Abstract
Trimetazidine dihydrochloride is an effective anti-anginal agent; however, it is freely soluble in water and suffers from a relatively short half-life. To solve this encumbrance, it is a prospective candidate for fabricating trimetazidine extended-release formulations. Trimetazidine extended-release floating tablets were prepared using different hydrophilic matrix forming polymers including HPMC 4000 cps, carbopol 971P, polycarbophil, and guar gum. The tablets were fabricated by dry coating technique. In vitro evaluation of the prepared tablets was performed by the determination of the hardness, friability, content uniformity, and weight variation. The floating lag time and floating duration were also evaluated. Release profile of the prepared tablets was performed and analyzed. Furthermore, a stability study of the floating tablets was carried out at three different temperatures over 12 weeks. Finally, in vivo bioavailability study was done on human volunteers. All tablet formulas achieved <0.5 min of floating lag time, more than 12 h of floating duration, and extended t1/2. The drug release in all formulas followed zero-order kinetics. T4 and T8 tablets contained the least polymer concentration and complied with the dissolution requirements for controlled-release dosage forms. These two formulas were selected for further stability studies. T8 exhibited longer expiration date and was chosen for in vivo studies. T8 floating tablets showed an improvement in the drug bioavailability compared to immediate-release tablets (Vastrel® 20 mg).
KEY WORDS: effervescent, extended release, floating tablets, oral drug delivery, trimetazidine dihydrochloride
INTRODUCTION
Various physical and chemical approaches have been applied to produce a well-characterized dosage form that controls drug input into the body within the specifications of the desired release profile. This is generally accomplished by attempting to obtain “zero-order” release from the dosage form, i.e., the rate of drug release is independent of the drug concentration. Extended-release systems generally do not attain this type of release and usually try to mimic zero-order release (1). Gastroretentive systems can remain in the gastric region for several hours and hence significantly prolong the gastric residence time of drugs and produce retardation in drug release. Gastric retention helps provide better availability of new products with new therapeutic possibilities and substantial benefits for patients (2,3). The controlled gastric retention of solid dosage forms may be achieved by the mechanisms of mucoadhesion (4,5), flotation (6), sedimentation (7,8), expansion (9,10), modified shape systems (11,12), or by the simultaneous administration of pharmacological agents that delay gastric emptying (13–16).
Trimetazidine dihydrochloride is a clinically effective anti-anginal agent that has been used in the prophylaxis and management of angina pectoris and in ischemia of neurosensorial tissues also in Meniere’s disease (17). It is freely soluble in water, is rapidly absorbed, and its half-life is relatively short (t1/2 = 6.0 ± 1.4 h). Therefore, it is a potential candidate for extended-release formulations; however, controlling its release is a challenging task due to its high water solubility (18). The USP and the FDA identify specific release requirements for extended-release dosage forms. The current requirements for one product may be the same or may differ between regulatory bodies. Releasing of 20–50% of the drug after 3 h assures that there is no dose dumping from the dosage form. The intermediate specification assures that drug release (45–75%) over the 3- to 6-h period occurs neither too slowly nor too rapidly, while the purpose of the last specification is to assure the complete dissolution of drug. From a practical standpoint, sampling through 8 h for a twice daily product and through 12 h for once daily product may be adequate, provided that not <75–80% of the drug is to be released (19).
The purpose of this work was to prepare dry coated floating tablets of trimetazidine using certain hydrophilic polymers in order to control the release of this highly water-soluble drug. The polymers used in the coating layers were a mixture of HPMC K4M/carbopol 971P or polycarbophil in a ratio of 7:1. Evaluation of the prepared tablets was performed by determination of the hardness, friability, content uniformity, weight variation, floating lag time, and floating duration. The in vitro release studies for the determination of trimetazidine released from the tablet formulations were performed and analyzed. The effect of the storage at high temperatures, namely, 40°C, 50°C, and 60°C for a period of 12 weeks on the chemical stability of the selected tablets and prediction of the shelf life was also assessed. In addition, the effect of storage at high temperature and humidity on the release of the drug from the selected formulas was evaluated. Finally, the bioaviability and pharmacokinetic parameters of trimetazidine from the selected floating tablet formula T8 were conducted in human volunteers and compared to commercially available tablets (Vastarel® 20 mg; Servier Pharmaceutical Company, Egypt).
MATERIALS AND METHODS
Materials
Trimetazidine dihydrochloride (TMZ.2HCL; Batch no. SBML/TMZ/708030, Exp. date 7/2012) was kindly supplied by Global Napi Pharmaceutical Company, Egypt. Hydroxypropylmethy cellulose (HPMC 2910, 15 cps; HPMC K4M, 4000 cps) were gift samples from SEPPIC, France. Carbopol 971P and Polycarbophil were gift samples from Lubrizol, Belgium. Guar gum was purchased from Sigma-Aldrich, USA. Sodium bicarbonate and triethylamine were purchased from Loba Chemie, Mumbai, India. Acetonitrile, methanol, and orthophosphoric acid (HPLC grade) were purchased from E-Merck, Germany. Trichloroacetic acid was purchased from Fluka, USA. Dibasic calcium phosphate (Emcompress®), sodium chloride, spray-dried lactose, potassium dihydrogen phosphate, sodium dihydrogen phosphate, and magnesium stearate were purchased from ADWEC, Egypt. Double-distilled water was used throughout the study.
Formulation of Dry Coated Floating Tablets
Preparation of Core Tablets
Different formulations were prepared according to Table I. The calculated amounts of the drug (25 mg) and the fillers, namely, spray-dried lactose, dibasic calcium phosphate, HPMC 15 cps, or guar gum, were mixed together and then 0.5% magnesium stearate was added and mixed well. Tablets of 50 mg were prepared by means of single-punch machine with flat 5-mm punch and die set (single-punch tablet press; model TDP, SHANGHAI TIANHE, Pharmaceutical Machinery Factory, China). The target tablet hardness was adjusted to be in the range of 40–60 N (tablet hardness tester; DR-SCHLENGER Pharmaton, USA).
Table I.
Composition of Different Trimetazidine Tablet Formulas Prepared with Different Polymers
| Formula no. | Core tablet | Coat layer | |||||
|---|---|---|---|---|---|---|---|
| Drug (mg) | Filler up to 50 mg | Drug (mg) | Matrix former | Sodium bicarbonate (mg) | Spray-dried lactose (mg) | ||
| Type | Percenta | ||||||
| T1 | 25 | Spray-dried lactose | 15 | HPMC K4M/Carbopol 971P (7:1 ) | 30 | 20 | Up to 150 |
| T2 | 25 | 15 | 40 | 20 | |||
| T3 | 25 | 15 | 50 | 20 | |||
| T4 | 25 | Spray-dried lactose | 15 | HPMC K4M/Polycarbophil (7:1) | 30 | 20 | Up to 150 |
| T5 | 25 | 15 | 40 | 20 | |||
| T6 | 25 | 15 | 50 | 20 | |||
| T7 | 25 | Emcompress | 15 | HPMC K4M/Carbopol 971P (7:1) | 30 | 20 | Up to 150 |
| T8 | 25 | 15 | 40 | 20 | |||
| T9 | 25 | 15 | 50 | 20 | |||
| T10 | 25 | HPMC 15 cps | 15 | HPMC K4M/Carbopol 971P (7:1) | 30 | 20 | Up to 150 |
| T11 | 25 | 15 | 40 | 20 | |||
| T12 | 25 | 15 | 50 | 20 | |||
| T13 | 25 | Guar gum | 15 | HPMC K4M/Carbopol 971P (7:1) | 30 | 20 | Up to 150 |
| T14 | 25 | 15 | 40 | 20 | |||
| T15 | 25 | 15 | 50 | 20 | |||
aPercent from total tablet weight (200 mg)
Preparation of Coat Layer
A mixture of hydrophilic polymers including HPMC K4M/carbopol 971P or HPMC K4M/polycarbophil in a ratio of 7:1 was used for the formulation of the coat layer. The calculated amounts of the drug (15 mg) and the hydrophilic polymers were mixed followed by the addition of sodium bicarbonate and the filler spray-dried lactose. At last, 0.5% magnesium stearate was added and mixed well.
Fabrication of Dry Coated Tablets
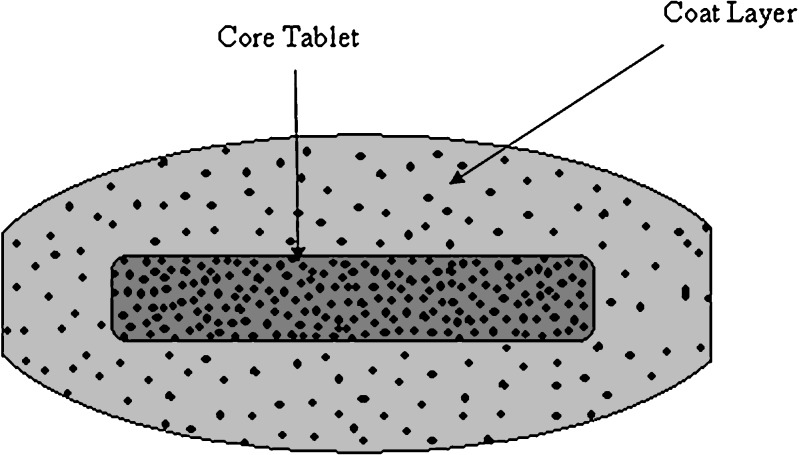
The tablets were fabricated by dry coating technique [20]. Using single-punch machine and concave 8-mm punch and die set, one third of the amount of the coating layer was placed in the die then the upper punch was descended to compress the powder into a thin disc. Then, the core tablet was placed in the middle of the die over the disc. The remaining two thirds of the amount of the coating layer was added above the core tablet in the die, then the upper punch was descended again to compress the powder into the tablet. A diagrammatic sketch of the dry coated tablets is shown in Fig. 1.
Fig. 1.
Diagrammatic sketch of dry coated tablet
Characterization of the Prepared Tablets
Weight Variation Test
Twenty tablets of each formula were individually weighed accurately and their average weight calculated.
Uniformity of Tablet Thickness and Diameter
The diameter and thickness of ten randomly selected tablets of each formula were measured using a Vernier caliper (Shanghai, China). Results were reported as the mean (±SD) of three measurements.
Content Uniformity
Twenty tablets of each formula were individually analyzed for initial drug content by dissolving each tablet in 100 mL distilled water. The mixture was then filtered through Whatman filter membrane (0.45 µm) prior to drug analysis spectrophotometrically at 231 nm (UV–VIS spectrophotometer; Jasco, V-530, Japan).
Friability Test
Ten tablets of each formula were accurately weighed (W1) and placed in the drum of the friabilator (Pharma Test, Germany). They were rotated at 25 rpm for a period of 4 min and then reweighed (W2). The percent loss in weights was calculated from the following equation and taken as a measure of friability (21).
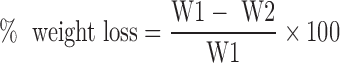 |
1 |
Hardness
From each formula, ten tablets were tested for their hardness using a hardness tester. The mean hardness (±SD) in newton of each formula was determined (21).
Determination of Floating Lag Time and Floating Duration of the Prepared Tablets
The time required for the tablets to emerge into the water surface (floating lag time) and the time taken for the tablets to remain floating on the water surface (floating duration) were inspected visually in a dissolution vessel filled with 500 mL of 0.1 N HCl, pH 1.2, at 37 ± 0.5°C rotated at a constant speed (50 rpm) (22). All the data were the average of three determinations.
In Vitro Release Studies
The release of trimetazidine dihydrochloride from the prepared formulations was performed according to the USP XXIV dissolution tester apparatus 2 (paddle method; Hanson Research, SR 8 plus model, USA). Studies were carried out at 37 ± 0.5°C in 500 mL of 0.1 N HCl, pH 1.2, as dissolution medium for a period of 8 h. Rotation speed was 50 rpm. At predetermined time intervals, aliquots (5 mL) were withdrawn and replaced with fresh medium to maintain constant dissolution volume. Samples were filtered through Whatman filter membrane (0.45 µm), diluted appropriately, and analyzed for the percent trimetazidine released [23]. All experiments were done in triplicate. The obtained data were subsequently analyzed to determine the order of release.
Accelerated Stability Testing
The accelerated stability testing was performed on the selected formulas (T4 and T8) which gave the most optimum results in all previous tests. The test was carried out by placing the tablets of each selected formula in dark closed glass container and stored in thermostatically controlled ovens adjusted at different temperatures, namely, 40°C, 50°C, and 60 ± 0.5°C with relative humidity 75% (maintained using a saturated solution of NaCl) for a period of 12 weeks. The stored tablets were examined visually for any changes in color and/or appearance every week. Three tablets from each formula were taken from the ovens after 1, 2, 4, 6, 8, and 12 weeks and analyzed for the determination of the amount of drug retained in each formula using an HPLC stability-indicating method (24,25). The unknown concentration of trimetazidine in each formula was calculated.
The stability data were kinetically analyzed to determine the order of drug degradation according to zero- and first-order kinetics. The rate constant of the reaction (k) was calculated according to a determined order at each of the three temperatures. The logarithmic K values at different temperatures were plotted against the reciprocal of the corresponding temperature according to Arrhenius plot for the determination of the expiration date (26–28).
HPLC Analysis of Trimetazidine
Quantification of trimetazidine samples were analyzed using a reverse-phase HPLC. The HPLC system consisted of a solvent delivery pump (Shimadzu L-7110, Hitachi Ltd., Japan), a controller (SCL-10A), and a UV/VIS detector (SPD-10A). The peak areas were integrated using Shimadzu C-R6A chromatopac, Hitachi Ltd. The drug was separated on a C18 column packed with Nucleosil 120 5 μ, 0.46 I.D × 25 mm, Teknokroma, Barcelona, Spain. Standards and samples were prepared in MillQ water. Mobile phase consisted of a mixture of acetonitrile/water/triethylamine (10:90:0.1%, v/v/v) and adjusted to pH 3 with orthophosphoric acid. The drug was eluted isocratically at a mobile phase flow rate of 1.2 mL/min and monitored with a UV detector operating at 205 nm. The run time for the assay was 10 min, and the retention time for the drug was 8.12 ± 0.2 min (25).
Method Validation
Standard samples were prepared to provide final concentrations of trimetazidine in the range of 2–10 µg/mL. The peak areas of the drug were plotted against the concentrations. The least square linear regression analysis was used to determine the slope, Y-intercept, and the correlation coefficient (R) of the standard plot.
Recovery
Recovery percent of trimetazidine from different samples was calculated and the relative standard deviation (%RSD) value calculated in each case.
Accuracy and Precision
The accuracy of the assay method was evaluated at three concentration levels, 2, 6, and 10 µg/mL, of trimetazidine three times and injecting into the system. Precision was determined by repeatability of injecting the same concentration solution three times into the chromatographic system, and %RSD of the peak area was calculated.
Intra-day and the Inter-day Variations
The intra-day and inter-day variations were established by analyzing samples in the range of 2–10 µg/mL drug solutions in triplicate on the same day and on three consecutive days, respectively, then, the %RSD of the slope and R were calculated in each case. A two-way analysis of variance (ANOVA) test was performed to determine the significance of difference between the results (p = 0.05).
Effect of Storage at High Temperature and Humidity (40°C+75% Relative Humidity) on the Drug Release
Aiming to study the effect of storage at high temperature and humidity on the release of trimetazidine from the selected formulas, a release study has been conducted on the samples taken from the stored formulas at 40°C and 75% relative humidity after 4, 8, and 12 weeks as previously mentioned.
Bioavailability Studies
Trimetazidine floating formula T8 was selected for the bioavailability study. The criteria for the selection of this formula were based on the results of all previous tests. Also, the relative bioavailability of the drug from this formula was computed to the commercially available Vastarel® tablets (20 mg).
Subjects
Twelve healthy male volunteers (60–70 kg, age between 25 and 30 years) participated in the study. All selected volunteers were nonsmokers and non-alcoholics. The biochemical examination of the volunteers revealed normal kidney and liver functions. The nature and purpose of the study were fully explained to them. An informed written consent was obtained from every volunteer. None of the volunteers was on drug treatment 1 week prior to the participation of the study. Volunteers were free to withdraw from the study under their own discretion. The study was approved by the Ethics Committee of the Faculty of Pharmacy, Cairo University.
Protocol
The study design was a single-dose, fasting, three-treatment parallel design comparing equal doses of the two formulas. An equal number of human volunteers were randomly assigned to the dosing sequence. One tablet formula T4 containing 40 mg trimetazidine and two commercially available Vastarel® tablets (20 mg drug in each tablet) were administered orally to the volunteers with 200 mL of water. Food and drinks were withheld for 2 h after dosing. Blood samples were collected from each volunteer’s forearm cubital vein via a syringe over a period of 48 h at time intervals of 0, 0.5, 1, 2, 4, 8, 12, 18, 24, 36, and 48 h. The blood samples were immediately centrifuged at 4,000 rpm and then the plasma was separated and stored at −10°C until analysis by HPLC assay.
HPLC Determination of Trimetazidine in the Plasma Samples
A sample (0.5 mL) of blood plasma was transferred to a 10-mL glass tube with a Teflon cap. Two hundred microliters of 10% (w/v) trichloroacetic acid was added and mixed for 5 min by means of a vortex mixer (Thermolyne Maxi Mix II, USA). The resultant mixture was centrifuged at 4,000 rpm for 15 min and then filtered through a membrane filter of 0.45-µm pore size and injected to the HPLC column. The concentration of drug in each sample was determined as previously mentioned.
Pharmacokinetic Analysis of the Data and Determination of Relative Bioavailability
The individual pharmacokinetic parameters of the drug were calculated by one-compartmental analysis. These parameters included the peak plasma concentration (Cmax) and the time to reach the maximum plasma concentration (Tmax). The areas under the plasma trimetazidine concentration–time curves AUC(0–48) and 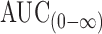 also were calculated by the linear trapezoidal rule and extrapolation to infinity, respectively. In addition, the overall elimination rate constant (Ke) and elimination half-life (t1/2) were determined. The percent relative bioavailability of the drug from floating tablet formula T8 in comparison to reference formulation (immediate release Vastarel® 20 mg) was calculated with respect to Cmax, AUC(0–48) and
also were calculated by the linear trapezoidal rule and extrapolation to infinity, respectively. In addition, the overall elimination rate constant (Ke) and elimination half-life (t1/2) were determined. The percent relative bioavailability of the drug from floating tablet formula T8 in comparison to reference formulation (immediate release Vastarel® 20 mg) was calculated with respect to Cmax, AUC(0–48) and 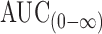 (29).
(29).
Statistical Studies
Two-way ANOVA was performed to determine the significance of difference between the pharmacokinetic parameters among groups. The level of significance was set at P value of <0.05 using SPSS version 12.0 software computer program.
Results and Discussion
Fabrication and In Vitro Characterization of Trimetazidine Dihydrochloride Floating Tablets
Dry coated floating tablets of trimetazidine dihydrochloride were prepared using certain hydrophilic polymers in order to control the release of this highly water-soluble drug. The polymers used in the coating layers were a mixture of HPMC K4M/carbopol 971P or polycarbophil. Several preliminary optimization studies were performed using different polymer ratios such as 1:1, 2:1, 7:1, and 6:2. These studies indicated that increasing the ratio of carbopol or polycarbophil in the mixture led to a dramatic lowering in the drug release to ∼20% over 8 h. Accordingly, the optimal ratio for HPMC K4M/carbopol 971P or polycarbophil was 7:1. Incorporation of 10% sodium bicarbonate (from the total tablet weight) in the coating layer produced an optimized floating layer. Sodium bicarbonate generates CO2 in the presence of a dissolution medium (0.1 N HCl, pH 1.2). The generated gas is trapped and protected within the gel formed by hydration of polymer, thus decreasing the density of the tablet. As the density of the tablet falls below 1 g/cm3, the tablet becomes buoyant (2).
All the prepared trimetazidine floating tablets showed an acceptable weight variation range from 198 to 203 mg with standard deviation <2%, and not more than two tablets were allowed to deviate from the average by more than twice the previous percentage (30). The prepared tablets showed a uniformity of thickness and diameter. The values of tablet thickness were in the range of 3.6–4 mm and the diameter in the range of 8–8.04 mm. The drug concentration was not <97% and did not exceed 103% of the labeled potency with standard deviation <2%. This result indicates that all the prepared formulations complied with the pharmacopoeial limits for content uniformity (31).
Friability and Hardness
Tablets showed a percentage fine ranging around the acceptable limit and did not exceed the permissible limit of 1% (32). For hydrophilic matrix tablets, friability did not vary significantly as a function of both polymer concentration and polymer type. Such findings are in agreement with the work of Dopeshwarker et al. (33). The hardness values ranged from 48 to 62 N with standard deviation <2% trimetazidine for all the prepared tablets.
Floating Lag Time and Floating Duration of the Tablets
Rapid system floatation is highly desirable for gastroretentive dosage forms and can potentially prolong gastric residence time and improve the bioavailability of drug substances with a narrow window of absorption (34). Incorporation of 10% sodium bicarbonate in the coating layer produced an optimized floating layer. Sodium bicarbonate generated carbon dioxide in the presence of 0.1 N HCl, pH 1.2, as a dissolution medium. The generated carbon dioxide was trapped and protected within the gel formed by hydration of polymer, thus decreasing the density of the tablet. As the density of the tablet falls below 1 g/cm3, the tablet became buoyant. All tablet formulas showed a floating lag time of <0.5 min (SD < 0.01), good matrix integrity, and floating duration of more than 12 h.
In Vitro Release Studies
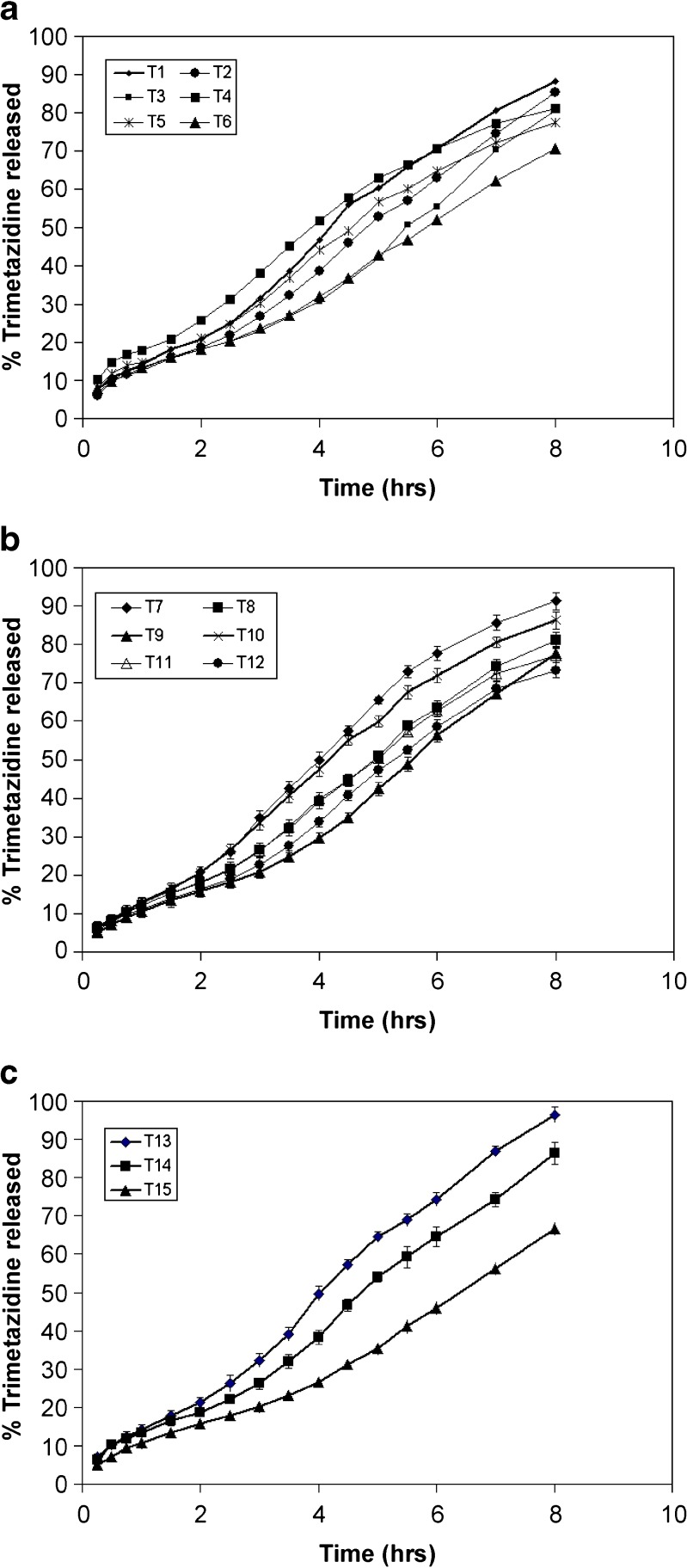
The percentage of trimetazidine released after 8 h (Q8 h) from the prepared floating tablet formulations are shown in Table II. The in vitro release of trimetazidine from the floating matrix tablets formulas T1–T3 showed that the extent of drug release was 88%, 85%, and 81%, respectively (Fig. 2). This may indicate that formula T3 complied with the dissolution specifications for controlled-release products: 75–80% over 8 h (19).
Table II.
Percentage of Trimetazidine Released After 8 h (Q 8 h) from the Prepared Floating Tablet Formulations
| Formulations | Q 8 h a (% ± SDb) |
|---|---|
| T1 | 88.5 ± 1.4 |
| T2 | 88.3 ± 0.89 |
| T3 | 80.1 ± 1.06 |
| T4 | 80.1 ± 1.86 |
| T5 | 77.5 ± 1.12 |
| T6 | 70.5 ± 2.21 |
| T7 | 91.3 ± 1.88 |
| T8 | 81.2 ± 1.76 |
| T9 | 77.7 ± 1.83 |
| T10 | 75.2 ± 2.17 |
| T11 | 77.1 ± 1.92 |
| T12 | 73.3 ± 1.26 |
| T13 | 96.3 ± 2.17 |
| T14 | 86.4 ± 2.85 |
| T15 | 66.7 ± 1.89 |
a Q 8h: % trimetazidine released after 8 h
bSD: Standard deviation
Fig. 2.
Release profile of trimetazidine from different floating tablet formulas
On the other hand, formulas T4–T6 were prepared by replacing carbopol 971P in the coating layer with polycarbophil at the same percents. The extent of the drug release from these formulations was 80%, 78%, and 71%, respectively (Fig. 2). Formula T4 complied with the release requirements for extended-release products: 45–75% within 6 h and 75–80% over 8 h. These results may signify that polycarbophil was successful in controlling the drug release and produced more retardation in the drug release when compared to carbopol. When replacing spray-dried lactose with Emcompress® in the core tablet for T7–T9 formulas, the drug achieved 91%, 81%, and 78% release, respectively, within 8 h (Fig. 2). The water solubility of the filler in the core may have a little effect on the release behavior of trimetazidine from the matrices. It is also obvious that formula T8 complied with the dissolution specifications for controlled-release products compared with the other two formulas.
For the tablets (T10–T12) prepared using HPMC 15 cps as a retarding polymer in the core instead of spray-dried lactose, the extent of the drug release was 75%, 77%, and 73% of the labeled amount after 8 h, respectively (Fig. 2). It is important to note that the use of HPMC 15 cps in the core tablet resulted in a retardation in the drug release. This could be explained on the basis of the formation of a viscous gel inside the tablet and the drug dissolves in the gel layer and slowly diffuses into the surrounding coating layer. Concerning the tablet formulations (T13–T15) prepared using guar gum in the core tablet, the release of the drug was found to be 96%, 86%, and 67% after 8 h, respectively (Fig. 2).
All the above results indicated that the drug release from all the prepared dry coated floating tablets showed an extended release of the drug over time. The release of trimetazidine from the tablets prepared using polycarbophil in the coat layer showed a marked decrease compared to those prepared with carbopol 971P. Hydrophilicity or hydrophobicity of the filler in the core tablet had a little effect on the release behavior of trimetazidine from the matrices. Replacing the soluble filler in the core tablet with gel-forming polymers as HPMC 15 cps or guar gum resulted in a decrease in the extent of drug release.
The release data were subjected to kinetic analysis using linear regression according to zero-order, first-order, and Higuchi diffusion model to determine the mechanism of the drug release. The drug release from all formulas was found to follow zero-order kinetics. The t1/2 values of the prepared floating tablets are in the range of 3.9–6.7 h. Formula T7 showed the lowest value of t1/2, while formula T15 exhibited the highest value. A two-way ANOVA was performed to determine the significance of difference in trimetazidine release profiles. Significant differences (p < 0.05) existed in the release profiles among all tablet formulations.
Accelerated Stability Testing
None of the stored formulas showed any changes in color or appearance throughout the storage period of 12 weeks under different temperatures with relative humidity 75%. The typical chromatogram of trimetazidine obtained following the analysis under the chromatographic conditions previously described is shown in Fig. 3. The drug showed a sharp and symmetrical peak with good baseline resolution and minimum tailing, thus facilitating the accurate measurement of the peak area.
Fig. 3.
HPLC chromatogram of trimetazidine in the mobile phase
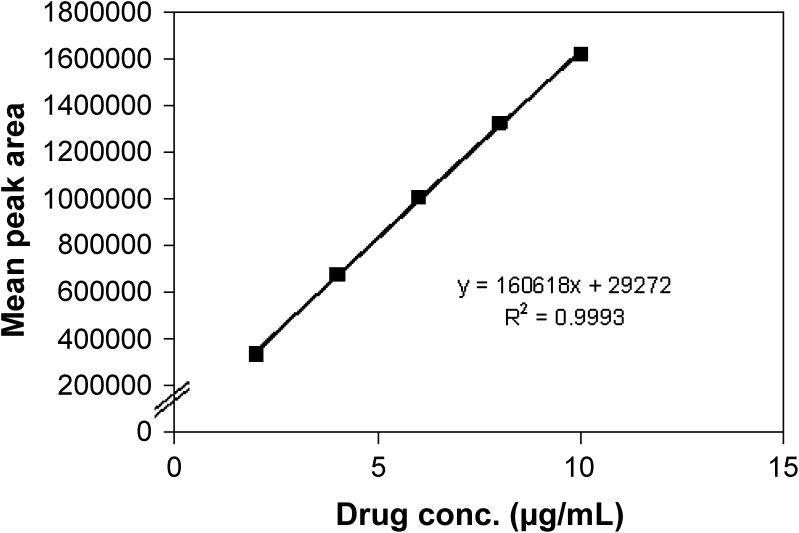
Figure 4 demonstrated that the calibration plot for the peak areas of varying trimetazidine concentrations was highly linear (R = 0.9998), and this result indicated that there was an excellent correlation between the peak area and the concentration. The mean percent recoveries were found to be very high for all trimetazidine concentrations (2–10 µg/mL) with relative standard deviation (%RSD) values of <0.438% (Table III). The %RSD of the assay of trimetazidine during the assay method was <0.75% for the peak area of the drug. In addition, the %RSD value for repeatability was <0.72%. These results may indicate that the developed method was precise (Table IV).
Fig. 4.
Calibration plot for the peak areas of different concentrations of trimetazidine
Table III.
Percent Recovery of Different Drug Concentrations
| Drug conc. (µg/mL) | Recovery (%) | %RSD | |||
|---|---|---|---|---|---|
| 1 | 2 | 3 | Mean | ||
| 2 | 95.9 | 95.9 | 95.8 | 95.9 | 0.11 |
| 4 | 100.8 | 101 | 100.8 | 100.9 | 0.13 |
| 6 | 101.4 | 101.6 | 100.8 | 101.3 | 0.44 |
| 8 | 100.5 | 101.1 | 101 | 100.9 | 0.35 |
| 10 | 99 | 98.9 | 99 | 99 | 0.03 |
RSD relative standard deviation
Table IV.
Accuracy and Precision of the HPLC Stability-Indicating Assay Method
| Conc. (µg/mL) | Mean peak area | SD | RSD (%) | |
|---|---|---|---|---|
| Accuracy | 2 | 336,750 | 799.3 | 0.24 |
| 6 | 1,005,154.3 | 7,528.1 | 0.75 | |
| 10 | 1,626,148.7 | 5,818.8 | 0.36 | |
| Precision | 2 | 339,981.3 | 2,448.1 | 0.72 |
| 6 | 1,001,411.7 | 6,225.1 | 0.62 | |
| 10 | 1,620,453.3 | 8,867.9 | 0.55 |
SD standard deviation, RSD relative standard deviation
The intra-day and inter-day variations were determined using concentrations in the range of 2–10 µg/mL. Each concentration was analyzed in triplicate and the %RSD of intra-day precision was found to be 0.11% and 0.02% for the slope and R, respectively. The inter-day precision showed %RSD of 0.41% and 0.05% for the slope and R, respectively (Tables V and VI). From the ANOVA results, there was no significant difference between the results of the intra-day variation since the p value is equal to 0.39 (i.e., >0.05). Furthermore, there was no significant difference between the results of the inter-day variation where the p value equals 0.74 (i.e., >0.05).
Table V.
Intra-day Precision Data of the HPLC Stability-Indicating Assay Method
| Conc. (µg/mL) | Intra-day precision | ||||
|---|---|---|---|---|---|
| Morning | Afternoon | Night | Mean | SD | |
| 2 | 337,601 | 337,634 | 337,015 | 337,416.67 | 348.24 |
| 4 | 676,802 | 678,297 | 676,834 | 677,311.00 | 854.05 |
| 6 | 1,006,523 | 1,008,577 | 1,000,363 | 1,005,154.33 | 4,274.62 |
| 8 | 1,320,312 | 1,328,858 | 1,327,470 | 1,325,546.67 | 4586.17 |
| 10 | 1,619,451 | 1,619,034 | 1,619,961 | 1,619,482.00 | 464.28 |
| Slope (±SD) | 160,613 ± 233.5 | ||||
| RSD (%) = 0.15 | |||||
| R (±SD) | 0.9996 ± 0.00021 | ||||
| RSD (%) = 0.021 | |||||
R correlation coefficient, SD standard deviation, RSD relative standard deviation
Table VI.
Intra-day Precision Data of the HPLC Stability-Indicating Assay Method
| Conc. (µg/mL) | Inter-day precision | ||||
|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | Mean | SD | |
| 2 | 337,601 | 332,492 | 339,851 | 336,648 | 3,770.92 |
| 4 | 676,802 | 673,062 | 675,027 | 674,963.67 | 1,870.80 |
| 6 | 1,006,523 | 1,006,634 | 1,003,078 | 1,005,411.6 | 2,021.78 |
| 8 | 1,320,312 | 1,321,855 | 1,326,038 | 1,322,735.0 | 2,962.69 |
| 10 | 1,619,451 | 1,618,152 | 1,610,757 | 1,616,120.0 | 4,689.69 |
| Slope (±SD) | 160,333 ± 680 | ||||
| RSD (%) = 0.4 | |||||
| R (±SD) | 0.9988 ± 0.0005 | ||||
| RSD (%) = 0.05 | |||||
R correlation coefficient, SD standard deviation, RSD relative standard deviation
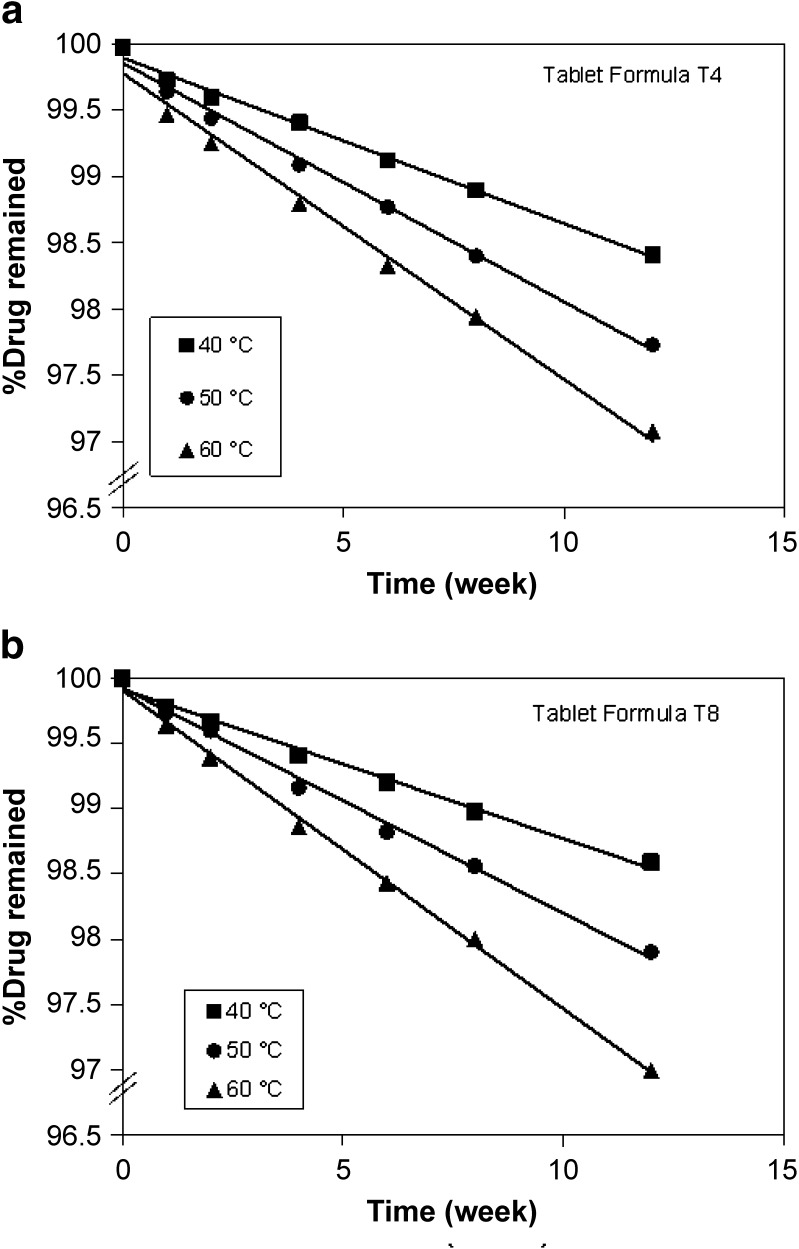
The chemical stability results of trimetazidine formulations demonstrated that the percent drug remaining after storage for a period of 12 weeks was found to be 98%, 98%, and 97% for formula T4 and 99%, 98%, and 97% for formula T8 at the three elevated temperatures, respectively (Fig. 5). It is worth noting that floating tablet formulations showed a very low rate of degradation after storage at the three temperatures for 12 weeks. Regression analysis of stability data indicated that the decomposition of the drug followed first-order kinetics. The degradation rate constant (K25) for each formula was calculated and the expiration dates were determined according to the Garret and Karper equation, which states that: t90% = 0.105/K25, where t90% is the time at which the percent remaining is 90% (26). The expiration dates were 3.3 and 3.6 years for T4 and T8, respectively. Furthermore, the release of the drug from the stored formulas did not exhibit any change after storage at 40°C and 75% relative humidity for 12 weeks.
Fig. 5.
Stability of floating tablet formulas T4 and T5 at different storage temperatures (40°C, 50°C, and 60°C)
Bioavailability Study
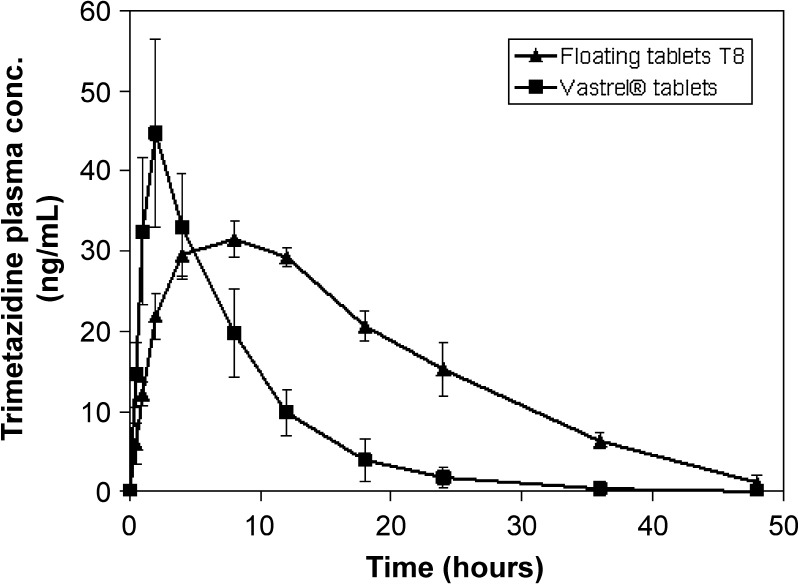
In vivo study demonstrated that the floating tablet formula T8 achieved lower Cmax and Tmax values compared to that of the commercially available tablet (Table VII and Fig. 6). Furthermore, the prepared formula exhibited a prolonged mean elimination half-life. t1/2 was found to be 9.565 h; however, the immediate-release Vastarel® tablets attained a t1/2 of 4.626 h.
Table VII.
Mean Pharmacokinetic Parameters for the Volunteers Following Oral Administration of Floating Tablet Formula T8 and Immediate-Release Vastarel® (20 mg) Tablets
| Subject no. | Pharmacokinetic parameters | |||||
|---|---|---|---|---|---|---|
| T max (h) | C max (ng/mL) | AUC(0–48) (ng h−1 m−1L) |
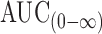 (ng h−1 m−1L) (ng h−1 m−1L) |
K e (h−1) | t 1/2 (h) | |
| T8 (SD) | 6.7 ± 2 | 32.5 ± 1 | 748.8 ± 31 | 1856 ± 31 | 0.07 ± 0.01 | 9.6 ± 1.4 |
| Vastarel® (SD) | 2.7 ± 1 | 47 ± 9 | 369.8 ± 76 | 789.6 ± 165 | 0.16 ± 0.04 | 4.6 ± 1.3 |
SD standard deviation
Fig. 6.
Trimetazidine plasma concentrations following the oral administration of floating tablet formula T8 and immediate-release Vastrel® tablets (20 mg)
Krishnaiah et al. (35) studied the bioavailability of trimetazidine from guar gum-based three-layer matrix tablets. They reported that the delayed Tmax, decreased Cmax, unaltered bioavailability, and prolonged t1/2 indicated a slow and prolonged release of the drug from guar gum three-layer matrix tablets in comparison with the immediate-release tablet dosage form. The obtained pharmacokinetic results of T8 floating tablets may have provided prolonged blood levels of the drug in human volunteers due to the slow release of the drug in the stomach and small intestine and slow absorption of the drug in the colon, resulting in a prolonged elimination half-life of the drug from guar gum tablets after oral administration As a result, the formulated trimetazidine floating tablets may be useful in providing constant drug delivery with minimum fluctuations.
Statistical analysis of pharmacokinetic parameters showed that there was a significant difference between the values of t1/2, AUC0–48, and 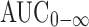 of the tablet formulation T8 when compared to immediate-release Vastarel® tablets. The mean percent relative bioavailability of the prepared floating tablet formula T8 was 69.12% with respect to Cmax, 202.52% with respect to AUC(0–48), and 235.06% with respect to
of the tablet formulation T8 when compared to immediate-release Vastarel® tablets. The mean percent relative bioavailability of the prepared floating tablet formula T8 was 69.12% with respect to Cmax, 202.52% with respect to AUC(0–48), and 235.06% with respect to 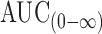 . As a result, T8 floating tablets created a 0.68-fold increase in the bioavailability compared to immediate-release tablets (Vastarel® 20 mg). It was clear that T8 generated 0.68-, 2.35-, and 2.02-fold increase in the bioavailability regarding the Cmax,
. As a result, T8 floating tablets created a 0.68-fold increase in the bioavailability compared to immediate-release tablets (Vastarel® 20 mg). It was clear that T8 generated 0.68-, 2.35-, and 2.02-fold increase in the bioavailability regarding the Cmax, 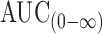 , and AUC(0–48), respectively, compared to immediate-release tablets.
, and AUC(0–48), respectively, compared to immediate-release tablets.
CONCLUSION
Trimetazidine dihydrochloride dry coated floating tablets were successfully prepared by dry coating technique using different polymers. These formulations achieved short floating lag time and long floating duration. The drug release from all the prepared dry coated floating tablets effectively exhibited an extended release of the drug over a prolonged period of time. The drug release in all formulas followed zero-order kinetics. There was no change in the color or physical appearance of selected floating tablets until the end of the storage period. The expiration date of the prepared formulas T4 and T8 were calculated to be 3.3 and 3.7 years, respectively. During the storage period, the release of the drug from the stored formulas did not show any change. T8 floating tablets demonstrated a great enhancement in the drug bioavailability compared to immediate-release tablets (Vastarel® 20 mg). Extended-release floating tablets may be considered as a promising technique for oral delivery of trimetazidine.
Acknowledgments
We would like to thank Global Napi Pharmaceutical Company, Egypt for the provision of trimetazidine dihydrochloride. We also thank SEPPIC, France and Lubrizol, Belgium for supplying gift samples of HPMC, carbopol 971P, and Polycarbophil.
References
- 1.Hirtz J. The gastrointestinal absorption of drugs in man: a review of current concepts and methods of investigation. Br J Clin Pharmacol. 1985;19:77S–83S. doi: 10.1111/j.1365-2125.1985.tb02746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arora S, Ali J, Ahuja A, Khar RK, Baboota S. Floating drug delivery systems: a review. AAPS PharmSciTech. 2005;6(3):E372–E390. doi: 10.1208/pt060347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kerc J, Opara J. A new amoxicillin/clavulanate therapeutic system: preparation, in vitro and pharmacokinetic evaluation. Int J Pharm. 2007;335(1–2):106–113. doi: 10.1016/j.ijpharm.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 4.Lenaerts VM, Gurny R. Gastrointestinal tract—physiological variables affecting the performance of oral sustained release dosage forms. In: Lenaerts V, Gurny R, editors. Bioadhesive drug delivery systems. Boca Raton: CRC; 1990. [Google Scholar]
- 5.Ponchel G, Irache J. Specific and non-specific bioadhesive particulate systems for oral delivery to the gastrointestinal tract. Adv Drug Deliv Rev. 1998;34(2–3):191–219. doi: 10.1016/S0169-409X(98)00040-4. [DOI] [PubMed] [Google Scholar]
- 6.Deshpande AA, Shah NH, Rhodes CT, Malick W. Development of a novel controlled-release system for gastric retention. Pharm Res. 1997;14(6):815–819. doi: 10.1023/A:1012171010492. [DOI] [PubMed] [Google Scholar]
- 7.Davis SS, Stockwell AF, Taylor MJ, Hardy JG, Whalley DR, Wilson CG, et al. The effect of density on the gastric emptying of single- and multiple-unit dosage forms. Pharm Res. 1986;3(4):208–213. doi: 10.1023/A:1016334629169. [DOI] [PubMed] [Google Scholar]
- 8.Rednick AB, Tucker SJ. Sustained release bolus for animal husbandry. US Patent 3,507,952; 1970.
- 9.Mamajek RC, Moyer ES. Drug dispensing device and method. US Patent 4,207,890; 1980.
- 10.Urguhart J, Theeuwes F (1994) Drug delivery system comprising a reservoir containing a plurality of tiny pills. US Patent 4,434,153
- 11.Fix JA, Cargill R, Engle K. Controlled gastric emptying. III. Gastric residence time of a nondisintegrating geometric shape in human volunteers. Pharm Res. 1993;10(7):1087–1089. doi: 10.1023/A:1018939512213. [DOI] [PubMed] [Google Scholar]
- 12.Kedzierewicz F, Thouvenot P, Lemut J, Etienne A, Hoffman M, Maincent P. Evaluation of peroral silicone dosage forms in humans by gamma-scintigraphy. J Control Release. 1999;58(2):195–205. doi: 10.1016/S0168-3659(98)00154-0. [DOI] [PubMed] [Google Scholar]
- 13.Groning R, Cloer C, Georgarakis M, Muller RS. Compressed collagen sponges as gastroretentive dosage forms: in vitro and in vivo studies. Eur J Pharm Sci. 2007;30(1):1–6. doi: 10.1016/j.ejps.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Groning R, Cloer C, Muller RS. Development and in vitro evaluation of expandable gastroretentive dosage forms based on compressed collagen sponges. Pharmazie. 2006;61(7):608–612. [PubMed] [Google Scholar]
- 15.Groning R, Heun G. Oral dosage forms with controlled gastrointestinal transit. Drug Dev Ind Pharm. 1984;10:527–539. doi: 10.3109/03639048409041405. [DOI] [Google Scholar]
- 16.Groning R, Heun G. Dosage forms with controlled gastrointestinal passage studies on the absorption of nitrofurantoin. Int J Pharm. 1989;56:111–116. doi: 10.1016/0378-5173(89)90003-3. [DOI] [Google Scholar]
- 17.McClellan KJ, Plosker GL. Trimetazidine. A review of its use in stable angina pectoris and other coronary conditions. Drugs. 1999;58(1):143–157. doi: 10.2165/00003495-199958010-00016. [DOI] [PubMed] [Google Scholar]
- 18.Moffat AC, Jackson JV, Widdop B. Thin layer chromatography. In: Moffat AC, editor. Clarke’s isolation and identification of drugs. 2. London: The Pharmaceutical Press; 1986. p. 1676. [Google Scholar]
- 19.Sievert B, Siewert M. Dissolution test for extended release products. In: Dressman JB, Lennernäs H, editors. Oral drug absorption: prediction and assessment. New York: Marcel Dekker; 2000. pp. 183–195. [Google Scholar]
- 20.Rahman Z, Ali M, Khar R. Design and evaluation of bilayer floating tablets of captopril. Acta Pharm. 2006;56(1):49–57. [PubMed] [Google Scholar]
- 21.Banker GS, Anderson NR. Tablets. In: Lachman L, Liberman HA, Kanig JL, editors. The theory and practice of industrial pharmacy. 3. Philadelphia: Lea and Febiger; 1986. pp. 293–345. [Google Scholar]
- 22.Narendra C, Srinath MS, Babu G. Optimization of bilayer floating tablet containing metoprolol tartrate as a model drug for gastric retention. AAPS PharmSciTech. 2006;7(2):E34. doi: 10.1208/pt070234. [DOI] [PubMed] [Google Scholar]
- 23.Rouge N, Cole ET, Doelker E, Buri P. Buoyancy and drug release patterns of floating minitablets containing piretanide and atenolol as model drugs. Pharm Dev Technol. 1998;3(1):73–84. doi: 10.3109/10837459809028481. [DOI] [PubMed] [Google Scholar]
- 24.Thoppil SO, Amin PD. Trimetazidine: stability indicating RPLC assay method. J Pharm Biomed Anal. 2001;25(2):191–195. doi: 10.1016/S0731-7085(00)00483-0. [DOI] [PubMed] [Google Scholar]
- 25.Thoppil SO, Cardoza RM, Amin PD. Stability indicating HPTLC determination of trimetazidine as bulk drug and in pharmaceutical formulations. J Pharm Biomed Anal. 2001;25(1):15–20. doi: 10.1016/S0731-7085(00)00440-4. [DOI] [PubMed] [Google Scholar]
- 26.Anderson G, Scott M. Determination of product shelf life and activation energy for five drugs of abuse. Clin Chem. 1991;37(3):398–402. [PubMed] [Google Scholar]
- 27.Oliva A, Fariña JB, Llabrés M. Influence of temperature and shaking on stability of insulin preparations: degradation kinetics. Int J Pharm. 1996;143(2):163–170. doi: 10.1016/S0378-5173(96)04700-X. [DOI] [Google Scholar]
- 28.Slater JG, Stone HA, Palermo BT, Duvall RN. Reliability of Arrhenius equation in predicting vitamin A stability in multivitamin tablets. J Pharm Sci. 1979;68(1):49–52. doi: 10.1002/jps.2600680117. [DOI] [PubMed] [Google Scholar]
- 29.Rouge N, Allemann E, Gex-Fabry M, Balant L, Cole ET, Buri P, et al. Comparative pharmacokinetic study of a floating multiple-unit capsule, a high-density multiple-unit capsule and an immediate-release tablet containing 25 mg atenolol. Pharm Acta Helv. 1998;73(2):81–87. doi: 10.1016/S0031-6865(97)00050-2. [DOI] [PubMed] [Google Scholar]
- 30.The British pharmacopoeia. London: The Pharmaceutical Press; 1998. p. 242.
- 31.The Pharmacopoeia of the United States of America 27, The National Formulary 22, Asian Edition. Rockville, MD: Pharmacopoeial Convention, Inc.; 2004. p. 454–8.
- 32.Bakan JA. Capsule part III, Microencapsulation. In: Lachmann L, Lieberman HA, Kanig JL, editors. The theory and practice of industrial pharmacy. 3. Philadelphia: Lea and Febiger; 1986. pp. 412–429. [Google Scholar]
- 33.Dopeshwarkar U, Zata J. Evaluation of xanthan gum in the preparation of sustained release matrix tablets. Drug Dev Ind Pharm. 1993;19(9):999–1017. doi: 10.3109/03639049309062997. [DOI] [Google Scholar]
- 34.Steingoetter A, Weishaupt D, Kunz P, Mader K, Lengsfeld H, Thumshirn M, et al. Magnetic resonance imaging for the in vivo evaluation of gastric-retentive tablets. Pharm Res. 2003;20(12):2001–2007. doi: 10.1023/B:PHAM.0000008049.40370.5a. [DOI] [PubMed] [Google Scholar]
- 35.Krishnaiah YS, Karthikeyan RS, Bhaskar P, Satyanarayana V. Bioavailability studies on guar gum-based three-layer matrix tablets of trimetazidine dihydrochloride in human volunteers. J Control Release. 2002;83(2):231–239. doi: 10.1016/S0168-3659(02)00215-8. [DOI] [PubMed] [Google Scholar]