Abstract
Drug-loaded calcium pectinate gel (CaPG) beads were prepared by either mixing, absorption, or swelling method. The effects of drug loading method as well as the drug loading factors (i.e., drug concentration, soaking time in drug solution, type of solvent) on drug content and drug release were investigated. The amount of drug uptake (i.e., drug content) into CaPG beads increased as the initial drug concentration increased and varied depending on the loading method. The in vitro release studies in 0.1 N hydrochloric acid (HCl) and pH 6.8 buffer indicated that the drug loading method affected drug release and release parameter, time for 50% of drug release (T50). The mixing method provided a faster drug release and lower T50 than the absorption method and swelling method, respectively. This is probably due to higher drug content in CaPG beads. The increased concentration of drug in soaking solution and soaking time resulted in higher drug content and thus faster drug release (lower in T50 values). When using 0.1 N HCl as solvent for soaking instead of water, the drug release was slower owing to the increase in molecular tortuosity of CaPG beads. The drug release was also affected by pH of the release medium in which drug release in 0.1 N HCl was faster than in pH 6.8 buffer.
Key words: beads, calcium pectinate, drug loading, drug release, pectin
Introduction
Although, the oral administration of drug products is the most convenient and commonly employed route of drug delivery, it is not always the most suitable route for some active compounds which cause gastric mucosal damage or poorly absorbed drugs, for example peptides or proteins. Furthermore, the possibility of controlling drug delivery after oral administration is very limited since it depends on the residence time of the dosage form in the gastrointestinal tract. In fact, the drug will follow the gastric emptying rate, a physiological parameter that is subject to significant inter-individual variability (1). Therefore, new excipients for developing drug carrier systems to control drug release after oral administration have been extensively researched. In the last decade, many controlled-release formulations based on natural water-soluble polymers have been developed (2). Pectin has been one of the successful choices for this purpose. The lack of toxicity and the low production costs of pectin make them useful for the formulation of controlled drug release dosage forms for oral administration.
Pectin, naturally occurring in fruits, has been commonly used as a food additive, a thickening agent and a gelling agent. It is a polysaccharide extracted industrially from citrus peels, sugar beet roots or apple pomace. Structurally, pectin composes of α-d-galacturonic acid units connected with 1–4 linkages (3,4), which may be interrupted by some rhamnogalacturonan segments (5). The galacturonic acid of the backbone is partly esterified by the methanol. Low methoxy pectin, which less than 50% of the galacturonic acid residues are esterified, can form gels by the action of di- or trivalent cations (6). Calcium-crosslinked pectin hydrogels have been investigated as a potential carrier material for different dosage forms or drug delivery systems.
Recently, calcium pectinate gel (CaPG) beads prepared by the ionotropic gelation method (7) have been developed as a carrier for drug delivery. Various applications for oral drug delivery have been investigated using CaPG beads, for example, for sustained release of drugs (7–9), for targeting drugs to the colon (10,11), or as gastroretentive dosage forms (12). Many factors (i.e., pectin type, concentration of calcium ions, amount of drug loading, drying condition, use of hardening agent, concentration of hardening agent and hardening time, etc.) can influence the bead formation as well as the release characteristics of the drug from the CaPG beads (8,9,13). In the present work, we further studied the effect of different drug loading methods on the drug content and release properties of drug-loaded CaPG beads. The effects of concentration and solvent used to prepare drug solution, soaking time, and dissolution medium on the drug content and drug release were also investigated.
Materials and Methods
Materials
Amidated low methoxy pectin with DE of 28% (GENU pectin type LM104 AS-FS) was obtained from Copenhagen Pectin A/S (Lille Skensved, Denmark). Theophylline anhydrous and calcium acetate monohydrate were purchased from P.C. Drug Center (Bangkok, Thailand) and Polskie od Czynniki Chemiczne (Gliwice, Poland), respectively. All other materials were used as supplied without further purification.
Preparation of Drug-Loaded CaPG Beads
Drug-loaded CaPG beads were prepared using three different methods. Formulations prepared from these methods were summarized in Table I.
Table I.
Summary of Formulations Tested
| Formulation | Loading method | Theophylline concentration (% w/w) | Soaking time (min) | Solventa | Code |
|---|---|---|---|---|---|
| 1 | – | – | – | – | Blank |
| 2 | Mixing | 0.125 | – | – | M-low |
| 3 | Mixing | 1.25 | – | – | M-high |
| 4 | Absorption | 0.125 | 20 | DW | A-low-20 |
| 5 | Absorption | 0.125 | 120 | DW | A-low-120 |
| 6 | Absorption | 1.25 | 20 | DW | A-high-20 |
| 7 | Absorption | 1.25 | 120 | DW | A-high-120 |
| 8 | Swelling | 0.125 | 20 | DW | S-low-20-w |
| 9 | Swelling | 0.125 | 120 | DW | S-low-120-w |
| 10 | Swelling | 0.125 | 20 | HCl | S-low-20-a |
| 11 | Swelling | 0.125 | 120 | HCl | S-low-120-a |
| 12 | Swelling | 1.25 | 20 | DW | S-high-20-w |
| 13 | Swelling | 1.25 | 120 | DW | S-high-120-w |
| 14 | Swelling | 1.25 | 20 | HCl | S-high-20-a |
| 15 | Swelling | 1.25 | 120 | HCl | S-high-120-a |
DW distilled water, HCl 0.1 N hydrochloric acid
aSolvent used to dissolve theophylline for preparing drug solution
Mixing (Conventional) Method
The drug-loaded CaPG beads were prepared according to the method described in previous study (8). Theophylline (80-mesh sieved) was dispersed in 5% w/w aqueous solution of low methoxy pectin. The dispersions were extruded using a nozzle of 0.80-mm inner diameter into a solution of calcium acetate (0.3 M) with a gentle agitation at room temperature. The CaPG beads formed were allowed to stand in the solution for 20 min, separated, and washed with distilled water. The suspension was filtered, rewashed with water, then filtered, and dried at 37°C for 12 h.
Absorption Method
The blank CaPG beads were prepared in the same manner as the conventional method, except the drug was not mixed into pectin solution. The resultant wet beads, those formed in the solution of calcium acetate, were separated, washed, and then soaked in an aqueous solution of theophylline (0.125% or 0.25% w/v). After 20 min or 2 h, the beads were then collected and washed twice with distilled water. The beads were consequently dried at 37°C for 12 h.
Swelling Method
The blank CaPG beads were prepared in the same manner as mentioned above and subsequently dried at 37°C for 12 h. The dried beads were soaked in theophylline solution for 20 min or 2 h. Theophylline solutions were prepared in either distilled water or 0.1 M hydrochloric acid (HCl) at the concentration of 0.125% or 0.25% w/v. The soaked beads were washed twice in distilled water and then dried again at 37°C for 12 h.
Characterization of the CaPG Beads
Particle Size
The mean diameter of 50 dried beads was determined by microscopic method, using an optical microscope (BH-2, Olympus, Japan). The microscope eyepiece was fitted with a micrometer by which the size of the beads can be measured.
Drug Content
Prior to the determination of theophylline content, the CaPG beads were dissolved in 2% w/v trisodium citrate. The content of theophylline was later assayed by UV-spectrophotometer (Hitachi U-2000, Japan) in 2% w/v trisodium citrate at 272 nm. The determinations were made in triplicate.
Swelling Studies
Twenty blank CaPG beads were accurately weighed and placed in small vials containing 10 mL of 0.1 M HCl or pH 6.8 Tris buffer. They were shaken (100 rpm) in an orbital shaker incubator (Model ES-20, Biosan, Latvia) at 37°C. The beads were suspended in the media in such a way that swelling could occur three-dimensionally with water penetrating all sides of the gel beads. At different time intervals, the beads were gently removed from the hydration medium, surface dried with filter paper, and reweighed on an analytical balance (Sartorius Type 1702, Germany). The same beads were used for all investigated times. The swelling ratio of the gel beads was calculated by Eq. 1 as follows:
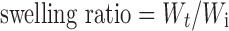 |
1 |
where Wt is the wet weight of the gel beads at time t and Wi is the initial dry weight of the beads. The experiments were performed in triplicate.
In Vitro Drug Release Studies
The in vitro release of theophylline from CaPG beads was determined using a USP dissolution apparatus I (Pharmatest™, Germany) in 900 mL of either 0.1 M HCl (pH 1.2) or pH 6.8 Tris buffer with the basket rotation at 100 rpm. The temperature was controlled at 37°C. The analytical wavelength was 272 nm and Beer’s law was obeyed over the range of 0–100 mg/L. Drug release was measured from accurately weighed amounts of the CaPG beads, such that 100 mg of theophylline was present in each dissolution pot. All dissolution runs were performed in triplicate.
The time for 50% release of the drug (T50) was employed as a response parameter. Analysis of variance and Levene’s test for homogeneity of variance were performed using SPSS version 10.0 for Windows (SPSS Inc., USA). Post hoc testing (p < 0.05) of the multiple comparisons was performed by either the Scheffé or Games–Howell test depending on whether Levene’s test was insignificant or significant, respectively.
Results and Discussion
Characterization of the CaPG Beads
The CaPG beads were successfully prepared by dropping the solution of pectin into the solution of calcium salt. The droplets instantaneously formed gelled spheres by ionotropic gelation (8,14,15). The CaPG beads were easily manufactured without any sophisticated equipment. The average diameter of the CaPG beads prepared by conventional mixing method and then air drying was about 1.64–1.65 mm while that of the freshly prepared beads ranged between 3.24 and 3.33 mm (Table II). The size of the beads prepared by absorption method at higher concentration of drug was slightly larger than the beads prepared by mixing method; however, after drying, the size was not statistically significantly different. By using the swelling method, the blank beads were dried and subsequently rehydrated in the drug solution. Most beads swelled or rehydrated to a size which was less than their original wet size. The size of rehydrated beads was not significantly different (p > 0.05) except those rehydrated in acid for 120 min in which the size was significantly increased. This may be because the swelling of dried CaPG beads reached a maximum within 2 h (16). The re-dried beads were almost the same size irrespective of the drug concentration or soaking time, and much smaller than the beads dried only once.
Table II.
Evaluation of CaPG Beads
| Formulation code | Size (mm), n = 50 | Drug content (mg/g of bead), n = 3 | T50 (min), n = 3 | ||
|---|---|---|---|---|---|
| Fresh beads | Dried beads | in 0.1 N HCl | in Tris buffer pH 6.8 | ||
| Blank | 3.24 ± 0.11 | 1.64 ± 0.08 | – | – | – |
| M-low | 3.29 ± 0.11 | 1.65 ± 0.09 | 27.97 ± 2.66 | 3.23 ± 0.61 | 3.42 ± 0.33 |
| M-high | 3.33 ± 0.11 | 1.64 ± 0.08 | 29.15 ± 1.57 | 2.95 ± 0.37 | 3.22 ± 0.18 |
| A-low-20 | 3.11 ± 0.08 | 1.49 ± 0.05 | 5.88 ± 0.10 | 4.76 ± 0.31 | 5.11 ± 0.64 |
| A-low-120 | 3.10 ± 0.09 | 1.51 ± 0.06 | 7.14 ± 0.18 | 4.03 ± 0.03 | 4.27 ± 0.47 |
| A-high-20 | 3.77 ± 0.14 | 1.50 ± 0.05 | 17.84 ± 3.78 | 3.10 ± 1.07 | 3.87 ± 0.23 |
| A-high-120 | 3.73 ± 0.14 | 1.66 ± 0.07 | 36.58 ± 3.62 | 3.72 ± 0.38 | 4.07 ± 0.48 |
| S-low-20-w | 1.59a ± 0.13 | 1.29b ± 0.12 | 1.09 ± 0.02 | 8.88 ± 0.69 | 10.42 ± 0.41 |
| S-low-120-w | 1.80a ± 0.11 | 1.48b ± 0.14 | 1.14 ± 0.20 | 6.98 ± 0.55 | 7.42 ± 0.47 |
| S-low-20-a | 1.70a ± 0.11 | 1.34b ± 0.08 | 0.97 ± 0.02 | 12.83 ± 2.74 | 15.25 ± 0.64 |
| S-low-120-a | 2.12a ± 0.14 | 1.39b ± 0.09 | 1.29 ± 0.01 | 7.53 ± 1.04 | 9.43 ± 1.65 |
| S-high-20-w | 1.48a ± 0.12 | 1.34b ± 0.06 | 2.61 ± 0.01 | 5.97 ± 0.50 | 6.24 ± 0.42 |
| S-high-120-w | 1.83a ± 0.10 | 1.53b ± 0.15 | 4.24 ± 0.07 | 5.25 ± 0.43 | 6.44 ± 0.50 |
| S-high-20-a | 1.59a ± 0.11 | 1.33b ± 0.06 | 2.71 ± 0.02 | 6.22 ± 1.24 | 7.05 ± 1.17 |
| S-high-120-a | 2.47a ± 0.13 | 1.40b ± 0.09 | 4.37 ± 0.09 | 5.70 ± 1.99 | 7.25 ± 0.40 |
aParticle size of the blank beads those rehydrated/swelled in drug solution, in which the original size of the wet and dried beads was 3.24 ± 0.11 and 1.64 ± 0.08, respectively
bParticle size of the re-dried beads
Drug content is an important parameter to evaluate the properties of the drug-loaded particles. As shown in Table II, the drug content (in milligram per gram of bead) of different batches of CaPG beads was carried out. It can be seen that the drug loading method has a significant impact on drug content of the CaPG beads. The absorption process using 0.25% w/v theophylline and 120-min soaking time increased the content of theophylline in the CaPG beads; the drug content (36.58%) reached the maximum value. This is probably due to a higher concentration of drug in the solution (16) and a longer soaking time. Similar results were reported that the absorption process increases the drug load into microcapsules of alginate/poly-l-lysine/chitosan ternary complex (17). At a lower theophylline concentration (0.125% w/v), the drug content in the CaPG beads prepared by absorption method was significantly lower. Swelling method also resulted in lower drug content in the CaPG beads, compared to other drug loading methods. Using the swelling, it is apparent that the drug content was higher when the concentration of theophylline used during soaking was higher. Moreover, the results obviously show that the lower drug content was found in the case of using lower concentration of drug solution regardless of the drug loading method.
Swelling Studies
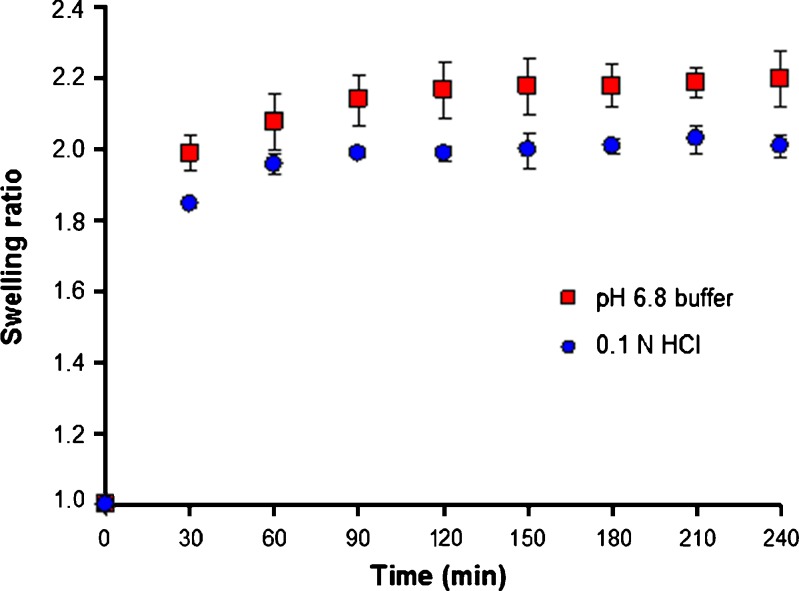
After rehydration in 0.1 M HCl or pH 6.8 Tris buffer, the CaPG beads swelled to different sizes. The swelling ratio of blank CaPG beads in both media at different times is shown in Fig. 1. It is apparent that the swelling of dried CaPG beads occurred quite rapidly. The swelling behavior can be justified due to the fact that beads tend to absorb water (bulk or free water) in order to fill the void regions of the polymer network within the beads that dehydrated, until they reach the equilibrium state (18). The phenomenon is provoked by the relaxation of the polymer network at the presence of osmotic pressure. Swelling of the CaPG beads lasts for about 60–90 min until the osmotic pressure equals the forces of the cross-linking bonds that maintain the structure of the polymer network stable. When these two forces are equal, no further water gaining from the beads is observed.
Fig. 1.
Swelling behavior of blank CaPG beads in 0.1 N HCl and pH 6.8 Tris buffer (n = 3)
The CaPG beads exhibit a swelling ratio of 2.0 and 2.2 in acidic medium and pH 6.8 buffer, respectively. The beads swelled less when exposed to the acidic environment of 0.1 N HCl than in neutral pH medium. The pKa of pectin ranges between 3 and 4, depending on the type and source of pectin. Therefore, a low pH of 1.2 influenced beads hydration due to the ready interconversion of carboxylate anions (pectin salt) to free carboxyl groups (pectinic acid), as the concentration of hydrogen ions increased (19).
In Vitro Drug Release Studies
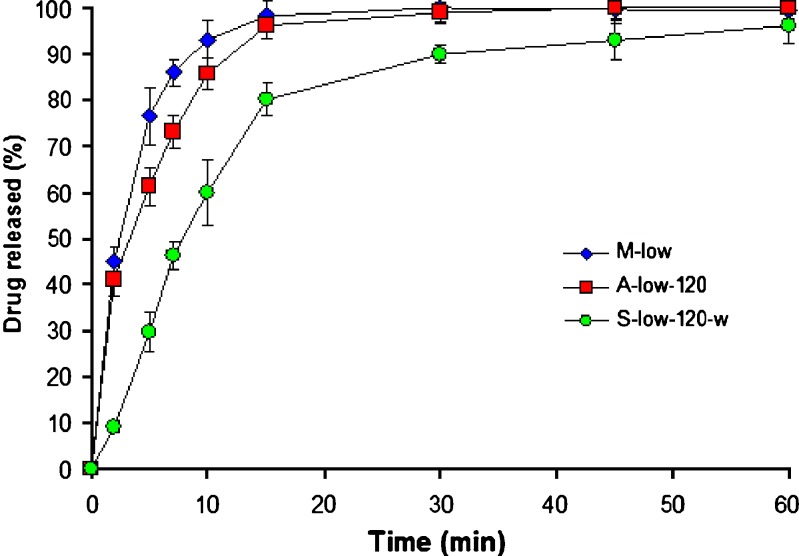
The release profiles, in pH 6.8 buffer, of theophylline from CaPG beads prepared from different drug loading methods are shown in Fig. 2. The release profiles are characterized by a biphasic behavior, fast release period, and declining rate phase. The first phase includes two apparent mechanisms that govern the drug release out from the beads—swelling and diffusion. During the second phase, the swelling of the beads is constant and, hence, does not affect the drug release. In addition, the T50 values for CaPG beads in 0.1 M HCl and pH 6.8 buffer are shown in Table II. The drug release was relatively influenced by pH of the release media which was in good agreement with a previous report (20) and the T50 values in 0.1 N HCl were lower than in pH 6.8 buffer. This is expected since it has been shown that CaPG can be converted to physically stable acid gels, and these gels were considerably more permeable than the original CaPG (20). The release rates of theophylline were also influenced by the drug loading method. The beads prepared from mixing method demonstrated the fastest drug release, followed by those prepared from absorption method and swelling method, respectively. The faster in drug release from CaPG beads, which contain higher drug content, may result from the lower in the polymer-to-drug ratio (8).
Fig. 2.
Effect of drug loading method on drug release, in pH 6.8 Tris buffer, from CaPG beads using 20-min soaking time and 0.125% w/w theophylline (n = 3)
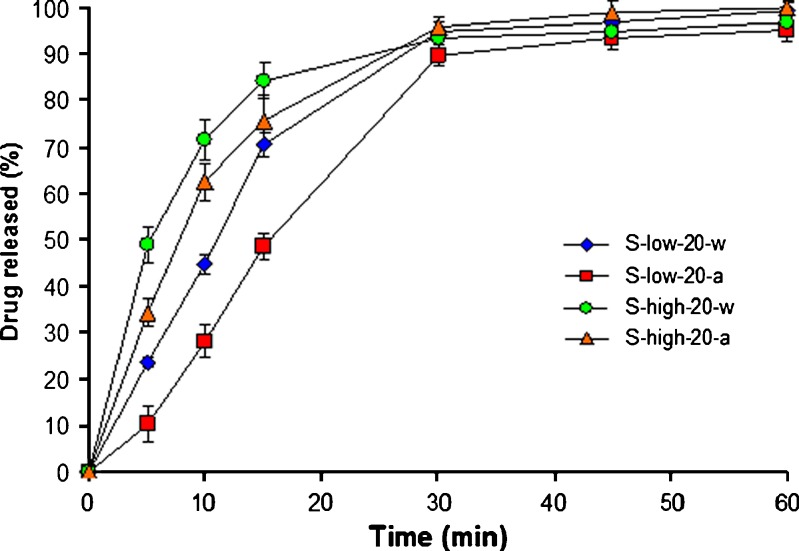
Figure 3 shows the effect of drug concentration and solvent on drug release, in pH 6.8 buffer, from CaPG beads prepared by swelling method. It is evident that the use of high concentration of theophylline in soaking solution resulted in faster drug release. This is probably due to a lower polymer-to-drug ratio resulting from higher drug content, similar to the beads prepared by absorption method (as discussed above). The drug release, in both 0.1 N HCl and pH 6.8 buffer, was slower when using 0.1 N HCl as solvent for soaking instead of water. It is likely that the acid impeded the swelling of CaPG beads during soaking in drug solution and, therefore, resulted in smaller particle size with greater molecular tortuosity. The increase of soaking time from 20 to 120 min resulted in higher drug content and thus faster drug release or lower in T50 values.
Fig. 3.
Effect of drug concentration and solvent on drug release, in pH 6.8 Tris buffer, from CaPG beads prepared by soaking method (n = 3)
Conclusion
The results of this study enable us to state that the drug loading method has a great impact on drug content and drug release from CaPG beads. The absorption method greatly increased the drug content in CaPG beads, while in using the swelling method, the drug content was lower but the drug release was prolonged.
Acknowledgments
This work was financially supported by the Silpakorn University Research and Development Institute, under the research program “Production of pectin from pomelo (Citrus maxima) peel and application in pharmaceutical industry.” The authors thank Food & Cosmetic Systems Co., Ltd. (Bangkok) that kindly provided pectin samples manufactured by CP Kelco. Thanks are also due to J. Chun-on, J. Thaicharoen, T. Yingkamhaeng, N. Worachun, and S. Sae-ieo for the laboratory assistance.
References
- 1.Gibaldi M. Biopharmaceutics and clinical pharmacokinetics. 4. Philadelphia: Lea and Lea Febiger; 1991. [Google Scholar]
- 2.Graham NB, McNeill ME. Hydrogels for controlled drug delivery. Biomaterials. 1984;5:27–36. doi: 10.1016/0142-9612(84)90063-2. [DOI] [PubMed] [Google Scholar]
- 3.Rolin C. Pectin. In: Whistler RL, Bemiller JN, editors. Industrial gums: Polysaccharides and their derivatives. New York: Academic; 1993. pp. 257–93. [Google Scholar]
- 4.Sriamornsak P. Chemistry of pectin and its pharmaceutical uses: a review. Silpakorn Univ Int J. 2003;3:206–28. [Google Scholar]
- 5.Schols HA, Voragen AGJ. Complex pectin: structure elucidation using enzymes. In: Visser J, Voragen AGJ, editors. Progress in biotechnology: pectin and pectinases, Vol. 14. Amsterdam: Elsevier; 1996. pp. 3–19. [Google Scholar]
- 6.Axelos MAV, Thibault JF. The chemistry of low-methoxy pectin gelation. In: Walter RH, editor. The chemistry and technology of pectin. New York: Academic; 1991. pp. 109–18. [Google Scholar]
- 7.Aydin Z, Akbuga J. Preparation and evaluation of pectin beads. Int J Pharm. 1996;137:133–6. doi: 10.1016/0378-5173(95)04458-2. [DOI] [Google Scholar]
- 8.Sriamornsak P, Nunthanid J. Calcium pectinate gel beads for controlled release drug delivery: I. Preparation and in vitro release studies. Int J Pharm. 1998;160:207–12. doi: 10.1016/S0378-5173(97)00310-4. [DOI] [Google Scholar]
- 9.Sriamornsak P, Nunthanid J. Calcium pectinate gel beads for controlled release drug delivery: II. Effect of formulation and processing variables on drug release. J Microencapsul. 1999;16:303–13. doi: 10.1080/026520499289031. [DOI] [PubMed] [Google Scholar]
- 10.Sriamornsak P. Investigation of pectin as a carrier for oral delivery of proteins using calcium pectinate gel beads. Int J Pharm. 1998;169:213–20. doi: 10.1016/S0378-5173(98)00129-X. [DOI] [Google Scholar]
- 11.Sriamornsak P. Effect of calcium concentration, hardening agent and drying condition on release characteristics of oral proteins from calcium pectinate gel beads. Eur J Pharm Sci. 1999;8:221–7. doi: 10.1016/S0928-0987(99)00010-X. [DOI] [PubMed] [Google Scholar]
- 12.Sriamornsak P, Thirawong N, Puttipipatkhachorn S. Emulsion gel beads of calcium pectinate capable of floating on the gastric fluid: effect of some additives, hardening agent or coating on release behavior of metronidazole. Eur J Pharm Sci. 2004;24:363–73. doi: 10.1016/j.ejps.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Sriamornsak P, Burapapadh K, Nunthanid J, Puttipipatkhachorn S. Effect of acidic medium on swelling and release of indomethacin from chitosan-reinforced calcium pectinate gel beads. Silpakorn Univ Sci Technol J. 2008;2:37–44. [Google Scholar]
- 14.Aslani P, Kennedy RA. Effect of gelation conditions and dissolution media on the release of paracetamol from alginate gel beads. J Microencapsul. 1996;13:601–14. doi: 10.3109/02652049609026044. [DOI] [PubMed] [Google Scholar]
- 15.Sriamornsak P, Thirawong N, Cheewatanakornkool K, Burapapadh K, Sae-Ngow W. Cryo-scanning electron microscopy (cryo-SEM) as a tool for studying the ultrastructure during bead formation by ionotropic gelation of calcium pectinate. Int J Pharm. 2008;352:115–22. doi: 10.1016/j.ijpharm.2007.10.038. [DOI] [PubMed] [Google Scholar]
- 16.Sriamornsak P, Kennedy RA. Effect of a small molecule on diffusion and swelling properties of selected polysaccharide gel beads. Carbohydr Polym. 2010;79:219–23. doi: 10.1016/j.carbpol.2009.07.059. [DOI] [Google Scholar]
- 17.Wang SB, Chen AZ, Weng LJ, Chen MY, Xie XL. Effect of drug loading methods on drug load, encapsulation efficiency and release properites of alginate/poly-L-lysin/chitosan ternary complex microcapsules. Macromol Biosci. 2004;4:27–30. doi: 10.1002/mabi.200300043. [DOI] [PubMed] [Google Scholar]
- 18.Hoffman AS. Hydrogels for biomedical applications. Adv Drug Deliv Rev. 2002;43:3–12. doi: 10.1016/S0169-409X(01)00239-3. [DOI] [PubMed] [Google Scholar]
- 19.Sriamornsak P, Thirawong N, Weerapol Y, Nunthanid J, Sungthongjeen S. Swelling and erosion of pectin matrix tablets and their impact on drug release behavior. Eur J Pharm Biopharm. 2007;67:211–9. doi: 10.1016/j.ejpb.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 20.Sriamornsak P, Kennedy RA. Effect of drug solubility on release behavior of calcium polysaccharide gel-coated pellets. Eur J Pharm Sci. 2007;32:231–9. doi: 10.1016/j.ejps.2007.08.001. [DOI] [PubMed] [Google Scholar]