gollisch and Meister 2008). The differences in firing rate, firing timescales, or distribution of firing events in spike trains affect the overall pattern similarity so that measurement of pattern correlation may reflect the remarkable difference between stimuli.
Firstly, pattern correlation and timing correlation are not isolated, because if two spike trains are highly synchronized, their patterns will be similar as well, although not necessarily reach the maximum similarity relatively. Thus, is the property of firing pattern in our results brought by timing correlation? For comparison, high pattern correlation between two neurons does not directly equal to high timing correlation (Fig. 5c). Moreover, the results of timing correlations just show stimulus difference subtly in neurons’ population performance (Fig. 6a) and fail in single neuron’s response (Fig. 3a). The different performance of two correlations demonstrates that the observed meaningful stimulus-related pattern correlation is not entirely caused by spike timing correlation. Firing patterns could give us additional insight in exploring neural coding mechanisms.
Next question is why the timing correlations fail to reflect stimulus difference in single neurons’ responses. Considering the precise temporal coding theory in visual systems, the precise temporal firing provides a substrate for unambiguous representations of complex stimuli (Butts et al. 2007; Theunissen and Miller 1995; Gerstner et al. 1997; Rullen and Thorpe 2001). This means the precise firing time is very sensitive to any subtle stimuli information, so that the neuron can code stimulus accurately. In response to identical stimulus trials, individual neurons have highly repeatable responses. In this paper, the scenes that locate in one neuron’s receptive field at the same time in three natural movies are subtly different and their temporal correlations are also diverse. Small difference in stimulus results in largely various firing time, so the stimulus-aroused timing correlation of individual neurons is weakened. As a result, even though the natural movies and checkerboard are quite different, we still can not tell their difference distinguished from that between natural movies by comparing each spike’s firing time exactly.
From another point of view, for the measurement of population correlation in Fig. 6a, timing correlation between neurons can more or less reflect the difference between two kind stimuli. So it may also consolidate that single neural coding is sometimes insufficient to express stimulus information while population coding can represent finer details of spatial information (Schnitzer and Meister 2003; Frechette et al. 2005).
Last but not least, why does the pattern correlation seem related to stimulus more closely than timing correlation in neurons’ population activities? Correlated firing in neuron population has been widely studied about the mechanism and function in neural coding. The timing correlation may be caused by two aspects: generated intrinsically by the neural circuitry (DeVries 1999; Brivanlou et al. 1998; Meister et al. 1995) or aroused by external stimulus.
In classical correlation analysis, direct inference was made from cross-correlogram to the underlying connectivity patterns. Experiment on salamander ganglion cells reveals that there are three types of correlated firings in ganglion cells according to the peak range in the cross-correlogram: broad (40–100 ms), medium (10–50 ms) and narrow (<5 ms) correlations were attributed respectively to shared signal from photoreceptor, amacrine cell and transferred by gap junctions between ganglion cells (Brivanlou et al. 1998).
However, the synchronization mode is not absolutely fixed by neurons’ connectivity circuitry (Schnitzer and Meister 2003). The timing correlation between neurons can be enhanced by receiving synchronized stimulus or weakened vice versa. The timing correlation among the same neurons can change dynamically towards the stimulus. In cats’ visual cortex, when two neurons with different feature preference were respectively optimally and suboptimally driven by certain stimulus, the stimulus-dependent time delay was observed in their cross-correlograms (König et al. 1995). Moreover, the result is further enhanced that the time course of significant timing correlation patterns among two or more neurons partially followed the temporal rhythm of the stimulation by using NeuroXidence analysis (Pipa et al. 2008). Thus, the dynamical varying interactions between neurons is stimulus-specific, indicating that synchronization among neurons could be a way of population coding to represent stimulus features (Singer 2009).
As a result, the timing correlation across neurons could be simultaneously affected by stimulus and physiological connectivity. As Fig. 5c shows, the synchronized neuron pairs do not remain the same across all the stimulus. Also in Fig. 6a, neurons’ timing correlations are slightly higher in response to natural stimulus which have more spatial correlations. However, in our results these stimulus-induced changes in timing correlation are inconspicuous, while the functional connectivity seems dominate the correlation patterns. In Fig. 5b, c, most of the highly coordinated neurons fire with high timing correlation whatever in response to natural movies or to checkerboard, being recorded by neighboring electrodes. During the natural movies, after a time delay the same object and scene may move from one to another neuron’s receptive field, arousing similar firing activities of these two neurons. But we did not find neuron pairs that have high spike timing correlation at the corresponding time delays. So we speculate that these neurons’ concerted firing is mainly affected by physiological connections. In fact, many researches have shown that correlated firing timing always happen in adjacent neurons (Shlens et al. 2009; Meister et al. 1995; Meister 1996; DeVries 1999; Mastronarde 1983; Puchalla et al. 2005).
Nevertheless, Fig. 5d shows that pattern correlation is not restricted in neuron locations, and the correlation distribution is totally different compared to timing correlations. Spike patterns may be mostly determined by the joint effect of each neuron’s individual coding property and the stimulus features, while spike timing correlation is easily affected by shared input signal through gap junctions among surround neurons or chemical synapse connections in the pathway.
For further research more evidence will be required in other stimuli with simple parameters so that detailed mechanism of firing pattern coding can be examined. The formation and decoding principle of neural firing patterns need to be explained combined with physiological meanings. Also, the effect and the coding information brought by overall patterns and precise spike timing respectively need to be further discussed. As the comprehensive representation of stimulus information, it seems that firing patterns are always distinct and reliable in response to certain stimulus. Therefore, the overall firing patterns may provide more convictive and adequate information than separate spikes, which helps us to distinguish the important content in stimulus that is coded by neurons.
Acknowledgments
This work was supported by the grants from the National Natural Science Foundation of China (No. 60775034) and National Basic Research Programme of China (No. 2005CB724301).
References
- Amigo JM. Estimating the entropy rate of spike trains via Lempel-Ziv complexity. Neural Comput. 2004;16:717–736. doi: 10.1162/089976604322860677. [DOI] [PubMed] [Google Scholar]
- Berry MM. Refractoriness and neural precision. J Neurosci. 1998;18(6):2200–2211. doi: 10.1523/JNEUROSCI.18-06-02200.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry MJ, Warland DK, Meister M. The structure and precision of retinal spike trains. Proc Natl Acad Sci USA. 1997;94:5411–5416. doi: 10.1073/pnas.94.10.5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brivanlou IH, Warland DK, Meister M. Mechanisms of concerted firing among retinal ganglion cells. Neuron. 1998;20:527–539. doi: 10.1016/S0896-6273(00)80992-7. [DOI] [PubMed] [Google Scholar]
- Butts DA, Weng C, Jin J, Yeh CI, Lesica NA, Alonso JM, Stanley GB. Temporal precision in the neural code and the timescales of natural vision. Nature. 2007;449(6):92–96. doi: 10.1038/nature06105. [DOI] [PubMed] [Google Scholar]
- Cai CF, Zhang YY, Liu X, Liang PJ, Zhang PM. Detecting determinism in firing activities of retinal ganglion cells during response to complex stimuli. Chin Phys Lett. 2008;25(5):1595–1598. doi: 10.1088/0256-307X/25/5/020. [DOI] [Google Scholar]
- Christen M, Kohn A, Ott T, Stoop R. Measuring spike pattern reliability with the Lempel-Ziv-distance. J Neurosci Meth. 2006;156:342–350. doi: 10.1016/j.jneumeth.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Christen M, Nicol A, Kendrick K, Ott T, Stoop R. Odour encoding in olfactory neuronal networks beyond synchronization. NeuroReport. 2006;17(14):1499–1502. doi: 10.1097/01.wnr.0000234750.58065.99. [DOI] [PubMed] [Google Scholar]
- Dan Y, Alonso JM, Usrey WM, Reid RC. Coding of visual information by precisely correlated spikes in the lateral geniculate nucleus. Nat Neurosci. 1998;1:501–507. doi: 10.1038/2217. [DOI] [PubMed] [Google Scholar]
- Boer E, Jongkees LB. On cochlear sharpening and cross-correlation methods. Acta Oto-laryngol. 1968;65(1):97–104. doi: 10.3109/00016486809120947. [DOI] [PubMed] [Google Scholar]
- Desbordes G, Jin J, Weng C, Lesica NA, Stanley GB, Alonso JM. Timing precision in population coding of natural scenes in the early visual system. PLoS Biol. 2008;6(12):e324. doi: 10.1371/journal.pbio.0060324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devries SH. Correlated firing in rabbit retinal ganglion cells. J Neurophysiol. 1999;81:908–920. doi: 10.1152/jn.1999.81.2.908. [DOI] [PubMed] [Google Scholar]
- Dong DW, Atick JJ. Statistics of natural time-varying images. Netw Comput Neural Syst. 1995;6(3):345–358. doi: 10.1088/0954-898X/6/3/003. [DOI] [Google Scholar]
- Frechette ES, Sher A, Grivich MI, Petrusca D, Litke AM, Chichilnisky EJ. Fidelity of the ensemble code for visual motion in primate retina. J Neurophysiol. 2005;94:119–135. doi: 10.1152/jn.01175.2004. [DOI] [PubMed] [Google Scholar]
- Gerstner W, Kreiter AK, Markram H, Herz AVM. Neural codes: firing rates and beyond. Proc Natl Acad Sci USA. 1997;94:12740–12741. doi: 10.1073/pnas.94.24.12740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollisch T, Meister M. Rapid neural coding in the retina with relative spike latencies. Science. 2008;319:1108–1111. doi: 10.1126/science.1149639. [DOI] [PubMed] [Google Scholar]
- Kaspar F, Schuster HG. Easily calculable measure for the complexity of spatiotemporal patterns. Phys Rev. 1987;36(2):842–848. doi: 10.1103/PhysRevA.36.842. [DOI] [PubMed] [Google Scholar]
- König P, Engel AK, Roelfsema PR, Singer W. How precise is neuronal synchronization? Neural Comput. 1995;7:469–485. doi: 10.1162/neco.1995.7.3.469. [DOI] [PubMed] [Google Scholar]
- Kreuz T, Haas JS, Morelli A, Abarbanel HDI, Politi A. Measuring spike train synchrony. J Neurosci Meth. 2007;165:151–161. doi: 10.1016/j.jneumeth.2007.05.031. [DOI] [PubMed] [Google Scholar]
- Lesica NA, Stanley GB. Encoding of natural scene movies by tonic and burst spikes in the lateral geniculate nucleus. J Neurosci. 2004;24(47):10731–10740. doi: 10.1523/JNEUROSCI.3059-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zhou Y, Gong HQ, Liang PJ. Contribution of the GABAergic pathway(s) to the correlated activities of chicken retinal ganglion cells. Brain Res. 2007;1177:37–46. doi: 10.1016/j.brainres.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Mastronarde DN. Correlated firing of cat retinal ganglion cells. I. Spontaneously active inputs to X- and Y-cells. J Neurophysiol. 1983;49(2):303–324. doi: 10.1152/jn.1983.49.2.303. [DOI] [PubMed] [Google Scholar]
- Meister M. Multineuronal codes in retinal signaling. Proc Natl Acad Sci USA. 1996;93:609–614. doi: 10.1073/pnas.93.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister M, Lagnado L, Baylor DA. Concerted signaling by retinal ganglion cells. Science. 1995;270(17):1207–1210. doi: 10.1126/science.270.5239.1207. [DOI] [PubMed] [Google Scholar]
- Nirenberg S, Carcieri SM, Jacobs AL, Latham PE. Retinal ganglion cells act largely as independent encoders. Nature. 2001;411:698–701. doi: 10.1038/35079612. [DOI] [PubMed] [Google Scholar]
- Perkel DH, Gerstein GL, Moore GP. Neuronal spike trains and stochastic point processes: II. Simultaneous spike trains. Biophys J. 1967;7(4):419–440. doi: 10.1016/S0006-3495(67)86597-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillow JW, Shlens J, Paninski L, Sher A, Litke AM, Chichilnisky EJ, Simoncelli EP. Spatio-temporal correlations and visual signalling in a complete neuronal population. Nature. 2008;454(7207):995–999. doi: 10.1038/nature07140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipa G, Wheeler D, Singer W, Nikolic D. NeuroXidence: reliable and efficient analysis of an excess or deficiency of joint-spike events. J Comput Neurosci. 2008;25:64–88. doi: 10.1007/s10827-007-0065-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puchalla JL, Schneidman E, Harris RA, Berry MJ. Redundancy in the population code of the retina. Neuron. 2005;46:493–504. doi: 10.1016/j.neuron.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Rullen RV, Thorpe SJ. Rate coding versus temporal order coding: what the retinal ganglion cells tell the visual cortex. Neural Comput. 2001;13:1255–1283. doi: 10.1162/08997660152002852. [DOI] [PubMed] [Google Scholar]
- Schneidman E, Berry MJ, Segev R, Bialek W. Weak pairwise correlations imply strongly correlated network states in a neural population. Nature. 2006;440(20):1007–1012. doi: 10.1038/nature04701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzer MJ, Meister M. Multineuronal firing patterns in the signal from eye to brain. Neuron. 2003;37:499–511. doi: 10.1016/S0896-6273(03)00004-7. [DOI] [PubMed] [Google Scholar]
- Shlens J, Field GD, Gauthier JL, Greschner M, Sher S, Litke AM, Chichilnisky EJ. The structure of large-scale synchronized firing in primate retina. J Neurosci. 2009;29(15):5022–5031. doi: 10.1523/JNEUROSCI.5187-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer W. Distributed processing and temporal codes in neuronal networks. Cogn Neurodyn. 2009;3:189–196. doi: 10.1007/s11571-009-9087-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Kohn A. Spatial and temporal scales of neuronal correlation in primary visual cortex. J Neurosci. 2008;28(48):12591–12603. doi: 10.1523/JNEUROSCI.2929-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theunissen T, Miller JP. Temporal encoding in nervous systems: a rigorous definition. J Comput Neurosci. 1995;2:149–162. doi: 10.1007/BF00961885. [DOI] [PubMed] [Google Scholar]
- Uzzell VJ, Chichilnisky EJ. Precision of spike trains in primate retinal ganglion cells. J Neurophysiol. 2004;92:780–789. doi: 10.1152/jn.01171.2003. [DOI] [PubMed] [Google Scholar]
- Hateren JH, Schaaf A. Independent component filters of natural images compared with simple cells in primary visual cortex. Proc R Soc Lond B. 1998;265:359–366. doi: 10.1098/rspb.1998.0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossum HCW. A novel spike distance. Neural Comput. 2001;13(4):751–763. doi: 10.1162/089976601300014321. [DOI] [PubMed] [Google Scholar]
- Steveninck RRR, Lewen GD, Strong SP, Koberle R, Bialek W. Reproducibility and variability in neural spike trains. Science. 1997;275:1805–1808. doi: 10.1126/science.275.5307.1805. [DOI] [PubMed] [Google Scholar]
- Victor JD, Purpura KP. Nature and precision of temporal coding in visual cortex: a metric-space analysis. J Neurophysiol. 1996;76(2):1310–1326. doi: 10.1152/jn.1996.76.2.1310. [DOI] [PubMed] [Google Scholar]
- Wang GL, Huang SY, Zhang YY, Liang PJ. Contrast adaptation decreases complexity in retinal ganglion cell spike trains. Chin Phys Lett. 2007;24(1):271–274. doi: 10.1088/0256-307X/24/1/074. [DOI] [Google Scholar]
- Willmore B, Tolhurst DJ. Characterizing the sparseness of neural codes. Network Comp Neural. 2001;12:255–270. [PubMed] [Google Scholar]
- Zhang PM, Wu JY, Zhou Y, Liang PJ, Yuan JQ. Spike sorting based on automatic template reconstruction with a partial solution to the overlapping problem. J Neurosci Meth. 2004;135:55–65. doi: 10.1016/j.jneumeth.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Zhang YY, Jin X, Gong HQ, Liang PJ. Temporal and spatial patterns of retinal ganglion cells in response to natural stimuli. Prog Biochem Biophys. 2010;37(4):389–396. [Google Scholar]
- Ziv J, Lempel A. Compression of individual sequences via variable-rate coding. IEEE T Inform Theory. 1978;24(5):530–536. doi: 10.1109/TIT.1978.1055934. [DOI] [Google Scholar]