Abstract
A novel transmembrane pH gradient active loading method to prepare alkaloids binary ethosomes was developed in this work. Using this novel method, binary ethosomes containing total alkaloids extracted from Sophora alopecuroides (TASA) were prepared successfully at the temperature below the phase transition temperature (Tc) of the phosphatidyl choline (PC). Several factors affecting this method were investigated. The qualities of the TASA binary ethosomes were characterized by the shape, particle size, and encapsulation efficiency (EE). The percutaneous absorption study of TASA binary ethosomes was performed using confocal laser scanning microscopy and Franz diffusion cells. The results showed that more than 90% sophoridine, 47% matrine, 35% sophocarpine, and 32% lemannine in TASA were entrapped within 1 h at 40°C, with an efficiency improvement of 8.87, 8.10, 7.63, and 7.78-fold than those observed in passive loading method. Transdermal experiments showed that the penetration depth and fluorescence intensity of Rhodamine B from binary ethosome prepared by pH gradient active loading method were much greater than that from binary ethosome prepared by passive loading method or hydroalcoholic solution. These results suggested transmembrane pH gradient active loading method may be an effective method to prepare alkaloids ethosomal systems at the temperatures below the Tc of PC.
Key words: alkaloids, ethosomes, pH gradient, Sophora alopecuroides, transdermal delivery
INTRODUCTION
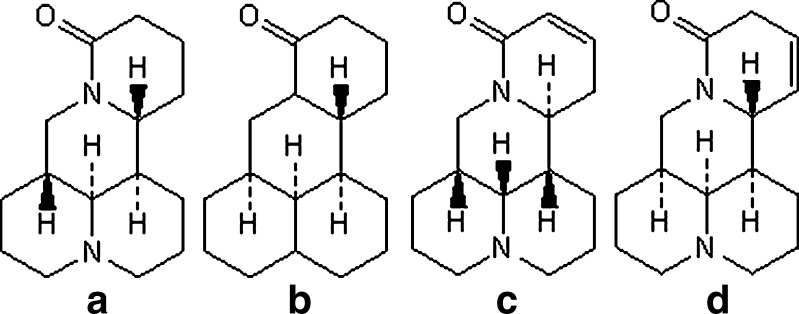
Sophora alopecuroides as an important Chinese herbal is widely distributed in the northwestern region of China. Total alkaloids extracted from S. alopecuroides (TASA), which contain many different ingredients like sophocarpine, matrine, oxymatrine, sophoridine, sophocarpine, sophoramine, aloperine and so on, are posses remarkable pharmacological activities, such as antiendotoxic, anticancer, and anti-inflammatory (1–3). It has been proved that TASA are nitrogen-containing heterocyclic compounds, which belong to the quinolizidine alkaloids, and very soluble in water and ethanol (4–6). The taste of TASA is very bitter. Among of TASA, sophoridine (SR), matrine (MA), sophocarpine (SC), and lemannine (LMN; structures shown in Fig. 1) comprise the largest group of alkaloids in TASA (4). Recent studies have also demonstrated that TASA were useful in the treatment of oral herpes catarrhalis, dental ulcer and herpes zoster (5,6). However, the use of TASA external preparation is limited by its low percutaneous penetration and bitter taste.
Fig. 1.
Structures of main alkaloids extracted from Sophora alopecuroides. a Sophoridine, b matrine, c sophocarpine, d lemannine
Ethosome delivery system is an interesting and innovative vesicular system embodying ethanol in relatively high concentration. It is a soft and malleable vesicle for enhancing delivery of active agents through the stratum corneum barrier into the deep layer of the skin (7,8). Therefore, that TASA were entrapped by the ethosome could reduce the bitter taste and make possible the transporting of active substances through the stratum corneum barrier into the deep layer of the skin. It could improve the compliance, increase selectivity for disease tissue, reduce the dose, and improve therapeutic index.
In the present study, the binary ethosomes of TASA were successfully prepared by using a novel transmembrane pH gradient active loading method at the temperature below the phase transition temperature (Tc) of the phosphatidyl choline (PC). In order to obtain the best performance, several factors affecting the encapsulation efficiency (EE) of TASA binary ethosome were investigated. Namely, the internal and external phase pH values of TASA binary ethosomes, the percentage of binary alcohol phase (comprised ethanol and propylene glycol, BAP) in the formulation, the incubation temperature, and the incubation period. Moreover, the binary ethosomes prepared by this novel method were characterized by transmission electron microscopy (TEM), the percutaneous absorption of TASA binary ethosomes was evaluated by confocal laser scanning microscopy (CLSM) and Franz diffusion cells.
MATERIALS AND METHODS
Chemicals and Reagents
Total alkaloids extracted from S. alopecuroides and the standard substances of SR, MA, SC, and LMN were provided by Zijinhua Pharmaceutical Factory (Ningxia, China). High purity PC (98%), phosphotungstic acid (PTA), Triton–X 100, and Sephadex G–50 were purchased from Sigma Chemicals (Missouri, USA). Rhodamine B (C28H31N2O3, [9-(2-carboxyhenyl)-6-diethylamino-3-xanthenylidene]-diethylammo nium chloride) and propylene glycol were purchased from Pengcheng Chemical Co., Ltd., (Lanzhou, China). All other solvents were of high-performance liquid chromatography (HPLC) grade, and double-distilled water was used wherever required.
Animals
Male Kunming mice (20–22 g) were obtained from the Laboratory Animal Center of Lanzhou University and bred for 2 days. Animal experimental protocols were reviewed and approved by the Institutional Animal Experimentation Committee of Lanzhou University.
Preparation of TASA Binary Ethosomes by the Passive Loading Method
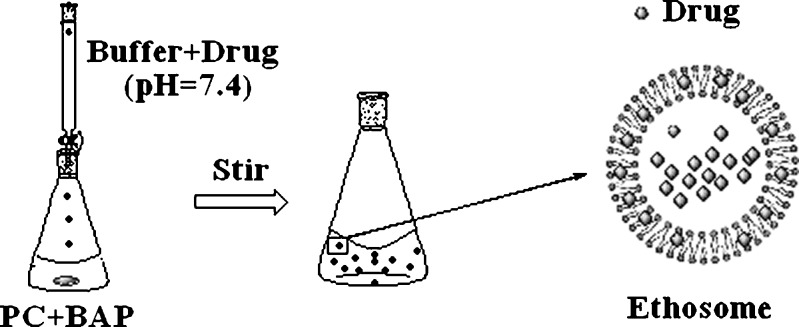
TASA binary ethosomes were prepared by the classic passive loading method as elsewhere reported (7,9). The diagram of this method was shown in Fig. 2. Briefly, PC was dissolved in BAP. Citrate buffer (pH = 7.4) with TASA was slowly added into the PC hydroalcoholic solution in dropwise with constant stirring (700 rpm) in a closed container at 30 ± 1°C. Mixing was continued for an additional 5 min, and then left to cool at room temperature for 30 min.
Fig. 2.
The formation of binary ethosomes containing drug by the passive loading method
Preparation of TASA Binary Ethosomes by the Active Loading Method
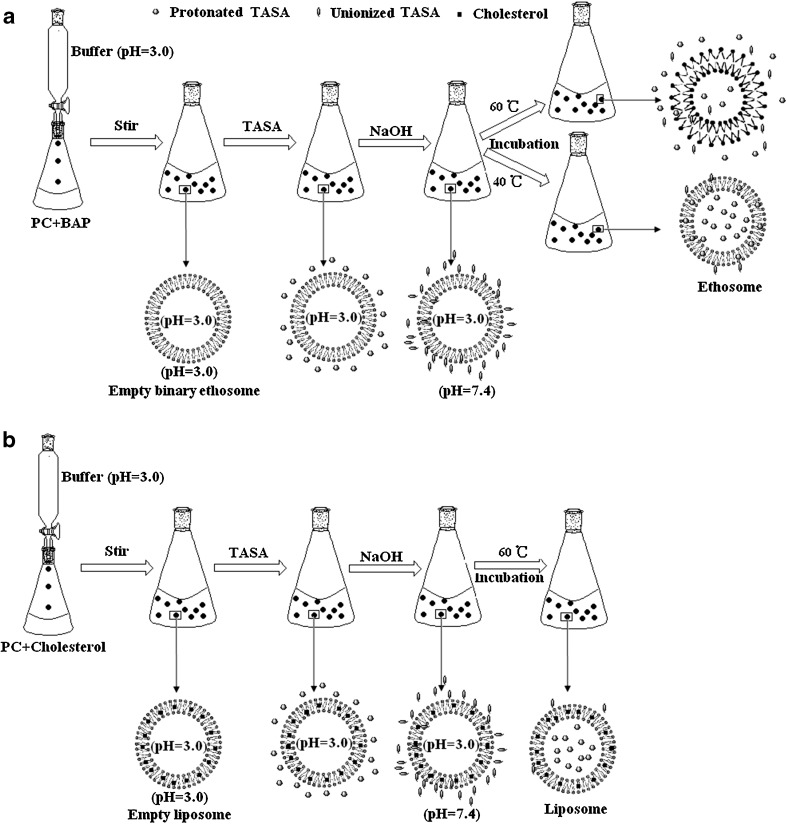
The diagram of the preparation procedure of TASA binary ethosomes were illustrated in Fig. 3a. This method included two steps. The first step was the formation of empty binary ethosomes. Briefly, PC was dissolved in BAP (comprised ethanol and propylene glycol). Citrate buffer was slowly added into the PC hydroalcoholic solution dropwise with constant stirring at 700 rpm in a closed container. Mixing was continued for an additional 5 min. The system was kept at 30 ± 1°C throughout the preparation, subsequently cooled at room temperature and the empty binary ethosomes were prepared (7,10). The second step was drug loading. Briefly, 1% (w/v) TASA was added into the empty binary ethosomes prepared in the first step, and the system was stirred at 700 rpm in a closed container to disperse and dissolve the TASA. In order to form the transmembrane pH gradient between the external phase (alkaline) and internal phase (acid) of ethosome, NaOH solution (0.5 M) was added into the system to adjust the external pH of ethosomes. Then, the system was incubated under an adequate time and temperature. The unionized drugs could across the lipid bilayer and were entrapped into the vesicles.
Fig. 3.
Diagrams of the process and mechanism for pH gradient active loading of drugs into a binary ethosomes and b traditional liposomes. Empty lipid vesicles were obtained by passive loading method in citrate buffer (pH = 3.0). After adjusting the external pH of lipid vesicles with 0.5 M NaOH solution to form ΔpH (inside acidic) and shift the alkaloids from protonated form to unionized form, the neutral drugs can across the lipid bilayer by incubation and can be trapped into the vesicles and protonated again
In order to investigate the percutaneous absorption of binary ethosomes visually, 0.1% (w/v) Rhodamine B binary ethosomes were prepared. The preparation procedure of Rhodamine B binary ethosomes was same, except for the drug (Rhodamine B was used instead of TASA).
Preparation of Hydroalcoholic Solution with TASA or Rhodamine B
Hydroalcoholic solution (with 45% BAP, the volume ratio of ethanol to propylene 1:1) with TASA (1%, w/v) or Rhodamine B (0.1%, w/v) was prepared to be used as a control.
Visualization of Binary Ethosomes by TEM
TEM was used as a visualizing aid for vesicles. Samples were negatively stained with a 1% aqueous solution of PTA. Vesicular suspension samples were dried on a carbon-coated grid for staining. The excess solution was removed by blotting. After drying, the specimen was viewed under the microscope at 10–100 k-fold enlargements at an accelerating voltage of 120 kV.
Determination of EE to Four Marker Components SR, MA, SC, and LMN in TASA by HPLC
The HPLC system was carried out using a Shimadzu LC-20A HPLC system (Kyoto, Japan), which consisted of a binary gradient pump (model LC-20A), a SPD-20MA diode-array detector, a SiL-20A auto sampler, a DGU-20A3 degasser and CTO-20A column oven. The apparatus was interfaced to a Dell PC compatible computer using LC solution software.
SR, MA, SC, and LMN comprise the largest groups of alkaloids in TASA, which were quantified simultaneously by HPLC with a reverse phase column (Cosmosil–ODS C18, 5 μm, 4.6 × 150 mm) to investigate the EE of TASA binary ethosomes. The mobile phase consisted of methanol–acetonitrile–0.02 M ammonium acetate buffer–triethylamine (5:16:79:0.05, v/v/v/v). Prior to use, the mobile phase was filtered through a 0.45 μm membrane filter. The mobile phase was delivered at a flow rate of 1.0 ml/min. Detection was performed at a wavelength of 220 nm at 40°C. The sample injection volume was 20 μl (11,12).
TASA binary ethosomes were separated from the free (unentrapped) drug by a Sephadex G–50 column. The experiment was repeated at least three times. The vesicles were demolished by Triton X–100 (0.5% w/w), and the entrapped drug was determined using HPLC as described above. The EE of the drug molecule was calculated according to the equation as follows:
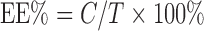 |
1 |
Where T is the total amount of drug that is detected in vesicular suspension and C is the amount of entrapped drug that is separated by a Sephadex G–50 column.
Skin Deposition of TASA Binary Ethosomes vs. TASA Hydroalcoholic Solution
Percutaneous experiments of TASA binary ethosomes were carried out by using Franz diffusion cells with an effective permeation area and receptor cell volume of 2.8 cm2 and 6.5 ml, respectively. The hair of 5–6-weeks-old Kunming mice was trimmed carefully (<2 mm), a patch of skin was excised from the back region of each sacrificed mice and the subcutaneous fat was removed. Then, the skins were washed and examined for integrity. The 300 μl TASA binary ethosomes or TASA hydroalcoholic solution (with 45% BAP) were applied on the skin in the donor compartment. The skin was removed from Franz diffusion after 24 h, placed on aluminum foil, and wiped promptly with cotton swabs soaked in methanol. Then, the skin was cut, and the drug in the skin was extracted by ultrasonic extraction in methanol solution. The extract solution was centrifuged for 10 min at 7,000 rpm, and the supernatant was analyzed for drug content by HPLC. Samples (200 μL) were withdrawn through the sampling port of the diffusion cell, and were analyzed for the accumulated amount of drug in the donor solution by HPLC. Triplicate experiments were conducted for each study and the average values were calculated.
Depth of Rhodamine B Skin Penetration from Binary Ethosomes and Hydroalcoholic Solution Visualized by CLSM
Depth of skin penetration of Rhodamine B-loaded binary ethosomes was investigated using CLSM (Leica SP2, Germany). The hair of test animals were carefully trimmed short (<2 mm) with a pair of scissors firstly. The Rhodamine B-loaded binary ethosomes were applied non-occlusively for 4 h to the dorsal skin of 5–6-weeks-old Kunming mice. Treated animals were kept in the separate cages and supplied with food and water freely under laboratory conditions. The dorsal skin was moved from animals, placed on aluminum foil, and wiped promptly with cotton swabs soaked in methanol. The excised skin was then placed on aluminum foil and the dermal side of the skin was gently teased off any adhering fat and subcutaneous tissue. The skin was sectioned into the pieces of 1 mm2 size and evaluated for depth of probe penetration. The full skin thickness was optically scanned at different increments through the z–axis of a CLSM. Optical excitations were carried out with a 554 nm argon laser beam and fluorescence emission was detected 575 nm for fluorescence.
Statistical Analysis
All the results were expressed as mean ± standard deviation (n = 3 independent samples). Statistical analysis was carried out employing the Student’s t test using the software SPSS12.0. A value of P < 0.05 was considered statistically significant.
RESULTS AND DISCUSSION
In general, the classic ethosomes are composed of phospholipids, ethanol (20~45%), and water. They are often prepared by passive loading method, namely, the formation of ethosomes and drug loading by one step, as shown in Fig. 2 (7,9). However, classic passive loading method is not very good to entrap hydrosoluble drugs. Moreover, ethosomes containing hydrosoluble molecules were readily leaky (7,8). So it is very difficult to prepare high EE, stable TASA ethosomes by classic passive loading method due to the fact that alkaloids extracted from S. alopecuroides are hydrosoluble.
However, a variety of amino-containing drugs can be efficiently encapsulated into liposomes (ethanol in liposomal formulation less than 10%) by the use of transmembrane pH gradient active loading method, as presented in Fig. 3b (13,14). This classic pH gradient active loading method relied on incubation temperatures above the Tc of the PC to promote drug loading (13,15,16). Nevertheless, the permeability of PC bilayer without cholesterol is enhanced at this temperature, which can result in the collapse of the pH gradient and the unstable loading (10). So ethosomes also could not be prepared by the classic pH gradient loading methods because there was no cholesterol in ethosomal formulation. However, Dos Santos et al. (10) suggested that ethanol addition was an effective method to achieve efficient pH gradient dependent loading of cholesterol–free-lipid vesicles at temperatures below the Tc of the PC. Inspired by this idea, TASA could be efficiently entrapped into ethosomes by pH gradient active loading method at temperatures below the Tc of the PC in theory, because ethosome as a lipid carrier just without cholesterol contain ethanol in relatively high concentration and TASA are a group of amino-containing compounds.
In this study, TASA binary ethosomes were prepared by classic passive loading method (without transmembrane pH gradient, pH 7.0 inside, 7.0 outside) and pH gradient active loading method (with transmembrane pH gradient, pH 3.0 inside, pH 7.0 outside), respectively. The results showed that greater than 90% SR, 47% MA, 35% SC, and 32% LMN in TASA were encapsulated by pH gradient active loading method, which was 8.87, 8.10, 7.63, and 7.78-fold for traditional passive loading method, respectively. This suggested that the pH gradient play an important role in the entrapment of TASA.
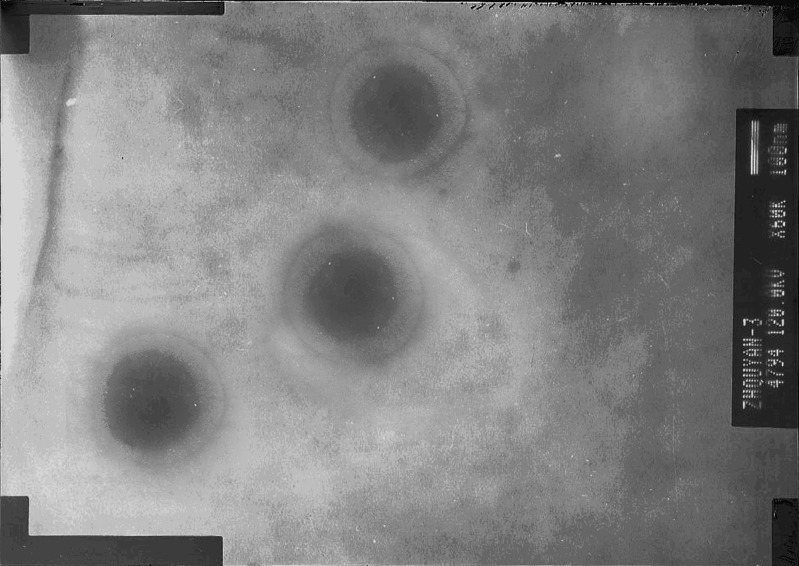
The appearance of TASA binary ethosomes prepared by pH gradient active loading method (bearing 45% BAP, with the volume ratio of ethanol to propylene glycol 1:1, pH 3.0 inside, pH 7.4 outside, incubated at 40°C) was observed by TEM and shown in Fig. 4. It showed that the binary ethosomes had intact spherical shape and lipid bilayer. The mean size of ethosomes is 142 ± 15.5 nm. These indicated that ethosomes prepared by this novel method can also keep intact lipid bilayer with high BAP concentration. Therefore, it is feasible to prepare ethosomes by pH gradient loading method at temperatures below the Tc of the PC.
Fig. 4.
Visualization of TASA binary ethosomes by TEM (the microscope at 50 k-fold enlargements at an accelerating voltage of 120 kV)
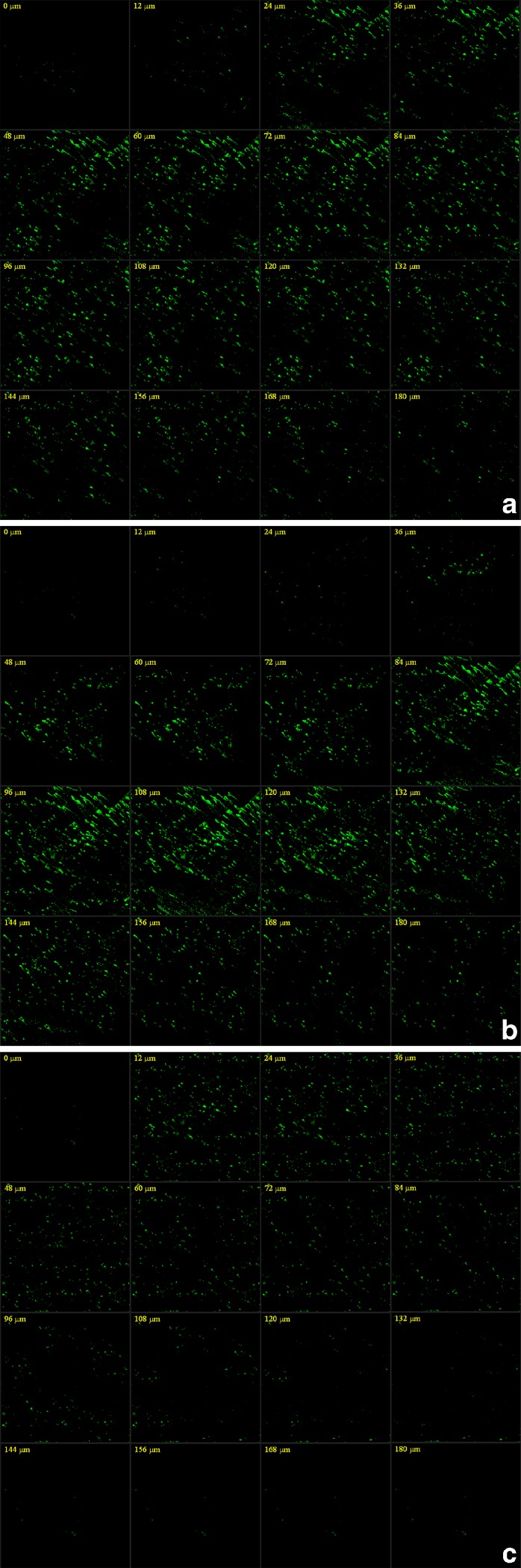
The skin penetration of TASA binary ethosomes were investigated by using Franz diffusion cells. Percutaneous experiments were carried out from the three systems (hydroalcoholic solution with TASA; TASA binary ethosomes prepared by passive loading method; TASA binary ethosomes prepared by pH gradient active loading method). The results had been shown in the Table I. It can be seen that TASA binary ethosomes prepared by pH gradient active loading method markedly increased the amount of TASA in the skin and in the donor solution. In order to investigate the percutaneous absorption of binary ethosomes more visually, 0.1% (w/v) Rhodamine B binary ethosomes was prepared by the pH gradient active loading method and classical passive loading methd, respectively. CSLM of skin after 4 h application of Rhodamine B from either binary ethosomes or hydroalcoholic solution were shown in Fig. 5a–c, respectively. Using hydroalcoholic solution of Rhodamine B, the penetration depth was up to about 120 μm, but of relatively very low fluorescence intensity. However, the use of fluorescein binary ethosomal system resulted in an increase in both the depth (up to about 180 μm) of penetration and the fluorescence intensity. The fluorescence intensity (range from 84 to 180 nm) from binary ethosomes prepared by pH gradient active loading method was significantly stronger than that from ethosomes prepared by passive loading method. So TASA entrapped by binary ethosmes could easily penetrate into deeper layers of the skin and increase the amount of drug in the skin. These results might be caused by the flexible characteristics of binary ethosomal system, and the higher EE of ethosomes binary ethosomes prepared by pH gradient active loading method because Elsayed et al. (17) suggested that ethosomes were not able to improve skin delivery of a non-entrapped drug.
Table I.
Comparison of the Percutaneous Absorption of Drug from Different Systems After 24 h (mean ± SD, n = 3)
| Qs (mg/cm2) | Percent deposition amount of drug in the skin (24 h) | J (mg/cm2/h) | Percent accumulated amount of drug in the donor solution (24 h) | ||
|---|---|---|---|---|---|
| Hydroalcoholic solution | SR | 7.19 | 11.24 | 0.71 | 26.64 |
| MA | 1.39 | 7.62 | 0.14 | 18.06 | |
| SC | 0.70 | 6.13 | 0.07 | 14.53 | |
| LMN | 0.12 | 5.05 | 0.01 | 11.97 | |
| Ethosome prepared by passive loading method | SR | 8.17 | 12.78 | 0.93 | 34.89 |
| MA | 1.58 | 8.66 | 0.18 | 23.65 | |
| SC | 0.80 | 6.97 | 0.09 | 19.03 | |
| LMN | 0.14 | 5.74 | 0.02 | 15.68 | |
| Ethosomes prepared by pH gradient active loading method | SR | 14.85 | 23.21 | 1.47 | 55.00 |
| MA | 2.43 | 13.32 | 0.24 | 31.57 | |
| SC | 1.26 | 11.02 | 0.12 | 26.12 | |
| LMN | 0.25 | 10.03 | 0.02 | 23.77 | |
Qs represents the quantity of drugs in the skin, J represents the fluxes of drug
Fig. 5.
CLSM micrographs of mouse skin, after application of the Rhodamine B from: a binary ethosomes prepared by the classical passive loading method, b binary ethosomes prepared by the pH gradient active loading method, and c hydroalcoholic solution (optical excitation was carried out with a 554 nm argon laser beam and fluorescence emission was detected 575 nm)
In summary, the binary ethosomes prepared by this novel method also were soft and malleable vesicle for enhancing delivery of active agents, which could be the very promising carriers for the topical administration. In order to obtain the best performance, several factors affecting the pH gradient active loading method were investigated and discussed as follow. Namely, the internal and external phase pH values of binary ethosomes, the BAP in the formulation, the incubation temperature, and the incubation period.
Effect of Internal Phase pH Values on the EE of TASA Binary Ethosomes
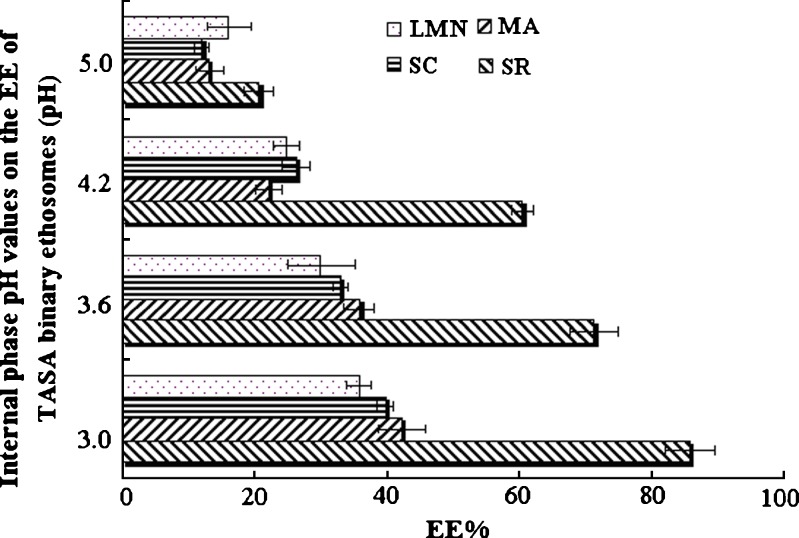
TASA binary ethosomes were prepared by pH gradient active loading method with Citrate buffer (0.10 M) at different pH values (3.0, 3.6, 4.2, and 5.0) as internal aqueous phase. As presented in Fig. 6, the EE of SR, MA, SC, and LMN in TASA increased with the decrease of the internal phase pH values. The EE of SR was significantly high than those of MA, SC, and LMN in ethosomes. In fact, SR, MA, SC, and LMN extracted from S. alopecuroides exhibited similar structures (presented in Fig. 1). All of them should have similar EE in theory. These differences of EE may be caused by the different dissociation levels of SR, MA, SC, and LMN. The dissociation constants (pKa) of SR, MA, SC, and LMN are 6.51, 7.72, 7.22, and 7.97 (18,19), respectively. Thus the alkalinity of SR is the strongest among the four kinds of alkaloids in TASA. When the external pH was adjusted by 0.5 M NaOH, SR might be liberated preferentially. In addition, the contents of above four alkaloids in TASA are SR > MA > SC > LMN. Large SR would be entrapped into binary ethosomes, which induced the increase of interior pH. Accordingly, the protonating ability of internal phase decreased and the EE of MA, SC, and LMN were lower than that of SR in binary ethosomes. Although the lower pH value inside the binary ethosomes can result in the higher EE, the further decrease of internal phase pH exacerbated lipid stability due to hydrolysis (13). Therefore, the optimum internal phase pH was 3.0.
Fig. 6.
Internal phase pH values on the EE of TASA binary ethosomes
Effect of External Phase pH Values on the EE of TASA Binary Ethosomes
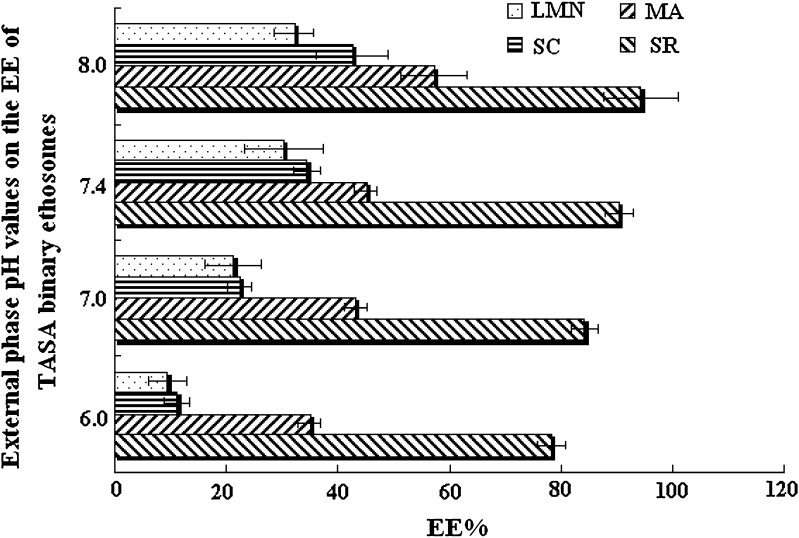
The external pH of ethosome was adjusted by 0.5 M NaOH to pH 6.0, 7.0, 7.4, and 8.0, respectively. The result was shown in Fig. 7. With increasing the ΔpH unit from 3.0 (internal pH 3.0, external pH 6.0) to 5.0 (internal pH 3.0, external pH 8.0), the EE of SR, MA, SC, and LMN was increased 1.21, 1.59, 4.76, and 4.04 times, respectively. However, there was no significant difference between the ethosomal system with external pH 7.4 and the ethosomal system with external pH 8.0 (P > 0.05). The standard deviation of EE was significantly increased when the ethosomal system with external pH 8.0. This might be caused by the collapse of lipid bilayer. Considering the instable entrapment of the ethosomal system with external pH 8.0, the optimum external phase pH was fixed as 7.4.
Fig. 7.
External phase pH values on the EE of TASA binary ethosomes
Influence of Incubation Temperature and Incubation Period on TASA Loading in Binary Ethosomes
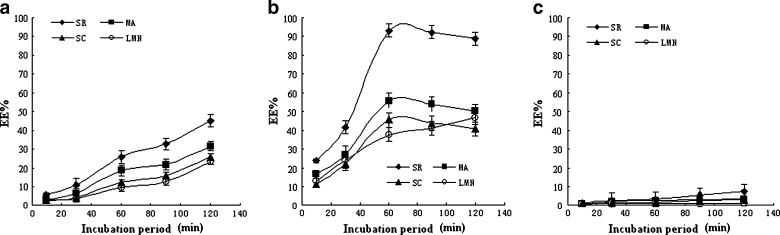
The novel method to prepare binary ethosomes should be below the Tc of PC. The Tc of PC is more than 40°C (15). Thus, the incubation temperature at 30°C, 40°C, and 60°C was investigated in this work, as presented in Fig. 8a–c. When the incubation was performed at 30°C, it would take more than 120 min to reach the maximum drug loading. The EE of SR, MA, SC, and LMN was less than 45%, 30%, 25%, and 23% after 120 min. When the incubation was performed at 40°C, the drug uptake into the ethosomes could reach a maximum EE after 60 min. However, when the incubation period was further prolonged, the EE of alkaloids except for LMN decreased. No measurable drug uptake could be observed when the samples were incubated at 60°C. These results indicated that the importance of maintaining optimal temperature and time in this novel method. When the temperature was too low, the foreign substances were difficult to overcome the activation energy into binary ethosomes. However, high temperature could result in the collapse of the pH gradient and unstable loading (10). Furthermore, if the incubation period was too short, KTA could not be entrapped into the ethosomes as much as possible. If the incubation period was too long, KTA could leak from the ethosomes. Therefore, a period of 60 min for loading at 40°C was proper. Because the intercalation of ethanol into the polar head group environment can result in an increase in the membrane permeability, effective entrapment can be completed at the incubation temperature below Tc (7, 10, 20). The internal aqueous was buffer, but it was not adequately buffered when large alkaloids had been captured into ethosomes. Accordingly, the protonating ability of internal phase decreased when the internal phase pH increased. Furthermore, the difference of concentration (ΔC) between the internal phase and external phase of ethosomes were decreased simultaneously. These results may be unfavorable for entrapment. Therefore, the drugs entrapped tend to leak when the incubation period was further prolonged at 40°C. It was possible that the ΔC of LMN was not low enough to leak because the EE of LMN was the lowest among the four alkaloids at 60 min.
Fig. 8.
Influence of temperature, time on the EE of TASA binary ethosomes. a TASA binary ethosomes were incubated at 30°C. b TASA binary ethosomes were incubated at 40°C. c TASA binary ethosomes were incubated at 60°C
The Volume Ratio of Ethanol to Propylene Glycol on TASA Loading in Binary Ethosomes
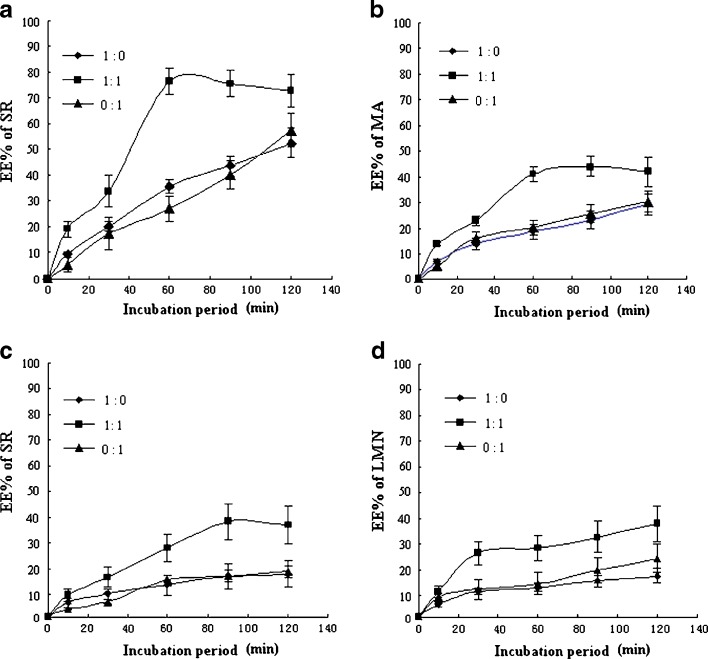
When the ethosomal system was stored, ethanol in the formulation volatilized easily and it was easy to precipitate. Furthermore, the low pH value inside the ethosomes can exacerbate lipid stability due to hydrolysis (13). Therefore, propylene glycol was added in the ethosomal formulation due to its anti-hydrolysis and viscidity (21). On the basis of propylene glycol, the hydrolysis of PC, volatilization of ethosomal system, and precipitation could be reduced. TASA ethosomes were prepared with the volume ratio of ethanol to propylene glycol 1:1, 1:0, and 0:1, respectively. The influence of the volume ratio of ethanol to propylene glycol on the uptake of SR, MA, SC, and LMN extracted from S. alopecuroides into ethosomes was investigated, respectively, as presented in Fig. 9.
Fig. 9.
Influence of the volume ratio of ethanol to propylene glycol (1:0, 1:1, 0:1 v/v) on the EE of SR, MA, SC and LMN in TASA at different time points. a Influence of the volume ratio of ethanol to propylene glycol on the EE of SR in TASA at different time points. b Influence of the volume ratio of ethanol to propylene glycol on the EE of MA in TASA at different time points. c Influence of the volume ratio of ethanol to propylene glycol on the EE of SC in TASA at different time points. d Influence of the volume ratio of ethanol to propylene glycol on the EE of LMN in TASA at different time points
These results illustrated that ethosomal system with the BAP significantly improved the EE of SR, MA, SC, and LMN in TASA relative to the ethosomal system only with the ethanol or propylene glycol in the formulation. However, there was no significant difference between the ethosomal system only with the ethanol or propylene glycol (P > 0.05). Moreover, the ethosomal systems only containing ethanol or propylene glycol would take more than 120 min to achieve maximal drug loading at 40°C. However, the incubation period was less than 60 min in the binary ethosomal system with the volume ratio of ethanol to propylene glycol 1:1. These results suggested that the binary combination of the ethanol and propylene glycol could synergistically increase the rate of drug into ethosomes. In fact, a longer incubation period was adverse to the lipid stability due to hydrolysis. Therefore, the volume ratio of ethanol to propylene glycol 1:1 was more suitable in this system.
The Percent of BAP in Formulation of TASA Binary Ethosomes
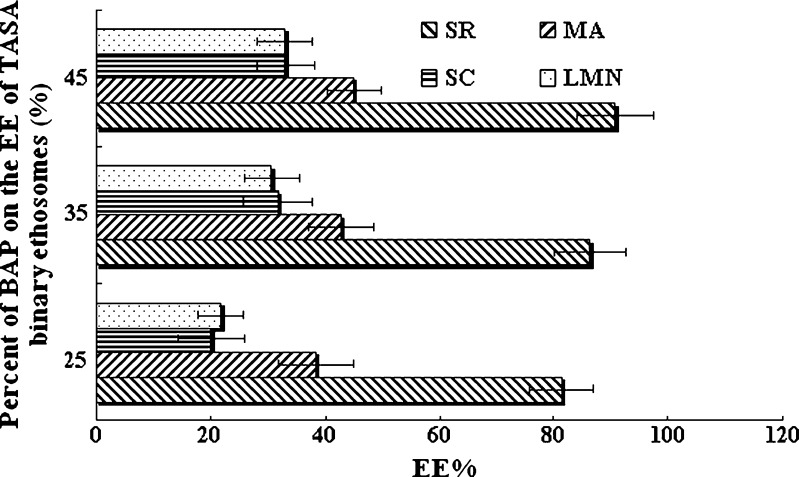
Due to the interdigitation effect of ethanol on lipid bilayers, it was commonly believed that vesicles cannot coexist with high concentrations of ethanol (7). Dos Santos et al. (10) suggested that high ethanol concentrations dissolved the lipid bilayer of liposomes. But the ethosomes containing PC and ethanol in relatively high concentration can still exist predominantly in the form of closed vesicles. The behavior of PC in 30–45% ethanol were investigated by the paramagnetic-ion NMR technique and 31P NMR spectra, which indicated that ethosomal lipids, in dispersions of up to 45% ethanol, were organized in phospholipid bilayers (7). The percent range 25–45% of BAP (the volume ratio of ethanol to propylene glycol 1:1) in formulation was investigated. As summarized in Fig. 10, these results suggested that the EE of the TASA binary ethosomal vesicles increased with increasing percent of BAP. Probably, ethanol causes a modification of the net charge of the system and confers it some degree of steric stabilization that may finally lead to a decrease in the mean particle size and an increase in the EE of the TASA binary ethosomes (7,9). Therefore, it is feasible to prepare ethosomes with high percent of BAP by pH gradient loading method.
Fig. 10.
Influence of the percent of BAP on the EE of TASA binary ethosomes
Stability of TASA Binary Ethosome
Primary study showed that the binary ethosomes (containing ethanol and propylene glycol in formulation) were more stable than ethosomes (only containing ethanol in formulation) at 4°C. The EE and the pH of the binary ethosomal system had no significant change (SR from 96.6% to 94.2%, MA from 57.1% to 54.3%, SC from 44.5% to 42.5%, and LMN from 41.2% to 40.9%) at 4°C within 2 months. The EE of ethosomes only containing ethanol had reduced greatly (SR from 91.8% to 62.6%, MA from 51.4% to 32.3%, SC from 37.5% to 22.5%, and LMN from 32.2% to 27.2%) and the pH of this system had a significant decrease (from 7.4 to 5.2). This may be caused by the hydrolysis of PC. The appearance of TASA binary ethosomes bearing 45% BAP kept intact spherical shape and lipid bilayer. Therefore, the addition of propylene glycol could efficaciously improve the formulation stability.
CONCLUSIONS
TASA were successfully encapsulated into binary ethosomes by pH gradient active-loading method at temperature below Tc of the PC. The results showed that this novel technology might be a more appropriate technique to load alkaloid into ethosomes. Furthermore, the addition of propylene glycol could efficaciously improve the formulation stability. The in vitro permeation experiments were very encouraging due to the enhanced delivery of drugs through the stratum corneum barrier into the deep layer of the skin. These indicated the ethosomes prepared by this novel way are also very promising carriers for the topical administration.
Acknowledgments
The authors thank Prof. ZD Hu (Department of chemistry, LanZhou University of China) for his foundation.
Abbreviations
- CLSM
Confocal laser scanning microscopy
- EE
Encapsulation efficiency
- LMN
Lemannine
- MA
Matrine
- PC
Phosphatidyl choline
- PTA
Phosphotungstic acid
- SC
Sophocarpine
- SR
Sophoridine
- TASA
Total alkaloids extracted from Sophora alopecuroides
- Tc
The phase transition temperature
- TEM
Transmission electron microscopy
References
- 1.Han Y, Zhou Y, Liu Q. Antiendotoxic effects of Sophora alopecuroides L. Zhong Yao Cai. 2006;29(10):1066–9. [PubMed] [Google Scholar]
- 2.Zhang L, Li G, Houghton PJ, Jackson S, Twentyman PR. Alkaloids in Sophora alopecuroides seed and relevant tests for activity. Zhongguo Zhong Yao Za Zhi. 1997;22(12):740–3. [PubMed] [Google Scholar]
- 3.Zhou Y, Wang H, Liang L, Zhao W, Chen Y, Deng H. Total alkaloids of Sophora alopecuroides increases the expression of CD4+ CD25+ Tregs and IL-10 in rats with experimental colitis. Am J Chin Med (AJCM) 2010;38(2):265–77. doi: 10.1142/S0192415X1000783X. [DOI] [PubMed] [Google Scholar]
- 4.Zhao G, Du W, Wei Y, Qin H, Wu X. An HPLC method for the determination and pharmacokinetic study of lehmannine in rat plasma. Biomed Chromatogr. 2008;22(10):1149–54. doi: 10.1002/bmc.1038. [DOI] [PubMed] [Google Scholar]
- 5.Wu X. The application of total alkaloids of Sophora alopecuroides in the treatment drug of oral herpes catarrhalis. In: Center CPI, editor. A61K 36/489;A61K 9/06;A61K 9/08;A61P 31/22;A61P 1/02 ed. China: Zhongyi Yang; 2006. [Google Scholar]
- 6.Wu X. The application of total alkaloids of Sophora alopecuroides in the treatment drug of oral herpes catarrhalis. In: Center CPI, editor. A61K 36/489;A61K 9/06;A61K 9/08;A61P 31/22 ed. China: Jichong Liu; 2006. [Google Scholar]
- 7.Touitou E, Dayan N, Bergelson L, Godin B, Eliaz M. Ethosomes—novel vesicular carriers for enhanced delivery: characterization and skin penetration properties. J Control Release. 2000;65(3):403–18. doi: 10.1016/S0168-3659(99)00222-9. [DOI] [PubMed] [Google Scholar]
- 8.Dayan N, Touitou E. Carriers for skin delivery of trihexyphenidyl HCl: ethosomes vs. liposomes. Biomaterials. 2000;21(18):1879–85. doi: 10.1016/S0142-9612(00)00063-6. [DOI] [PubMed] [Google Scholar]
- 9.Jain S, Tiwary AK, Sapra B, Jain NK. Formulation and evaluation of ethosomes for transdermal delivery of lamivudine. AAPS PharmSciTech. 2007;8(4):E111. doi: 10.1208/pt0804111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dos Santos N, Cox KA, McKenzie CA, van Baarda F, Gallagher RC, Karlsson G, et al. pH gradient loading of anthracyclines into cholesterol-free liposomes: enhancing drug loading rates through use of ethanol. Biochim Biophys Acta. 2004;1661(1):47–60. doi: 10.1016/j.bbamem.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 11.Song Y, Wei Y, Wu X. Determination of sophoridine, matrine and sophocarpine in Saphora alopeurodies injection by RP-HPLC. J Lanzhou Univ (Med Sci) 2007;33(4):24–6. [Google Scholar]
- 12.Song Y, Wei Y, Liu W, Wu X. Simultaneous determination of sophoridine, matrine and sophocarpine in sophora alopecuroides cream by HPLC. China Pharmcy. 2008;19(24):1878–9. [Google Scholar]
- 13.Qiu L, Jing N, Jin Y. Preparation and in vitro evaluation of liposomal chloroquine diphosphate loaded by a transmembrane pH-gradient method. Int J Pharm. 2008;361(1–2):56–63. doi: 10.1016/j.ijpharm.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 14.Maurer-Spurej E, Wong KF, Maurer N, Fenske DB, Cullis PR. Factors influencing uptake and retention of amino-containing drugs in large unilamellar vesicles exhibiting transmembrane pH gradients. Biochim Biophys Acta. 1999;1416(1–2):1–10. doi: 10.1016/s0005-2736(98)00204-1. [DOI] [PubMed] [Google Scholar]
- 15.Leonenko ZV, Finot E, Ma H, Dahms TE, Cramb DT. Investigation of temperature-induced phase transitions in DOPC and DPPC phospholipid bilayers using temperature-controlled scanning force microscopy. Biophys J. 2004;86(6):3783–93. doi: 10.1529/biophysj.103.036681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li C, Cui J, Li Y, Wang C, Zhang L, Guo W, et al. Copper ion-mediated liposomal encapsulation of mitoxantrone: the role of anions in drug loading, retention and release. Eur J Pharm Sci. 2008;34(4–5):333–44. doi: 10.1016/j.ejps.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 17.Elsayed MM, Abdallah OY, Naggar VF, Khalafallah NM. Deformable liposomes and ethosomes: mechanism of enhanced skin delivery. Int J Pharm. 2006;322(1–2):60–6. doi: 10.1016/j.ijpharm.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 18.Gong S, Su X, Bo T, Zhang X, Liu H, Li KA. Determination of dissociation constants of ten alkaloids by capillary zone electrophoresis. J Sep Sci. 2003;26(6–7):549–54. doi: 10.1002/jssc.200390075. [DOI] [Google Scholar]
- 19.Wang G, Yu D, Zhou R. Determination of pKa of sophoridine and lehmannine using potentiometric titration. Journal of Shengyang College of Pharmacy. 1991;8(3):215–6. [Google Scholar]
- 20.Elsayed MM, Abdallah OY, Naggar VF, Khalafallah NM. Lipid vesicles for skin delivery of drugs: reviewing three decades of research. Int J Pharm. 2007;332(1–2):1–16. doi: 10.1016/j.ijpharm.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 21.Cui F. Liquid preparations. Pharmaceutics. 5. Beijing: Pepole’s Medical Publishing House; 2007. p. 17. [Google Scholar]