Abstract
Buccal patches for the delivery of atenolol using sodium alginate with various hydrophilic polymers like carbopol 934 P, sodium carboxymethyl cellulose, and hydroxypropyl methylcellulose in various proportions and combinations were fabricated by solvent casting technique. Various physicomechanical parameters like weight variation, thickness, folding endurance, drug content, moisture content, moisture absorption, and various ex vivo mucoadhesion parameters like mucoadhesive strength, force of adhesion, and bond strength were evaluated. An in vitro drug release study was designed, and it was carried out using commercial semipermeable membrane. All these fabricated patches were sustained for 24 h and obeyed first-order release kinetics. Ex vivo drug permeation study was also performed using porcine buccal mucosa, and various drug permeation parameters like flux and lag time were determined.
KEY WORDS: atenolol, buccal delivery, buccal patches, controlled delivery
INTRODUCTION
Extensive research efforts have recently been focused on placing a drug delivery system in a particular region of the body for maximizing biological drug availability and minimizing dose-dependent side effects. Buccal delivery of drugs provides an attractive alternate to other conventional methods of systemic drug administration, since buccal mucosa is relatively permeable with rich blood supply and acts as an excellent site for the absorption of drugs (1,2). The administration of drugs via buccal route facilitates a direct entry of drug molecules into the systemic circulation, avoiding the first-pass metabolism and drug degradation in the harsh gastrointestinal environment, which are often associated with oral administration (3–5). The buccal cavity is easily accessible for self medication, and hence it is safe and well accepted by patients, since buccal patches can be very easily administered and even removed from the application site, terminating the input of drug whenever desired. Moreover, buccal patches provide more flexibility than other drug deliveries.
Atenolol is a β1-receptor selective antagonist and is mainly used in treating hypertension, angina, heart failure, and myocardial infarction; chemically, it is 4-(2-hydroxyl-3-isopropyl aminopropoxy) phenylacetamide (6,7). The physicochemical properties of atenolol, i.e., slight water solubility, low molecular weight (266.336), and its suitable elimination half-life (t1/2 = 6–7 h) (7), make it a suitable candidate for administration by buccal route.
During last few decades, mucoadhesive polymers received considerable attention as platforms for buccal delivery of drugs due to their ability to localize the dosage form in the specific regions to enhance drug bioavailability (8). Verma et al. have studied mucoadhesive buccal patches of atenolol formulated by solvent casting technique using different mucoadhesive polymers, namely polyvinyl alcohol (PVA), sodium carboxymethyl cellulose (NaCMC), and chitosan (9). Jug et al. have developed novel bioadhesive films for atenolol buccal delivery containing drug/cyclodextrin inclusion by casting method using ethyl cellulose (EC), PVA, and hydroxypropyl methyl cellulose (HPMC) (10). Satishbabu et al. have formulated buccoadhesive films of atenolol by a casting/solvent evaporation technique using only sodium alginate (SA) and carbopol 934 P (CP 934 P) as mucoadhesive polymers and a backing layer of EC (11). However, in previous literature, no attempt has been taken to formulate atenolol buccal patches using SA along with two other mucoadhesive polymers, NaCMC and HPMC. NaCMC and HPMC are both release-retardant polymers. So, they will provide delayed release of drug from buccal patches for long time, if buccal patches are formulated using these polymers. In the present investigation, we made an attempt to formulate atenolol buccal patches using SA along with various hydrophilic and mucoadhesive polymers like CP 934 P, NaCMC, and HPMC in various proportions and combinations to ensure sustained drug release for prolonged periods with satisfactory mucoadhesive properties. The aim of the present investigation was to formulate and evaluate buccal patches comprising drug (atenolol)-containing mucoadhesive polymeric layer (using SA, CP 934 P, NaCMC, and HPMC) and drug-free backing membrane composed of PVA-aluminum foil. Aluminum foil was used with adhesive polymer PVA to prevent back release of the drug from the buccal patches. The influence of various proportions and combinations of polymers used in the study (SA, CP 934 P, NaCMC, and HPMC) on physicomechanical properties, mucoadhesive characteristics, in vitro drug release, and ex vivo drug permeation were investigated. Such buccal patches of atenolol may provide sustained buccal delivery of atenolol for a long period and can be a good way to bypass the extensive hepatic first-pass metabolism in the management of hypertension.
MATERIALS AND METHODS
Materials
The following chemicals were obtained from different sources and used as received. Atenolol was a gift sample from M/S. P.D.I.L, Baddi, India; sodium alginate, CP 934 P, NaCMC, HPMC, PVA, and glycerine were obtained from commercial sources. All other chemicals and reagents used were of analytical grade; double-distilled water was used throughout.
Preparation of Atenolol-Containing Buccal Patches
A series of buccal patches composed of different proportions and combinations of SA (600 to 900 mg), HPMC (100 to 300 mg), CP 934 P (100 to 300 mg), and NaCMC (100 to 300 mg) containing atenolol (50 mg) were prepared using a 54-cm2 petri dish by solvent casting technique. Glycerin was incorporated as a plasticizer at a concentration of 15% w/w of dry weight of polymers. Backing membrane was casted by pouring 4% w/v aqueous solution of PVA on aluminum foil in petri dishes at 42°C and left for 10 h. Phosphate buffer saline, pH 6.8, was used as solvent in the casting method.
Fifty milligrams of atenolol was incorporated in mixtures containing different ratios and combinations of polymers and plasticizer. The matrices were prepared by pouring 40 ml of the homogeneous solutions on the PVA-aluminum foil backing membrane. Then, these buccal patches were dried at 42°C in an incubator (Yorco International Pvt. Ltd., India). After 24 h, the dried patches were removed from the petri dishes and kept in desiccators until use.
Table I shows the composition of different buccal patches containing atenolol.
Table I.
Formulation Chart of Atenolol Buccal Patches
| Formulation water | SA (mg) | HPMC (mg) | CP 934 P (mg) | NaCMC (mg) | Atenolol (mg) | Glycerine (%) | Distilled (ml) |
|---|---|---|---|---|---|---|---|
| F 1 | 900 | 100 | – | – | 50 | 15 | 40 |
| F 2 | 800 | 200 | – | – | 50 | 15 | 40 |
| F 3 | 700 | 300 | – | – | 50 | 15 | 40 |
| F 4 | 900 | – | 100 | – | 50 | 15 | 40 |
| F 5 | 800 | – | 200 | – | 50 | 15 | 40 |
| F 6 | 700 | – | 300 | – | 50 | 15 | 40 |
| F 7 | 700 | 200 | 100 | – | 50 | 15 | 40 |
| F 8 | 600 | 100 | 300 | – | 50 | 15 | 40 |
| F 9 | 700 | 100 | 200 | – | 50 | 15 | 40 |
| F 10 | 900 | – | – | 100 | 50 | 15 | 40 |
| F 11 | 800 | – | – | 200 | 50 | 15 | 40 |
| F 12 | 800 | – | 100 | 100 | 50 | 15 | 40 |
| F 13 | 700 | 200 | – | 100 | 50 | 15 | 40 |
| F 14 | 600 | 100 | – | 300 | 50 | 15 | 40 |
SA sodium alginate, CP 934 P carbopol 934 P, NaCMC sodium carboxymethyl cellulose, HPMC hydroxypropyl methylcellulose
Measurement of Weight Variation and Thickness
The thickness of the patches was assessed at six different points of the patch using a thickness gauze (Mitutoyo, Japan). For each formulation, three randomly selected patches were used (9). Six films from each batch, as a whole (54 cm2), were weighed individually, and the average weights were calculated.
Measurement of Folding Endurance
The folding endurance was determined manually for the prepared films by repeatedly folding the film at the same place until it broke. The number of times the film could be folded at the same place without breaking or cracking gave the value of folding endurance (12).
Determination of Drug Content
The drug contents in the buccal patches were determined by dissolving 1 cm2 patch in 100 ml phosphate buffer saline (pH = 6.8) and shaken vigorously for 24 h at room temperature. These solutions were filtered through Whatman® filter paper (No. 42). After proper dilution, optical density was measured spectrophotometrically using a UV–VIS spectrophotometer (UV-1700 Double beam spectrophotometer, SHIMADZU Corporation, Japan) at 274 nm against a blank. The drug content was estimated from the calibration curve, which was constructed between 1 and 5 µg/ml concentration ranges. The method was validated for linearity, accuracy, and precision. The regression equation for the calibration curve was Y = 0.048X + 0.002, R2 = 0.9990.
Determination of Moisture Content and Moisture Absorption
The buccal patches were weighed accurately and kept in desiccators containing anhydrous calcium chloride. After 3 days, the patches were taken out and weighed (13). The moisture content (%) was determined by calculating moisture loss (%) using the formula:
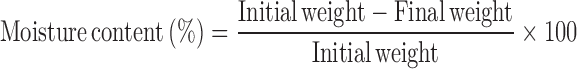 |
1 |
The buccal patches were weighed accurately and placed in the desiccators containing 100 ml of saturated solution of aluminum chloride, which maintains 76% and 86% relative humidity (RH). After 3 days, the films were taken out and weighed. The percentage moisture absorption was calculated using the formula:
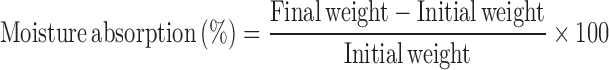 |
2 |
Scanning Electron Microscopy Observation
The cross section of these films was examined by scanning electron microscopy (SEM). The dried films were coated with gold sputter and then observed under scanning electron microscope (JEOL, JSM 840, Japan).
Preparation of Porcine Buccal Mucosa
The porcine buccal mucosa excised from porcine cheek pouch was obtained within 2 h of its death from the slaughter house and immediately transported to the laboratory in phosphate buffer solution. The buccal mucosa was separated from the full thickness of the tissue after immersion in distilled water and then in isotonic phosphate buffer, pH 6.8, at 37 ± 1°C for 2 min. The fatty layers were removed by scalpel, and the buccal mucosa was isolated from the underlying tissue. Finally, the mucosa was washed with isotonic phosphate buffer, pH 6.8.
Ex Vivo Mucoadhesion Study
Mucoadhesive strength of all fabricated buccal patches was measured ex vivo (n = 3) on a modified physical balance using the method described by Gupta et al. (14). A piece of porcine buccal mucosa was tied to the open mouth of a glass vial filled completely with isotonic phosphate buffer, pH 6.8. The glass vial was tightly fitted in the center of a beaker filled with isotonic phosphate buffer (pH 6.8; temperature, 37 ± 1°C). The patches were stuck to the lower side of the rubber stopper with glue. The mass (in gram) required to detach the patches from the mucosal surface gave the measure of mucoadhesive strength (shear stress). The following parameters were calculated from mucoadhesive strength (2):
 |
3 |
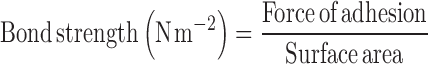 |
4 |
In Vitro Release Study
The commercially available dialysis membrane (obtained from Sigma Chemicals) was employed for the study (15), and the in vitro drug release study was carried out using a Franz diffusion cell. The effective diffusion area was 1.8 cm2. The receptor compartment (40 ml) was filled with phosphate buffer saline (PBS), pH 6.8. The patches were applied under occlusion on the dialysis membrane fitted between the donor and receptor compartments of the diffusion cell. The drug release was performed at 37 ± 0.5°C, at a stirring speed of 50 rpm using a magnetic stirrer. Five milliliters of the sample from receptor medium was withdrawn at regular intervals and replaced immediately with an equal volume of phosphate buffer saline, pH 6.8. The amount of atenolol released into the receptor medium was quantified by using UV–visible spectrophotometer at 274 nm against a blank.
Ex Vivo Permeability Study
The extent and rate of mucosal permeation of atenolol through the porcine buccal mucosa were carried out using Franz diffusion cell. The effective diffusion area was 1.8 cm2. The receptor compartment (40 ml) was filled with PBS, pH 6.8, and its temperature was maintained at 37 ± 0.5°C. A 50 rpm stirring speed was applied using a magnetic stirrer to simulate buccal cavity environment. The patch was applied under occlusion on the buccal mucosal surface of the goat fitted between the donor and receptor compartments of the diffusion cell. Five milliliters of the sample from receptor medium was withdrawn at regular intervals and replaced immediately with an equal volume of PBS, pH 6.8. The amount of atenolol released into the receptor medium was quantified by using UV–visible spectrophotometer at 274 nm against a blank.
RESULTS AND DISCUSSION
The main goal of the present investigation efforts was to develop and evaluate new buccal patches comprising a drug-containing mucoadhesive polymeric layer using polymers like sodium alginate, NaCMC, HPMC, and CP 934 P in various combinations and proportions and a drug-free PVA-aluminum foil backing membrane. The physicomechanical evaluation (Table II) indicates that the weight variation of these formulated buccal patches varied between 2.00 ± 0.05 (F 6) and 2.12 ± 0.08 g (F 14). The thickness of these patches varied between 0.51 ± 0.04 and 0.59 ± 0.05 mm, the thinnest being formulation F 13 and the thickest being formulation F 14. Folding endurance was measured manually. The highest folding endurance was observed in the case of F 11 (93) and the lowest in the case of F 4 and F 10 (80). The range of folding endurance study ensured flexibility of these formulated buccal patches. The drug content (%) in all formulations varied between the range 98.24 ± 0.12% and 99.77 ± 0.12%. This indicates that the drug dispersed uniformly throughout the polymeric film.
Table II.
Physicomechanical Evaluation of Atenolol Buccal Patches
| Formulations | Weight variationa (g) | Thicknessa (mm) | Folding endurance | Drug contenta (%) |
|---|---|---|---|---|
| F 1 | 2.06 ± 0.06 | 0.54 ± 0.02 | 81 | 99.50 ± 0.10 |
| F 2 | 2.09 ± 0.03 | 0.56 ± 0.04 | 83 | 98.61 ± 0.09 |
| F 3 | 2.10 ± 0.06 | 0.57 ± 0.04 | 88 | 99.14 ± 0.08 |
| F 4 | 2.03 ± 0.05 | 0.53 ± 0.05 | 80 | 99.42 ± 0.12 |
| F 5 | 2.02 ± 0.04 | 0.53 ± 0.03 | 86 | 98.87 ± 0.11 |
| F 6 | 2.00 ± 0.05 | 0.54 ± 0.01 | 91 | 98.43 ± 0.10 |
| F 7 | 2.09 ± 0.07 | 0.58 ± 0.02 | 83 | 98.24 ± 0.10 |
| F 8 | 2.07 ± 0.08 | 0.56 ± 0.02 | 84 | 98.43 ± 0.12 |
| F 9 | 2.10 ± 0.09 | 0.55 ± 0.05 | 82 | 98.61 ± 0.12 |
| F 10 | 2.10 ± 0.09 | 0.56 ± 0.03 | 80 | 99.77 ± 0.12 |
| F 11 | 2.11 ± 0.07 | 0.54 ± 0.04 | 93 | 99.60 ± 0.13 |
| F 12 | 2.09 ± 0.09 | 0.53 ± 0.04 | 90 | 98.88 ± 0.10 |
| F 13 | 2.06 ± 0.06 | 0.51 ± 0.04 | 92 | 99.51 ± 0.10 |
| F 14 | 2.12 ± 0.08 | 0.59 ± 0.05 | 86 | 99.77 ± 0.12 |
aAll values are mean ± SD, n = 3.
The moisture content (%) study was done for 3 days. The percentage of moisture content (%) is varied between 0.96 ± 0.01% (F 7) and 1.53 ± 0.03% (F 12) (Table III). In most cases, the moisture uptake content was found to increase with increasing concentration of polymers that are more hydrophilic in nature. The low moisture content in the formulation is highly appreciable to protect from microbial contaminations and bulkiness of the patches. Again, a low moisture content in formulations helps them to remain stable from being a completely dried and brittle film.
Table III.
Moisture Content (%) and Moisture Uptake (%) of Different Atenolol Buccal Patches
| Formulations | Moisture content (%)a | Moisture uptake (%)a | |
|---|---|---|---|
| (76% RH) | (86% RH) | ||
| F 1 | 1.32 ± 0.01 | 4.07 ± 0.06 | 6.20 ± 0.06 |
| F 2 | 1.43 ± 0.01 | 4.17 ± 0.10 | 6.22 ± 0.08 |
| F 3 | 1.67 ± 0.02 | 4.23 ± 0.09 | 6.33 ± 0.09 |
| F 4 | 1.47 ± 0.02 | 3.89 ± 0.08 | 5.84 ± 0.04 |
| F 5 | 1.03 ± 0.01 | 3.53 ± 0.07 | 4.84 ± 0.03 |
| F 6 | 1.04 ± 0.02 | 3.36 ± 0.06 | 4.53 ± 0.05 |
| F 7 | 0.96 ± 0.01 | 3.75 ± 0.11 | 4.86 ± 0.07 |
| F 8 | 0.98 ± 0.01 | 3.57 ± 0.07 | 4.97 ± 0.10 |
| F 9 | 1.04 ± 0.01 | 3.90 ± 0.05 | 5.16 ± 0.07 |
| F 10 | 1.22 ± 0.01 | 4.42 ± 0.12 | 5.27 ± 0.09 |
| F 11 | 1.46 ± 0.02 | 4.46 ± 0.13 | 5.35 ± 0.17 |
| F 12 | 1.53 ± 0.03 | 4.57 ± 0.09 | 5.12 ± 0.07 |
| F 13 | 1.16 ± 0.01 | 4.66 ± 0.17 | 5.10 ± 0.20 |
| F 14 | 1.23 ± 0.01 | 4.87 ± 0.20 | 6.78 ± 0.20 |
aAll values are mean ± SD, n = 3.
The moisture uptake (%) study of various films was done at high relative humidity like 76% and 86% for a period of 3 days (Table III). In all cases, the moisture uptake was found to increase with increase in relative humidity. The low moisture uptake by all these formulations was observed at various levels of relative humidity. This low moisture uptake (%) by atenolol buccal patches can help to retard any hydrolytic degradation, and patches will remain stable.
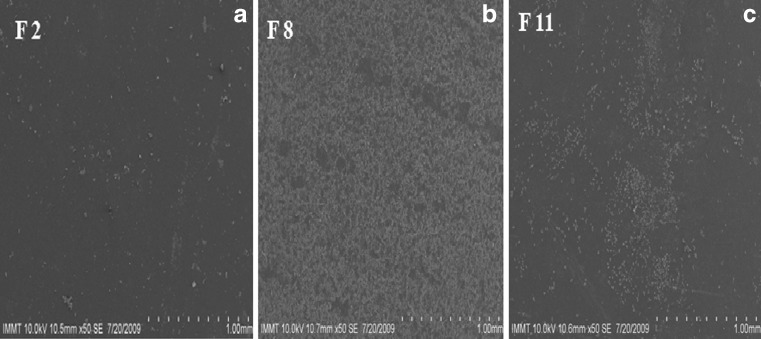
The cross sectional SEM microphotographs of various atenolol-containing buccal patches are shown in Fig. 1a–c. These microphotographs demonstrate that mucoadhesive materials like sodium alginate, HPMC, NaCMC, and CP 934 P were well laminated on to the PVA layer of PVA-aluminum foil backing. This confirms a perfect binding between the drug-containing mucoadhesive layer and the adhesive layer of backing membrane.
Fig. 1.
The cross sectional SEM microphotograph of various atenolol-containing buccal patches (a F 2, b F 8, and c F 11)
Mucoadhesion of buccal patches may be defined as the adhesion between buccal patches and buccal mucosa. The strength of mucoadhesion is affected by various factors like biological membrane used in the study, molecular mass, and swelling rate of polymers present in the formulation (16). In this study, fresh goat buccal mucosa was used as biological membrane. Various mucoadhesion parameters like mucoadhesive strength, force of adhesion, and bond strength exhibited by these patches was satisfactory for maintaining them in oral cavity (Table IV). Among all these formulated patches, formulation no. F 8 showed maximum mucoadhesive strength (32.45 ± 1.11 g), force of adhesion (0.32 ± 0.01 N), and bond strength (176.85 ± 5.11 N m−2).
Table IV.
Ex Vivo Mucoadhesive Characteristics of Atenolol Buccal Patches
| Formulations | Mucoadhesive strengtha (g) | Force of adhesiona (N) | Bond strengtha (N m−2) |
|---|---|---|---|
| F 1 | 25.33 ± 0.67 | 0.25 ± 0.01 | 138.05 ± 4.37 |
| F 2 | 25.68 ± 0.75 | 0.25 ± 0.01 | 139.96 ± 4.22 |
| F 3 | 26.09 ± 1.00 | 0.26 ± 0.01 | 142.19 ± 5.34 |
| F 4 | 28.98 ± 1.29 | 0.28 ± 0.01 | 157.94 ± 4.32 |
| F 5 | 29.27 ± 1.00 | 0.29 ± 0.01 | 159.52 ± 4.44 |
| F 6 | 30.88 ± 1.21 | 0.30 ± 0.02 | 168.30 ± 4.78 |
| F 7 | 26.56 ± 1.12 | 0.26 ± 0.01 | 144.75 ± 5.22 |
| F 8 | 32.45 ± 1.11 | 0.32 ± 0.01 | 176.85 ± 5.11 |
| F 9 | 29.89 ± 1.15 | 0.29 ± 0.01 | 162.90 ± 4.56 |
| F 10 | 20.00 ± 2.62 | 0.20 ± 0.02 | 109.00 ± 6.85 |
| F 11 | 20.12 ± 1.25 | 0.20 ± 0.01 | 109.65 ± 4.47 |
| F 12 | 24.88 ± 1.19 | 0.24 ± 0.01 | 135.60 ± 4.62 |
| F 13 | 23.06 ± 1.35 | 0.23 ± 0.02 | 125.68 ± 2.16 |
| F 14 | 22.11 ± 1.09 | 0.22 ± 0.01 | 120.50 ± 3.34 |
aAll values are mean ± SD, n = 3.
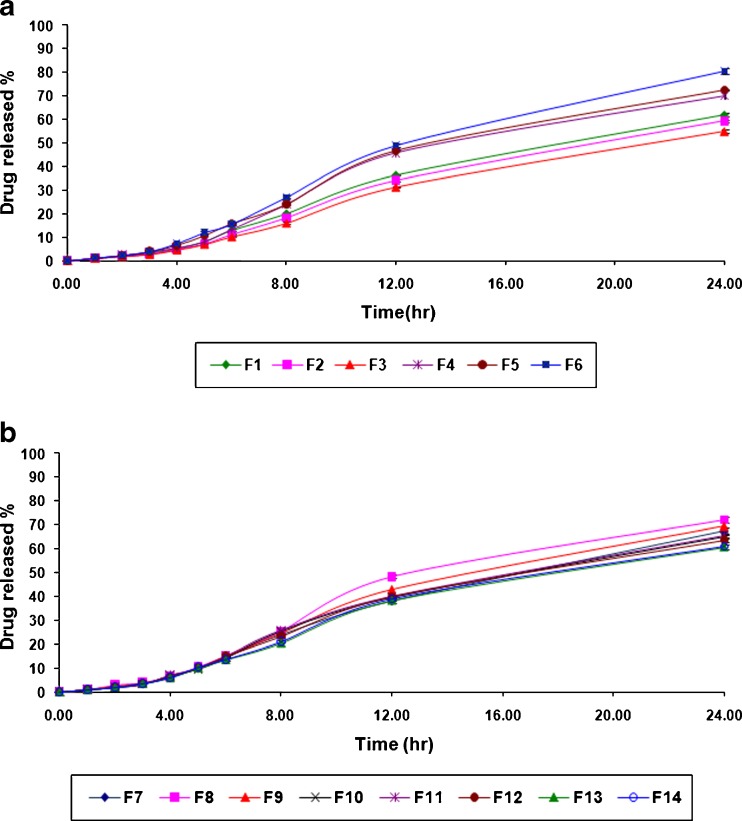
The in vitro drug release pattern of atenolol from formulated buccal patches is shown in Fig. 2a, b. All of these buccal patches slowly released the drug, incorporated and sustained over a period of 24 h. The drug release from buccal patches varied with respect to the polymer composition and nature. An increase in drug release from the buccal patches was found with increasing concentration of polymers that are more hydrophilic in nature. Among all formulations, the maximum in vitro drug release (72.03 ± 1.10%) over a period of 24 h was observed in the case of formulation no. F 8, while the minimum in vitro drug release (55.08 ± 0.57%) over a period of 24 h was found in the case of formulation no. F 3. The in vitro drug release was more sustained for the atenolol buccal patches which were composed with high proportion of HPMC.
Fig. 2.
a In vitro drug release profile of various atenolol-containing buccal patches (F 1 to F 6). b In vitro drug release profile of various atenolol-containing buccal patches (F 7 to F 14)
In order to predict and correlate the release behavior of atenolol from different patches, it is necessary to fit into a suitable mathematical model. The in vitro atenolol release data from buccal patches were evaluated kinetically using various mathematical models like zero-order, first-order, Higuchi, and Koresmeyer–Peppas model equations.
Zero-Order Kinetics
F = Kot, where F represents the fraction of drug released in time t, and Ko is the zero-order release constant.
First-Order Kinetics
ln (1 − F) = −K1t, where F represents the fraction of drug released in time t, and K1 is the first-order release constant.
Higuchi Model
F = KHt1/2, where F represents the fraction of drug released in time t, and KH is the Higuchi dissolution constant.
Koresmeyer–Peppas Model
F = Kptn, where F represents the fraction of drug released in time t, Kp is the Koresmeyer–Peppas release rate constant, and n is the diffusion exponent.
The results of curve fitting into these above-mentioned mathematical models indicates the drug release behavior from these formulated buccal patches of atenolol (Table V). When the release rate of atenolol and their respective correlation coefficients were compared, it was found to follow first-order release kinetics (R2 = 0.9866 to 0.9984).
Table V.
Results of Curve Fitting of the In Vitro Atenolol Release from Buccal Patches
| Zero-order | First-order | Higuchi | Koresmeyer–Peppas | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Formulation code | K o | R 2 | K 1 | R 2 | K H | R 2 | K P | n | R 2 |
| F 1 | 0.026 | 0.9736 | 0.042 | 0.9963 | 0.088 | 0.7122 | 99.232 | 1.434 | 0.9656 |
| F 2 | 0.024 | 0.9798 | 0.040 | 0.9910 | 0.084 | 0.6968 | 95.834 | 1.404 | 0.9776 |
| F 3 | 0.023 | 0.9798 | 0.038 | 0.9866 | 0.079 | 0.6977 | 91.182 | 1.427 | 0.9780 |
| F 4 | 0.031 | 0.9657 | 0.052 | 0.9933 | 0.092 | 0.6736 | 93.980 | 1.402 | 0.9638 |
| F 5 | 0.033 | 0.9783 | 0.055 | 0.9960 | 0.096 | 0.6923 | 95.735 | 1.409 | 0.9778 |
| F 6 | 0.035 | 0.9702 | 0.069 | 0.9897 | 0.107 | 0.6886 | 99.883 | 1.413 | 0.9734 |
| F 7 | 0.030 | 0.9766 | 0.048 | 0.9933 | 0.090 | 0.7210 | 98.371 | 1.408 | 0.9738 |
| F 8 | 0.032 | 0.9826 | 0.054 | 0.9976 | 0.095 | 0.6825 | 95.255 | 1.402 | 0.9866 |
| F 9 | 0.031 | 0.9839 | 0.051 | 0.9878 | 0.092 | 0.6999 | 96.184 | 1.404 | 0.9880 |
| F 10 | 0.029 | 0.9656 | 0.046 | 0.9945 | 0.090 | 0.6856 | 96.664 | 1.400 | 0.9333 |
| F 11 | 0.029 | 0.9742 | 0.048 | 0.9913 | 0.086 | 0.6843 | 96.335 | 1.398 | 0.9413 |
| F 12 | 0.028 | 0.9668 | 0.045 | 0.9955 | 0.089 | 0.6890 | 99.093 | 1.408 | 0.9766 |
| F 13 | 0.026 | 0.9679 | 0.040 | 0.9984 | 0.085 | 0.7762 | 99.011 | 1.443 | 0.9643 |
| F 14 | 0.026 | 0.9633 | 0.042 | 0.9965 | 0.086 | 0.7774 | 99.594 | 1.433 | 0.9723 |
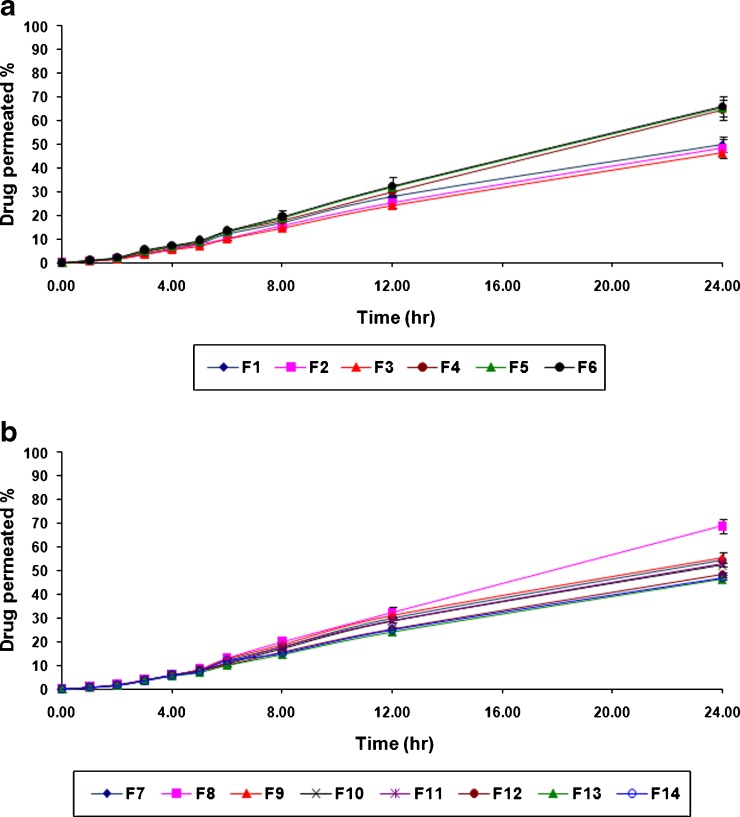
The ex vivo atenolol permeation from various formulations of buccal patches showed that the drug permeated well across porcine buccal mucosa over 24 h period and is shown in Fig. 3a, b. The maximum ex vivo drug permeation of 70.17 ± 2.28% was observed over a period of 24 h in the case of formulation no. F 8, while the minimum ex vivo drug release was found to be 51.02 ± 1.18% over a period of 24 h in the case of formulation no. F 3.
Fig. 3.
a Ex vivo drug permeation profile of various atenolol-containing buccal patches (F 1 to F 6). b Ex vivo drug permeation profile of various atenolol-containing buccal patches (F 7 to F 14)
The amounts of atenolol that permeated through goat buccal mucosa over a 24-h period were plotted against the function of time. The slope and intercept of the linear portion of plots were derived by regression. The permeation flux (J, µg/cm2/h) was calculated as the slope divided by the buccal mucosa surface area. The lag time (Lt, h) was determined by extrapolation of the linear portion of the plot on the abscissa (17). Table VI presents ex vivo permeation parameters of atenolol that permeated through goat buccal mucosa from various formulations of buccal patches. The result reveals the maximum permeation flux (30.83 ± 1.23 µg/cm2/h) with minimum lag time (0.95 ± 0.22 h) in the case of formulation no. F 8. This occurred due to more hydrophilic polymeric matrix composition.
Table VI.
Ex Vivo Permeation Characteristics of Atenolol Buccal Patches
| Formulations | Permeation fluxa (µg/cm2/h) | Lag timea (h) |
|---|---|---|
| F 1 | 24.98 ± 0.36 | 1.30 ± 0.17 |
| F 2 | 24.03 ± 0.23 | 1.40 ± 0.25 |
| F 3 | 23.19 ± 0.45 | 1.48 ± 0.29 |
| F 4 | 28.66 ± 0.56 | 1.07 ± 0.16 |
| F 5 | 28.98 ± 0.70 | 1.07 ± 0.16 |
| F 6 | 30.47 ± 0.33 | 0.98 ± 0.09 |
| F 7 | 27.95 ± 0.64 | 1.12 ± 0.18 |
| F 8 | 30.83 ± 1.23 | 0.95 ± 0.22 |
| F 9 | 28.46 ± 0.31 | 1.12 ± 0.20 |
| F 10 | 26.02 ± 0.22 | 1.27 ± 0.23 |
| F 11 | 26.50 ± 0.17 | 1.23 ± 0.23 |
| F 12 | 25.33 ± 0.23 | 1.30 ± 0.22 |
| F 13 | 21.32 ± 0.33 | 1.33 ± 0.17 |
| F 14 | 21.88 ± 0.35 | 1.36 ± 0.26 |
aAll values are mean ± SD, n = 3.
CONCLUSION
Buccal patches of atenolol using polymers like sodium alginate, NaCMC, HPMC, and CP 934 P in various proportions and combinations showed satisfactory physicomechanical and mucoadhesive characteristics. The proportional amounts of various hydrophilic polymers in various formulations have influence on drug release from these formulated atenolol buccal patches. From the present investigation, it can be concluded that such buccal patches of atenolol may provide sustained buccal delivery for prolonged periods in the management of hypertension, which can be a good way to bypass the extensive hepatic first-pass metabolism.
Acknowledgements
Sincere thanks to Principal (Dr. Dilipkumar Pal) and management of Seemanta Institute of Pharmaceutical Sciences, Jharpokharia, India for providing necessary facilities throughout this study.
References
- 1.Hoogstrate AJ, Verhoef JC, Tuk B, Pijpers A, van Leengoed LAMG, Verheijden JHM, et al. In vitro buccal delivery of fluorescein isothiocyanate-dextran 4400 with glycodeoxycholate as an absorption enhancer in pigs. J Pharm Sci. 1996;85:457–460. doi: 10.1021/js950129k. [DOI] [PubMed] [Google Scholar]
- 2.Patel VM, Prajapati BG, Patel MM. Design and characterization of chitosan-containing mucoadhesive buccal patches of propranolol hydrochloride. Acta Pharm. 2007;57:61–72. doi: 10.2478/v10007-007-0005-9. [DOI] [PubMed] [Google Scholar]
- 3.Vashmi Vishnu Y, Chandrasekhar K, Ramesh G, Madhusudan Rao Y. Development of mucoadhesive patches for buccal administration of carvedilol. Curr Drug Deliv. 2007;4:27–39. doi: 10.2174/156720107779314785. [DOI] [PubMed] [Google Scholar]
- 4.Khairnar A, Jain P, Baviskar D, Jain D. Development of mucoadhesive buccal patch containing aceclofenac: in-vitro evaluation. Int J Pharm Sci. 2009;1(1):91–95. [Google Scholar]
- 5.Hao J, Heng PWS. Buccal delivery systems. Drug Dev Ind Pharm. 2003;29(8):821–832. doi: 10.1081/DDC-120024178. [DOI] [PubMed] [Google Scholar]
- 6.Budavari S (ed). The Merck Index, 13th edn. Merck & Co. Inc. Whitehouse Station, NJ, 2001, p. 147.
- 7.The Indian Pharmacopoeia. The Controller of Publications. Ministry of Health. Govt. of India. New Delhi, 1996. Vol I. p. 72.
- 8.Gu JM, Robinson JR, Leung SHS. Binding of acyclic polymer to mucin/epithelial surfaces: structure–property relationships. CRC Crit Rev Ther. Drug Carrier Systems. 1988;21:21–67. [PubMed] [Google Scholar]
- 9.Verma N, Wahi AK, Verma A, Chattopadhayay P. Evaluation of a mucoadhesive buccal patch for delivery of atenolol: in vitro screening of bioadhesion. J Pure Appl Microbiol. 2007;1:115–118. [Google Scholar]
- 10.Jug M, Beirevi-Laan M, Bengez S. Novel cyclodextrin-based film formulation intended for buccal delivery of atenolol. Drug Dev Ind Pharm. 2009;35(7):796–807. doi: 10.1080/03639040802596212. [DOI] [PubMed] [Google Scholar]
- 11.Satishbabu BK, Srinivasan BP. Preparation and evaluation of buccoadhesive films of atenolol. Indian J Pharm Sci. 2008;70(2):175–179. doi: 10.4103/0250-474X.41451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nafee NA, Ahemed F, Borale A. Preparation and evaluation of mucoadhesive patches for delivery of cetylpyridinium chloride (CPC). Acta Pharma. 2003; 199–212.
- 13.Attama A, Akpa PA, Onugwu LE, Igwilo G. Novel buccoadhesive delivery system of hydrochlorothiazide formulated with ethyl cellulose hydroxypropyl methylcellulose interpolymer complex. Scientific Res Essay. 2008;3(6):26–33. [Google Scholar]
- 14.Gupta A, Garg S, Khar RK. Measurement of bioadhesion strength of muco-adhesive buccal tablet: design of an in vitro assembly. Indian Drugs. 1992;30:152–155. [Google Scholar]
- 15.Raghuraman S, Velrajan G, Ravi R, Jeyabalan B, Johnson DB, Sankar V. Design and evaluation of propranolol hydrochloride buccal films. Indian J Pharm Sci. 2002;64(1):32–36. [Google Scholar]
- 16.Park H, Robinson JR. Mechanism of bioadhesion of poly (acrylic acid) hydrogels. Pharm Res. 1987;4:457–464. doi: 10.1023/A:1016467219657. [DOI] [PubMed] [Google Scholar]
- 17.Shah JC. Analysis of permeation data: evaluation of lag time method. Int J Pharm. 1993;90:161–169. doi: 10.1016/0378-5173(93)90152-6. [DOI] [Google Scholar]