Abstract
It is thought that cyclodextrins, such as 2-hydroxypropyl-β-cyclodextrin (HPβCD), will at high concentration affect pharmacokinetics of drugs through competitive binding with plasma proteins. Albumin is the major component of plasma proteins responsible for plasma protein binding. The purpose of this study was to evaluate in vitro the competitive binding of drugs between human serum albumin (HSA) and HPβCD in isotonic pH 7.4 phosphate buffer saline solution (PBS) at ambient temperature. Eight model drugs were selected based on their physicochemical properties and ability to form complexes with HSA and HPβCD. The drug/HPβCD stability constants (K1:1) were determined by the phase-solubility method and HSA/HPβCD competitive binding determined by an equilibrium dialysis method. Protein binding of drugs that are both strongly protein bound and have high affinity to HPβCD (i.e., have high K1:1 value) is most likely to be affected by parenterally administered HPβCD. However, this in vitro study indicates that even for those drugs single parenteral dose of HPβCD has to be as high as 70 g to have detectable effect on their protein binding. Weakly protein bound drugs and drugs with low affinity towards HPβCD are insensitive to the cyclodextrin presence regardless their lipophilic properties.
KEY WORDS: 2-hydroxypropyl-β-cyclodextrin, competitive binding, equilibrium dialysis, human serum albumin, parenteral delivery, stability constant
INTRODUCTION
Cyclodextrins are pharmaceutical excipients that are mainly used as solubilizing complexing agents in solid dosage forms, eye drops, and parenteral solutions (1,2). Cyclodextrins are cyclic oligosaccharides with a hydrophilic outer surface and a somewhat lipophilic central cavity that are able to form water-soluble inclusion complexes by taking up some lipophilic moiety of a poorly soluble drug into the cavity. Although such inclusion complexes are the most common types of cyclodextrin complexes, especially in dilute solutions, various types of non-inclusion complexes and cyclodextrin aggregates are also known to exist (1,3,4). No covalent bonds are formed during the complexation, and in aqueous complexation media bound drug molecules are in dynamic equilibrium with free molecules in solution. The major driving force for drug release from the complexes is simple dilution although other mechanism, such as drug-protein binding and direct drug partition from the complex to tissue, do contribute to rapid drug release from the complexes (2,5–7). Two cyclodextrins, 2-hydroxypropyl-β-cyclodextrin (HPβCD) and sulfobutylether-β-cyclodextrin (SBEβCD), have been approved for use in parenterally administered drug formulations. Both HPβCD and SBEβCD have relatively small volume of distribution (VD ≈ 0.2 l/kg) and short half-life (t1/2 ≈ 1.7 h), and both are mainly excreted unchanged with urine after parenteral administration to humans (6,8). Several studies in both animals and humans have indicated that drug/HPβCD and drug/SBEβCD complexes have negligible effect on drug pharmacokinetics (9–16). In fact, it has been shown that the binding constants of drug/HPβCD and drug/SBEβCD complexes must be greater than about 105 M−1 to have some effect on the drug pharmacokinetics (6). Most commonly drug/cyclodextrin binding constants have values between 10 and 2,000 M−1 and binding constants much greater than 5,000 M−1 are rarely observed. There are however a couple of exceptions. Sugammadex is a γ-cyclodextrin derivative that was designed to specifically bind rocuronium, a neuromuscular blocking agent. The binding constant of the rocuronium/sugammadex complex has been determined to be 1.8 × 107 M−1, and sugammadex is able to reverse rocuronium-induced neuromuscular blockade after intravenous administration (17,18). In other words, sugammadex does affect the pharmacokinetics of rocuronium. The other example is complexes of some ozonide antimalarial drug candidates with SBEβCD possessing binding constants of about 106 M−1 (19). The pharmacokinetics of these drug candidates in rats have been shown to be affected by the SBEβCD complexation (20).
Aqueous parenteral drug/cyclodextrin solutions are mixed rapidly with blood plasma after intravenous injection and the plasma proteins with subsequent formation of drug/plasma protein complexes (i.e., drug-protein binding). Albumin is the major component of plasma proteins responsible for plasma protein binding and the normal average albumin concentration in plasma is 40 mg/ml corresponding to 5.88 × 10−4 M (21). The purpose of this study was to evaluate in vitro the competitive binding of drugs between human serum albumin (HSA) and HPβCD. The model drugs were selected with regard to their physicochemical properties and ability to form complexes with HSA and HPβCD (Table I). The complexation media (i.e., the simulated plasma) was isotonic pH 7.4 PBS solution containing 5.88·10−4 M or 40 mg/ml of HSA.
Table I.
Structures and Some Physicochemical Properties of the Drugs Studied. Log D7.4 is the Logarithmic Value of the Partition Coefficient between n-octanol and Aqueous pH 7.4 Buffer Solution (22–29)

aFree acid was used in this study extracted as described elsewhere (26)
BACKGROUND
Three types of complexes can be formed in aqueous solution containing drug (D), cyclodextrin (CD), and protein (P), i.e., drug/cyclodextrin complex (D/CD), drug/protein complex (D/P) and cyclodextrin/protein complex (CD/P):
 |
1 |
 |
2 |
 |
3 |
assuming 1:1 complex stoichiometry. The relative concentration of these three complexes depends on the concentration of components (i.e., D, P, and CD) and on the value of the constants KCD, KP, and KCD/P. D/CD complexes are most frequently inclusion complexes although other types of D/CD complexes are also known (30). Stability constants (KCD) of D/CD complexes commonly range between 10 and 1,000 M−1. Formation and dissociation of both CD and P complexes is diffusion-controlled and thus the bound molecules release up on dilution of the complexation media (6).
Most drugs are to some extent bound to plasma proteins (i.e., form D/P complexes) in the systemic blood circulation. The major component (~60%) of plasma proteins responsible for reversible drug binding is albumin, but other proteins such as globulins do also participate in plasma protein binding of drugs. HSA is a water-soluble monomeric polypeptide containing 585 amino acidic residues that form nine loops fixed by 17 disulphide bridges. The HSA chain forms three domains. HSA has at least six binding regions that are able to bind wide spectrum of drugs through various types of non-covalent interactions (31). However, the binding regions are usually somewhat specific and hence each drug only binds to one or two of the regions. Formation of D/P complexes is sensitive to various external factors, such as competitive binding (32,33) and in vitro experimental conditions (i.e., pH, temperature, ionic strength) (34,35).
It is known that cyclodextrins interact with proteins. Matsuyama et al. (36) used calorimetry to show that native cyclodextrins interact with aromatic amino acids such as those found in HSA. The strongest interaction was observed between the natural β-cyclodextrin (βCD) and tryptophan. However, the interaction was very weak. Brewster et al. (37) found that HPβCD can solubilize several different proteins with MW between 6,000 and 20,000 Da, as well as prevent protein aggregation and preserve biological potency. Katakam and Banga (38) observed that HPβCD stabilizes bovine serum albumin and γ-globulin and suggested that hydrophobic side chains of amino acids were entrapped in the cyclodextrin cavity. However, these observations were made at relatively high cyclodextrin and protein concentrations and the interactions appear to be relatively weak. Finally, Sideris et al. (39) proposed, after potentiometric studies of diflunisal-albumin binding in the presence of HPβCD, that there is no significant interaction between HPβCD and albumin in aqueous solutions. Other studies have indicated that SBEβCD is only negligible protein bound in human plasma.
MATERIALS AND METHODS
HPβCD with molar substitution of 0.64 (Kleptose HPB, MW 1400) was purchased from Roquette (Lestrem, France). HSA (fraction V, assay ≥85%, MW ~ 68,000) was purchased from Sigma-Aldrich, Inc. (USA). Acetazolamide, amoxicillin, atropin, diclofenac sodium, ketoprofen and ketorolac were purchased from Sigma-Aldrich, Inc. (USA), lidocaine from ICN Biomedicals, Inc. (USA), and paracetamol from Norsk Medisinaldepot (Norway). All other chemicals used were commercially available products of special reagent grade.
Solubility
The solubilities of drugs in PBS and HPβCD solutions were determined by the previously described heating method (40). Briefly, sealed vials containing excess amount of the solid drug to be tested suspended in isotonic pH 7.4 PBS were heated in autoclave (121°C for 20 min). After cooling to room temperature, the vials were opened and small amount of the solid drug added in order to provoke precipitation. Then the samples were equilibrated in a shaker at ambient temperature (23 ± 1°C) for 7 days. If the drug was chemically unstable, heating in an autoclave was replaced by sonication in an ultrasonic bath (60–70°C for 60 min). The degradation of drugs in studied solutions was monitored by HPLC and did not exceed 1%. After equilibration the samples were filtered through 0.45 μm membrane filter and the concentration of dissolved drug was determined by HPLC. At least three parallel experiments were carried out for each study condition.
The stability constants of drug/cyclodextrin complexes were calculated from the linear slopes of the phase-solubility diagrams (41):
 |
4 |
where KCD is the stability constant of a 1:1 stoichiometry complex, Slope is the slope of a linear phase-solubility diagram, and S0 is the determined intrinsic solubility of a drug in the aqueous complexation medium (PBS).
Protein Binding
The in vitro protein binding of the drugs was determined in the presence of HPβCD by equilibrium dialysis at room temperature (23 ± 1°C). The dialysis system consisted of Franz diffusion cells (FDC 400 15 FF, Vangard International, Neptune, NJ, USA) where a semi-permeable cellophane membrane (Spectra/Por® Dialysis Tubing from regenerated cellulose with MWCO 12,000–14,000, Spectrum Laboratories, USA) was sandwiched between 12 ml stirred receptor chamber and an unstirred donor chamber. The donor phase consisted of 0% to 0.5% (w/v) HPβCD and a given model drug dissolved in PBS. The receptor phase consisted of HSA dissolved in PBS that contained identical amount of HPβCD or 0% to 0.5% (w/v). The stirring rate of the receptor phase was 300 rpm. To prevent protein degradation (such as conformational changes or decomposition) the HSA solutions were prepared immediately prior to use. The concentration of the protein solutions was 40 mg/ml. The concentration of the model drugs in the donor phase was limited by their solubilities and the HPLC detection limits, and varied from 0.7·10−5 to 2.6·10−3 M (or from 0.023 to 0.65 mg/ml) for the different drugs. During dialysis, the solutions were stirred until drug concentration equilibrium between the two chambers had been reached. For each drug, the equilibrium time was determined from permeation profile such as the one shown in Fig. 1.
Fig. 1.
Typical kinetic curve for determination of equilibration time for the protein binding studies using ketoprofen as a sample drug
In all cases equilibrium was reached within 24 h. Due to osmotic phenomena small volume shift, ∆V, took place during dialysis. It was corrected for using the following equation:
 |
5 |
where c (unbound) is the true equilibrium concentration of protein-unbound drug, c (observed) is the apparent equilibrium concentration of protein-unbound drug after volume shift,  is the volume of donor phase after volume shift, and V (donor) is the donor phase volume before dialysis.
is the volume of donor phase after volume shift, and V (donor) is the donor phase volume before dialysis.
After equilibration had been reached at least four aliquots were taken from donor phase in every cell and concentrations were determined by HPLC. The protein-bound fraction of a drug was calculated by the following equation:
 |
6 |
where f (bound) is the drug protein-bound fraction, c (total) is the initial concentration of a drug in donor phase, and c (unbound) is the equilibrium concentration of protein-unbound drug. The absence of drug adsorption to the membrane and the glass cell surface during experiments was checked as described elsewhere (42). Each experiment was repeated three times, and the values given are the mean values ± standard deviation.
Quantitative Determinations
The quantitative determinations of the drugs studied were performed using a reverse phase HPLC component system from Dionex Softron GmbH (Germering, Germany) Ultimate 3000 Series, consisting of a P680 pump with a DG-1210 degasser, an ASI-100 autosampler, a VWD-3400 UV–vis detector, and Phenomenex Luna 5 μm C18 reverse-phase column (150 × 4.6 mm).
RESULTS AND DISUSSION
Drug selected for this study can be divided into four groups based on their physicochemical properties. The first group (amoxicillin, atropine and lidocaine) has low affinity for both HPβCD and plasma protein, the second group (acetazolamide and diclofenac) has low affinity for HPβCD but high affinity for protein, the third group (paracetamol) has high affinity for HPβCD but low affinity for protein, and the fourth group (ketoprofen and ketorolac) has high affinity for both HPβCD and protein. The KCD values of the drugs studied are shown in Table II. The increasing affinity of the drugs to HPβCD is as follows:  .
.
Table II.
Drug-HSA and Drug-HPβCD Binding Parameters (Protein Bound Fraction, f p, and Cyclodextrin Complex Stability Constant, K CD) with Some Pharmacokinetic Data (See Text for Symbol Decryption) (43–45)
| Compound |
 (%) (%) |
K CD (M −1)a | D b (mg) | τb (h) | V D (liters/kg) | t 1/2 (h) |
|---|---|---|---|---|---|---|
| Low HPβCD affinity, low HAS affinity | ||||||
| Amoxicillin | 20 | 2.5 | 500 | 8 | 0.3 | 1.7 |
| Atropine | 50 | 65 | 0.3 | 12 | 2.0 | 3.5 |
| Lidocaine | 70 | 17 | 100 | 1 | 1.1 | 1.8 |
| Low HPβCD affinity, medium or high HAS affinity | ||||||
| Acetazolamide | 95 | 60 | 500 | 24 | 0.2 | 13 |
| Diclofenac Na | 99.5 | –c | 50 | 12 | 0.17 | 1.1 |
| High HPβCD affinity, low HAS affinity | ||||||
| Paracetamol | <20 | –c | 500 | 6 | 0.95 | 2 |
| High HPβCD affinity, medium or high HAS affinity | ||||||
| Ketoprofen | 95 | 110 | 50 | 8 | 0.15 | 1.8 |
| Ketorolac | 99.2 | 270 | 10 | 6 | 0.21 | 5.3 |
| Cyclodextrin | ||||||
| HPβCD | – | – | 8,000d | 12d | 0.2 | 1.7 |
aExperimental values from the present work
bExample of a dosage regiment
cThe slope of the phase-solubility diagram was greater than unity indicating that the drug has high affinity for HPβCD but that it is not forming 1:1 drug/HPβCD complex (forming higher order complexes or mixtures of inclusion and non-inclusion complexes)
dDosage of HPβCD in Sporanox® parenteral solution
The drugs shown in parentheses give phase-solubility diagrams with slopes greater than unity and, consequently, it was not possible to estimate their KCD value or complexation efficiency, but the high slope indicates that the drugs have high affinity to HPβCD and can form inclusion complexes and/or aggregates of higher stoichiometry. Interestingly, direct relationship appears to be between the drug/HPβCD complex stability constants, KCD, and drug/HSA bound fraction values taken from literature,  (Fig. 2).
(Fig. 2).
Fig. 2.
Relationship between the drug-HPβCD complex stability coefficient, K
CD, and drug fraction bound to HSA,  . Numbering coincides with Table I
. Numbering coincides with Table I
It is thought that the affinity of a given drug to the cyclodextrin cavity is determined by the drug’s lipophilicity and molecular structure (i.e., how well a given drug moiety fits into the cavity). Apparently, similar criteria apply to drug-HSA interactions. Moreover, exponential relationship between KCD and  indicates that the drug protein binding is much more sensitive towards mentioned drug properties, especially towards lipophilic (van der Waals) interactions. In general, negatively charged and neutral drugs possess comparable affinity for HSA despite of the fact that HSA has a net negative charge at pH 7.4 (31). However, no linear correlation was observed between Log D7.4 and
indicates that the drug protein binding is much more sensitive towards mentioned drug properties, especially towards lipophilic (van der Waals) interactions. In general, negatively charged and neutral drugs possess comparable affinity for HSA despite of the fact that HSA has a net negative charge at pH 7.4 (31). However, no linear correlation was observed between Log D7.4 and  indicating that drug-HSA interactions are composed of complex set of factors.
indicating that drug-HSA interactions are composed of complex set of factors.
Figure 3 shows the results of the competitive binding studies. The figures show the fraction of drug bound to HSA (y-axis) as a function of total drug concentration, i.e., bound and free drug (x-axis). The binding profile of each drug was determined at three different HPβCD concentrations, i.e., 0.00%, 0.05%, and 0.50% (w/v). For ketoprofen the binding profile was also determined at 0.005% (w/v) HPβCD due to the drug’s anomalous sensitivity to the cyclodextrin coexistence. The affinity of the drugs tested towards HSA is given by the profiles obtained when no HPβCD is present and although the absolute values are not identical to their degree of protein binding in vivo in humans (i.e., the  values) their ranking is approximately the same. Amoxicillin, atropine, lidocaine and paracetamol have low affinity to HSA with less than 25% binding in vitro and less than 70% binding in vivo. Acetazolamide and ketoprofen have medium affinity to HSA with 50% to 65% binding in vitro but about 95% protein binding in vivo. Finally, diclofenac sodium and ketorolac have high affinity to HSA with over 70% binding in vitro and over 99% protein binding in vivo. The in vitro values, obtained in isotonic pH 7.4 PBS solution at room temperature, are all lower than in vivo values. Similar observations have been reported by other investigators (21). The drugs with medium and high affinity for HSA, i.e., acetazolamide, ketoprofen, diclofenac sodium, and ketorolac, demonstrate decreasing HSA binding with increasing total drug concentration which most likely is due to saturation of the binding sites.
values) their ranking is approximately the same. Amoxicillin, atropine, lidocaine and paracetamol have low affinity to HSA with less than 25% binding in vitro and less than 70% binding in vivo. Acetazolamide and ketoprofen have medium affinity to HSA with 50% to 65% binding in vitro but about 95% protein binding in vivo. Finally, diclofenac sodium and ketorolac have high affinity to HSA with over 70% binding in vitro and over 99% protein binding in vivo. The in vitro values, obtained in isotonic pH 7.4 PBS solution at room temperature, are all lower than in vivo values. Similar observations have been reported by other investigators (21). The drugs with medium and high affinity for HSA, i.e., acetazolamide, ketoprofen, diclofenac sodium, and ketorolac, demonstrate decreasing HSA binding with increasing total drug concentration which most likely is due to saturation of the binding sites.
Fig. 3.
Binding profiles of studied drugs to 4% w/v HSA at various concentrations of HPβCD. The error bars represent standard deviation
HSA binding of five of the eight drugs tested are not affected by HPβCD, i.e., acetazolamide, amoxicillin, atropine, lidocaine, and paracetamol, while three show some effect, i.e., diclofenac sodium, ketoprofen and ketorolac (Fig. 3). Acetazolamide, amoxicillin, atropine, lidocaine, and paracetamol have low affinity to HSA and although paracetamol has high affinity for HPβCD, presence of HPβCD has little or no effect on the relatively small fraction of the drug that is bound to HSA. In case of acetazolamide, which has medium affinity towards HSA, the affinity for the HPβCD cavity is not sufficient to affect its binding to HSA. HSA binding of three of the drugs tested, i.e., diclofenac sodium, ketoprofen, and ketorolac, are affected by HPβCD but only at HPβCD concentrations of 0.05% (w/v) or higher for ketoprofen and 0.5% (w/v) or higher for diclofenac sodium and ketorolac. The greatest decrease in HSA binding was observed for ketorolac, which has high affinity for both HPβCD and HSA, followed by diclofenac sodium and ketoprofen. The most likely mechanism is competitive drug/HPβCD and drug/HSA complex formation. However, an alternative mechanism could be competitive HPβCD/HSA and drug/HSA complex formation.
Although these studies of competitive binding were performed at room temperature (23 ± 1°C) they are still valid at physiologic temperature (37°C). Due to exothermicity of both drug/cyclodextrin and drug/HSA complexation processes the value of the stability constants of both complexes will decrease when the temperature is increased from room temperature to 37°C but the decrease is only moderate. For example, Tanaka et al. (46) have shown that binding of the nonsteroidal anti-inflammatory drug ibuprofen to bovine serum albumin decreased by only 14% when the temperature was increased from 25°C to 37°C. In addition, experiments such as these performed at elevated temperatures are known to give higher experimental error due to the temperature gradient between thermostated sample and laboratory environment as well as protein denaturation (39,47).
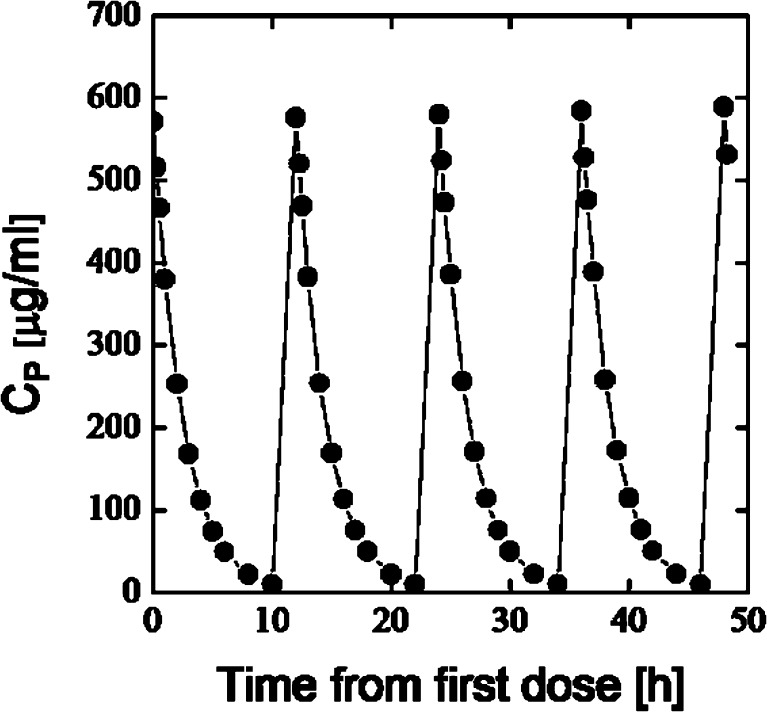
HPβCD has a small volume of distribution (VD ≈ 0.2 l/kg) and short half-life (t1/2 ≈ 1.7 h), and is mainly excreted unchanged with the urine after parenteral administration to humans (Table II) (6,8). Highest dose of HPβCD in currently marketed product is in Sporanox® (itraconazole) parenteral solution. Single dose of Sporanox® parenteral solution contains 8 g of HPβCD given intravenously twice a day (Fig. 4).
Fig. 4.
Simulated plasma concentration (C P)—time profile in man (70 kg) after intravenous administration of 8 g of HPβCD twice a day for 4 days. One compartment open model; V D = 0.2 l/kg; t 1/2 = 1.7 h
The maximum and minimum HPβCD plasma concentrations are approximately 580 and 4 μg/ml, respectively. Thus, maximum concentration of HPβCD in plasma will be less than 0.06% (w/v) and HPβCD will be eliminated almost completely from the blood circulation before the next dose is given 12 h later. Consequently, HPβCD will not accumulate in the body.
CONCLUSION
Published in vivo studies have indicated that drug/HPβCD and drug/SBEβCD complexes have negligible effect on drug pharmacokinetics (9–16). Present results show that this can be explained by competitive drug binding between cyclodextrin and plasma proteins. The competitive binding studies show that protein binding of drugs that are both highly protein bound and have high affinity to HPβCD (i.e., have high K1:1 value) is most likely to be affected by parenterally administered HPβCD. Furthermore, the HPβCD concentration has to be relatively high to affect the drug protein binding.
Acknowledgements
The financial support provided by Javelin Pharmaceuticals, Inc. (Cambridge, USA) is gratefully acknowledged by the authors.
Contributor Information
Sergey V. Kurkov, Email: kurkov@hi.is
Thorsteinn Loftsson, Phone: +354-525-4464, FAX: +354-525-4071, Email: thorstlo@hi.is.
Martin Messner, Email: messner@hi.is.
Donna Madden, Email: dmadden@javelinpharma.com.
References
- 1.Loftsson T, Duchêne D. Cyclodextrins and their pharmaceutical applications. Int J Pharm. 2007;329:1–11. doi: 10.1016/j.ijpharm.2006.10.044. [DOI] [PubMed] [Google Scholar]
- 2.Brewster ME, Loftsson T. Cyclodextrins as pharmaceutical solubilizers. Adv Drug Deliv Rev. 2007;59:645–666. doi: 10.1016/j.addr.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 3.Loftsson T, Másson M, Brewster ME. Self-association of cyclodextrins and cyclodextrin complexes. J Pharm Sci. 2004;93:1091–1099. doi: 10.1002/jps.20047. [DOI] [PubMed] [Google Scholar]
- 4.Jansook P, Kurkov SV, Loftsson T. Cyclodextrins as solubilizers: formation of complex aggregates. J Pharm Sci. 2010;99(2):719–729. doi: 10.1002/jps.21861. [DOI] [PubMed] [Google Scholar]
- 5.Stella VJ, Rajewski RA. Cyclodextrins: their future in drug formulation and delivery. Pharm Res. 1997;14(5):556–567. doi: 10.1023/A:1012136608249. [DOI] [PubMed] [Google Scholar]
- 6.Stella VJ, He Q. Cyclodextrins. Tox Pathol. 2008;36:30–42. doi: 10.1177/0192623307310945. [DOI] [PubMed] [Google Scholar]
- 7.Uekama K. Design and evaluation of cyclodextrin-based drug formulation. Chem Pharm Bull. 2004;52:900–915. doi: 10.1248/cpb.52.900. [DOI] [PubMed] [Google Scholar]
- 8.Zhou H, Goldman M, Wu J, Woestenborghs R, Hassell AE, Lee P, et al. A pharmacokinetic study of intravenous intraconazole followed by oral administration of intraconazole capsules in patients with advanced human immunodeficiency virus infection. J Clin Pharmacol. 1998;38:593–602. doi: 10.1002/j.1552-4604.1998.tb04465.x. [DOI] [PubMed] [Google Scholar]
- 9.Piel G, Evrard B, Van Hees T, Delattre L. Comparison of the IV pharmacokinetics in sheep of miconazole–cyclodextrin solutions and a micellar solution. Int J Pharm. 1999;180:41–45. doi: 10.1016/S0378-5173(98)00403-7. [DOI] [PubMed] [Google Scholar]
- 10.Liu J, Qiu L, Gao J, Jin Y. Preparation, characterization and in vivo evaluation of formulation of baicalein with hydroxypropyl-β-cyclodextrin. Int J Pharm. 2006;312:137–143. doi: 10.1016/j.ijpharm.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 11.Muller BW, Alberts E. Effect of hydrotropic substances on the complexation of sparingly soluble drugs with cyclodextrin derivatives and the influence of cyclodextrin complexation on the pharmacokinetics of the drugs. J Pharm Sci. 1991;80:599–604. doi: 10.1002/jps.2600800620. [DOI] [PubMed] [Google Scholar]
- 12.Egan TD, Kern SE, Johnson KB, Pace NL. The pharmacokinetics and pharmacodynamics of propofol in modified cyclodextrin formulation (Captisol®) versus propofol in a lipid formulation (Diprivan®): an electroencephalographic and hemodynamic study in a porcine model. Anesth Analg. 2003;97:72–79. doi: 10.1213/01.ANE.0000066019.42467.7A. [DOI] [PubMed] [Google Scholar]
- 13.Buggins TR, Dickinson PA, Taylor G. The effects of pharmaceutical excipients on drug disposition. Adv Drug Deliv Rev. 2007;59:1482–1503. doi: 10.1016/j.addr.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 14.Stella VJ, Lee HK, Thompson DO. The effect of SBE4-β-CD on i.m. prednisolone pharmacokinetics and tissue damage in rabbits: comparison to a co-solvent solution and a water-soluble prodrug. Int J Pharm. 1995;120:197–204. doi: 10.1016/0378-5173(94)00405-T. [DOI] [Google Scholar]
- 15.McIntosh MP, Schwarting N, Rajewski RA. In vitro and in vivo evaluation of a sulfobutyl ether β-cyclodextrin enabled etomidate formulation. J Pharm Sci. 2004;93:2585–2594. doi: 10.1002/jps.20160. [DOI] [PubMed] [Google Scholar]
- 16.Zuo Z, Tam YK, Diakur J, Wiebe LI. Hydroxypropyl-β-cyclodextrin-flutamide inclusion complex. II. Oral and intravenous pharmacokinetics of flutamide in the rat. J Pharm Pharm Sci. 2002;5:300–306. [PubMed] [Google Scholar]
- 17.Adam JM, Bennett DJ, Bom A, Clark JK, Feilden H, Hutchinson EJ, et al. Cyclodextrin-derived host molecules as reversal agents for the neuromuscular blocker rocuronium bromide: synthesis and structure-activity relationships. J Med Chem. 2002;45:1806–1816. doi: 10.1021/jm011107f. [DOI] [PubMed] [Google Scholar]
- 18.Welliver M. New drug sugammadex: a selective relaxant binding agent. AANA J. 2006;74:357–363. [PubMed] [Google Scholar]
- 19.Perry CS, Charman SA, Prankerd RJ, Chiu FCK, Scanlon MJ, Chalmers D, et al. The binding interaction of synthetic ozonide antimalarials with natural and modified β-cyclodextrins. J Pharm Sci. 2006;95:146–158. doi: 10.1002/jps.20525. [DOI] [PubMed] [Google Scholar]
- 20.Charman SA, Perry CS, Chiu FCK, McIntosh KA, Prankerd RJ, Charman WN. Alteration of the intravenous pharmacokinetics of a synthetic ozonide antimalarial in the presence of a modified cyclodextrin. J Pharm Sci. 2006;95:256–267. doi: 10.1002/jps.20534. [DOI] [PubMed] [Google Scholar]
- 21.Dubois N, Lapicque F, Abiteboul M, Netter P. Stereoselective protein binding of ketoprofen: effect of albumin concentration and of the biological system. Chirality. 1993;5:126–134. doi: 10.1002/chir.530050305. [DOI] [PubMed] [Google Scholar]
- 22.Flieger J, Swieboda R, Tatarczak M. Chemometric analysis of retention data from salting-out thin-layer chromatography in relation to structural parameters and biological activity of chosen sulphonamides. J Chrom B. 2007;846:334–340. doi: 10.1016/j.jchromb.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 23.Mikolasch A, Niedermeyer THJ, Lalk M, Witt S, Seefeldt S, Hammer E, et al. Novel penicillins synthesized by biotransformation using laccase from Trametes spec. Chem Pharm Bull. 2006;54:632–638. doi: 10.1248/cpb.54.632. [DOI] [PubMed] [Google Scholar]
- 24.Alimuddin M, Grant D, Bulloch D, Lee N, Peacock M, Dahl R. Determination of log D via automated microfluidic liquid-liquid extraction. J Med Chem. 2008;51:5140–5142. doi: 10.1021/jm8005228. [DOI] [PubMed] [Google Scholar]
- 25.Barbato F, La Rotonda MI, Quaglia F. Interactions of nonsteroidal antiinflammatory drugs with phospholipids: comparison between octanol/buffer partition coefficients and chromatographic indexes on immobilized artificial membranes. J Pharm Sci. 1997;86(2):225–229. doi: 10.1021/js960233h. [DOI] [PubMed] [Google Scholar]
- 26.Malhotra M, Majumdar DK. In vitro transcorneal permeation of ketorolac from oil based ocular drops and ophthalmic ointment. Ind J Exp Biol. 1997;35:1324–1330. [PubMed] [Google Scholar]
- 27.Puglia C, Filosa R, Peduto A, de Caprariis P, Boatto G, Nieddu M, et al. Synthesis, physicochemical properties and in vitro permeation studies of new ketorolac ester derivatives. Curr Drug Deliv. 2007;4:205–210. doi: 10.2174/156720107781023893. [DOI] [PubMed] [Google Scholar]
- 28.Wilson DM, Wang X, Walsh E, Rourick RA. High throughput log D determination using liquid chromatography-mass spectrometry. Comb Chem High Throughput Screen. 2001;4:511–519. doi: 10.2174/1386207013330913. [DOI] [PubMed] [Google Scholar]
- 29.Giaginis C, Theocharis S, Tsantili-Kakoulidou A. Contribution to the standardization of the chromatographic conditions for the lipophilicity assessment of neutral and basic drugs. Anal Chim Acta. 2006;573–574:311–318. doi: 10.1016/j.aca.2006.03.074. [DOI] [PubMed] [Google Scholar]
- 30.Magnusdottir A, Masson M, Loftsson T. Self association and cyclodextrin solubilization of NSAIDs. J Incl Phen Macro Chem. 2002;44:213–218. doi: 10.1023/A:1023079322024. [DOI] [Google Scholar]
- 31.Kragh-Hansen U. Molecular aspects of ligand binding to serum albumin. Pharm Rev. 1981;33:17–53. [PubMed] [Google Scholar]
- 32.Sun SF, Hsiao CL. Qualitative study of the competition of drugs in binding to serum albumin. Chromatographia. 1993;37:329–335. doi: 10.1007/BF02278643. [DOI] [Google Scholar]
- 33.Rahman MM, Rahman MH, Rahman NN. Competitive binding of ibuprofen and naproxen to bovine serum albumin: modified form of drug-drug displacement interaction at the binding site. Pak J Pharm Sci. 2005;18:43–47. [PubMed] [Google Scholar]
- 34.Kochansky CJ, McMasters DR, Lu P, Koeplinger KA, Kerr HH, Shou M, et al. Impact of pH on plasma protein binding in equilibrium dialysis. Mol Pharm. 2008;5:438–448. doi: 10.1021/mp800004s. [DOI] [PubMed] [Google Scholar]
- 35.Dutta SK, Basu SK, Sen KK. Binding of diclofenac sodium with bovine serum albumin at different temperatures, pH and ionic strengths. Ind J Exp Biol. 2006;44:123–127. [PubMed] [Google Scholar]
- 36.Matsuyama K, El-Gizawy S, Perrin JH. Thermodynamics of binding of aromatic amino acids to α-, β- and γ-cyclodextrins. Drug Dev Ind Pharm. 1987;13:2687–2691. doi: 10.3109/03639048709022084. [DOI] [Google Scholar]
- 37.Brewster ME, Hora MS, Simpkins JW, Bodor N. Use of 2-hydroxypropyl β-cyclodextrin as a solubilizing and stabilizing excipient for protein drugs. Pharm Res. 1991;8:792–795. doi: 10.1023/A:1015870521744. [DOI] [PubMed] [Google Scholar]
- 38.Katakam M, Banga AK. Aggregation of proteins and its prevention by carbohydrate excipients: albumins and γ-globulin. J Pharm Pharm. 1995;47:103–107. doi: 10.1111/j.2042-7158.1995.tb05759.x. [DOI] [PubMed] [Google Scholar]
- 39.Sideris EE, Koupparis MA, Macheras PE. Effect of cyclodextrins on protein binding of drugs: the diflunisal/hydroxypropyl β-cyclodextrin model case. Pharm Res. 1994;11(1):90–95. doi: 10.1023/A:1018901912619. [DOI] [PubMed] [Google Scholar]
- 40.Loftsson T, Hreinsdottir D, Masson M. Evaluation of cyclodextrin solubilization of drugs. Int J Pharm. 2005;302:18–28. doi: 10.1016/j.ijpharm.2005.05.042. [DOI] [PubMed] [Google Scholar]
- 41.Higuchi T, Connors KA. Phase-solubility techniques. Adv Anal Chem Instrum. 1965;4:117–212. [Google Scholar]
- 42.Deschamps-Labat L, Pehourcq F, Jagou M, Bannwarth B. Relationship between lipophilicity and binding to human serum albumin of arylpropionic acid nonsteroidal anti-inflammatory drugs. J Pharm Biomed Anal. 1997;16:223–229. doi: 10.1016/S0731-7085(97)00017-4. [DOI] [PubMed] [Google Scholar]
- 43.Hardman JG, Limbird LE, editors. Goodman & Gilman's the pharmacological basis of therapeutics. 10. New York: McGraw-Hill; 2001. [Google Scholar]
- 44.Moffat AC, Osselton MD, Widdop B, editors. Clarke's analysis of drugs and poisons. 3. London: The Pharmaceutical Press; 2004. [Google Scholar]
- 45.Sweetman SC, editor. Martindale, the complete drug reference. 33. London: The Pharmaceutical Press; 2002. [Google Scholar]
- 46.Tanaka M, Asahi Y, Masuda S, Ota T. Interaction between drugs and water-soluble polymers. Part III. Binding position of ibuprofen with bovine serum albumin determined by measuring nuclear magnetic resonance relaxation time. Chem Pharm Bull. 1991;39:1–4. doi: 10.1248/cpb.39.2771. [DOI] [PubMed] [Google Scholar]
- 47.Eriksson MAL, Gabrielsson J, Nisson LB. Studies of drug binding to plasma proteins using a variant of equilibrium dialysis. J Pharm Biomed Anal. 2005;38:381–389. doi: 10.1016/j.jpba.2005.01.015. [DOI] [PubMed] [Google Scholar]