Abstract
The objective of the present study was to prepare mucoadhesive in situ nasal gels with mucilage isolated from fig fruits (Ficus carica, family: Moraceae) containing midazolam hydrochloride. Nasal gels of midazolam were prepared using three different concentrations (0.5%, 1.0% and 1.5% w/v) of F. carica mucilage (FCM) and synthetic polymers (hydroxypropylmethyl cellulose and Carbopol 934). Evaluation of FCM showed that it was as safe as the synthetic polymers for nasal administration. In situ gels were prepared with mixture Pluronic F127 and mucoadhesive agents. Evaluation of the prepared gels was carried out, including determination of viscosity, texture profile analysis and mucoadhesive strength. In vitro drug permeation study was conducted with the gels prepared with and without permeation enhancer (0.5% w/v sodium taurocholate) using excised goat nasal mucosa. In vitro permeation profiles were evaluated, and histological study of nasal mucosae before and after permeation study was also conducted to determine histological change, if any. In vivo experiments conducted in rabbits further confirmed that in situ nasal gels provided better bioavailability of midazolam than the gels prepared from synthetic mucoadhesive polymers. It was observed that the nasal gel containing 0.5% FCM and 0.5% sodium taurocholate exhibited appropriate rheological, mechanical and mucoadhesive properties and showed better drug release profiles. Moreover, this formulation produced no damage to the nasal mucosa that was used for the permeation study, and absolute bioavailability was also higher compared to gels prepared from synthetic polymers.
KEY WORDS: midazolam hydrochloride, mucoadhesive, nasal, permeation enhancer
INTRODUCTION
Midazolam hydrochloride is a potent water soluble benzodiazepine with rapid onset and short duration of action. It has an excellent safety profile with minimal cardiovascular and respiratory side effects (1). It is an important drug that is used in the treatment of status epilepticus (2). Midazolam is usually given in the form of intravenous (i.v.) or intramuscular injections, but parenteral route has minimal patient compliance. Oral administration of midazolam is preferable, but it undergoes extensive first pass metabolism in the liver (3), owing to which its oral bioavailability ranges from 15% to 27% in children and from 31% to 72% in adults (4). In that case, administration of midazolam via transmucosal route is advantageous. Sublingual and buccal administration of midazolam has been studied (5–7), but nasal route is most important among them in terms of drug absorption and availability of the drug for systemic effect (8–10).
Administration of midazolam via the nasal route has proved to be quite effective in the treatment of epileptic seizures (11–19). For that purpose, various nasal midazolam formulations were developed like solutions and sprays (20,21). However, these systems suffer from a number of disadvantages. Solutions are cleared off rapidly from nasal cavity and do not allow prolonged residence of the drug in the cavity. Parenteral solution of midazolam for intranasal administration caused irritation that is produced by its acidic pH and the relatively large volume that has to be administered. These problems could be reduced by using aerosols containing a solution of midazolam in cyclodextrin, which accomplishes a greater concentration with a pH that is less acidic (21). It has been reported that nasal spray prepared by Knoester et al. (20) caused nasal irritation, lacrimation and irritation in throat of almost all the subjects. So the idea of administration of midazolam via nasal gels was conceived, but conventional gels pose difficulty in administering accurate dose of the drug owing to their high viscosity. This problem could, however, be overcome by formulating in situ mucoadhesive gels which are liquid at room temperature but change to a firm gel at the temperature of the nasal cavity. Pluronic F 127 is widely used as a thermosensitive gelling agent since it is non-toxic, non-irritating, poses good release characteristics and biocompatible with other chemicals (22–25).
In the present investigation, mucilage isolated from fig fruits (Ficus carica) was used as a mucoadhesive agent along with Pluronic F127 to prepare mucoadhesive in situ nasal gels containing midazolam hydrochloride and to characterise them accordingly, in terms of texture profile analysis, viscosity, mucoadhesive strength, in vitro release profile, in vivo absorption kinetics and histological studies. Mucilage isolated from F. carica used in formulations is compared with synthetic polymers (hydroxypropylmethyl cellulose (HPMC) and Carbopol 934). This mucilage is biocompatible and biodegradable since it is edible in nature.
EXPERIMENTAL
Materials
Midazolam hydrochloride was obtained as a gift from Sun Pharmaceutical Industries Ltd., Gujarat, India. F. carica fruits were purchased from local market. Pluronic F127 was purchased from Sigma Aldrich Pvt. Ltd., India. Ruthenium red, HPMC, Carbopol 934 and Sodium taurocholate were purchased from Loba Chemie Pvt. Ltd., Mumbai, India. All other reagents and chemicals used were of analytical grade.
METHODS
Extraction of Mucilage from F. carica Fruits
F. carica fruits were washed with double distilled water to remove any adherent material. About three times its volume of water was added and heated at 60 ± 1°C on a water bath for about 4 h until the slurry was prepared. The viscous solution was then filtered, and the filtrate was diluted with three times its volume of water and kept undisturbed overnight in a refrigerator so that most of the undissolved portion settled down. In the following day, the clear supernatant portion was decanted and concentrated at 60 ± 1°C in a rotary vacuum evaporator. The concentrate was cooled to room temperature and precipitated in about three times its volume of acetone. The precipitate was washed repeatedly with acetone and dried at 60 ± 1°C. The dried material was ground by a mechanical grinder and passed through # 80 mesh sieve and kept in a desiccator till further use.
CHARACTERISATION OF MUCILAGE
Chemical Identification of F. carica Mucilage
F. carica mucilage was tested for the presence of carbohydrates and reducing sugars with Molisch reagent and Fehling’s solution, respectively (26). The mucilage was treated with α-naphthol and concentrated sulphuric acid which gave purple colour. For detecting reducing sugars, the mucilage is treated with Fehling’s solution A and B. After heating, brick-red precipitate was obtained. The mucilage was further treated with ferric chloride solution for detection of tannins, but no precipitate was observed. Further presence of mucilage was tested by treating the mucilage with 0.1% w/v ruthenium red solution (26). Presence of protein was detected by Biuret reagent. No test for determination of ficin was done.
DETERMINATION OF pH
pH of 1% (w/v) aqueous solution of F. carica mucilage (FCM) was measured using a Toshniwal pH meter (Toshniwal Inst. Mfg. Pvt. Ltd., Ajmer, India).
SWELLING CAPACITY
Swelling capacity of FCM was determined by keeping 1 g of the mucilage in 20 ml water in a measuring cylinder for 24 h (27). Swollen volume was recorded and determined as follows:
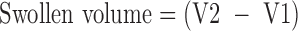 |
1 |
where V1 and V2 are initial volume of the material prior to hydration and volume of the hydrated material, respectively.
DETERMINATION OF ZETA POTENTIAL
Zeta potential of the mucilage was measured with the help of a zetasizer (Malvern Instruments Limited, UK). One millilitre of the mucilage solution was taken in a zeta potential cell and placed inside the instrument.
DETERMINATION OF VISCOSITY
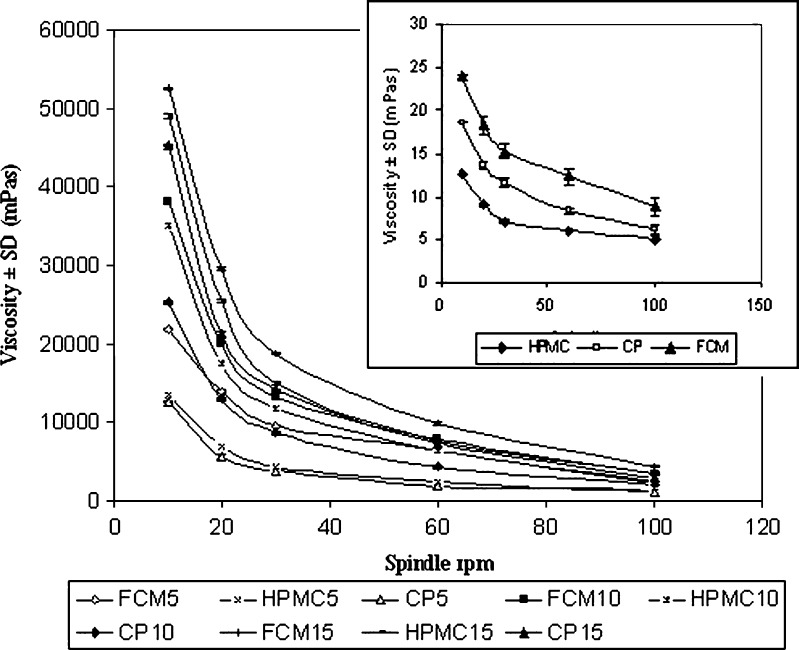
Viscosity of 1% aqueous solution of FCM was studied using Toki Sangyo Viscometer TV-10 (Toki Sangyo Co. Ltd., Japan) at five different speeds of 10, 20, 30, 60 and 100 rpm, respectively using spindle M4 and cord no. 23 at 34 ± 1°C (Fig. 1).
Fig. 1.
Comparative result of viscosities of 1% aqueous solutions of mucoadhesive agents and of in situ gels measured at 34 ± 1°C. Data represent mean values ± SD (n = 6)
EVALUATION OF MUCOADHESIVE STRENGTH
Mucoadhesive strength of 1% aqueous solution of FCM was studied with QTS-25 Texture Analyser (Brookfield Engineering Labs., USA). Freshly excised goat nasal membrane was attached to the upper probe of the instrument, and drop of FCM solution was kept below that. The upper probe was then lowered at a speed of 10 mm/min. to touch the surface of the solution. A force of 0.1 N was applied for 5, 10, 15, 20 and 30 min, respectively, to ensure intimate contact between the membrane and the gel. The surface area of exposed mucous membrane was 1.13 cm2.
The above studies were also conducted for HPMC and Carbopol 934, and results were compared.
PREPARATION OF MUCOADHESIVE NASAL GEL CONTAINING MIDAZOLAM
In situ gels are prepared by cold method (28). A 16% w/v Pluronic F127 was prepared in a mixture of propylene glycol/water (45:55), and then the mucoadhesive agents (FCM, HPMC and Carbopol 934) were added to it in three different concentrations of 0.5%, 1.0% and 1.5% w/v, respectively. Midazolam was then added to the dispersion, which was kept at 4°C overnight for complete swelling to form a homogenous gel. Gels containing enhancer (0.5% w/v sodium taurocholate) were also prepared to study the effect of enhancer on drug release pattern. Formulation codes of the gels are provided in Table I.
Table I.
Formulation Codes of the Nasal Gels
| Formulation code | FCM 5 | FCM 10 | FCM 15 | HPMC 5 | HPMC 10 | HPMC 15 | CP 5 | CP 10 | CP 15 |
|---|---|---|---|---|---|---|---|---|---|
| Midazolam hydrochloride (%w/v) | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Pluronic F127 (%w/v) | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 |
| FCM (%w/v) | 0.5 | 1.0 | 1.5 | – | – | – | – | – | – |
| HPMC (%w/v) | – | – | – | 0.5 | 1.0 | 1.5 | – | – | – |
| Carbopol 934 (%w/v) | – | – | – | – | – | – | 0.5 | 1.0 | 1.5 |
FCM Ficus carica mucilage, HPMC hydroxypropylmethyl cellulose, CP Carbopol 934
PHYSICOCHEMICAL CHARACTERISATION OF NASAL GELS
Determination of Viscosity
Viscosity of different formulations were measured with the same viscometer at five different speeds of 10, 20, 30, 60 and 100 rpm, respectively, using spindle M4 and cord no. 23 at 34 ± 1°C. The corresponding viscosities were plotted against spindle rpm as depicted in Fig. 1.
Gelation Temperature Measurement
Gelation temperature was determined by pouring 5 ml formulation in a 20-ml beaker containing magnetic bar and placing it on a hot plate. A thermometer was immersed in the gel, which was heated at a rate of 2°C/min with constant stirring at 20 rpm. When the bar stopped moving due to gelation, the temperature was recorded as the gelation temperature (29).
Texture Profile Analysis
Texture profile analyses of the prepared gels were evaluated using QTS-25 Texture Analyser to determine the mechanical parameters like hardness, cohesiveness and adhesiveness. An analytical probe of diameter 1.2 cm was depressed twice into each sample to a defined depth (15 mm), at a defined rate (30 mm/min), with a defined recovery period (15 s), between the end of the first compression and the beginning of the second. A trigger force of 4 g was applied. At least six replicate analyses of each sample were performed at 34 ± 1°C. Data collection and calculation were done by Texture pro software, version 2.1 (30).
The analysis was done using a 5-kg load cell instrument. Validation of the method was done by calibrating the instrument for distance and load cell. While calibrating the instrument in terms of distance, the force required to travel a particular distance (15 mm) was measured. For calibrating the instrument for load cell, distance travelled by the probe to attain a particular force (5 kg) was determined. The other parameters like probe speed of 30 mm/min before and after the test and trigger force of 4 g were kept constant. In both the cases, net work done was calculated from area under the curve. Intraday and interday variability were determined, and for both, the methods, accuracy and precision were determined by performing the experiment on six successive days and six times in a day.
EVALUATION OF MUCOADHESIVE STRENGTH
Mucoadhesive strength of each formulation was determined by measuring force required to detach nasal mucous membrane from the formulation using the same texture analyser. Freshly excised goat nasal membrane was attached to the upper probe of the instrument, and fixed amount of gel was kept below that. The upper probe was then lowered at a speed of 10 mm/min to touch the surface of the gel. A force of 0.1 N was applied for 5 min to ensure intimate contact between the membrane and the gel. The surface area of exposed mucous membrane was 1.13 cm2.
IN VITRO PERMEATION STUDY
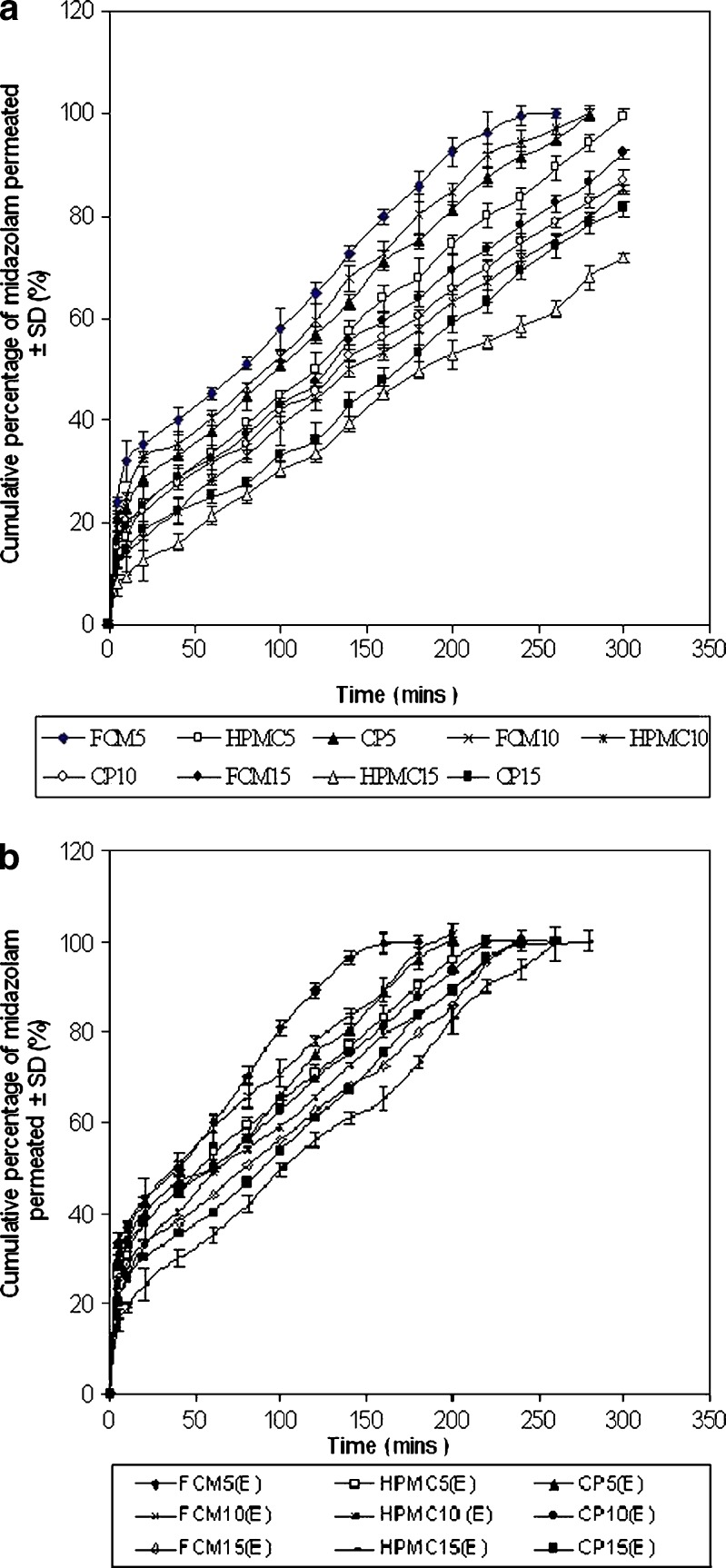
In vitro permeation study was conducted using a Franz diffusion cell containing 100 ml of phosphate buffer (pH 6, 0.1 M) using an excised goat nasal mucosa. The goat nose was obtained from local slaughterhouse within 15 min after the goat was sacrificed. After removing the skin, the nose was stored on ice cold phosphate buffer (pH 7.4, 0.05 M). The septum was fully exposed, and nasal mucosa was carefully removed using forceps and surgical scissors. The mucosal tissues were immediately immersed in Ringer’s solution. The freshly excised nasal mucosa was mounted on the diffusion cell, and 100 μl of gel containing 5 mg midazolam was placed on it. Throughout the study, the buffer solution in the chamber was maintained at 34 ± 1°C by connecting the Franz diffusion cell with water bath. At predetermined time intervals, 1 ml of the samples was withdrawn at a time and replenished with an equal amount of phosphate buffer. The samples were diluted appropriately and filtered. Absorbances of the samples were measured spectrophotometrically at 218 nm using Jasco V-550 UV/Vis Spectrophotometer (Tokyo, Japan), taking phosphate buffer (pH 6) as the blank. The amount of drug permeated was calculated from the calibration curve (linearity range = 2.4 to 120 μg/ml; r2 = 0.9996). The mean cumulative percentage of drug permeated was plotted against time (Fig. 2). Permeation area was 2.54 cm2.
Fig. 2.
a Comparative result of in vitro release profile of midazolam hydrochloride from the nasal gels prepared with F. carica mucilage, HPMC and Carbopol 934, without 0.5% w/v of sodium taurocholate (E) in phosphate buffer (pH 6) at 34 ± 1°C. Data represent mean values ± SD (n = 6). b Comparative result of in vitro release profile of midazolam hydrochloride from the nasal gels prepared with F. carica mucilage, HPMC and Carbopol 934, with 0.5% w/v of sodium taurocholate (E) in phosphate buffer (pH 6) at 34 ± 1°C. Data represent mean values ± SD (n = 6)
HISTOLOGICAL EVALUATION OF NASAL MUCOSA
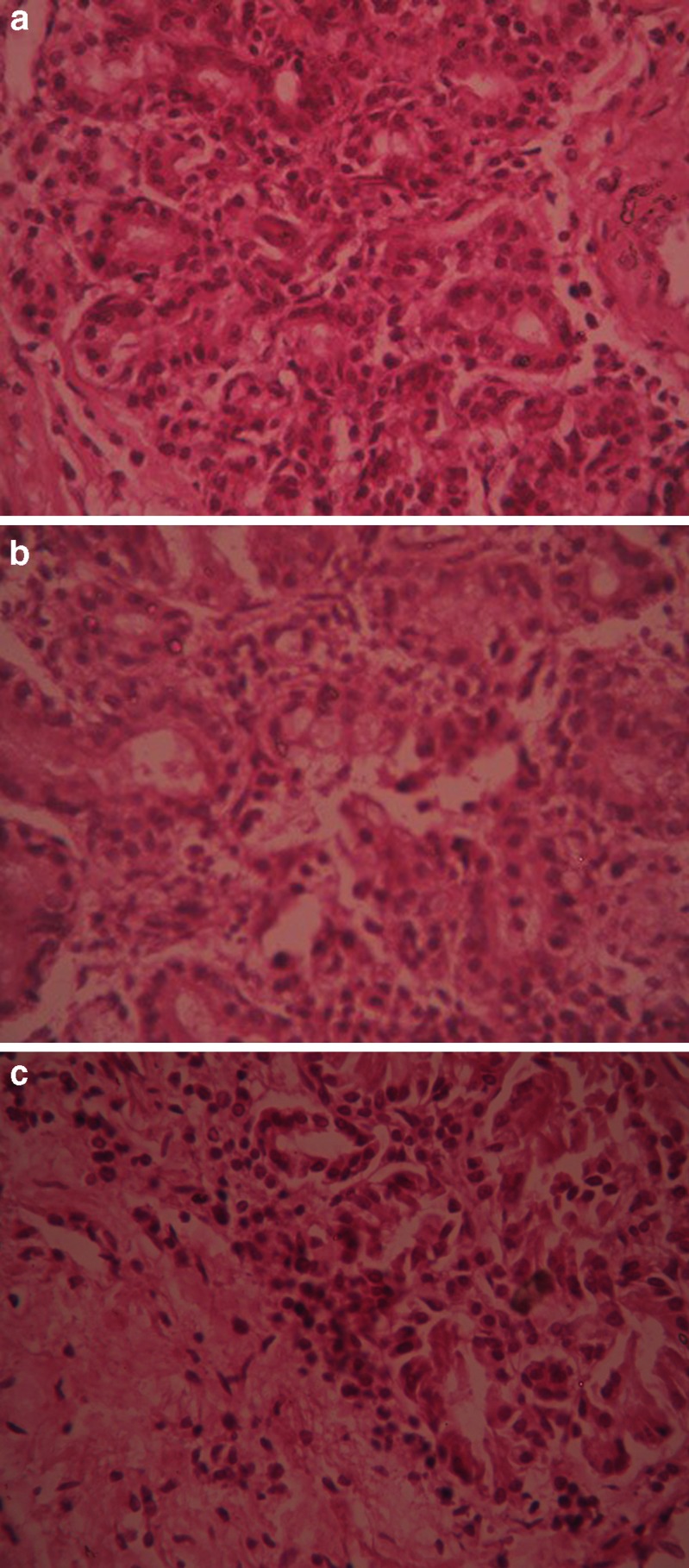
Histological study of excised nasal mucosa was conducted by comparing a fresh mucosa with the mucosae used for 5 h in the in vitro permeation study to detect if any significant histological change has occurred during the experiment. After permeation study, nasal mucosa was cleared off the gel, sectioned with a rotary microtome (Model 1090 A, The Western Electric and Scientific Works, India) and fixed in 10% formalin solution. The sectioned tissue was then stained with hematoxylin and eosin. Another normal mucosa was taken as a control and treated similarly. Tissue sections were observed under Olympus CKX41 optical microscope (Olympus Optical Co. Ltd., Tokyo, Japan). The photographs were taken with an Olympus-SC 35 camera (Fig. 3).
Fig. 3.
Histological photomicrographs of eosin–hematoxylin-stained nasal mucosa (40 × 0.55 μ PHP magnification) a Control—mucosa without application of nasal gel. b Nasal mucosa after application of FCM nasal gel. c Nasal mucosa after application of FCM nasal gel containing 0.5% sodium taurocholate
IN VIVO DRUG ABSORPTION STUDY
In vivo studies were conducted on 12 New Zealand albino male rabbits weighing between 1.5 and 2 kg. Based on texture profile analysis and drug release pattern of the formulations, FCM5, HPMC5 and CP5 containing 0.5% sodium taurocholate were selected for in vivo study. The animals were kept in individual metal cages and maintained at 25°C for 10 days prior to the experiment. They were provided with standard diet and water ad libitum. The approval of the Institutional Animal Ethics Committee was obtained before starting the study, and it was conducted according to the institutional guidelines. The rabbits were kept in fasting condition for 24 h before the experiment commenced. The rabbits were grouped into four (group I, II, III and IV), each group containing three rabbits. Group I was administered intravenous bolus injection of midazolam. Groups II, III and IV were administered nasal gels of FCM, HPMC and Carbopol 934. Single dose of midazolam (2 mg/kg body weight of rabbit) was administrated intravenously to compare the pharmacokinetic parameters. No anaesthesia was used for the intravenous study. Midazolam was injected through cannulated marginal ear vein. Two millilitres of blood samples each time were collected before intravenous injection and then at 5-, 10-, 15-, 20-, 30-, 45-, 60-, 90-, 120-, 180-, 240- and 300-min intervals in eppendorfs containing heparin sodium (100 U/ml). In case of nasal gels, the dose of midazolam that was administered was also 2 mg/kg body weight of rabbit. Before application of gel, each rabbit was lightly anaesthetised by intramuscular injection of a mixture of xylazine (3 mg/kg) and ketamine (35 mg/kg). Following induction of anaesthesia, a catheter was fixed into the central artery for blood sample collection. About 2 ml blood sample was collected prior to the application of gel and then at 5-, 10-, 15-, 20-, 30-, 45-, 60-, 90-, 120-, 180-, 240- and 300-min intervals in eppendorfs as above. After every 20 min, each rabbit was administered one-third of the initial dose of xylazine and ketamine intramuscularly to maintain a light plane of anaesthesia. The blood samples were kept on ice and centrifuged at 3,000 rpm for 10 min immediately after collection to separate the plasma and stored at −20°C until the time of analysis. Immediately after each blood sample collection, the catheter was flushed with 0.2 ml of a 10% (v/v) heparin/normal saline solution to prevent blood clotting inside the catheter.
All animal experiments are performed as per the standard norms and guidelines of the Animal Ethics Committee of Dr. B. C. Roy College of Pharmacy and Allied Health Sciences, West Bengal University of Technology, as recognised by the Committee for the Purpose of Control and Supervision on Experiments on Animals, India.
HPLC ANALYSIS
Reverse phase HPLC was used to quantitate midazolam in plasma samples. Midazolam was extracted with 3 ml of cyclohexane/diethyl ether (3:7) after the addition of 10 μl of 2% sodium hydroxide (31). The organic phase was removed and evaporated to dryness under nitrogen, and the residue was reconstituted in 200 μl of the mobile phase (10 mM phosphate buffer (pH 6.0)/acetonitrile, 80:20). Of the mixture, 100 μl was injected for chromatographic analysis. The mobile phase consisted of phosphate buffer/acetonitrile (80:20) v/v. The mobile phase was delivered into the HPLC apparatus at a flow rate of 1 ml/min (isocratic pump, Model LC-10AS, Jasco, Japan). The detection wavelength was 218 nm (ultraviolet variable wavelength detector, Model SPD-10A), and a C18 column was used. All assays were performed at ambient temperature.
Calibration curve of midazolam hydrochloride prepared in rabbit plasma was found to be linear over the concentration range of 10–1,000 ng/ml (r2 = 0.9999). The experiment was repeated six times a day and for six consecutive days. Interday and intraday accuracy and precision values are displayed in Table II.
Table II.
Interday and Intraday Accuracy and Precision Data for Quantitation of Midazolam in Rabbit Plasma
| Amount of drug added (ng/ml) | Concentration in plasma (ng/ml) | Accuracy | Precision (% CV) | |||
|---|---|---|---|---|---|---|
| Intraday | Interday | Intraday | Interday | Intraday | Interday | |
| 10 | 9.91 ± 0.24 | 9.55 ± 0.38 | 99.10 | 95.50 | 2.42 | 3.97 |
| 200 | 199.05 ± 2.15 | 195.99 ± 4.51 | 99.53 | 98.46 | 1.08 | 2.30 |
| 600 | 599.30 ± 2.65 | 596.08 ± 6.58 | 99.88 | 99.34 | 0.44 | 1.10 |
| 1,000 | 998.05 ± 3.95 | 994.09 ± 5.01 | 99.80 | 99.41 | 0.39 | 0.50 |
Data represent mean ± SD (n = 6)
CV coefficient of variation
PHARMACOKINETIC ANALYSIS
Pharmacokinetic parameters like peak plasma concentration (Cmax), time to reach peak plasma concentration (tmax), area under the (concentration–time) curve (AUC), mean residence time (MRT), elimination half-life (t1/2) and total body clearance (CL) were calculated following non-compartment model by Kinetica 4.4, PK/PD Analysis, Thermoelectron Corporation. All the parameters were calculated for i.v. bolus injection of midazolam and in situ nasal gels.
Fraction of dose absorbed (F) was calculated by the equation
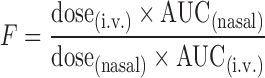 |
2 |
where dose(iv) = dose of midazolam given as i.v. solution, dose(nasal) = dose of midazolam in nasal gels, AUC(i.v.) = AUC after i.v. administration of midazolam and AUC(nasal) = AUC after nasal administration of midazolam.
STATISTICS
Data were analysed by one-way ANOVA followed by Tukey’s HSD test using Vassar stat software (USA). p < 0.01 has been considered to be significant statistically.
RESULTS
Chemical identification of mucoadhesive agent isolated from F. carica revealed that it consisted of carbohydrates, reducing sugar and protein but no tannin. Treatment of the material with ruthenium red confirmed that it was a mucilage and not gum.
Table III depicts the values of pH and swollen volumes of the mucoadhesive agents. pH of F. carica mucilage was found to be 7.04 which is within the pH range of the nasal cavity (5.5–7.5) and also comparable with the synthetic polymers like HPMC and Carbopol 934 that are widely used in formulation of nasal gels.
Table III.
Values of pH, Swollen Volumes, Zeta Potentials and Mucoadhesive Strengths of Mucoadhesive Agents
| Mucoadhesive agent | pH | Swollen volume (cm3/g) ± SD (n = 6) | Zeta potential (mV) ± SD (n = 6) | Mucoadhesive strength (g) ± SD (n = 6) | ||||
|---|---|---|---|---|---|---|---|---|
| 5 min | 10 min | 15 min | 20 min | 30 min | ||||
| FCM | 7.02 | 8.04 ± 0.33 | −20.54 ± 0.05 | 5.50 ± 0.39 | 5.86 ± 0.36 | 6.46 ± 0.44 | 6.89 ± 0.52 | 7.52 ± 0.61 |
| HPMC | 6.73 | 7.85 ± 0.95 | 0.00 ± 0.00 | 3.63 ± 0.15 | 4.15 ± 0.09 | 4.44 ± 0.06 | 4.76 ± 0.03 | 5.25 ± 0.04 |
| CP | 3.27 | 15.75 ± 0.65 | −23.64 ± 0.03 | 4.24 ± 0.08 | 4.72 ± 0.05 | 5.20 ± 0.06 | 5.36 ± 0.08 | 5.74 ± 0.09 |
FCM Ficus carica mucilage, HPMC hydroxypropylmethyl cellulose, CP Carbopol 934, SD standard deviation
Swollen volume of FCM (8.04 ± 0.80 cm3/g) was found to be slightly greater than that of HPMC (7.89 ± 0.95 cm3/g) but significantly less than that of Carbopol 934 (15.57 ± 0.65 cm3/g).
Values of zeta potential also are presented in Table III. Zeta potential of FCM, HPMC and Carbopol are reported to be –20.54 ± 0.05, 0.00 ± 0.00 and −23.64 ± 0.03 mV, respectively.
Viscosity plot of 1% aqueous solutions of FCM, HPMC and Carbopol 934 at five different speeds of 10, 20, 30, 60 and 100 rpm (Fig. 1) shows that viscosities of 1% aqueous solutions of HPMC, Carbopol 934 and FCM were in the range of 5–12, 6–18 and 8–28 mPa, respectively.
Table III depicts the mucoadhesive strengths of 1% solutions of FCM, HPMC and Carbopol 934. FCM was found to exhibit greater mucoadhesive strength compared to the synthetic polymers.
Regarding formulation development, various concentrations of Pluronic in a mixture of propylene glycol/water (45:55) were evaluated from 15% to 18% w/v, prior to selecting the optimum concentration of Pluronic necessary for the present study. Solutions containing 15%, 16%, 17% and 18% w/v of Pluronic had gelation temperatures of 38.5°C, 29.8°C, 24.5°C and 23.8°C, respectively. Only 16% w/v Pluronic F127 in a mixture of propylene glycol/water (45:55) was selected for further investigation. Addition of mucoadhesive agents lowered the gelation temperature. The temperature range of the nasal cavity is within 32–35°C (31).
Gelation temperatures of the formulations, prepared with or without sodium taurocholate, are displayed in Table IV. The concentration of mucoadhesive agent at which gelation of formulation occurs at the lower range of nasal temperature is found to be optimum. From the table, it is evident that gelation temperatures of gel containing FCM and synthetic polymers were comparable in all possible concentrations. It is observed that addition of sodium taurocholate lowered the gelation temperature in the case of all the formulations.
Table IV.
Values of Gelation Temperature and Mechanical Properties of the Gels
| Formulation code | Gelation temperature (°C) | Hardness (g) | Adhesiveness (g) | Cohesiveness | Mucoadhesive strength (g) |
|---|---|---|---|---|---|
| FCM 5 | 27.20 ± 0.33 | 141.67 ± 2.08 | −2,560.96 ± 12.71 | 1.07 ± 0.05 | 67.56 ± 0.34 |
| FCM 5(E) | 26.50 ± 0.21 | – | – | – | – |
| FCM 10 | 24.60 ± 0.24 | 245.67 ± 2.62 | −3,628.05 ± 22.87 | 0.96 ± 0.02 | 75.25 ± 0.55 |
| FCM 10(E) | 24.20 ± 0.31 | – | – | – | – |
| FCM 15 | 22.80 ± 0.25 | 468.11 ± 2.60 | −6,644.66 ± 20.29 | 0.92 ± 0.07 | 83.75 ± 0.65 |
| FCM 15(E) | 22.40 ± 0.11 | – | – | – | – |
| CP 5 | 28.50 ± 0.02 | 135.50 ± 2.91 | −2,269.82 ± 17.67 | 1.08 ± 0.08 | 44.27 ± 0.33 |
| CP 5 (E) | 28.00 ± 0.04 | – | – | – | – |
| CP 10 | 25.50 ± 0.03 | 204.00 ± 2.24 | −3,628.09 ± 12.26 | 1.03 ± 0.02 | 62.08 ± 0.98 |
| CP 10 (E) | 25.00 ± 0.05 | – | – | – | – |
| CP 15 | 23.30 ± 0.04 | 335.00 ± 1.85 | −5,508.83 ± 9.90 | 0.93 ± 0.02 | 73.26 ± 0.53 |
| CP 15(E) | 22.80 ± 0.06 | – | – | – | – |
| HPMC 5 | 29.50 ± 0.05 | 118.00 ± 0.82 | −2,158.04 ± 17.67 | 1.04 ± 0.14 | 10.23 ± 0.95 |
| HPMC 5(E) | 29.00 ± 0.03 | – | – | – | – |
| HPMC 10 | 27.80 ± 0.11 | 175.67 ± 3.62 | −3,084.31 ± 25.92 | 1.03 ± 0.03 | 30.67 ± 1.15 |
| HPMC 10(E) | 27.20 ± 0.05 | – | – | – | – |
| HPMC 15 | 25.20 ± 0.15 | 295.00 ± 2.85 | −4,438.49 ± 30.26 | 0.96 ± 0.04 | 43.67 ± 1.13 |
| HPMC 15(E) | 24.60 ± 0.04 | – | – | – | – |
Data shows mean ± SD (n = 6)
FCM Ficus carica mucilage, HPMC hydroxypropylmethyl cellulose, CP Carbopol 934
Viscosities of different formulations at different spindle rpm at 34 ± 1°C are shown in Fig. 1. All the formulations exhibited pseudoplastic behaviour. It was observed that all the FCM gels showed higher viscosity than the gels prepared from synthetic polymers.
Regarding validation of the texture profile analysis method, in terms of distance, interday and intraday accuracy were 97.50% and 98.25%, respectively, while interday and intraday precision were 2.17% and 1.15%, respectively. Regarding validation of the instrument in terms of load cell, interday and intraday accuracy were 96.5% and 98.99%, respectively, whereas interday and intraday precision were 3.15% and 2.74%, respectively.
Texture profile analyses of the gels were studied at 34 ± 1°C. The results of texture profile analysis were displayed in Table IV. Minimum hardness was shown by HPMC5 gel, and maximum was shown by FCM15 gel. Adhesiveness of the formulations showed that with the increase in concentration of FCM from 0.5% to 1.5%, adhesiveness increased by 2.6 times (from −2,560.96 ± 12.71 to −6,644.66 ± 20.29 g). For HPMC gels, with the increase in concentration from 0.5% to 1.5%, it increased by 2.05 times (from −2,158.04 ± 17.67 to −4,438.49 ± 30.26 g). For Carbopol gels, it was enhanced by 2.1 times (from −2,269.82 ± 17.67 to −5,508.83 ± 9.90 g). Cohesiveness was found to decrease as the concentration of the mucoadhesive agents increased from 0.5% to 1.5% w/v. Maximum cohesiveness was observed in HPMC 5 gel and minimum in FCM 15 gel. However, the formulations containing sodium taurocholate did not show any change in the values of hardness, adhesiveness and cohesiveness.
Mucoadhesive strengths of the gels were also measured in texture analyzer. Results are displayed in Table IV which shows that mucoadhesive strength of FCM gels was higher than those prepared with synthetic mucoadhesive polymers and it increased with corresponding increase in concentration of mucoadhesive agent used.
In vitro release profiles of midazolam from various gels were studied in phosphate buffer (pH 6.0). Figure 2a depicts in vitro release profiles of midazolam from various formulations. Results reveal that drug release from FCM gels was faster than the synthetic ones. In Fig. 2b, it is shown that drug release from gels containing 0.5% sodium taurocholate as enhancer was faster in comparison to gels without enhancer.
Safety of a nasal formulation can be assessed by a comparative histological study of nasal mucosae after the in vivo study and normal mucosae (control). The histological micrographs (Fig. 3) revealed that no significant alteration of nasal epithelium and/or necrosis occurred. This implies that FCM is as safe as HPMC and Carbopol 934 to be used as a mucoadhesive agent for nasal administration, and the concentration of sodium taurocholate used is also safe.
The accuracy and precision values of the HPLC method displayed in Table II show that inter- and intraday coefficients of variation were found to be less than 4%, and accuracy varied from 95.50% to 99.88%. The limit of quantification was 10 ng/ml.
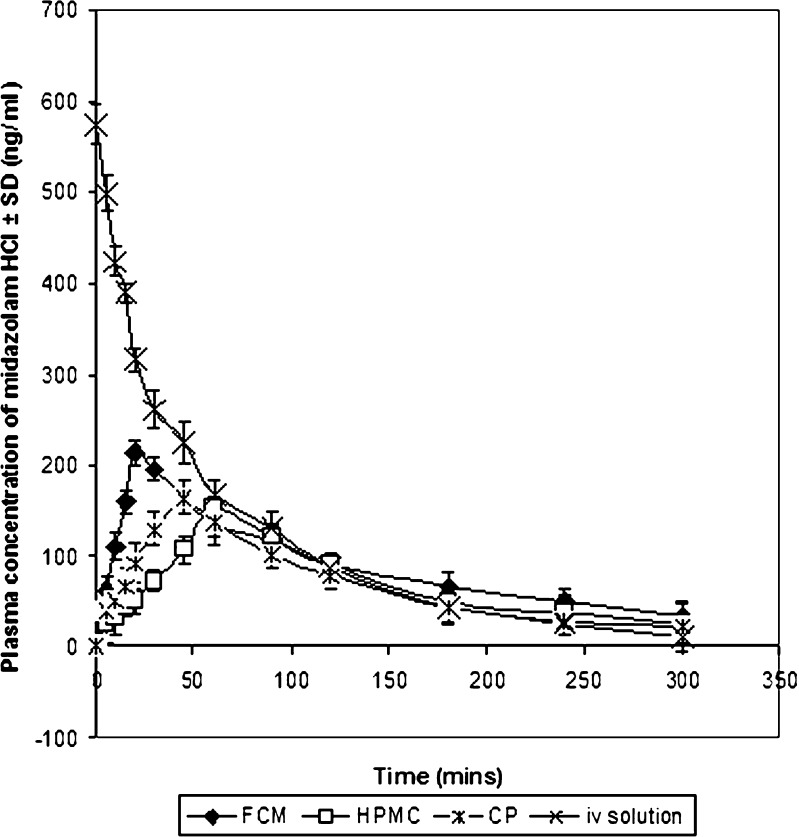
Plasma concentration–time profiles of midazolam after administration of i.v. solution and the nasal gels are shown in Fig. 4. Pharmacokinetic parameters were displayed in Table V. Cmax values of i.v. injection, FCM, HPMC and CP gels were 573.64 ± 5.23, 199.59 ± 9.28, 151.47 ± 7.22 and 164.07 ± 6.85 ng/ml, respectively. tmax values of i.v. injection, FCM, HPMC and CP gels were 0.00, 20.00 ± 5.11, 60.00 ± 5.68 and 45.00 ± 3.66 min, respectively. t1/2 values of i.v. injection, FCM, HPMC and CP gels were 58.78 ± 6.32, 133.52 ± 8.07, 68.44 ± 5.28 and 99.68 ± 4.89 min, respectively.
Fig. 4.
Plasma concentration–time profiles of midazolam after administration of intravenous solution and the in situ nasal gels in rabbits. Data represent mean ± SD (n = 3)
Table V.
Comparative Pharmacokinetic Parameters of Midazolam Hydrochloride Following Administration of Intravenous and Nasal In Situ Gels in Rabbits (dose = 2 mg/kg)
| Parameter | Intravenous solution | FCM gel | HPMC gel | Carbopol gel |
|---|---|---|---|---|
| C max (ng/ml) | 73.64 ± 5.23 | 199.59 ± 9.28 | 151.47 ± 7.22 | 164.07 ± 6.85 |
| t max (min) | 0.00 | 20.00 ± 5.11 | 60.00 ± 5.68 | 45.00 ± 3.66 |
| AUC(0–t) (min(ng/ml)) | 32,671.50 ± 90.56 | 25,311.30 ± 115.05 | 18,034.30 ± 101.45 | 22,074.10 ± 96.72 |
| AUC(t–∞) (min(ng/ml)) | 865.87 ± 37.25 | 6,907.46 ± 55.41 | 1,191.83 ± 45.26 | 3,684.71 ± 60.58 |
| AUCtotal (min(ng/ml)) | 33,537.40 ± 101.25 | 32,218.80 ± 86.25 | 19,226.10 ± 97.25 | 25,758.90 ± 95.46 |
| t 1/2 (min) | 58.78 ± 6.32 | 133.52 ± 8.07 | 68.44 ± 5.28 | 99.68 ± 4.89 |
| MRT (min) | 71.61 ± 5.05 | 196.20 ± 5.35 | 130.16 ± 6.58 | 162.50 ± 7.25 |
| CL (×10−5) (mg/kg/(ng/ml)min) | 5.79 ± 6.95 | 6.02 ± 7.58 | 10.93 ± 8.15 | 7.53 ± 5.66 |
Data represent mean ± SD (n = 3)
DISCUSSION
Chemical identification of mucoadhesive agent isolated from F. carica fruits shows that it consists of reducing sugars, protein and mucilage, but no tannin is present.
The pH of the mucilage isolated from F. carica was found to be consistent with that of the nasal mucosa as shown in Table III. Hence, FCM can be effectively used in the formulation of nasal mucoadhesive gels.
Swellability is an indispensable property of mucoadhesive polymers. There is a critical degree of swelling that is necessary for optimum bioadhesion (8). However, excessive swelling converts the gel network to a slippery surface that causes a decrease in bioadhesion. From Table III, it is found that swollen volume of Carbopol 934 was greater than HPMC, but Carbopol has very high percentage of carboxyl groups which form hydrogen bonding with sugar residues in oligosaccharide chains in the mucus membrane leading to formation of a strong network between the polymer and mucus membrane (24). As a result, Carbopol 934 is found to provide better mucoadhesive property than HPMC. However, it is observed that FCM exhibited moderate swellability compared to HPMC and Carbopol suggesting better mucoadhesion.
The negative values of zeta potential displayed in Table III indicate that in colloidal condition repulsive force prevails between particles of mucoadhesive agents in aqueous solution, which helps the mucilage preparation to settle down quickly.
Viscosity of FCM was found to be higher than that of HPMC and Carbopol 934 at the same concentration as is depicted in Fig. 1. Hence, FCM can be considered to be a better gel-forming agent than synthetic polymers.
Determination of mucoadhesive strength showed that FCM has better bioadhesive strength than synthetic polymers. From Table III, it is observed that the greater the contact time, the higher is the force of adhesion and, hence, the greater the mucoadhesive strength. It can be concluded that the isolated mucilage with higher viscosity and moderate swellability has better mucoadhesive property compared to synthetic polymers. This may be due to presence of certain functional groups in the mucilage that were able to establish a more intimate contact with mucin of the mucosa.
The aim of the present study was to prepare nasal in situ gels within a temperature range of 25–34°C so that the preparation is liquid at room temperature for easy administration and providing accurate dose of the drug but forms a firm gel at the temperature of the nasal cavity. The lowering of gelation temperature with addition of mucoadhesive agents attributed to the fact that the mucoadhesive agents formed hydrogen bonds with Pluronic to promote dehydration leading to increased entanglement of adjacent molecules and thereby enhancing micellar association (32,33).
Mucoadhesive agents contributed to increased viscosity of the formulations which is evident from Fig. 1. Viscosity of the gels was enhanced with increase in concentration of the mucoadhesive agents.
The validation confirms that the texture analyzer is suitable for use and produces results that are accurate and precise.
Texture profile analysis was used to evaluate the mechanical properties of the gels, like hardness, adhesiveness and cohesiveness. Hardness is the maximum force during first compression cycle. It has been found to increase with the increase in the amount of mucoadhesive agent. Adhesiveness is an important parameter since it determines proper gel contact and retention at mucosal surface thereby leading to enhanced bioavailability of the drug (34). Results show that adhesiveness was augmented significantly with increase in amount of the mucoadhesive agent. Cohesiveness is the ratio of area of the curve during second compression to that during first compression of the gel with the probe. It was observed to be reduced with increase in amount of mucoadhesive agents. With increase in amount of dispersed solids, that is the mucoadhesive agents, semisolid nature of the gels increased, which caused the gel to become less coherent (34,35).
Results show that 5-min contact time was enough to achieve optimum mucoadhesive strength. However, increase in contact time did not affect mucoadhesive strength. It is expected that presence of numerous carboxyl and hydroxyl groups of carbohydrates present in FCM contributed to enhanced mucoadhesive strength of the FCM gels than those prepared from synthetic polymers. This shows that FCM has better mucoadhesive properties than HPMC and Carbopol 934.
Drug release pattern from in situ gels showed that with the increase in concentration of mucoadhesive agents, drug release was retarded, but Carbopol gels were exceptional. Since Carbopol itself has a penetration-enhancing effect, hence, drug release was enhanced with increase in amount of Carbopol. However, drug release from gels containing enhancer was relatively faster than the gels containing no enhancer.
Histopathological study of the nasal mucosae suggests that FCM nasal gel prepared with enhancer was safe for nasal administration after 5-h permeation study when compared with the normal mucosa. Bile salts like sodium taurocholate enhance paracellulaar transport of drugs by opening the tight junctions of the nasal mucosa owing to bile salt-induced calcium complexation. However, this change is temporary and reversible (36). Since no necrosis or removal of epithelial layer was observed, hence, it can be inferred that no major histological change has occurred.
Higher plasma concentration of midazolam hydrochloride is observed in case of FCM gels in comparison to HPMC and Carbopol 934 gels (Fig. 4), and accordingly, absolute bioavailabilities of midazolam from FCM, HPMC and Carbopol gels were reported to be 96.07 ± 3.45%, 57.33 ± 5.85% and 76.81 ± 5.09%, respectively.
Mucilages obtained from F. carica fruits were collected from plants of different places. They, however, exhibited similar basic physical and chemical characteristics, and it is observed that there were certain variations in results of viscosity and mucoadhesive strength, but the deviations were within 10%. However, when gels were prepared from such maucilages, the concentration of FCM was very small (0.5–1.5% w/v) compared to that of the Pluronic F 127 (16% w/v), owing to which the results of viscosity, texture analysis and mucoadhesive strengths showed minimal deviations.
CONCLUSION
The mucilage extracted from F. carica fruits was found to possess better mucoadhesive properties than the synthetic polymers HPMC and Carbopol 934 that are widely used in preparation of nasal gels. The in situ nasal gels prepared from FCM showed better rheological, mechanical and mucoadhesive properties than the gels prepared from synthetic polymers. Histopathological study confirmed that this natural mucilage had no adverse impact on the structural integrity of the nasal mucosa. Further, in vivo study proves that bioavailability of midazolam from FCM gels was far better than those prepared from HPMC and Carbopol 934 gels. Results obtained from the present study show that nasal gel prepared from 0.5% w/v F. carica mucilage containing 0.5% sodium taurocholate as enhancer gave reproducible results. This type of nasal in situ gel of midazolam prepared from natural mucilage extracted from F. carica fruits will undoubtedly provide a cheaper dosage form of midazolam and will thus add a new dimension in this regard.
Acknowledgement
The authors are grateful to AICTE National Doctoral Fellowship for providing financial assistance for carrying out the research work.
References
- 1.Wikipedia. The free encyclopedia. http://en.wikipedia.org/wiki/. Accessed 20 July 2007.
- 2.DeLorenzo RJ, Pellock JM, Towne AR, Boggs JG. Epidemiology of status epilepticus. J Clin Neurophysiol. 1995;12:316–25. [PubMed] [Google Scholar]
- 3.Goldman RD. Intranasal drug delivery for children with acute illness. Curr Drug Ther. 2006;1:127–30. doi: 10.2174/157488506775268470. [DOI] [Google Scholar]
- 4.Pecking M, Montestruc F, Marquet P, Wodey E, Homery MC, Dostert P. Absolute bioavailability of midazolam after subcutaneous administration to healthy volunteers. Br J Clin Pharmacol. 2002;54:357–62. doi: 10.1046/j.1365-2125.2002.01665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geldner G, Hubmann M, Knoll R, Jacobi K. Comparison between three transmucosal routes of administration of midazolam in children. Paediatr Anaesth. 1997;7:103–9. doi: 10.1046/j.1460-9592.1997.d01-57.x. [DOI] [PubMed] [Google Scholar]
- 6.Scott RC, Besag FM, Neville BG. Buccal midazolam and rectal diazepam for treatment of prolonged seizures in childhood and adolescence: a randomised trial. Lancet. 1999;353:623–6. doi: 10.1016/S0140-6736(98)06425-3. [DOI] [PubMed] [Google Scholar]
- 7.Schwagmeier R, Alincic S, Striebel HW. Midazolam pharmacokinetics following intravenous and buccal administration. Br J Clin Pharmacol. 1998;46:203–6. doi: 10.1046/j.1365-2125.1998.00781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ugwoke MI, Verbeke N, Kinget R. The biopharmaceutical aspects of nasal mucoadhesive drug delivery. J Pharm Pharmcol. 2001;53:3–22. doi: 10.1211/0022357011775145. [DOI] [PubMed] [Google Scholar]
- 9.Illum L. Nasal drug delivery: new developments and strategies. Drug Discov Today. 2002;7:184–9. doi: 10.1016/S1359-6446(02)02529-1. [DOI] [PubMed] [Google Scholar]
- 10.Türker S, Onur E, Özer Y. Nasal route and drug delivery systems. Pharm World Sci. 2004;26:137–42. doi: 10.1023/B:PHAR.0000026823.82950.ff. [DOI] [PubMed] [Google Scholar]
- 11.Jeannet PY, et al. Home and hospital treatment of acute seizures in children with nasal midazolam. Eur J Paediatr Neurol. 1999;3:73–7. doi: 10.1016/S1090-3798(99)80016-6. [DOI] [PubMed] [Google Scholar]
- 12.Fisgin T, Gurer Y, Tezic T, Senbil N, Zorlu P, Okuyaz C, et al. Effects of intranasal midazolam and rectal diazepam on acute convulsions in children: prospective randomized study. J Child Neurol. 2002;17:123–6. doi: 10.1177/088307380201700206. [DOI] [PubMed] [Google Scholar]
- 13.Lahat E, Goldman M, Barr J, Bistritzer T, Berkovitch M. Comparison of intranasal midazolam with intravenous diazepam for treating febrile seizures in children: prospective randomized study. BMJ. 2000;21:83–6. doi: 10.1136/bmj.321.7253.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Armijo JA, Herranz JL, Pena Pardo MA, Adin J. Intranasal and buccal midazolam in the treatment of acute seizures. Rev Neurol. 2004;38:458–68. [PubMed] [Google Scholar]
- 15.Mahmoudian T, Zadeh MM. Comparison of intranasal midazolam with intravenous diazepam for treating acute seizures in children. Epilepsy Behav. 2004;5:253–5. doi: 10.1016/j.yebeh.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Wilson MT, Macleod S, O'Regan ME. Nasal/buccal midazolam use in the community. Arch Dis Child. 2004;89:50–1. doi: 10.1136/adc.2002.019836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhattacharyya M, Kalra V, Gulati S. Intranasal midazolam vs rectal diazepam in acute childhood seizures. Pediatr Neurol. 2006;34:355–9. doi: 10.1016/j.pediatrneurol.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 18.Wolfe TR, Macfarlane TC. Intranasal midazolam therapy for pediatric status epilepticus. Am J Emerg Med. 2006;24:343–6. doi: 10.1016/j.ajem.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 19.Holsti M, Sill BL, Firth SD, Filloux FM, Joyce SM, Furnival RA. Prehospital intranasal midazolam for the treatment of pediatric seizures. Pediatr Emerg Care. 2007;23:148–53. doi: 10.1097/PEC.0b013e3180328c92. [DOI] [PubMed] [Google Scholar]
- 20.Knoester PD, Jonker DM, van der Hoeven RTM, Vermeij TAC, Edelbroek PM, Brekelmans GJ, de Haan GJ. Pharmacokinetics and pharmacodynamics of midazolam administered as a concentrated intranasal spray. A study in healthy volunteers. Br J Clin Pharmacol. 2002;53:501–7. doi: 10.1046/j.1365-2125.2002.01588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loftsson TH, Guðmundsdo´ ttir H, Sigurjo´nsdo´ ttir JF, Sigurðsson HH, Sigfu´sson SD, Ma´sson M, Stefa´nsson E. Cyclodextrin solubilization of benzodiazepines: formulation of midazolam nasal spray. Int J Pharm. 2001;212:29–40. doi: 10.1016/S0378-5173(00)00580-9. [DOI] [PubMed] [Google Scholar]
- 22.Miyazaki S, Takeuchi S, Yokouchi C, Takada M. Pluronic F-127 gels as a vehicle for topical administration of anticancer agents. Chem Pharm Bull. 1984;10:4205–8. doi: 10.1248/cpb.32.4205. [DOI] [PubMed] [Google Scholar]
- 23.Miyazaki S, Nakamura T, Yokouchi C, Takada M. Effect of Pluronic gels on the rectal absorption of indomethacin in rabbits. Chem Pharm Bull. 1987;35:1243–8. doi: 10.1248/cpb.35.1243. [DOI] [PubMed] [Google Scholar]
- 24.Majithiya RJ, Ghosh, PK, Umrethia ML, Murthy RS. Thermoreversible-mucoadhesive gel for nasal delivery of sumatriptan. AAPS PharmSciTech. 2006;7(3):Article 67. [DOI] [PMC free article] [PubMed]
- 25.Zhang L, Parsons DL, Navarre C, Kompella UB. Development and in-vitro evaluation of sustained release Poloxamer 407 (P407) gel formulations of ceftiofur. J Control Release. 2002;85:73–81. doi: 10.1016/S0168-3659(02)00273-0. [DOI] [PubMed] [Google Scholar]
- 26.Tease WB. Trease and Evans’ pharmacognosy. 15. London: WB Saunders Company Ltd; 2002. [Google Scholar]
- 27.Park H, Robinson JR. Mechanisms of mucoadhesion of poly (acrylic acid) hydrogels. Pharm Res. 1987;4:457–64. doi: 10.1023/A:1016467219657. [DOI] [PubMed] [Google Scholar]
- 28.Schmolka IR. Artificial skin. I. Preparation and properties of Pluronic F-127 gels for the treatment of burns. J Biomed Mater Res. 1972;6:571–82. doi: 10.1002/jbm.820060609. [DOI] [PubMed] [Google Scholar]
- 29.Choi HK, Jung JH, Ryu JM, Yoon SJ, Oh YK, Kim CK. Development of in-situ gelling and mucoadhesive acetaminophen liquid suppository. Int J Pharm. 1998;165:33–44. doi: 10.1016/S0378-5173(97)00386-4. [DOI] [Google Scholar]
- 30.Cevher E, Taha MAM, Orlu M, Araman A. Evaluation of mechanical and mucoadhesive properties of clomiphene citrate gel formulations containing carbomers and their thiolated derivatives. Drug Dev. 2008;15:57–67. doi: 10.1080/10717540701829234. [DOI] [PubMed] [Google Scholar]
- 31.Shih PE, Huang JD. Pharmacokinetics of midazolam and 1-hydroxymidazolam in Chinese with different cyp3a5 genotypes. Drug Metab Dispos. 2002;30:1491–6. doi: 10.1124/dmd.30.12.1491. [DOI] [PubMed] [Google Scholar]
- 32.Marttin E, Shipper NGM, Verhoef JC, Merkus FWHM. Nasal mucociliary clearance as a factor in nasal drug delivery. Adv Drug Deliv Rev. 1998;29:13–38. doi: 10.1016/S0169-409X(97)00059-8. [DOI] [PubMed] [Google Scholar]
- 33.Wei G, Xu H, Ding PT, Li SM, Zheng JM. Thermosetting gels with modulated gelation temperature for ophthalmic use: the rheological and gamma scintigraphic studies. J Control Release. 2002;83:65–74. doi: 10.1016/S0168-3659(02)00175-X. [DOI] [PubMed] [Google Scholar]
- 34.Bansal K, Rawat MK, Jain A, Rajput A, Chaturvedi TP, Singh S. Development of satranidazole mucoadhesive gel for the treatment of periodontitis. AAPS PharmSciTech. 2009;10(3):716–23. doi: 10.1208/s12249-009-9260-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tan YTF, Peh KK, Al-Hanbali O. Effect of Carbopol and polyvinylpyrrolidone on the mechanical, rheological and release properties of bioadhesive polyethylene glycol gels. AAPS PharmSciTech. 2000;1:Article 24. [DOI] [PMC free article] [PubMed]
- 36.Lin H, Gebhardt M, Bian S, et al. Enhancing effect of surfactants on fexofenadine HCl transport across the human nasal epithelial cell monolayer. Int J Pharm. 2007;330:23–31. doi: 10.1016/j.ijpharm.2006.08.043. [DOI] [PubMed] [Google Scholar]