Abstract
The objective of the present research was to evaluate the physicochemical characteristics of berberine chloride and to assess the complexation of drug with 2-hydroxypropyl-β-cyclodextrin (HPβCD), a first step towards solution dosage form development. The parameters such as log P value were determined experimentally and compared with predicted values. The pH-dependent aqueous solubility and stability were investigated following standard protocols at 25°C and 37°C. Drug solubility enhancement was attempted utilizing both surfactants and cyclodextrins (CDs), and the drug/CD complexation was studied employing various techniques such as differential scanning calorimetry, Fourier transform infrared, nuclear magnetic resonance, and scanning electron microscopy. The experimental log P value suggested that the compound is fairly hydrophilic. Berberine chloride was found to be very stable up to 6 months at all pH and temperature conditions tested. Aqueous solubility of the drug was temperature dependent and exhibited highest solubility of 4.05 ± 0.09 mM in phosphate buffer (pH 7.0) at 25°C, demonstrating the effect of buffer salts on drug solubility. Decreased drug solubility was observed with increasing concentrations of ionic surfactants such as sodium lauryl sulfate and cetyl trimethyl ammonium bromide. Phase solubility studies demonstrated the formation of berberine chloride–HPβCD inclusion complex with 1:1 stoichiometry, and the aqueous solubility of the drug improved almost 4.5-fold in the presence of 20% HPβCD. The complexation efficiency values indicated that the drug has at least threefold greater affinity for hydroxypropyl-β-CD compared to randomly methylated-β-CD. The characterization techniques confirmed inclusion complex formation between berberine chloride and HPβCD and demonstrated the feasibility of developing an oral solution dosage form of the drug.
KEY WORDS: berberine chloride, complexation, cyclodextrin, solubility, surfactants
INTRODUCTION
Berberine, a quaternary protoberberine isoquinoline alkaloid, is a well-known naturally occurring medicine derived from the root and the stem bark of numerous clinically important medicinal plants such as Hydrastis canadensis (goldenseal), Berberis aquifolium (Oregone grape), Berberis aristata (tree turmeric), Berberis vulgaris (barbery), and many other plants (1). It occurs as a yellowish crystalline powder that is odorless, or has a faint characteristic odor, with a bitter taste. It is sparingly soluble in methanol, slightly soluble in ethanol, and very slightly soluble in water; however, the salt forms are relatively more soluble and contribute to the tissue coloring (2). Berberine has demonstrated significant antimicrobial activity towards a variety of organisms including bacteria, fungi, protozoans, viruses, chlamydia, and helminthes and is used to treat several skin and eye ailments (3,4). It has been mainly used for the treatment of diarrhea and gastroenteritis for centuries in traditional Eastern medicine (5). It has also been reported to have a multitude of biological effects, including anti-malarial (6), anti-hypertensive (7), anti-arrhythmic (8,9), anti-hyperglycemic (10), anti-tumor (11,12), anti-inflammatory (13,14), anti-fungal (15), anti-oxidative (16), and cerebro-protective (17) activities. Recently, it has been reported that berberine helps in reducing cholesterol and lipid accumulations in both the plasma and in the liver (18).
Although berberine has wide-ranging therapeutic potential, in vivo pharmacokinetic studies demonstrate that its apparent permeability co-efficient (Papp) across the intestinal tissue is in the order of only 10−7 cm/s (19). The poor absorption characteristic is probably because of the P-gp expressed in intestinal cells and the significant first-pass metabolism by CYP 450-dependent processes (20,21). Literature precedents suggest that its metabolism in humans is primarily based on phase I demethylation and phase II glucuronidation and/or sulfate conjugation (9,22). Higher levels of berberine chloride in the plasma are essential when the drug is intended for treating systemic disorders. The scope of the current work, however, entails developing a solution dosage form for oral administration of the active, for the effective treatment of bacterial gastroenteritis.
Bacterial gastroenteritis is a common disorder, usually associated with the symptoms such as vomiting, diarrhea, and abdominal discomfort. In the USA, it is a very frequent problem, especially with children under 5 years of age and accounts for as many as 5% of pediatric office visits and 10% of hospitalizations in this age group (23,24). Worldwide, millions of children and adults are affected by diarrhea each year, and appropriate sanitation measures are necessary to prevent significant mortality rates due to gastroenteritis, especially in the elderly (25). In the management of such a clinical condition, solid dosage forms present significant administration challenges in pediatric and geriatric patient populations. Pediatric patients may suffer from ingestion problems as a result of underdeveloped muscular and nervous control, whereas the physiological and neurological conditions of the elderly, such as dysphagia, risk of choking, and hand tremors, may lead them to be uncooperative in the self-administration of conventional solid oral dosage forms (26). In an attempt to overcome these limitations, a solution dosage form of berberine chloride has been investigated to facilitate rapid onset of action.
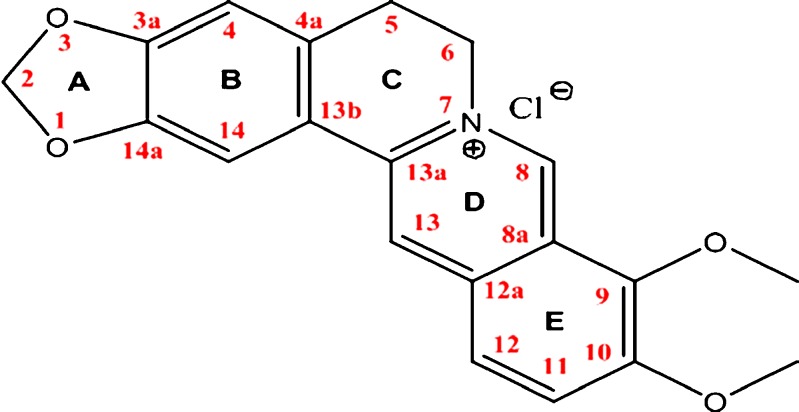
In clinical practice, berberine chloride (Fig. 1) and berberine sulfate are the commonly used salt forms of berberine, and the chloride form is available, either alone or in combination, as an immediate release tablet and/or capsule (current dosage being 100 mg, three to four times a day). Lately, a cream formulation of berberine hydrochloride, for topical antimicrobial and anti-inflammatory applications (27), has been developed and investigated by Patel et al. However, from a formulation perspective till date, the physicochemical properties of berberine chloride have not been studied in depth, and no attempt has been made to develop a solution dosage form utilizing various solubilizers which might help in both solubilization of high drug dose (∼100 mg) and taste masking. The current research focuses on improving patient compliance, especially in pediatric and elderly patient populations, by developing a solution dosage form. Different techniques such as micellar solubilization and complexation utilizing CDs were evaluated to enhance the drug solubility. The objective of the present work was to establish the physicochemical characteristics of berberine chloride and to characterize its complexation with 2-hydroxypropyl-β-cyclodextrin, a first step towards solution dosage form development.
Fig. 1.
Structure of berberine chloride with assigned proton signals
MATERIALS AND METHODS
Materials
Berberine chloride, randomly methylated-β-cyclodextrin (RMβCD; MW: 1310), and hydroxypropyl-β-cyclodextrin (HPβCD; MW: 1380) were purchased from Sigma Aldrich (St. Louis, MO, USA); cetyl trimethylammonium bromide (CTAB), sodium lauryl sulfate (SLS), pluronic® F 127 (Poloxamer 407), tween® 80, cremophor® RH 40, potassium phosphate monobasic, orthophosphoric acid, and sodium hydroxide were obtained from Fisher Scientific (Fair Lawn, NJ, USA); d-α tocopheryl polyethylene glycol 1000 succinate (TPGS) was purchased from the Peboc Division of Eastman (Llangefni, UK); and diethylene glycol monoethyl ether (Transcutol® HP) was obtained from Gattefosse (Saint-Priest, France). High performance liquid chromatography (HPLC) grade water was freshly prepared in the laboratory by Nanopure systems (Barnstead, Dubuque, IA, USA). All solvents utilized in the study were of analytical grade and obtained from Fisher Scientific (Fair Lawn, NJ, USA).
Analytical Method
A reverse phase-HPLC-based analytical method for determination/quantification of berberine chloride was developed. An HPLC equipped with UV detector, Waters Symmetry shield 5 μ C18 column (250 × 4.6 mm), and a gradient mode of elution with varying compositions of mobile phase (acetonitrile and 20 mM orthophosphoric acid in water) with a flow rate of 1.2 ml/min were employed to quantify the drug at a wavelength of (λmax) 346 nm. The acquired data was processed using Empower 2 build 2154 software (Waters Inc., Mount Holly, NJ, USA).
Evaluation of Physicochemical Properties
The various physicochemical properties of berberine chloride, such as octanol–water partition co-efficient (log P), percent hydrophilic surface area (%HSA), topological polar surface area (tPSA), and solubility parameter were determined based on the structure of the compound either experimentally or by utilizing computational softwares such as ChemBioDraw Ultra v 12.0, Molecular Modeling Pro™ and Advanced Chemistry Development (ACD) Lab/I-lab web service.
The log P was determined experimentally following the conventional shake flask method at 25°C and compared with the predicted log P values obtained from various other computational softwares. The solvents (octanol and water) were pre-saturated for a period of 24 h prior to the experiment to ensure equilibrium. A known amount of drug was added to the equilibrated solvent mixture and shaken for a period of 24 h. An aliquot of the aqueous phase was pipetted from the flask and analyzed for the drug content using UV–Vis spectrophotometer as described above. The experimental log P value was determined using the following equation:
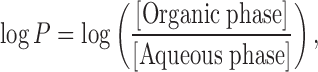 |
1 |
where “[ ]” represents the molar concentration.
Solution Stability
The chemical degradation of berberine chloride was determined as a function of pH at two different temperatures (25°C and 40°C). Buffers of different pH such as hydrochloric acid buffer (pH 1.2), phthalate buffer (pH 3.0 and 5.0), phosphate buffer (pH 7.0), and borate buffer (pH 9.0), each with a buffer strength of 200 mM, were prepared according to USP XXXI and employed in the study. Stability studies were initiated with the addition of 125 μL of a 1-mg/mL drug stock solution to 5 mL of the respective buffers previously equilibrated to the study temperatures, separately. The vials containing drug solution were placed at 25°C and 40°C for a period of 6 months. Aliquots (200 μL) were collected at predetermined time intervals (0, 0.5, 1, 2, 3, 4, 5, 6, 7, 14, 30, 60, 90, and 180 days) from each of the vials under study and were stored at −80°C until further analysis. The experiment was performed in triplicate, and the samples were analyzed for the drug content.
Aqueous and pH-Dependent Solubility
The solubility of berberine chloride was determined in both water and freshly prepared USP buffers of different pH ranging from 1.2 to 9.0, each with a buffer strength of 200 mM, following the standard shake flask method, wherein excess quantities of drug were added to 3 mL of solvent (water/buffer) in tightly capped glass vials. The buffers were prepared as described under the “Solution Stability” section. To achieve uniform mixing, samples were continuously agitated at 75 rpm at 25°C and 37°C, separately, for a period of 24 h in a reciprocal shaking water bath (Fisher Scientific, USA). At the end of 24 h, samples were centrifuged (accuSpin 17R, Fisher Scientific, USA) at 10,000 rpm for 10 min; the clear supernatant was suitably diluted and analyzed for drug content. Moreover, phosphate buffers of different pH (2.0 to 9.0) were also prepared utilizing either sodium hydroxide or phosphoric acid, and the drug solubility was estimated as per the above procedure at 25°C. All of the experiments were performed in triplicate, and the mean drug solubility was determined.
Solubility in Surfactants
Additionally, drug solubility was also screened in the presence of various commonly used surfactants and other solubilizers. Several surfactants, such as SLS (anionic), CTAB (cationic), Poloxamer 407, Tween® 80, Cremophor® RH 40 (non-ionic), and other solubilizers such as Transcutol® and TPGS, each at concentrations of 0.1% and 1% w/v, were employed in the study. The standard shake flask method was utilized to determine drug solubility at 25°C, and the processed samples were analyzed using HPLC as described in the “Analytical Method” section.
Phase Solubility Studies: Effect of Substituted β-CDs
The effects of different CDs such as HPβCD and RMβCD on solubility were evaluated. In both cases, concentrations between 0.2% to 20% w/v of CDs in water were used, and the phase solubility studies were carried out according to the method reported by Higuchi and Connors (28). An excess amount of the berberine chloride was added to the aqueous solutions containing increasing concentrations of RMβCD (1.52 to 152.67 mM) or HPβCD (1.44 to 144.92 mM). The flasks were sealed and shaken for 24 h to ensure equilibrium. The samples were centrifuged at 3,000 rpm for 10 min, filtered through a 0.45-μm membrane filter, and appropriately diluted and analyzed using a UV–Vis spectrophotometer. The equations below were used to calculate the parameters such as apparent stability constant (Ks) of the complex and complexation efficiency (CE) from the slope of the phase solubility diagram.
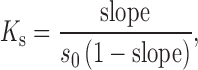 |
2 |
where “S0” is the intrinsic solubility of the drug,
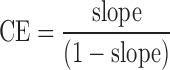 |
3 |
The study was conducted in triplicate, and the average drug solubility (mM) at varying concentrations of CDs (mM) was plotted.
Preparation of Inclusion Complex
A co-precipitation method was utilized in the preparation of the berberine–HPβCD inclusion complex. Both the drug and CD, in equal molar concentrations, were dissolved in a common solvent (methanol). The resultant solution was placed overnight in a vacuum oven so as to completely evaporate the methanol. The dried final residue (complex) thus obtained was further characterized for complexation utilizing various thermal and non-thermal techniques.
Characterization of Drug–CD complex
Differential Scanning Calorimetry (DSC) Analysis
The differential scanning calorimetry (DSC) analysis of berberine chloride, HPβCD, the physical mixture of drug and HPβCD (1:1 M ratio), and the inclusion complex were carried out using a Diamond Differential Scanning Calorimeter (Perkin-Elmer Life and Analytical Sciences, Shelton, CT, USA). Samples of approximately 2–3 mg were weighed and hermetically sealed in aluminum pans (Kit 0219-0062, Perkin-Elmer Instruments, Shelton, CT, USA). The samples were heated from 40°C to 280°C at a ramp rate of 10°C/min under a nitrogen purge at a flow rate of 20 mL/min. An empty aluminum pan was used as the reference.
Fourier Transform Infrared (FTIR) Spectroscopic Analysis
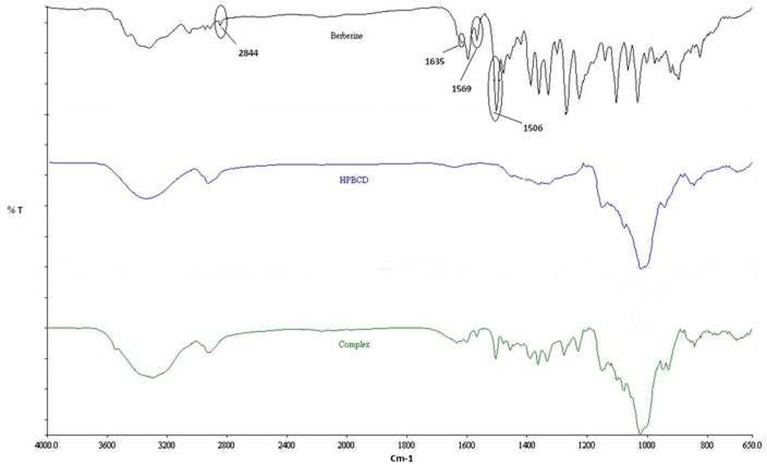
Fourier transform infrared (FTIR) spectra for the drug, HPβCD, and their complex were obtained using a Perkin-Elmer FTIR spectrometer (Perkin-Elmer Life and Analytical Sciences, Shelton, CT, USA). A spectrum was collected for each sample within the wave number region 4,000–400 cm−1. The spectra were analyzed for the absence or shift in the wave numbers of the characteristic peaks and reported.
Nuclear Magnetic Resonance (NMR) Spectroscopic Analysis
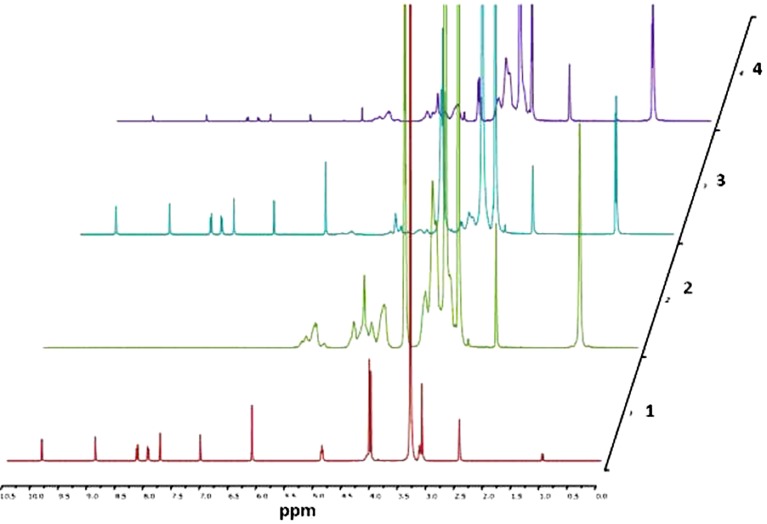
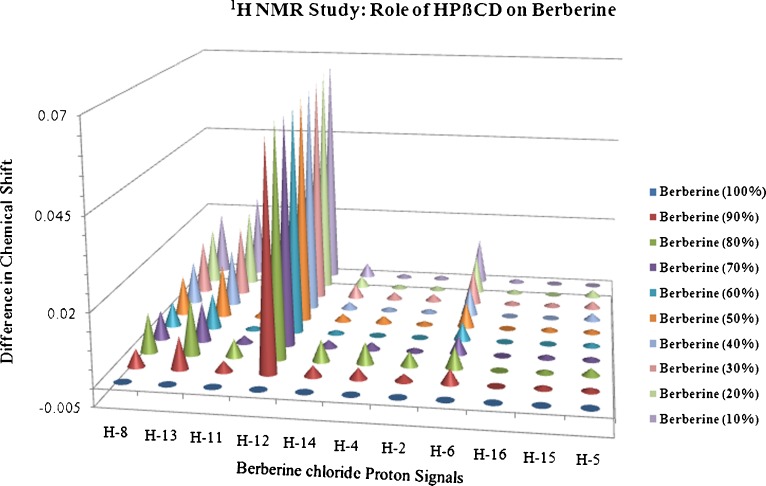
Nuclear magnetic resonance (NMR) studies were conducted to investigate the electronic interactions between berberine chloride and HPβCD. The pure samples along with the complex were prepared by mixing both the drug and HPβCD (25 mM each, previously dissolved in deuterated dimethyl sulfoxide; DMSO-d6) in the ratios of 100:0, 90:10, 80:20, 70:30, 60:40, 50:50, 40:60, 30:70, 20:80, 10:90, and 0:100 of drug:HPβCD followed by an overnight equilibration. Samples of 600 μL each were taken in 3-mm NMR glass tubes, and their proton NMR (1 H NMR) spectra in DMSO-d6 were recorded at 21°C on a 400-MHz Bruker AV spectrometer (Bruker Ultrashield 400 PLUS, Germany) equipped with a 3-mm nalorac dual proton-optimized probe. The obtained spectra were analyzed for proton chemical shifts utilizing Bruker TOPSPIN 2.0 software. The difference in the chemical shifts of protons in berberine chloride was plotted as a function of varying concentrations of drug:HPβCD. The chemical shifts were reported in parts per million (ppm) and are referenced by the residual solvent peak at 2.503 ppm.
Scanning Electron Microscopy (SEM) Analysis
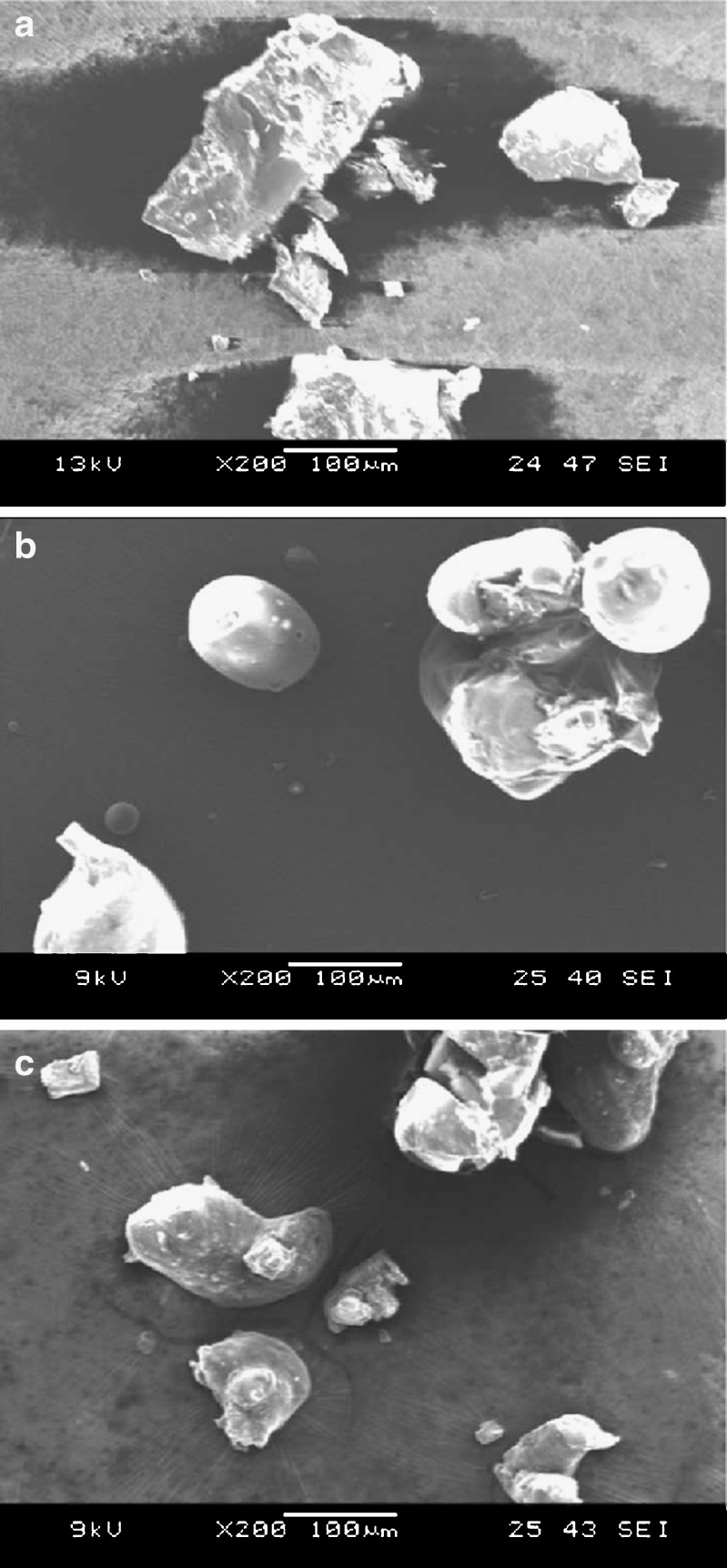
Morphology of the crystals of the inclusion complex obtained by co-precipitation technique was compared against the physical appearance of the pure drug and the HPβCD particles utilizing a scanning electron microscope. The samples were mounted onto an aluminum stage using adhesive carbon tape. Samples were then sputter coated with gold under an argon atmosphere using a Hummer™ 6.2 Sputter Coater (Ladd Research Industries, Williston, VT, USA) in a high vacuum evaporator equipped with an omni-rotary stage tray to produce uniformly coated irregular specimens without thermal damage. The processed samples were examined, and the images were captured using a JEOL JSM-5600 scanning electron microscope (JEOL USA, Inc., Waterford, VA, USA) operating at an accelerating voltage of 9–13 kV.
Statistical Analysis
In all cases, statistical analysis was performed utilizing one-way analysis of variance. A statistically significant difference was considered when p < 0.05.
RESULTS
Analytical Method
Validation was carried out according to ICH and FDA guidelines for chromatographic method. The linear calibration range for the detection of berberine chloride was found to be 1–100 μg/mL. The limit of detection and quantitation for the drug were 0.1 and 0.5 μg/mL, and the retention time for the drug under investigation was ∼8 min. The percent relative standard deviation (%RSD) within replicates (n = 3) was less than 2%, demonstrating reproducibility of the method. Precision was tested by preparing a single concentration of 10 μg/mL, and the readings were taken ten times. Excellent precision was observed based on the %RSD value, which was less than 0.5%.
Physicochemical Properties
The physicochemical characteristics of berberine chloride, such as %HSA and tPSA, were calculated using Molecular Modeling Pro™ and found to be 25.97% and 40.8 (A°)2, respectively. The average effective solubility parameter obtained from both Hansen's (three-dimensional (3-D)) and Van Krevelen and Hoftyzer (3-D) methods was 24.43 MPa1/2. The log P values were calculated by fragment-based and/or property-based method, utilizing various computational softwares such as ChemBioDraw Ultra v 12.0, Molecular Modeling Pro™, and ACD Lab/I-lab web service. Both the computed (from −0.9 to −2.2) and experimental (−1.5) log P values suggested that the compound is hydrophilic in nature.
Solution Stability
Berberine chloride was found to remain stable at all pH and temperature conditions tested, with less than 5% degradation being observed over a period of 6 months.
Aqueous and pH-Dependent Solubility
Aqueous and pH-dependent solubility of berberine chloride was temperature dependent and increased with an increase in temperature. The aqueous solubility of the drug at 25°C and 37°C was found to be 5.27 ± 0.29 and 8.50 ± 0.40 mM, respectively. Also, amongst all of the pHs tested, the maximum solubility of the drug was observed in phosphate buffer pH 7.0 (4.05 ± 0.09 and 9.69 ± 0.37 mM at 25°C and 37°C, respectively). The solubility of berberine chloride at pH 1.2 (HCl), 3.0, and 5.0 (phthalate buffer) and pH 9.0 (borate buffer) was nearly 20-fold lower than that at pH 7.0 (phosphate buffer), at 25°C. Interestingly, when all the buffers were prepared using phosphate salts (pH 2.0 to 9.0), solubility was observed to be pH independent (data not shown).
Solubility in Surfactants
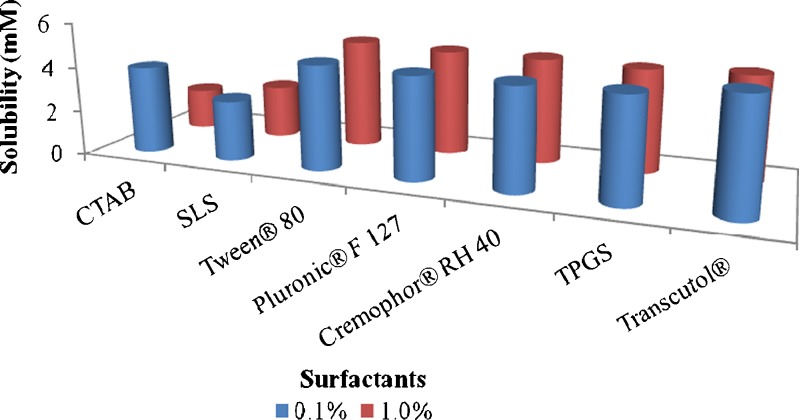
All of the non-ionic surfactants and/or solubilizing agents investigated exhibited drug solubility of 4.6–4.8 mM (Fig. 2), comparable to that in water. Moreover, they did not demonstrate any remarkable improvement in solubility as a function of surfactant concentration. Also, despite the toxicity issues posed by the ionic surfactants, they were utilized in the current research to explore their efficiency in the solubilization of berberine. A remarkable decrease in solubility was noticed in the presence of the employed ionic surfactants, SLS and CTAB. The data represents average drug solubility (mM) ± SD in different surfactants.
Fig. 2.
Influence of surfactants at different concentrations on solubility of berberine chloride. Data represent the mean value (n = 3)
Phase Solubility Studies: Effect of CDs
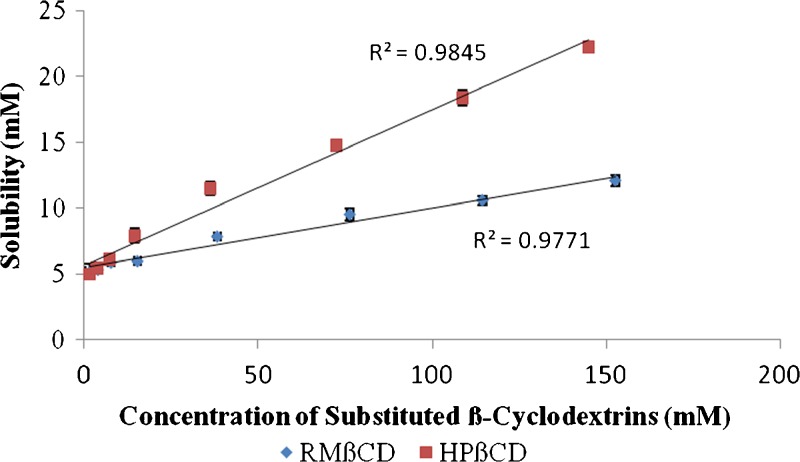
Amongst the two substituted β-CDs employed, HPβCD produced greater drug solubility in comparison to RMβCD. Moreover, the solubility of berberine chloride increased linearly as a function of HPβCD concentration, and the shape of the phase solubility diagram suggested an AL-type system (Fig. 3). From the slope values of the phase solubility studies and the drug's intrinsic solubility in water “S0,” the apparent stability constant (Ks) for both RMβCD and HPβCD was calculated as 9.1 and 25.7 M−1, respectively. Similarly, the complexation efficiency (CE) values were calculated and reported as 0.048 and 0.134 for both the cyclodextrins studied. Further evidence of complex formation was obtained by DSC, FTIR spectroscopy, NMR spectroscopy, and scanning electron microscopy (SEM) analysis.
Fig. 3.
Phase solubility diagram of berberine chloride in water. Values represent mean ± SD (n = 3)
Characterization of Drug–CD complex
Differential Scanning Calorimetry (DSC) Analysis
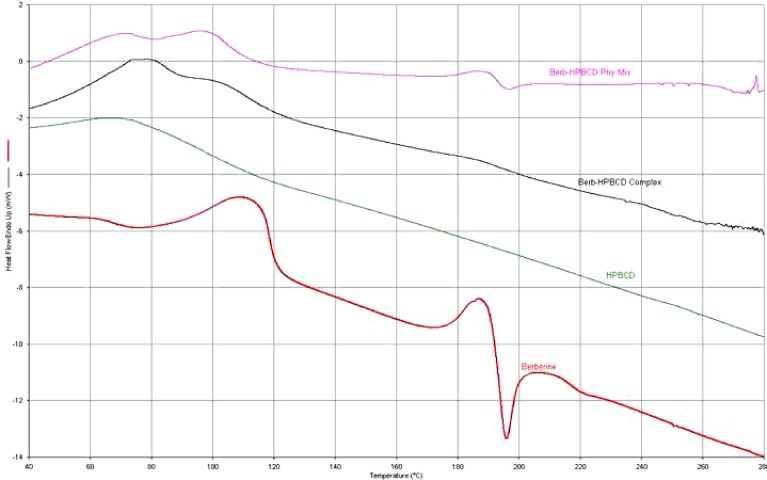
The complexation of the berberine chloride with HPβCD was investigated using Perkin-Elmer diamond DSC. In the thermograms shown in Fig. 4, temperature is plotted on the x-axis and heat flow (Endotherm up) on the y-axis. It is observed that the drug exhibits a peak melting endotherm at ∼196°C, while the thermal behavior of HPβCD exhibited no such phenomenon at any temperature intervals. The appearance of a peak corresponding to the melting of berberine chloride was also evident in the thermogram of the physical mixture, whereas it disappeared in the complex.
Fig. 4.
An overlay of DSC thermograms of berberine chloride, HPβCD, the inclusion complex, and their corresponding physical mixture
Fourier Transform Infrared (FTIR) Spectroscopic Analysis
In the spectra obtained (Fig. 5), wave numbers (cm−1) on the x-axis are plotted against percent transmittance on the y-axis. The IR spectrum of berberine chloride exhibited specific absorptions at 2,844, 1,635, 1,569, and 1,506 cm−1, identified by circles (29), whereas the FTIR spectrum of HPβCD presented a profile without distinctly sharp peaks. From the results, it is obvious that the peak at 2,844 cm−1 in the spectrum of berberine chloride disappeared in the complex. Additionally, other characteristic peaks of the drug present in the range of 2,000–1,500 cm−1 were reduced in their intensities, and/or exhibited a shift in the IR spectrum, in the complex. This corroborates the DSC data, suggesting an interaction between the guest and the host molecules.
Fig. 5.
An overlay of FTIR spectra of berberine chloride, HPβCD, and the inclusion complex
Nuclear Magnetic Resonance (NMR) Spectroscopic Analysis
The proton signals for berberine chloride (Fig. 1) were assigned based on a report published by Tripathi et al. (30). Figure 6 demonstrates the 1HNMR spectra of berberine chloride, HPβCD, and the drug in the presence of varying amounts of HPβCD, and Fig. 7 represents the NMR chemical shifts and assignments, respectively. From both of these figures, it can be inferred that the H-8, H-12, and H-13 protons of berberine chloride may be located inside the cavity of HPβCD, and depending on the concentration of the host molecule, a change in up-field shift for each proton was observed. The remaining hydrogens of the guest molecule, berberine, did not show considerable changes in their chemical shifts (<0.01 ppm).
Fig. 6.
1HNMR spectra (400 MHz) of (1) berberine (25 mM), (2) HPβCD (25 mM), (3) berberine:HPβCD (4:1), and (4) berberine:HPβCD (1:4). Samples are dissolved in DMSO-d6 at 21°C
Fig. 7.
Graphical representation of effect of HPβCD on berberine chloride. The chemical shifts are referenced by the residual solvent peak at 2.503 ppm
Scanning Electron Microscopy (SEM) Analysis
The micrographs in Fig. 8a–c represent pure drug, pure HPβCD, and drug–HPβCD complex (1:1), respectively. The pure drug is crystalline in nature and exists as loose particles with sharp edges, whereas the pure HPβCD is amorphous and appears as near spherical particles with smooth surfaces. A clear difference in the morphology of the drug crystals, drug–HPβCD inclusion complex, and native HPβCD could be seen.
Fig. 8.
a Scanning electron micrographs of berberine chloride. b Scanning electron micrographs of HPβCD. c Scanning electron micrographs of the drug-cyclodextrin inclusion complex
DISCUSSION
The physicochemical profiling, in particular, the molecular descriptors such as %HSA, tPSA, log P, solubility, and ionization constant (pKa), of a drug molecule is very important in predicting the oral drug absorption (31). The %HSA and tPSA indicate the hydrophilicity of the compound, especially tPSA which is defined as “the sum of surfaces of polar atoms in a molecule,” and has also been used to investigate the membrane transport characteristics of a molecule. Recently, Fernandes et al. reported a good correlation between tPSA of drug molecules and their transport properties across biological membranes (32). On the other hand, the 3-D solubility parameter obtained from Molecular Modeling Pro™ represents dispersive, polar, and hydrogen-bonding interactions of a molecule, and it could be utilized to estimate the interactions between the formulation components (33). From a pharmaceutical applications perspective, this could be a very useful tool in predicting the drug–polymer miscibility in the solid dispersions and the drug–excipient interactions in the development of solid oral dosage forms.
Lipophilicity is considered as another important parameter that plays a vital role in regulating dynamic and kinetic aspects of drug action. The apparent log P values derived both computationally and experimentally (−1.51) were comparable and further demonstrated the hydrophilic nature of the compound. The different log P values obtained from each of these methods can be attributed to the varying molecular lipophilicity calculation approaches. For instance, ClogP (−0.90) and ACD/logP (−0.99) were estimated using the fragmental methods where the different fragments of the drug molecule and their intra-molecular attractions were considered in the calculation. On the contrary, mLogP (−2.20) was estimated from a property-based method considering the topological descriptors of the molecule (34).
Assessing the stability of the active ingredients is considered as an important criterion while formulating any solution dosage form. In this research, berberine chloride was prepared in buffers of varying pH, as stated earlier, and these solutions were exposed to different temperature conditions. No significant degradation of the drug was noticed at the end of 6 months, at all of the conditions tested, suggesting that berberine chloride does not undergo hydrolysis.
In the present research, solubility of berberine chloride in water and buffers of various pH have also been studied. The aqueous solubility of berberine chloride at 25°C was determined to be 1.96 ± 0.11 mg/mL (equivalent to 5.27 ± 0.30 mM), and the studies involving effect of pH on the drug solubility provided very interesting results. From the pKa standpoint, berberine is a permanently charged compound and has no ionizable groups. Thus, irrespective of pH of the buffer, drug solubility was expected to remain the same. However, the drug displayed a significantly higher solubility of ∼4.0 mM in the phosphate buffer (pH 7.0) at 25°C, whereas in all of the other buffers (pH 1.2, 3.0, 5.0, and 9.0), it exhibited a solubility of only 0.2–0.4 mM. In order to study this unusual behavior of berberine chloride at pH 7.0, the buffers within a pH range of 2.0–9.0 were prepared using phosphoric acid and its salts as per the procedure described in the “Materials and Methods” section, and the solubility of the active was studied at 25°C. Interestingly, the drug now exhibited remarkably high solubility (∼4.0–4.3 mM) at all of the pH conditions tested (data not shown). Hence, from this set of experiments, it can be inferred that the buffer salts selected make a significant difference to the drug solubility. Unlike other buffer salts, the increased solubility due to phosphates could be attributed to their interaction with the drug resulting in the formation of a more soluble complex.
Additionally, the effect of temperature has been investigated, and it demonstrated a significant impact on drug solubility in all of the solvents tested, under the employed conditions (15). Increase in temperature from 25°C to 37°C enhanced the aqueous solubility of berberine chloride by nearly 62%. This temperature dependence could be attributed to the lower exothermic energy compared to the endothermic energy involved in the kinetic event of drug solubilization. Hence, at the higher temperature, an excess amount of heat energy is provided to the system to break the bonds further, which facilitates increase in drug solubility.
Overcoming the challenge of incorporating the targeted drug dose of 100 mg in an acceptable volume of 1 tbsp. or less water was essential to develop a stable solution dosage form. Considering the stability and aqueous solubility of berberine chloride (∼2.0 mg/mL), it would require over 50 mL of water to dissolve the 100-mg dose, without any solubilizing aid. In an attempt to solve the stated problem, surfactants were utilized to improve the drug's aqueous solubility. All of the non-ionic surfactants tested in this study did not demonstrate any improvement in the aqueous solubility of berberine chloride. However, in the presence of the ionic surfactants, SLS and CTAB, drug solubility was significantly reduced, especially at higher surfactant concentrations. With regard to the anionic surfactant, altered drug solubility can be ascribed to the in situ formation and subsequent precipitation of the insoluble lauryl sulfate (estolate) salt of the drug, leading to a desolubilization phenomenon (35). This event could be reverted by increasing the SLS concentration further, which might solubilize the drug-estolate salt. On the other hand, decreased solubility in the presence of the cationic surfactant, CTAB, may be due to the electrostatic repulsions between the positively charged drug molecule and the cationic micellar system (36) or the drug might be displaced by the surfactant owing to its increased affinity to water.
As the drug's aqueous solubility could not be enhanced by the surfactants, a complexation technique was utilized involving CDs. The CDs have been extensively studied to improve the aqueous solubility, physical stability, and permeability of poorly soluble compounds (37,38) and to mask the bitter taste of the drugs (39,40) through the formation of inclusion complexes. The CDs (α, β, γ, and δ) are defined on the basis of their characteristics such as molecular weight, cavity size, and the aqueous solubility, and each of these CDs has their own advantages and disadvantages. The β-CDs accommodate most of the drugs in their cavity; however, they initially suffered from limited water solubility and toxicity problems which were overcome by the introduction of substituted β-CDs. HPβCD has been utilized at a concentration of 40% w/v to promote the solubility of Itraconazole in a marketed preparation intended for oral delivery (41). Moreover, CDs followed the rank order of α-CDs < γ-CDs < β-CDs in terms of taste masking efficiency (39). In the current research, RMβCD and HPβCD were thus evaluated with respect to solubility enhancement of berberine chloride.
The parameters such as Ks and CE for both RMβCD and HPβCD were obtained from the phase solubility diagram. The former parameter aids in comparing the affinity of a drug for different CDs or CD derivatives, whereas the latter helps in determining their solubilizing efficiency in the aqueous vehicle (42). The curves corresponding to the two substituted β-CDs presented in the diagram (Fig. 3) exhibited straight lines with positive slopes of less than unity and an intercept equal to S0, indicating the formation of an inclusion complex with 1:1 M ratio. However, equivalent concentrations of RMβCD demonstrated lower drug solubility. Additionally, the Ks values mentioned earlier indicated that the affinity of berberine chloride towards RMβCD is at least three times lower compared to that for HPβCD. Moreover, the CE value of HPβCD (∼0.14) indicates its high solubilizing efficiency compared to RMβCD (∼0.05) and means that on an average, about one out of every ten CD molecules in solution forms a water-soluble complex, assuming a 1:1 drug–CD complex formation. From the phase solubility data, it can be deduced that approximately 25% HPβCD will be needed to achieve a concentration of ∼10 mg/ml of berberine chloride. Hence, for a dose of 150 mg of the drug, 1 tbsp. of the formulation will be required.
Inclusion complex formation was further characterized by different thermal and non-thermal techniques such as DSC, FTIR, NMR and SEM. DSC is a qualitative analytical tool which can be used to identify complex formation. When complexation occurs, the inclusion complex so formed exhibits different characteristics in comparison to either pure drug or cyclodextrin-alone. In this research, the disappearance of the melting peak of drug (loss of crystallinity) in the newly formed inclusion complex might be attributed to the drug being embedded in the CD cavity.
The FTIR spectrum of berberine chloride revealed the existence of a methoxyl group (peak at 2,844 cm−1) (43), and the peak at 1,635 cm−1 is believed to correspond to the iminium (C = N+) double bond present in the molecule (44). Moreover, the signals at 1,569 and 1,506 cm−1 represent the aromatic C = C bending and the furyl group, respectively (45). However, the spectrum of the newly formed inclusion complex did not show any peak at 2,844 cm−1, corresponding to the methoxyl group stretching, indicating the interaction of the guest molecule in the host cavity.
Proton NMR studies play an important role in displaying the chemical shift of the protons inside the CD cavity and on the drug molecule during complexation (46). The results obtained from the NMR spectroscopic study demonstrated a chemical shift up-field for the specific proton signals at eighth, 12th, and 13th positions with regard to the host molecule concentration. Thus, it can be assumed that berberine chloride's “D” and “E” rings were preferentially inserted into the CD cavity. These results were in strong agreement with the IR data where the peak corresponding to methoxyl groups (E ring) disappeared in the formed inclusion complex.
SEM of the physical mixtures demonstrated that the structures of the particles in the mixture are similar to that of their individual molecules (image not shown), indicating no physical complexation. However, the morphology of the material produced by co-precipitation was found to be significantly different from the structures of either the free drug or the CD particles, further validating complex formation. Overall, the characterization techniques employed confirmed complexation, indicating a 1:1 stoichiometry between the drug and the CD moieties.
CONCLUSION
Berberine chloride exhibited excellent solution stability, and solubilization of the high drug dose (100 mg) in 2 tsp. of the final formulation could be achieved by employing the complexation technique utilizing approximately 25% HPβCD. The results from the characterization techniques substantiate complex formation between berberine chloride and HPβCD and demonstrate the feasibility of developing a solution dosage form. Complexation of berberine with cyclodextrins would also probably aid in masking the taste of the compound.
Acknowledgements
This project was supported by Grant No. 5P20RR021929 from the National Institute of Health/National Center for Research Resources (NIH/NCRR) and the Grant No. D1 BIT 16663 (DHHS/HRSA). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
References
- 1.Zuo F, Nakamura N, Akao T, Hattori M. Pharmacokinetics of berberine and its main metabolites in conventional and pseudo germ-free rats determined by liquid chromatography/ion trap mass spectrometry. Drug Metab Dispos. 2006;34(12):2064–2072. doi: 10.1124/dmd.106.011361. [DOI] [PubMed] [Google Scholar]
- 2.Dostál J, Man S, Seckárová P, Hulová D, Necas M, Potácek M, et al. Berberine and coptisine free bases. J Mol Struct. 2004;687(1–3):135–142. doi: 10.1016/j.molstruc.2003.09.018. [DOI] [Google Scholar]
- 3.Hayashi K, Minoda K, Nagaoka Y, Hayashi T, Uesato S. Antiviral activity of berberine and related compounds against human cytomegalovirus. Bioorg Med Chem Lett. 2007;17(6):1562–1564. doi: 10.1016/j.bmcl.2006.12.085. [DOI] [PubMed] [Google Scholar]
- 4.Birdsall TC, Kelly GS. Berberine: therapeutic potential of an alkaloid found in several medicinal plants. Altern Med Rev. 1997;2(2):94–103. [Google Scholar]
- 5.Taylor CT, Winter DC, Skelly MM, O'Donoghue DP, O'Sullivan GC, Harvey BJ, et al. Berberine inhibits ion transport in human colonic epithelia. Eur J Pharmacol. 1999;368(1):111–118. doi: 10.1016/S0014-2999(99)00023-0. [DOI] [PubMed] [Google Scholar]
- 6.Tran QL, Tezuka Y, Ueda JY, Nguyen NT, Maruyama Y, Begum K, et al. In vitro antiplasmodial activity of antimalarial medicinal plants used in Vietnamese traditional medicine. J Ethnopharmacol. 2003;86(2–3):249–252. doi: 10.1016/S0378-8741(03)00045-X. [DOI] [PubMed] [Google Scholar]
- 7.Ko WH, Yao XQ, Lau CW, Law WI, Chen ZY, Kwok W, et al. Vasorelaxant and antiproliferative effects of berberine. Eur J Pharmacol. 2000;399(2–3):187–196. doi: 10.1016/S0014-2999(00)00339-3. [DOI] [PubMed] [Google Scholar]
- 8.Sanchez-Chapula J. Increase in action potential duration and inhibition of the delayed rectifier outward current IK by berberine in cat ventricular myocytes. Br J Pharmacol. 1996;117(7):1427–1434. doi: 10.1111/j.1476-5381.1996.tb15302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsai PL, Tsai TH. Hepatobiliary excretion of berberine. Drug Metab Dispos. 2004;32(4):405–412. doi: 10.1124/dmd.32.4.405. [DOI] [PubMed] [Google Scholar]
- 10.Pan GY, Huang ZJ, Wang GJ, Fawcett JP, Liu XD, Zhao XC, et al. The antihyperglycaemic activity of berberine arises from a decrease of glucose absorption. Planta Med. 2003;69(7):632–636. doi: 10.1055/s-2003-41121. [DOI] [PubMed] [Google Scholar]
- 11.Jantova S, Cipak L, Cernakova M, Kost'alova D. Effect of berberine on proliferation, cell cycle and apoptosis in HeLa and L1210 cells. J Pharm Pharmacol. 2003;55(8):1143–1149. doi: 10.1211/002235703322277186. [DOI] [PubMed] [Google Scholar]
- 12.Kettmann V, Kosfalova D, Jantova S, Cernakova M, Drimal J. In vitro cytotoxicity of berberine against HeLa and L1210 cancer cell lines. Pharmazie. 2004;59(7):548–551. [PubMed] [Google Scholar]
- 13.Kupeli E, Kosar M, Yesilada E, Husnu K, Baser C. A comparative study on the anti-inflammatory, antinociceptive and antipyretic effects of isoquinoline alkaloids from the roots of Turkish Berberis species. Life Sci. 2002;72(6):645–657. doi: 10.1016/S0024-3205(02)02200-2. [DOI] [PubMed] [Google Scholar]
- 14.Yesilada E, Kupeli E. Berberis crataegina DC. root exhibits potent anti-inflammatory, analgesic and febrifuge effects in mice and rats. J Ethnopharmacol. 2002;79(2):237–248. doi: 10.1016/S0378-8741(01)00387-7. [DOI] [PubMed] [Google Scholar]
- 15.Lu Y-C, Lin Q, Luo G-S, Dai Y-Y. Solubility of berberine chloride in various solvents. J Chem Eng Data. 2006;51(2):642–644. doi: 10.1021/je0504360. [DOI] [Google Scholar]
- 16.Misik V, Bezakova L, Malekova L, Kostalova D. Lipoxygenase inhibition and antioxidant properties of protoberberine and aporphine alkaloids isolated from Mahonia aquifolium. Planta Med. 1995;61(4):372–373. doi: 10.1055/s-2006-958107. [DOI] [PubMed] [Google Scholar]
- 17.Ma L, Xiao P, Guo B, Wu J, Liang F, Dong S. Cerebral protective effects of some compounds isolated from traditional Chinese herbs. Zhongguo Zhong Yao Za Zhi. 1999;24(4):238–239. [PubMed] [Google Scholar]
- 18.Doggrell SA. Berberine−a novel approach to cholesterol lowering. Expert Opin Investig Drugs. 2005;14(5):683–685. doi: 10.1517/13543784.14.5.683. [DOI] [PubMed] [Google Scholar]
- 19.Alan WB, Cormac TT, David JB. Non-antibiotic anti-diarrhoeal drugs: factors affecting oral bioavailability of berberine and loperamide intestinal tissue. Adv Drug Deliv Rev. 1997;23:111–120. doi: 10.1016/S0169-409X(96)00429-2. [DOI] [Google Scholar]
- 20.Pan GY, Wang GJ, Liu XD, Fawcett JP, Xie YY. The involvement of P-glycoprotein in berberine absorption. Pharmacol Toxicol. 2002;91(4):193–197. doi: 10.1034/j.1600-0773.2002.t01-1-910403.x. [DOI] [PubMed] [Google Scholar]
- 21.Gurley BJ, Swain A, Hubbard MA, Williams DK, Barone G, Hartsfield F, et al. Clinical assessment of CYP2D6-mediated herb-drug interactions in humans: effects of milk thistle, black cohosh, goldenseal, kava kava, St. John's wort, and Echinacea. Mol Nutr Food Res. 2008;52(7):755–763. doi: 10.1002/mnfr.200600300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ye M, Fu S, Pi R, He F. Neuropharmacological and pharmacokinetic properties of berberine: a review of recent research. J Pharm Pharmacol. 2009;61(7):831–837. doi: 10.1211/jpp.61.07.0001. [DOI] [PubMed] [Google Scholar]
- 23.Hamer DHLGS. Infectious diarrhea and bacterial food poisoning. In: Feldman M, Scharschmidt BF, Sleisenger MH, editors. Sleisinger and Fordtran's gastrointestinaland liver disease. 6. Philadelphia: WB Saunders; 1998. pp. 1594–1632. [Google Scholar]
- 24.Liebelt EL. Clinical and laboratory evaluation and management of children with vomiting, diarrhea, and dehydration. Curr Opin Pediatr. 1998;10(5):461–469. doi: 10.1097/00008480-199810000-00002. [DOI] [PubMed] [Google Scholar]
- 25.Streit JM, Jones RN, Toleman MA, Stratchounski LS, Fritsche TR. Prevalence and antimicrobial susceptibility patterns among gastroenteritis-causing pathogens recovered in Europe and Latin America and Salmonella isolates recovered from bloodstream infections in North America and Latin America: report from the SENTRY Antimicrobial Surveillance Program (2003) Int J Antimicrob Agents. 2006;27(5):367–375. doi: 10.1016/j.ijantimicag.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 26.Battu SK, Repka MA, Majumdar S, Madhusudan RY. Formulation and evaluation of rapidly disintegrating fenoverine tablets: effect of superdisintegrants. Drug Dev Ind Pharm. 2007;33(11):1225–1232. doi: 10.1080/03639040701377888. [DOI] [PubMed] [Google Scholar]
- 27.Patel NA, Patel NJ, Patel RP, Patel RK. The formulation and evaluation of topical berberine hydrochloride products. Pharm Technol. 2010;34:60–69. [Google Scholar]
- 28.Higuchi T, Connors KA. Phase-solubility techniques. Adv Anal Chem Instrum. 1965;4:117–212. [Google Scholar]
- 29.Koide T, Iwata M, Maekawa K, Saito H, Tanimoto T, Okada S. [Berberine Hydrochloride Reference Standard (Control 001) of National Institute of Health Sciences]. Kokuritsu Iyakuhin Shokuhin Eisei Kenkyusho Hokoku. 2001(119):97–100. [PubMed]
- 30.Tripathi AN, Chauhan L, Thankachan PP, Barthwal R. Quantum chemical and nuclear magnetic resonance spectral studies on molecular properties and electronic structure of berberine and berberrubine. Magn Reson Chem. 2007;45(8):647–655. doi: 10.1002/mrc.2019. [DOI] [PubMed] [Google Scholar]
- 31.Avdeef A. Physicochemical profiling (solubility, permeability and charge state) Curr Top Med Chem. 2001;1(4):277–351. doi: 10.2174/1568026013395100. [DOI] [PubMed] [Google Scholar]
- 32.Fernandes J, Gattass CR. Topological polar surface area defines substrate transport by multidrug resistance associated protein 1 (MRP1/ABCC1) J Med Chem. 2009;52(4):1214–1218. doi: 10.1021/jm801389m. [DOI] [PubMed] [Google Scholar]
- 33.Hancock BC, York P, Rowe RC. The use of solubility parameters in pharmaceutical dosage form design. Int J Pharm. 1997;148:1–21. doi: 10.1016/S0378-5173(96)04828-4. [DOI] [Google Scholar]
- 34.Mannhold R, Poda GI, Ostermann C, Tetko IV. Calculation of molecular lipophilicity: state-of-the-art and comparison of log P methods on more than 96,000 compounds. J Pharm Sci. 2009;98(3):861–893. doi: 10.1002/jps.21494. [DOI] [PubMed] [Google Scholar]
- 35.Jain A, Ran Y, Yalkowsky SH. Effect of pH-sodium lauryl sulfate combination on solubilization of PG-300995 (an anti-HIV agent): a technical note. AAPS PharmSciTech. 2004;5(3):e45. doi: 10.1208/pt050345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rangel-Yagui CO, Hsu HWL, Pessoa A, Jr, Tavares LC. Micellar solubilization of ibuprofen—influence of surfactant head groups on the extent of solubilization. Braz J Pharm Sci. 2005;41(2):237–246. [Google Scholar]
- 37.Duchene D, Glomot FCV. Cyclodextrins and their industrial uses. In: Duchene D, editor. Pharmaceutical applications of cyclodextrins. 1. France: Paris; 1987. [Google Scholar]
- 38.Masson M, Loftsson T, Masson G, Stefansson E. Cyclodextrins as permeation enhancers: some theoretical evaluations and in vitro testing. J Control Release. 1999;59(1):107–118. doi: 10.1016/S0168-3659(98)00182-5. [DOI] [PubMed] [Google Scholar]
- 39.Wagh VD, Ghadlinge SV. Taste masking methods and techniques in oral pharmaceuticals: current perspectives. J Pharm Res. 2009;2(6):1049–1054. [Google Scholar]
- 40.Patel AR, Vavia PR. Preparation and evaluation of taste masked famotidine formulation using drug/beta-cyclodextrin/polymer ternary complexation approach. AAPS PharmSciTech. 2008;9(2):544–550. doi: 10.1208/s12249-008-9078-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nagase Y, Hirata M, Wada K, Arima H, Hirayama F, Irie T, et al. Improvement of some pharmaceutical properties of DY-9760e by sulfobutyl ether beta-cyclodextrin. Int J Pharm. 2001;229(1–2):163–172. doi: 10.1016/S0378-5173(01)00851-1. [DOI] [PubMed] [Google Scholar]
- 42.Loftsson T, Hreinsdottir D, Masson M. Evaluation of cyclodextrin solubilization of drugs. Int J Pharm. 2005;302(1–2):18–28. doi: 10.1016/j.ijpharm.2005.05.042. [DOI] [PubMed] [Google Scholar]
- 43.Sharma RK, Wooten JB, Baliga VL, Lin X, Chan WG, Hajaligol MR. Characterization of chars from pyrolysis of lignin. Fuel. 2004;83(11–12):1469–1482. doi: 10.1016/j.fuel.2003.11.015. [DOI] [Google Scholar]
- 44.Page PCB, Barros D, Buckley BR, Marples BA. Organocatalysis of asymmetric epoxidation mediated by iminium salts: comments on the mechanism. Tetrahedron: Assymetry. 2005;16(21):3488–3491. doi: 10.1016/j.tetasy.2005.08.053. [DOI] [Google Scholar]
- 45.Juan Hikawczuk VE, Rossomando PC, Giordano OS, Saad JR. neo-Clerodane diterpenoids from Baccharis flabellata. Phytochemistry. 2002;61(4):389–394. doi: 10.1016/S0031-9422(02)00241-8. [DOI] [PubMed] [Google Scholar]
- 46.Miller LA, Carrier RL, Ahmed I. Practical considerations in development of solid dosage forms that contain cyclodextrin. J Pharm Sci. 2007;96(7):1691–1707. doi: 10.1002/jps.20831. [DOI] [PubMed] [Google Scholar]