Abstract
Stability enhancement of protein-loaded chitosan microparticles under storage was investigated. Chitosan glutamate at 35 kDa and bovine serum albumin as model protein drug were used in this study. The chitosan microparticles were prepared by ionotropic gelation, and polyethylene glycol 200 (PEG 200) was applied after the formation of the particles. All chitosan microparticles were kept at 25°C for 28 days. A comparison was made between those preparations with PEG 200 and without PEG 200. The changes in the physicochemical properties of the microparticles such as size, zeta potential, pH, and percent loading capacity were investigated after 0, 3, 7, 14, and 28 days of storage. It was found that the stability decreased upon storage and the aggregation of microparticles could be observed for both preparations. The reduction in the zeta potential and the increase in the pH, size, and loading capacity were observed when they were kept at a longer period. The significant change of those preparations without PEG 200 was evident after 7 days of storage whereas those with PEG 200 underwent smaller changes with enhanced stability after 28 days of storage. Therefore, this investigation gave valuable information on the stability enhancement of the microparticles. Hence, enhanced stability of chitosan glutamate microparticles for the delivery of protein could be achieved by the application of PEG 200.
Key words: chitosan microparticles, polyethylene glycol 200, protein, stability
INTRODUCTION
Microparticles and nanoparticles have been widely studied for the delivery of macromolecular drugs, such as peptides, proteins, and genes, due to their ability to protect the labile drugs from enzymatic degradation (1) and the ability of permeability through the gastrointestinal membrane (1–5). There are several techniques for the preparation of a carrier for drug delivery system. One of the most popularly used techniques is ionic cross-linking (6–10) because it is simple to prepare and a wide range of polymer can be used. Chitosan is a polymer of choice due to biodegradation, mucoadhesion, and the ability of opening tight junctions (5,11–13). Chitosan microparticles are formed by ionic cross-linking between the positive charges of the amino groups of chitosan and the negative charges of tripolyphosphate ions (TPP; 6–10). In general, the chitosan base is used for drug delivery and a lot of work has been performed on microparticles prepared with chitosan base. Chitosan base is soluble in mild acids, such as acetic acid, citric acid, and glutamic acid, while chitosan salt is soluble in water, which can be an advantage in pharmaceutical processing for encapsulation of therapeutic proteins. From our previous study, protein-loaded microparticles could be prepared from chitosan salts. The higher encapsulation and release could be obtained from chitosan salts in comparison to chitosan base. The molecular weight of chitosan tended to affect their particles sizes and encapsulation efficiency. The smaller size was obtained from chitosan with a lower molecular weight (10).
Though microparticles have been widely studied, the problem of stability still remained unsolved. The microparticles tended to aggregate and lose their stability after a certain period of storage (14). The larger size and the change in the zeta potential were observed after 28 days of storage. It was found that the larger size was related to the change in pH of the chitosan solution. The stability of the microparticles could be due to the electrostatic charge of particles. The repulsion forces of the particles were attributed to the stability of small particles. The reduction of electrostatic charge until a certain extent could lead to the aggregation (15). Several studies were investigated to modify the surface charges of the microparticles in order to prevent opsonization or interactions with macrophages (16,17). In general, polyethyelene glycol (PEG) has been employed as a particle coating for the surface modification. In addition, PEG and PEGylation have been studied for the improvement of gene delivery system (18). However, the application of PEG to stabilize the chitosan microparticles upon storage as a result of the aggregation has been paid less attention and has never been thoroughly clarified. Therefore, the objective of this work was to enhance the stability of protein-loaded chitosan microparticles with the application of PEG 200. Chitosan glutamate at 35 kDa was chosen in this study as it had the higher encapsulation and release than the other salt and 35 kDa gave the lowest size which had a higher tendency to aggregate than the larger size obtained from the chitosan with a higher molecular weight. The effect of PEG 200 on the physicochemical properties of chitosan microparticles upon storage was explored to obtain insight information on the stability enhancement of protein-loaded chitosan microparticles. A comparison was made between the formulation with and without the application of PEG 200.
MATERIALS AND METHODS
Materials
Chitosan 35 kDa with 85% degree of deacetylation was purchased from Seafresh Chitosan Lab. Co. (Bangkok, Thailand). Bovine serum albumin (BSA) was from Sigma (St. Louis, Missouri, USA). Glutamic acid was from Fluka (Switzerland). Sodium tripolyphosphate (TPP) and PEG 200 were obtained from Fluka (Switzerland), and other reagents were all of analytical grade.
Preparation of Chitosan Glutamate
Chitosan glutamate was prepared by dissolving chitosan base in glutamic acid solutions. A minimum amount of glutamic acid was calculated as optimum to give an exact clear solution in order to avoid the excess glutamic acid. Hence, the calculated molar ratio of glucosamine and glutamic acids was 1:1 mole. The chitosan glutamate solution was then spray-dried by a spray dryer (Labquip SD06 Spray Dryer, England) with an inlet temperature of 125°C and a spraying rate of 6 ml/min.
Preparation of Chitosan Microparticles and BSA-Loaded Microparticles
Chitosan microparticles were prepared by ionotropic gelation with negatively charged TPP ions according to a previously reported procedure (10). An optimum concentration of chitosan and TPP was required to form suspended particles. The concentrations of chitosan and TPP were 0.4% w/v and 0.1% w/v, respectively. The microparticles were prepared by drop-wise addition of 5 ml of 0.1% w/v TPP in water to 10 ml of chitosan glutamate solution with stirring at 1,000 rpm (Corning Stirrer, USA). The microparticles were stirred for 30 min prior to further study. BSA-loaded microparticles were formed by the addition of 1 ml of BSA solution to the chitosan solution prior to the incorporation of TPP solution. The concentrations of BSA were 0, 1, and 2 mg/ml. After the formation of the microparticles, 1 ml of PEG 200 was added. All the samples (with and without PEG 200) were then kept at 25°C for a period of 28 days for stability study. A comparison was made between both preparations (chitosan microparticles with and without PEG 200). All the samples were evaluated for their particle size, zeta potential, pH, and percent loading capacity (LC). All parameters were detected after 0, 3, 7, 14, and 28 days.
Particle Size Measurement
The diameter of the microparticles was detected by the method of light diffraction (Horiba L 950, Japan). All analyses were performed in triplicate.
Zeta Potential Measurement
The zeta potential of the samples was measured at 25°C by a Brookhaven Zeta plus 90 (USA).
Determination of BSA-Loading Capacity of Microparticles
The percent loading capacity (%LC) of all microparticles was determined by high speed centrifugation at 20,000×g and 25°C for 30 min (Heraeus, Germany). The amount of BSA from the clear supernatant was detected by spectrophotometry according to the Lowry method (19). The LC of BSA was calculated using as the following equation and was performed with triplicate batches of samples.
 |
1 |
Fourier Transforms Infrared Spectroscopy
Transmission infrared spectra of the chitosan microparticles with PEG 200 and without PEG 200 after 0, 7, 14, and 28 days were measured by the KBr method using a Fourier transform infrared spectrophotometer (model Magna-IR system 750, Nicolet Biomedical Inc., USA). The prepared samples were dried under vacuum and mixed with KBr. The mixture was compressed into a disk. Spectral scanning was performed in the range between 4,000 and 400 cm−1.
Microscopy
The chitosan microparticles were dropped onto a microslide plate and covered with a slid. The picture was then taken by an inverted microscope (model Eclipse TE2000-S, Japan) after 0, 7, and 28 days for preparations with and without PEG 200.
Statistical Analysis
Data were expressed as the mean ± standard deviation. The statistical analysis was carried out using analysis of variance at the 0.01 significant levels.
RESULTS AND DISCUSSION
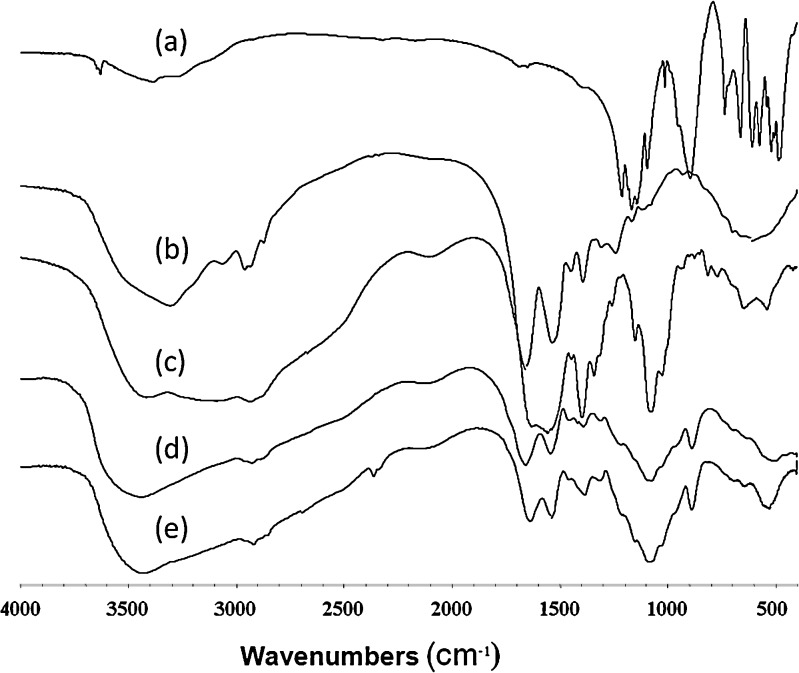
Nanoparticles and microparticles were successfully prepared from chitosan glutamate by the method of ionotropic gelation as described in our previous report (10). The ionic bond formation between the amino group of chitosan glutamate and TPP was confirmed by IR spectroscopy. Figure 1 shows the IR spectra of TPP, BSA, chitosan glutamate, chitosan microparticles, and BSA-loaded chitosan microparticles. BSA gave absorption bands in the amide I (∼1,650 cm−1) and amide II (∼1,540 cm−1) due to C = O stretching vibration. Chitosan glutamate showed a broad peak between 1,640 and 1,539 cm−1 which were attributed to the N–H bending of NH+3 whereas chitosan microparticles exhibited a sharp peak around 1,654 and 1,539 cm−1 indicating the formation of the microparticles. Therefore, the peaks at 1,654 and 1,539 cm−1could be an indicator for electrostatic bond formation. The result was in agreement with the work of Xu and Du (6) which found that the peak of –NH2 bending was shifted due to the electrostatic force between TPP and the ammonium group of the chitosan. The BSA-loaded chitosan microparticles also showed a peak similar to blank microparticles. The result was in accordance with the work of Sarmento et al. (14) which found a similar pattern for blank particles and insulin-loaded nanoparticles.
Fig. 1.
IR spectra of a TPP, b BSA, c chitosan, d blank chitosan microparticles, and e BSA-loaded chitosan microparticles
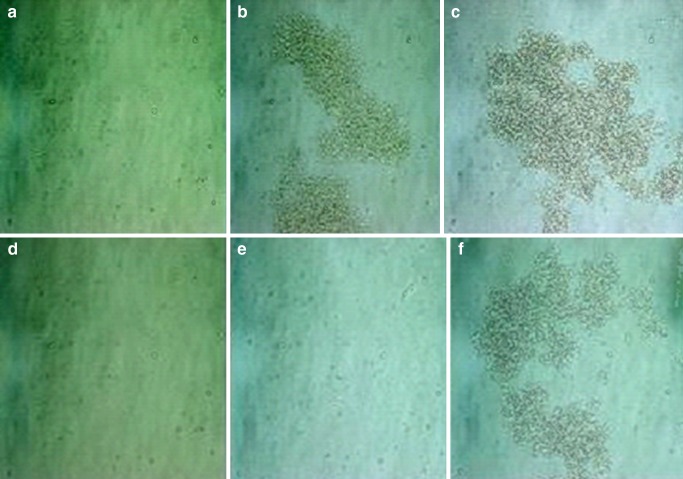
From our previous study, a smaller size was obtained from chitosan of low molecular weight because chitosan of higher molecular weight was more viscous in solution and the amino groups of the chitosan could become less accessible for TPP interaction in a viscous environment. Therefore, chitosan at 35 kDa was chosen in this study for the stability study as the smaller size tended to aggregate more than the larger size due to a higher surface area. Table I shows the effect of storage time on the particle size of the chitosan microparticle with and without PEG 200. The size of blank chitosan particles for those with and without PEG 200 was 65.02 ± 11.84 and 50.41 ± 1.16 μm, respectively. The addition of PEG 200 gave a slightly larger size for blank chitosan microparticles due to the coating effect of PEG 200 on the microparticles (20). The incorporation of BSA at 1 and 2 mg/ml showed a smaller size for both preparations. The smaller size could be due to the negative charge of BSA causing a tighter bonding between the amino groups of the chitosan and the carboxylic groups of BSA. The storage time had an influence on the particle size. Upon storage, all the microparticles tended to increase in the size. However, for those without BSA and PEG, the decrease in size was found after 7 days of storage and gradually increased on longer time of storage. The change in size was dependent on the change in the environment of microstructure. After a certain period of storage, the change in the size was observed for both preparations, i.e., after 7 and 28 days of storage for those without and with PEG, respectively. For those without PEG, the size of 1 and 2 mg/ml BSA-loaded microparticles increased from about 14 μm to be 27 and 35 μm, respectively, after 7 days of storage whereas for those with PEG 200 remained constant for 14 days of storage. However, the tremendous increase in size (p < 0.01) could be observed after 28 days of storage for those with PEG 200 at all concentrations of BSA. The significant increase in size was related to the aggregation and was confirmed by the photographs in Fig. 2. The aggregation could be seen after 7 days of storage for those without PEG 200 and 28 days for those with PEG 200. The individual single particles were still visible for the preparation with PEG 200 after 7 days of storage. Therefore, the addition of PEG 200 could prove to prolong the change in the particle size. This was the result of the steric hindrance of PEG 200, which could possibly protect the particles from aggregation.
Table I.
Effect of Storage Time on the Particle Size of Chitosan Microparticles without and with PEG 200
| Surfactant | Chitosan particle | Size (μm) | ||||
|---|---|---|---|---|---|---|
| Day 0 | Day 3 | Day 7 | Day 14 | Day 28 | ||
| Without PEG 200 | BSA 0 mg/ml | 50.41 ± 1.16 | 61.89 ± 3.03 | 31.50 ± 3.78 | 32.83 ± 0.12 | 37.12 ± 4.30* |
| BSA 1 mg/ml | 15.66 ± 0.84 | 14.24 ± 1.88 | 26.73 ± 5.06 | 60.58 ± 8.75 | 70.75 ± 8.61* | |
| BSA 2 mg/ml | 13.57 ± 1.81 | 13.68 ± 2.34 | 34.69 ± 1.74 | 84.19 ± 9.18 | 84.16 ± 8.69 | |
| With PEG 200 | BSA 0 mg/ml | 65.02 ± 11.84 | 78.10 ± 19.82 | 64.27 ± 7.78 | 65.05 ± 9.40 | 87.83 ± 3.27* |
| BSA 1 mg/ml | 13.51 ± 2.63 | 14.08 ± 0.98 | 12.34 ± 3.07 | 15.46 ± 1.45 | 51.47 ± 14.35* | |
| BSA 2 mg/ml | 15.36 ± 2.43 | 15.53 ± 5.86 | 11.23 ± 5.25 | 13.07 ± 2.04 | 130.82 ± 14.20* | |
BSA bovine serum albumin, PEG polyethyelene glycol
*p < 0.01
Fig. 2.
Pictures of microparticles without PEG 200 after storage for a 0, b 7, and c 28 days and with PEG 200 after storage for d 0, e 7, and f 28 days
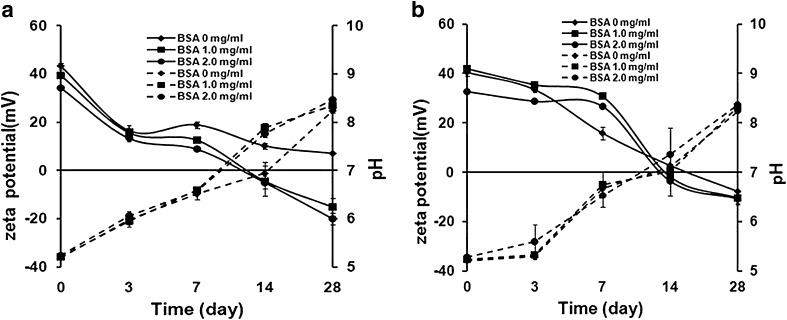
The zeta potential of the blank chitosan microparticles with and without PEG 200 was +40 and +43 mV, respectively. The addition of PEG 200 tended to lower the zeta potential. This may be due to the ability of the OH group of PEG 200 to counter the positive charge of the chitosan. Wu et al. (20) found that the increase in PEG concentration led to the decrease of the positive charge of chitosan nanoparticles. Figure 3 shows the effect of storage time on the zeta potential and pH for preparation without (a) and with PEG 200 (b). The positive charge of the particles was attributed to an unoccupied amine group of the chitosan. The addition of BSA resulted in a decrease in the zeta potential due to the load of negatively charged BSA causing the bonding between NH+3 from the chitosan and the COOH from the BSA. The result was in agreement with Chen et al. (21) which found that the zeta potential decreased as the BSA concentration increased. The longer the time of storage, the lower in the zeta potential was reported for all preparations, which was in accordance with the increase in size. The zeta potential was reduced such that the particles could come closer and a larger size was obtained.
Fig. 3.
Effect of storage time on the zeta potential (solid line) and pH (dashed line) of chitosan microparticles for preparation a without and b with PEG 200
The reduction of the zeta potential was from positive charges to the less positive charges for all preparations. For those without PEG 200 after 7 days of storage, the charge of particles loaded with BSA 2 mg/ml was reduced from +34 mV to be +8 mV while those with PEG 200 was reduced from +32 mV to be +26 mV. A similar incident was observed for 1 mg/ml BSA-loaded particles. The higher positive charge of those with PEG 200 was attributed to the higher protection from aggregation due to the steric hindrance effect. The BSA-loaded particles reached neutrality after 14 days of storage compared with 10 days of storage for those without PEG 200. The result was similar to those in a number of reports which found that the stabilization of the particles was due to the electrostatic nature of the chitosan particles (14,15,22–24). They found that the particles started to aggregate and lose their entity as the surface charge of the colloidal system approached neutrality. At neutral or lower charge, the electrostatic repulsion of the microparticles decreased, thereby increasing the probability of aggregation. Sarmento et al. (14) found that insulin-loaded chitosan nanoparticles changed in size and zeta potential due to the aggregation after 28 days of storage. The change in the zeta potential might be due to the more occupied amine group as a result of prolonged association time with BSA. The reduction in the zeta potential was also in accordance with the increase in pH for both preparations. The longer the storage time, the higher pH was found for all preparations and the change was statistically significant after 28 days of storage (p < 0.01). The increase in pH was a result of an increase in pH of the chitosan itself upon storage leading to the change in the structure of the microparticles. The structure change was reported in the work of Tsai et al. (25) and was subject to change with different environment condition such as pH and ionic strength of the solutions.
From the current study, the higher change in pH was reported for those preparations without PEG 200. The pH of 2 mg/ml BSA-loaded microparticles with PEG 200 increased from 5.28 to be 7.37, whereas those without PEG 200 increased from 5.24 to be 7.76 after 14 days of storage. A similar trend of change was observed for 0 and 1 mg/ml BSA-loaded particles. The increase in pH was also associated with the increase in size and the decreased zeta potential upon storage which was in agreement with another work (26) which found that the significant changes were observed between pH 6.4 and 7.0 where the size increased 50 times and the zeta potential changed from +30 to ±2.1 mV. At pH beyond the pKa of the chitosan (pKa = 6.3), it was in the unprotonated form and the chitosan was insoluble at this pH; the aggregation was then obtained. The sharp increase in size at pH > 6 suggested that the degree of protonization at the surface of the particles was reduced, decreasing the electrostatic repulsion between the particles thereby increasing the probability of particle aggregation. The result was in accordance with the work of Gan et al. (15) which found that the larger size was due to the aggregation of the particles.
Table II shows the effect of storage time on %LC for both preparations. The higher LC was obtained for the chitosan with PEG 200. The PEG 200 might entrap BSA leading to the higher LC. The result was not in agreement with other reports (6,20) as PEGs of different molecular weights (PEG 20000 and 2000) were used in their studies. The PEGs with higher molecular weights hinder BSA encapsulation. At a lower pH in the vicinity of the isoelectric point of the protein (PI = 4.7), BSA still remained in a compact 3D structure and less spread on the chitosan chain. Protein molecule attachment did not sufficiently suppress the positive charge (27). Therefore, the higher positive charge of the chitosan was obtained on the first day of both preparations. However, at higher pH upon storage, BSA underwent expansion, leading to a higher interaction with the amino group and greater reduction of the positive charge of the chitosan resulting in the higher LC (27). In addition, the higher LC was attributed to the longer association time with BSA and hence, the higher interaction between BSA and the chitosan. The work was in agreement with the other researchers (22,23) which reported the significant changes in the zeta potential and particle size at the isoelectirc point of the chitosan and the aggregation occurred. The significant increase in LC was reported after 28 days (p < 0.01) consistent with the changes in other properties such as size, pH, and zeta potential. The result was similar to the work of Gan and Wang (27) and Alsarra et al. (28) which found that the LC of peptides was dependent on the pH, with a consequential effect on increased negative surface charge of the protein which enhanced the electrostatic interaction between the chitosan and BSA molecules. The significant change (p < 0.01) of all parameters after 28 days such as size, pH, zeta potential, and LC were in accordance with the IR spectra as shown in Fig. 4 where the N–H bending was shifted from 1,539 to 1,592 cm−1 after 28 days for both preparations. Therefore, it could be concluded that the storage time had an influence on the physicochemical properties of the chitosan microparticles. The higher extent of change was reported for the chitosan microparticles without PEG 200. Therefore, the addition of PEG 200 has been proved to enhance the stability of protein-loaded chitosan microparticles due to the steric hindrance effect.
Table II.
Effect of Storage Time on %LC of Chitosan Microparticles without and with PEG 200
| Surfactant | Chitosan particle | Percent loading capacity | ||||
|---|---|---|---|---|---|---|
| Day 0 | Day 3 | Day 7 | Day 14 | Day 28 | ||
| Without PEG 200 | BSA 1 mg/ml | 7.61 ± 0.05 | 7.22 ± 0.10 | 11.34 ± 0.50 | 10.10 ± 0.56 | 18.31 ± 6.23* |
| BSA 2 mg/ml | 21.09 ± 0.28 | 19.35 ± 0.18 | 21.66 ± 0.37 | 24.17 ± 1.75 | 40.71 ± 3.35* | |
| With PEG 200 | BSA 1 mg/ml | 12.95 ± 0.37 | 13.21 ± 0.38 | 13.60 ± 0.95 | 10.10 ± 1.64 | 18.31 ± 6.23* |
| BSA 2 mg/ml | 24.26 ± 0.53 | 24.35 ± 0.74 | 25.68 ± 0.64 | 22.37 ± 1.83 | 40.71 ± 3.35* | |
BSA bovine serum albumin, PEG polyethyelene glycol, %LC percent loading capacity
*p < 0.01
Fig. 4.
IR spectra of chitosan microparticles a without and b with PEG 200 after storage for 0, 7, 14, and 28 days
CONCLUSIONS
The stability of chitosan microparticles was the drawback factor for applying them as a carrier for protein or peptide drug delivery system. The aggregation was often reported after longer periods of storage. In this study, the instability of the microparticles was observed after 1-week storage as indicated by the significant changes in the physicochemical properties of the microparticles such as size, pH, zeta potential, loading capacity, and chemical structure, and hence, the aggregation occurred. The stability of the microparticles was attributed to the electrostatic charge of the particles causing them to remain individual particles. The application of PEG 200 resulted in smaller changes in the physicochemical properties. The stabilization of PEG 200 could be due to the steric hindrance effect of PEG 200. This study, therefore, contributes to a deeper understanding of the stability enhancement of protein-loaded chitosan microparticles. Therefore, chitosan glutamate microparticles could be prepared and employed as a potential carrier of protein by the ionotropic gelation process. In addition, the understanding of the stability enhancement of the microparticles will be useful information for stabilization of nanoparticles. However, the drastic change exhibited after 28 days of storage for all preparations would require further studies to prolong their stability.
Acknowledgments
The author wishes to thank the Japan Society for the Promotion of Science (JSPS) for their financial support and the research and development institute, the University of Silpakorn, Thailand.
References
- 1.Sakuma S, Hayashi M, Akashi M. Design of nanoparticles composed of graft copolymers for oral peptide delivery. Adv Drug Delivery Rev. 2001;47:21–37. doi: 10.1016/S0169-409X(00)00119-8. [DOI] [PubMed] [Google Scholar]
- 2.McClean S, Prosser E, Meehan E, O’Malley D, Clarke N, Ramtoola Z, Brayden D. Binding and uptake of biodegradable poly-DL-lactide micro- and nanoparticles in intestinal epithelia. Eur J Pharm Sci. 1998;6:153–163. doi: 10.1016/S0928-0987(97)10007-0. [DOI] [PubMed] [Google Scholar]
- 3.Ahsan F, Rivas I, Khan M, Sharez AT. Targeting to macrophages: role of physicochemical properties of particulate carriers-liposomes and micropheres on the phagocytosis by macrophages. J Controlled Release. 2002;79:29–40. doi: 10.1016/S0168-3659(01)00549-1. [DOI] [PubMed] [Google Scholar]
- 4.Jung T, Kamm W, Breitenbaach A, Kaiserling E, Xio JX, Kissel T. Biodegradable nanoparticles for oral delivery of peptides: is there a role for polymers to affect mucosal uptake? Eur J Pharm Biopharm. 2000;50:147–160. doi: 10.1016/S0939-6411(00)00084-9. [DOI] [PubMed] [Google Scholar]
- 5.Pan Y, Li Y, Zhao H, Zheng J, Xu H, Wei G, Hao J, Cui F. Bioadhesive polysaccharide in protein delivery system: chitosan nanoparticles improve the intestinal absorption of insulin in vivo. Int J Pharm. 2002;249:139–147. doi: 10.1016/S0378-5173(02)00486-6. [DOI] [PubMed] [Google Scholar]
- 6.Xu Y, Du Y. Effect of molecular structure of chitosan on protein delivery properties of chitosan nanoparticles. Int J Pharm. 2003;250:215–226. doi: 10.1016/S0378-5173(02)00548-3. [DOI] [PubMed] [Google Scholar]
- 7.Ko JA, Park HJ, Hwang SJ, Park JB, Lee JS. Preparation and characterization of chitosan microparticles intended for controlled drug delivery. Int J Pharm. 2002;249:165–174. doi: 10.1016/S0378-5173(02)00487-8. [DOI] [PubMed] [Google Scholar]
- 8.Ma Z, Yeoh HH, Lim LY. Formulation pH modulates the interaction of insulin with chitosan nanoparticles. J Pharm Sci. 2002;91:1396–1404. doi: 10.1002/jps.10149. [DOI] [PubMed] [Google Scholar]
- 9.Shu XZ, Zhu KJ. Controlled drug release properties of ionically cross-linked chitosan beads: the influence of anion structure. Int J Pharm. 2002;233:217–225. doi: 10.1016/S0378-5173(01)00943-7. [DOI] [PubMed] [Google Scholar]
- 10.Luangtana-anan M, Opanasopit P, Ngawhirunpat T, Nunthanid T, Sriamornsak P, Limmatavapirat S, Lim LY. Effect of chitosan salts and molecular weight on a nanoparticulate carrier for therapeutic protein. Pharm Dev Technol. 2005;10:163–170. doi: 10.1081/pdt-54388. [DOI] [PubMed] [Google Scholar]
- 11.Karlsen J. Excipient properties of chitosan. Manuf Chemist. 1991;62:18–19. [Google Scholar]
- 12.Illum L. Chitosan and its use as a pharmaceutical excipient. Pharm Res. 1998;15:1326–1331. doi: 10.1023/A:1011929016601. [DOI] [PubMed] [Google Scholar]
- 13.Lim ST, Martin GP, Berry DJ, Brown MB. Preparation and evaluation of the in vitro drug release properties and mucoadhesion of novel microspheres of hyaluronic acid and chitosan. J Controlled Release. 2000;66:281–292. doi: 10.1016/S0168-3659(99)00285-0. [DOI] [PubMed] [Google Scholar]
- 14.Sarmento B, Ribeiro A, Veiga F, Ferreira D. Development and characterization of new insulin containing polysaccharide nanoparticles. Colloids Surf B Biointerfaces. 2006;53:193–202. doi: 10.1016/j.colsurfb.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 15.Gan Q, Wang T, Cochrane C, McCarron P. Modulation of surface charge, particle size and morphological properties of chitosan-TPP nanoparticles intended for gene delivery. Colloids Surf B Biointerfaces. 2005;44(2–3):65–73. doi: 10.1016/j.colsurfb.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 16.Cuña M, Alonso-Sandel M, Remuñán-López C, Pivel JP, Alonso-Lebrero JL, Alonso MJ. Development of phosphorylated glucomannan-coated chitosan nanoparticles as nanocarriers for protein delivery. J Nanosci Nanotechnol. 2006;6(9–10):2887–2895. doi: 10.1166/jnn.2006.435. [DOI] [PubMed] [Google Scholar]
- 17.Rieux A, Fievez V, Garinot M, Schneider Y, Préat V. Nanoparticles as potential oral delivery systems of proteins and vaccines: a mechanistic approach. J Controlled Release. 2006;116(1):1–27. doi: 10.1016/j.jconrel.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 18.Luo X, Pan S, Feng M, Wen Y, Zhang W. Stability of poly(ethylene glycol)-graft-polyethylenimine copolymer/DNA complexes: influences of PEG molecular weight and PEGylation degree. J Mater Sci Mater Med. 2010;21(2):597–607. doi: 10.1007/s10856-009-3903-1. [DOI] [PubMed] [Google Scholar]
- 19.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 20.Wu Y, Yang W, Wang C, Hu J, Fu S. Chitosan nanoparticles as a novel delivery system for ammonium glycyrrhizinate. Int J Pharm. 2005;295:235–245. doi: 10.1016/j.ijpharm.2005.01.042. [DOI] [PubMed] [Google Scholar]
- 21.Chen F, Zhang ZR, Huang Y. Evaluation and modification of N-trimethyl chitosan chloride nanoparticles as protein carriers. Int J Pharm. 2007;336(1):166–173. doi: 10.1016/j.ijpharm.2006.11.027. [DOI] [PubMed] [Google Scholar]
- 22.Sonvico F, Cagnani A, Rossi A, Motta S, Di Bari MT, Cavatorta F, Alonso MJ, Deriu A, Colombo P. Formation of self-organized nanoparticles by lecithin/chitosan ionic interaction. Int J Pharm. 2006;324(1):67–73. doi: 10.1016/j.ijpharm.2006.06.036. [DOI] [PubMed] [Google Scholar]
- 23.López-León T, Carvalho EL, Seijo B, Ortega-Vinuesa JL, Bastos-González D. Physicochemical characterization of chitosan nanoparticles: electrokinetic and stability behavior. J Colloid Interface Sci. 2005;283(2):344–351. doi: 10.1016/j.jcis.2004.08.186. [DOI] [PubMed] [Google Scholar]
- 24.Cai C, Bakowsky U, Rytting E, Schaper A, Kissel T. Charged nanoparticles as protein delivery systems: a feasibility study using lysozyme as model protein Eur. J Pharm Biopharm. 2008;69(1):31–42. doi: 10.1016/j.ejpb.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 25.Tsai ML, Chen RH, Bai SW, Chen WY. The storage stability of chitosan/tripolyphosphate nanoparticles in a phosphate buffer Carbohydrate Polymers. in press, Available online 29 April 2010
- 26.Sæther H, Holme H, Maurstad G, Smidsrød O, Stokke B. Polyelectrolyte complex formation using alginate and chitosan. Carbohydrate Polymers. 2008;74(4):813–821. doi: 10.1016/j.carbpol.2008.04.048. [DOI] [Google Scholar]
- 27.Gan Q, Wang T. Chitosan nanoparticle as protein delivery carrier—systematic examination of fabrication conditions for efficient loading and release. Colloids Surf B Biointerfaces. 2007;59:24–34. doi: 10.1016/j.colsurfb.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 28.Alsarraa IA, Neau SH, Howard MA. Effects of preparative parameters on the properties of chitosan hydrogel beads containing Candida rugosa lipase. Biomaterials. 2004;25(13):2645–2655. doi: 10.1016/j.biomaterials.2003.09.051. [DOI] [PubMed] [Google Scholar]