Abstract
Parasitic diseases are of immense global significance as around 30% of world’s population experiences parasitic infections. Among these, malaria is the most life-threatening disease. Various routes of administration have been explored for delivering antimalarial actives. The present investigation aims at formulating self-microemulsifying suppositories of β-artemether with faster onset of action and prolonged effect to be administered by rectal route. These were compared with conventional polyethylene glycol suppositories with respect to melting range, rheology, texture analysis, disintegration time, self microemulsification time, particle size, and drug content. In vitro drug release was studied by using USP apparatus II. Further, the suppositories were evaluated in murine model against virulent rodent malaria parasite Plasmodium berghei wherein the developed self-microemulsifying suppositories could sustain the activity (94%) for 20 days post infection. The survival of animals was also better as compared to the conventional formulation.
KEY WORDS: β-artemether, in vitro drug release, malaria, pharmacodynamic, suppository
INTRODUCTION
Malaria accounts for one to two million deaths around the globe every year. The tropical countries such as India are more prone to malaria and around two million cases of the disease are reported annually. In humans, malaria is caused by four distinct species of parasites, viz, Plasmodium vivax, Plasmodium falciparum, Plasmodium malariae, and Plasmodium ovale. Among these, the most severe malaria is caused by blood-borne apicomplexan parasite P. falciparum which is responsible for almost all the malaria-related deaths. Existing treatments for malaria include a limited number of clinically effective antimalarial agents (1). Children with severe malaria often experience unconsciousness, convulsions, and vomiting, which makes oral medication difficult. In many cases, parenteral administration is impossible, due to lack of equipment and adequately trained staff, or potentially hazardous if adequate sanitization is not maintained.
Rectal administration is often sought as an alternative route of administration to overcome the gastric irritation, nausea, and vomiting that may be associated with oral administration. Furthermore, drugs are administered rectally when the oral route is not convenient, as in infants and elderly patients. Moreover, this route offers additional advantages of ease and safety of administration. A generous blood supply from the middle and inferior hemorrhoid veins ensures rapid absorption of administered moieties so that much of the absorbed drug enters the systemic circulation directly, without passing through the portal circulation, thereby avoiding first pass effect. Additionally, of particular relevance to the third world countries is that rectal administration of antimalarial drugs may allow effective treatment to be instituted early in outlying rural areas, thus, providing the valuable time to seek appropriate medical care (2).
Conventional polyethylene glycol (PEG) based rectal suppository may soften or melt in the posterior rectum and vagina due to their relatively high melting point and cannot be rapidly absorbed in the mucous membranes. Moreover, they may also cause local irritation and mucosal damage. However, these problems of conventional solid suppositories may be overcome by developing self microemulsifying suppositories (SMES) (3).
SMES formulations are isotropic mixtures of a lipid base, surfactant/s, cosurfactant/s (or solubilizer/s), and a drug. Using gentle agitation, they are known to form instantaneous fine oil-in-water microemulsion in aqueous media, making them an ideal vehicle for rectal delivery of hydrophobic drugs having adequate oil solubility. The presence of drug in a solubilized form and the small size of the formed droplets provide a large interfacial surface area for drug absorption. Additionally, the presence of lipid in the formulation helps to improve the bioavailability of the administered moieties by enhancing the drug absorption. Thus, for drugs exhibiting dissolution rate-limited absorption, the SMES could prove to be a useful tool to enhance the rate and extent of absorption (4–6).
β-Artemether is a potent and rapidly acting antimalarial agent which has been enlisted in WHO list of essential medicines for the treatment of severe multiresistant malaria. It is active against P. vivax as well as chloroquine-sensitive and chloroquine-resistant strains of P. falciparum. It is also indicated in the treatment of cerebral malaria. Currently, β-artemether is available as tablets for oral therapy and as an intramuscular (IM) oily injection for the treatment of severe malarial infections. However, the oral bioavailability of β-artemether is low (~40%), which is ascribed to its poor aqueous solubility and degradation in stomach acids, ensuing significant reduction in its therapeutic efficacy (7).
Thus, the objective of the present investigation was to formulate and evaluate SMES of β-artemether as an alternative dosage form to overcome the disadvantages of both oral as well as IM routes of administration.
MATERIALS AND METHODS
Materials
β-Artemether was kindly provided by Ipca Laboratories Ltd., Mumbai, India. Suppocires® (saturated polyglycolysed glycosides semi-synthetic glycerideas of fatty bases), Gelucire® 44/14 (Lauroyl macrogol32glycerides), and Transcutol® P (Ethylene glycol monoethyl ether) were obtained as gift sample from Gattefosse, France, through Colorcon Asia Pvt Ltd., (Goa, India). Polyethylene glycol 400 (PEG 400) and Polyethylene glycol 4000 (PEG 4000; AR grade) were purchased from s.d. Fine Chemicals (Mumbai, India). All the other chemicals and solvents were purchased from Merck International and s.d. Fine Chemicals, Mumbai, India. Milli Q water (Bedford, MA, USA) was used wherever necessary.
HPLC Analysis of β-Artemether
The amount of β-artemether solubilized in various bases was determined using HPLC (8). The HPLC system consisted of Jasco PU 2080 Plus Intelligent HPLC Pump (Jasco, Japan), equipped with a Jasco UV 2075 Intelligent UV–VIS Detector (Jasco, Japan), and Rheodyne 7725 injector (Rheodyne, U.S.A). The compound was eluted using Lichrosphere 100 RP-18 (250 × 4 mm), 5-μm particle size column. Analysis was done by using Borwin Chromatography Software version 1.50 (Jasco, Japan). The mobile phase (acetonitrile/water, 75:25 v/v) was employed at a flow rate of 1 ml/min, and the detection of β-artemether was carried out at 214 nm, where the drug exhibited a retention time of 8.2 min.
Solubility Studies
The solubility of β-artemether in different bases and surfactants was determined by using shake flask method (9). Briefly, an excess of β-artemether was added individually to the Suppocire® bases, surfactants, and solubilizers (5 g each) in screw capped tubes. Mixtures were then shaken for 24 h in a water bath shaker (Remi, Mumbai, India) maintained at 37 ± 0.5°C. After 24 h, each sample was centrifuged (Eltek®, Research Centrifuge TC 4100D, Mumbai) at 1,677×g for 10 min. The supernatant (0.5 ml) was suitably diluted, and the amount of β-artemether present in the supernatant was analyzed by HPLC.
Preparation of Polyethylene Glycol (PEGs) Suppositories
The suppositories were prepared by molding technique (10). Briefly, PEG 400 and PEG 4000 (640 and 320 mg, respectively) were mixed and melted at 50°C to form a homogeneous blend. Subsequently, β-artemether (40 mg) was dissolved in this melt by vortexing. The resultant melt of the drug containing bases was then poured into the 1-g capacity suppository mold. The suppositories were allowed to set at room temperature for 5–10 min and further hardened for 30 min at 10 ± 2.0°C. The obtained suppository was assessed for its appearance, stability at room temperature, and ease of removal from the mold.
Preparation of Self-Microemulsifying Suppositories
The suppositories were prepared by an in-house molding technique. Briefly, Suppocire® AP (110 mg), Gelucire® 44/14 (800 mg), and Transcutol®P (50 mg) were mixed and melted at 50°C to form a homogenous lipid blend. Subsequently, β-artemether (40 mg) was dissolved in this mixture by vortexing. The resultant molten SMES blend was poured into suppository mold of 1-g capacity. The suppositories were allowed to set at room temperature for 5–10 min and further hardened for 30 min at 10 ± 2.0°C. The obtained SMES was assessed for its appearance, stability at room temperature, and ease of removal from the mold.
Determination of Melting Range of Suppositories
Melting range of suppositories and solubilized state of drug were evaluated by using Hot Stage Microscopy (Reachert Thermovar, Rechert-Jung, Austria) (11). The determinations were conducted by weighing 1 mg of sample on a glass slide which was mounted on the stage of microscope. The stage was gradually heated, and the temperature where the formulation melted was recorded using a thermometer. The physical state of drug at the melting point of the base was also recorded.
Rheology Studies
The rheology studies were performed in triplicate on both the PEG-based and SMES suppositories using rotational rheometer (Haake RT 10 Rheometer, Germany) employing parallel plate’s sensor of diameter 35 mm (12).The test was carried out in oscillatory mode at a temperature between 30°C and 40°C.
Texture Analysis
The texture was determined on a TA.XT2 Texture Analyzer (Stable Microsystems, UK). A flat end, round cylindrical stainless steel probe (Ø 2 mm × SSP/5S mm) was used to measure the distance that the probe traveled in the suppository layer (13). The probe traveled at a rate of 2 mm/s until the surface of the suppository was detected. The force of penetration was 0.7 g at the point at which the probe penetrated the suppository layer. Once the probe detected 500 g of the force, which was determined as the breaking force, the probe was withdrawn automatically out of the suppository layer at a rate of 0.2 mm/s, at 25°C. The texture analysis was performed in triplicate for each suppository.
Determination of Disintegration Time and Self-Microemulsification Time
The prepared suppositories were evaluated for disintegration as per the method described in British Pharmacopoeia (1998; volume II). The mean values were calculated from six parallel measurements. Self-microemulsification time was recorded as the time taken by the formulation to form a clear solution in water as medium used during the disintegration studies.
Particle Size Analysis
The particle size and polydispersity index of SMES were determined by photon correlation spectroscopy (Beckman N4 plus submicron particle size analyzer, Wipro, India). SMES were diluted with 3 ml of double distilled to ensure that the light scattering intensity (between 6e+004 and 1e+006) was within the instrument’s sensitivity range. Double distilled water was filtered through 0.45-μm membrane filters (Pall Life Sciences, Mumbai) prior to particle size determination. All the measurements were carried out in triplicate at a temperature of 25 ± 2°C and at an angle of 90° to the incident beam. The data obtained was analyzed by Counter program.
Similarly, for PEG-based suppositories, the particle size was measured on Mastersizer (Malvern instruments), based on the measurement of light intensity directly scattered by PEG based suppository particles.
Determination of Drug Content
Content uniformity of the developed suppositories was evaluated in triplicate as per the method described in British Pharmacopoeia. One suppository was transferred to 100 ml volumetric flask, and the volume was made up using Acetonitrile (HPLC grade). The resulting solution was filtered through 0.45-μm membrane and analyzed by HPLC as described earlier.
In Vitro Drug Release
In vitro drug release of both SMES and conventional PEG-based suppositories were compared using the USP XXII paddle method. The studies were conducted using 900 ml phosphate buffer (0.1 M) pH 7.4 maintained at 37 ± 0.5°C. The paddle speed was maintained at 50 rpm (14). At appropriate time intervals (5, 10, 15, 30 min),5 ml aliquots were withdrawn and filtered through 0.45-μm membrane and analyzed by HPLC as described earlier.
Pharmacodynamic Evaluation: In Vivo Antimalarial Efficacy Testing in Plasmodium berghei-Infected Mice
In vivo antimalarial efficacy of the developed SMES of β-artemether was comparatively evaluated with conventional PEG suppositories. The study protocol was approved by the Institutional Animal Ethical Committee of the Tata Institute of Fundamental Research (protocol no. TIFR/IAEC/2007 1, 2), and the work was carried out in the same premises. For this study, “Peter’s four day suppressive test” protocol was used (15) where ANKA strain of P. berghei was employed for inducing infection in the animals. In-house bred Swiss male mice (weighing around 25–30 g each) free of mycoplasma were infected by intra-peritoneal inoculation of donor mouse blood diluted in acid citrate buffer, containing approximately 106 infected RBCs on day 0. The animals were divided into five groups (n = 6) viz, group I (positive control, no drug treatment), group II (Blank SMES), group III (SMES, 4 mg/kg), group IV (conventional PEG-based suppositories, 4 mg/kg), and group V (blank conventional PEG-based suppositories). The animals were housed at conditions of 22°C/65% RH and were fed with a standard mouse diet and clean drinking water ad libitum. Starting from day 0 to 3 post infection, the suppositories (Ø 3 × 10 mm containing 4 mg/kg drug) were inserted into the rectum of mice lightly anesthetized using ketamine hydrochloride. After dosing, the anus was closed with surgical tape in order to prevent the leakage of suppositories. The parameters such as mean percentage parasitemia against time (in days), percent activity against time (in days), and the animal survival period were monitored to test the efficacy of the developed formulation. The parasite counts were made on days 2, 3, 4,8,16, and 20 from thin blood smears of the tail blood of mice, fixed with methanol and stained with Giemsa’s stain. Parasitemia was reported as mean percentage parasitemia after counting 1,000 RBCs from each slide. Percent antimalarial activity was calculated by using following formula (16).
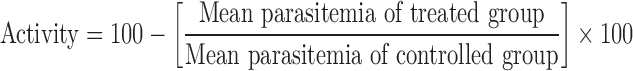 |
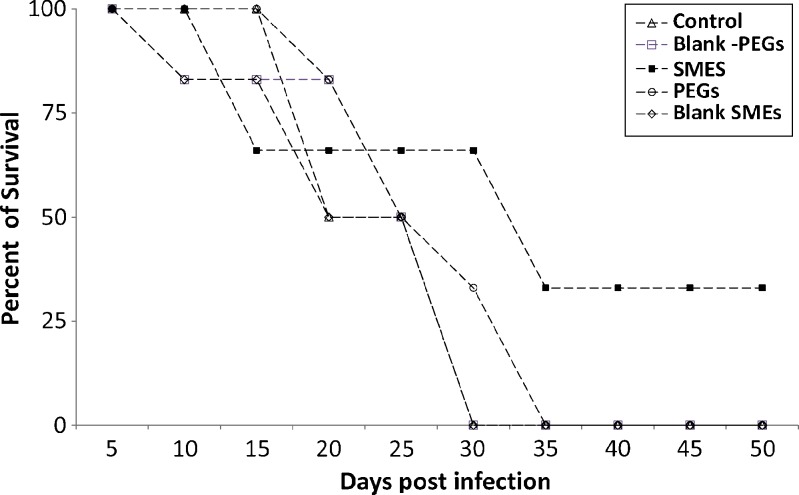
The animals in all groups were also observed for the survival trend for a period of 50 days.
Statistical Analysis
Data were expressed as mean ± SD, and parasitemia of the different groups were statistically assessed by unpaired t test using Graph pad Instat Demo version. Differences were considered significant at P < 0.05.
RESULTS AND DISCUSSION
Solubility Studies
Solubility studies were carried out to identify potential ingredients for the formulation of SMES (Table I). In the various surfactants/solubilizers tested, β-artemether presented the following solubility order: Tween®20 > Gelucire® 44/14 > Trancutol® P > Labrasol® > Tween®80 > PEG 400. Gelucire® 44/14 was finalized as one of the formulation ingredients since the excipient has been reported to exhibit good microemulsifying properties, serving as an interface between the hydrophobic portion of the system (oil and drug) and hydrophilic portion of system. Also, Gelucire® 44/14is a well-established bioavailability enhancer (1). Hence, presence of Gelucire® 44/14 could be hypothesized to aid in increasing the bioavailability of β-artemether. Furthermore, self-microemulsification was observed to be better using the combination of Gelucire® 44/14 and Transcutol® P.
Table I.
Solubility of β-Artemether in Different Surfactants and Solubilizers (n = 3; ±SD)
| Surfactant | Solubility (mg/g) |
|---|---|
| Tween 20 | 50 ± 2.4 |
| Tween 80 | 12 ± 9.4 |
| Solutol HS® 15 | 11 ± 6.9 |
| Labrasol® | 21 ± 2.1 |
| Gelucire® 44/14 | 44 ± 1.3 |
| Transcutol® P | 30 ± 3.4 |
| PEG 400 | 2 ± 4.6 |
Miscibility of suppository base with the emulsifying components is an essential performance characteristic. Various Suppocire® bases were screened for their miscibility with Gelucire® 44/14, Transcutol® P, and water. The results of the same have been enlisted in Table II. Suppocire® AP was miscible with Transcutol ® P and Gelucire® 44/14, showed no phase separation, and was dispersible in water. The combined melt of these components also exhibited good drug solubilizing capacity, i.e., 40 mg drug/600 mg base.
Table II.
Suppocire® Miscibility with Water Alone and in Combination with Transcutol® P or Gelucire® 44/14
| Suppocire® grade | Miscibility in water | Drug (40 mg) | ||
|---|---|---|---|---|
| Without surfactants/cosurfactants | Transcutol® P | Gelucire® 44/14 | ||
| Suppocire® A | Immiscible | Miscible | Immiscible | >700 mg |
| Suppocire® C | Immiscible | Miscible | Immiscible | >600 mg |
| Suppocire® D | Immiscible | Miscible | Immiscible | >500 mg |
| Suppocire® AP | Dispersible | Miscible | Miscible | >600 mg |
| Suppocire® BM | Immiscible | Miscible | Immiscible | >800 mg |
| Suppocire ®SNB | Immiscible | Miscible | Immiscible | >700 mg |
| Suppocire® NX | Immiscible | Miscible | Immiscible | >800 mg |
Melting Range of Suppositories
The melting ranges of PEG-based and SMES suppositories were found to be 38–48°C and 36–42°C, respectively. In comparison to conventional, SMES exhibited a narrow melting range as compared to the PEG-based suppositories. Narrow melting range is important for maintaining the shape of suppositories at ambient storage conditions and for controlling the melting time after insertion into the rectum. The drug was found to be completely soluble in both types of suppository bases.
Rheology Studies
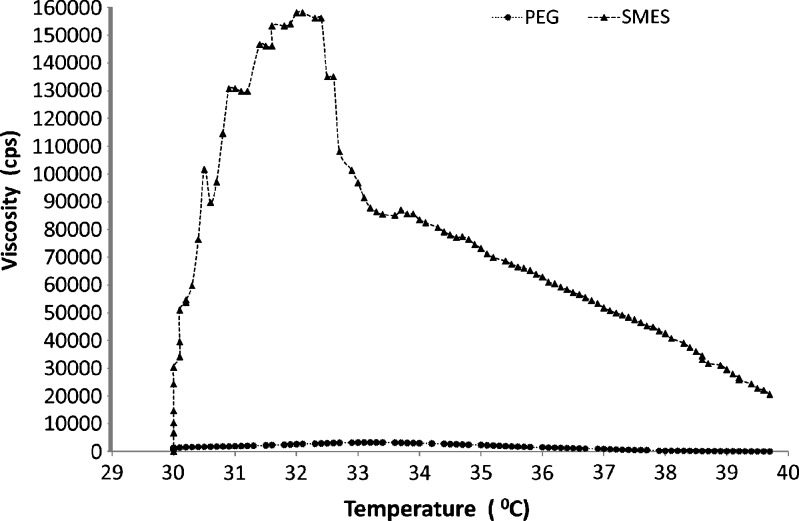
Rheology plays an important role in governing the residence time in the cavity and contributes to an important quality control tool to assess batch to batch uniformity of suppositories. The results of the rheology studies on suppositories were conducted at various temperatures between 30°C and 40°C, the results of which are represented in Fig. 1; viscosity of the developed SMES was found to be higher than PEG-based suppositories. This could be attributed to the composition of SMES which is a mixture of lipid Suppocire® AP (11% w/w), Gelucire® 44/14 (80% w/w), and Transcutol® P (5% w/w). Thus, a strong possibility arises that the blend of major components, Gelucire® 44/14 (M.P. 44°C) and Suppocire® AP (MP 33–35°C), would exhibit a semisolid consistency contributing to the drag effect, subsequently to high viscosity.
Fig. 1.
Rheology of suppositories
Texture Analysis
Texture analysis provides an insight into the performance of a suppository under a mechanical force. This aids in understanding the robustness of the product to withstand the mechanical stress incurred during shipment of the suppository (17).
Texture analysis was performed by inserting the instrument probe into the formulation with a force value of less than 1 kg. The probe was introduced at different positions in to the suppositories. The force required for PEG-based suppositories and SMES was 389 and 88.69 g, respectively. The PEG-based suppositories exhibited a higher requirement of force which may be attributed to the solid nature of PEG 4000 and its higher melting range. However, both the suppositories withstood the structural deformation on insertion into rectal cavity of the animal.
Determination of Disintegration Time and Self-Microemulsification Time
The disintegration time and the self-microemulsification time of the SMES (Table III) were in compliance with the pharmacopeial limits. The higher disintegration time is a comparative term. Self-emulsifying suppositories emulsify easily in disintegration medium while PEG suppositories slowly dissolve and melt. Hence, in comparison to developed SMES, PEG-based suppositories demonstrated a higher disintegration time which could also be ascribed to the higher melting points of the suppository base. Conversely, SMES showed rapid microemulsification time justifying its short disintegration time.
Table III.
Determination of Disintegration Time and Self-Microemulsifying Time (n = 6; ±SD)
| Type of system | Disintegration time | Self-microemulsifying time |
|---|---|---|
| PEG-based suppositories | 13 ± 0.5 min | – |
| SMES | 3 ± 0.28 min | 4.5 ± 0.5 min |
Particle Size Analysis
The globule size of SMES was observed to be 156.1 ± 9.3 nm. This may be attributed to the efficient emulsification due to employment of Gelucire® 44/14 and the homogenous dispersion of lipid in aqueous phase due to its self-microemulsifying nature. As reported in literature, the nanosized oil droplets could further aid in enhancing the absorption of the drug, consequently improving the bioavailability (18). In contrast to the SMES, the globule size of PEG suppository-based dispersion was observed to be 26.9 ± 12 μm.
Determination of Drug Content
The drug content of PEG-based suppositories and SMES has been stated in (Table IV). The drug content was found to be within the limits stated in British Pharmacopoeia.
Table IV.
Results of Drug Content (n = 3; ±SD)
| PEG-based suppositories | SMES |
|---|---|
| 103.16 ± 5% | 103.07 ± 3% |
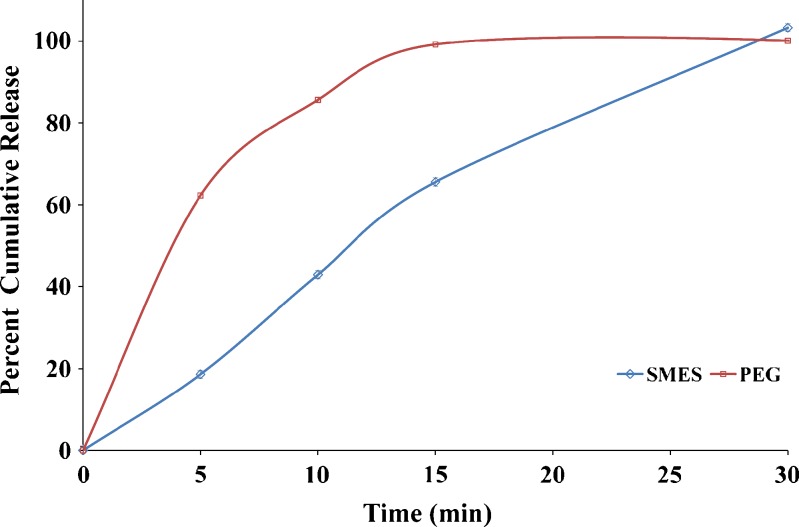
In Vitro Drug Release
The comparative dissolution profile of both the suppository formulations has been shown in (Fig. 2). PEG suppositories released drug at a faster rate (100% in 15 min) as compared to SMES (65% in 15 min). However, release at the end of 30 min was comparable for both the suppositories. The faster release rate of drug from the PEG base and SMES may be due to both the low affinity of the drug for the base and water solubility of the base, which allows the drug to be released by both diffusion and erosion mechanisms (19). Though SMES exhibited a faster emulsification; a delayed release was observed due to the lag time of the drug to get released from oily globules to bulk of the media. Furthermore, there is a possibility that the high content of Gelucire® 44/14 would result in gel formation at the site of administration, which would further sustain the release of the drug from the formulation. Together these two phenomena could contribute to prolong in vivo activity.
Fig. 2.
In vitro drug release profile of suppositories
Pharmacodynamic Evaluation: In Vivo Antimalarial Efficacy Testing in P. berghei-Infected Mice
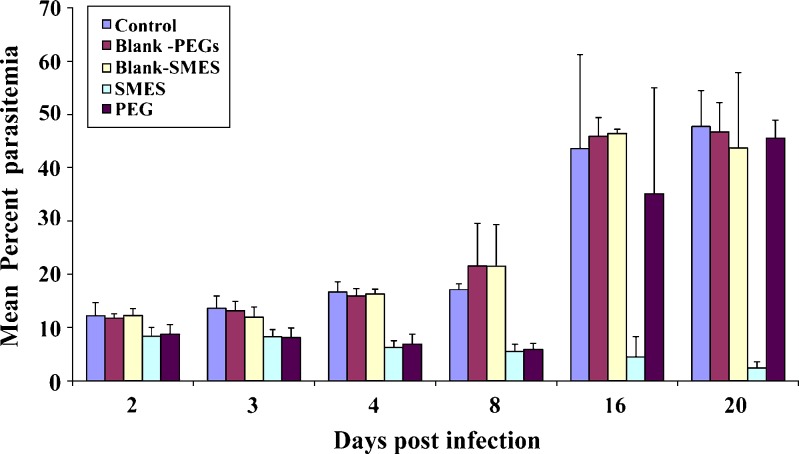
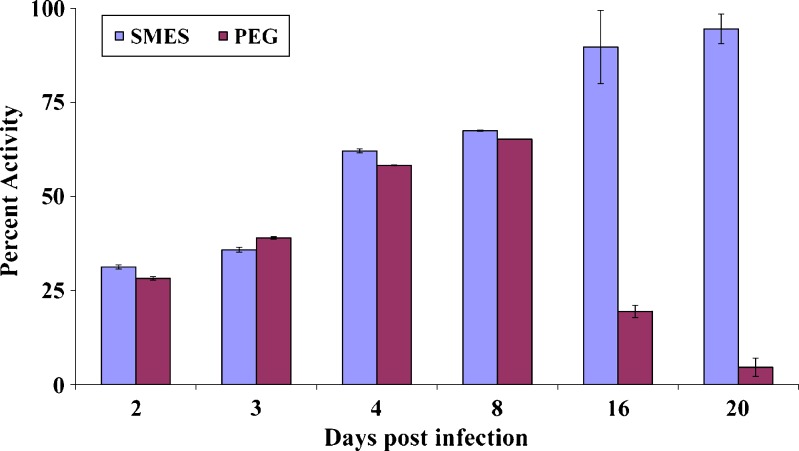
The developed SMES of β-artemether was comparatively evaluated with conventional PEG suppositories. Improved therapeutic efficacy of β-artemether may be attributed to higher and prolonged drug levels at the site of action, on incorporation in SMES. Figures 3 and 4 depict the mean percent parasitemia and percent activity of different groups at varying time points. SMES exhibited higher efficacy because of quicker disintegration resulting in lipid nanodispersion. The in vivo findings further reiterate the fact that high drug solubilization capacity and self-microemulsification efficiency by the SMES could have significantly contributed to the absorption. Moreover, Gelucire® 44/14 and Transcutol® P used to formulate SMES are known penetration enhancers (20). Further, the higher content of Gelucire® 44/14 would contribute to higher viscosity leading to delayed release subsequently leading to prolonged in vivo activity (5). This is also evident from the percent survival plot wherein SMES group was observed to be better as compared to other groups (Fig. 5). Two of the six mice in SMES group survived for 50 days post infection. This again affirms the superior antimalarial effect of SMES as compared to the conventional suppositories.
Fig. 3.
Mean percent parasitemia in P. berghei-infected mice
Fig. 4.
Percent activity of suppository formulations in P. berghei-infected mice
Fig. 5.
Survival plot for the various treatment groups
CONCLUSION
SMES of β-artemether were compared with conventional PEG-based suppositories. The results indicated that SMES possessed all the requisite physico-chemical characteristics of suppositories. Further, in vivo studies demonstrated higher antimalarial activity for SMES as compared to PEG-based suppositories. Additionally, the developed SMES of β-artemether could efficiently prolong the antimalarial activity as compared to PEG-based suppositories suggesting a strong rational for further evaluation in human trials toward commercialization of this formulation.
Acknowledgment
The authors are thankful to the University Grants Commission (New Delhi, India) for the funding required for executing this work. The authors are also thankful to IPCA Laboratories (Mumbai, India) for the gift sample of β-artemether, Colorcon Asia Pvt. Ltd. (Mumbai, India) for the gift samples of excipients.
References
- 1.Joshi M, Pathak S, Sharma S, Patravale V. Solid microemulsion preconcentrate (NanOsorb) of artemether for effective treatment of malaria. Int J Pharm. 2008;362:172–8. doi: 10.1016/j.ijpharm.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 2.Phuong CXT, Bethell DB, Phuong PT, Mail TTT, Nhu TT, Thuyl NTH, Thuyl TPT, Anh NTT, Day NJ, White NJ. Comparison of artemisinin suppositories, intramuscular artesunate and intravenous quinine for the treatment of severe childhood malaria. Trans R Soc Trop Med Hyg. 1997;9(1):335–42. doi: 10.1016/S0035-9203(97)90099-7. [DOI] [PubMed] [Google Scholar]
- 3.Chul SY, Jing JX, Seung HP, Yu KO, Jong SW, Mann HL, Jung AK, Han GC. Enhanced anti-tumor activity and alleviated hepatotoxicity of clotrimazole-loaded suppository using poloxamer–propylene glycol gel. Int J Pharm. 2006;321:56–61. doi: 10.1016/j.ijpharm.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 4.Patel RA, Vavia PR. Preparation and in vivo evaluation of SMEDDS (self-microemulsifying drug delivery system) containing fenofibrate. AAPS PharmSciTech. 2007;9(3):E344–52. doi: 10.1208/aapsj0903041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mandawgade SD, Pathak P, Sharma S, Patravale V. Development of SMEDDS using natural lipophile: application to artemether delivery. Int J Pharm. 2008;362:179–83. doi: 10.1016/j.ijpharm.2008.06.021. [DOI] [PubMed] [Google Scholar]
- 6.Kim JY, Ku YS. Enhanced absorption of indomethacin after oral or rectal administration of a self-emulsifying system containing indomethacin to rats. Int J Pharm. 2000;194:81–9. doi: 10.1016/S0378-5173(99)00367-1. [DOI] [PubMed] [Google Scholar]
- 7.Joshi M, Pathak S, Sharma S, Patravale V. Design and in vivo pharmacodynamic evaluation of nanostructured lipid carriers for parenteral delivery of artemether: nanoject. Int J Pharm. 2008;364:119–26. doi: 10.1016/j.ijpharm.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 8.Chimanuka B, Gabriels M, Detaevernier MR, Plaizier VJ. Preparation of artemether liposomes, their HPLC–UV evaluation and relevance for clearing recrudescent parasitaemia in Plasmodium chabaudi malaria-infected mice. J Pharm Biomed Anal. 2002;28:13–22. doi: 10.1016/S0731-7085(01)00611-2. [DOI] [PubMed] [Google Scholar]
- 9.Baka E, Comer JEA, Novak KT. Study of equilibrium solubility measurement by saturation shake-flask method using hydrochlorothiazide as model compound. J Pharm Biomed Anal. 2008;46:335–41. doi: 10.1016/j.jpba.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 10.Rajan GK, Kulkurni GT, Venkateswaran G, Samanta MK, Suresh B. Formulation and dissolution of meloxicam solid dispersion incorporated suppositories. Ind J Pharm Sci. 2002;64:525–8. [Google Scholar]
- 11.Babar A, Bellet T, Piakogiannis FM. Ketoprofen suppositories dosage forms: in vitro release and in vivo absorption studies in rabbits. Drug Dev Ind Pharm. 1999;25:241–5. doi: 10.1081/DDC-100102166. [DOI] [PubMed] [Google Scholar]
- 12.Victoria MM, David CJ. Thermal and rheological study of lipophilic ethosuximide suppositories. Eur J Pharm Sci. 2003;19:123–8. doi: 10.1016/S0928-0987(03)00071-X. [DOI] [PubMed] [Google Scholar]
- 13.Li H, Gu X. Correlation between drug dissolution and polymer hydration: a study using texture analysis. Int J Pharm. 2007;342:18–25. doi: 10.1016/j.ijpharm.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 14.Ahmed M, Jalil R, Islam M, Shaheen SM. Preparation and stability study of diclofenac sodium suppositories. Pak J Biol Sci. 2000;3(10):1755–7. doi: 10.3923/pjbs.2000.1755.1757. [DOI] [Google Scholar]
- 15.Peters W, Portus JH, Robinson BL. The chemotherapy of rodent malaria. XXII, the value of drug-resistant strains of P. berghei in screening of blood schizonticidal activity. J Trop Med Hyg. 1975;69:155–71. [PubMed] [Google Scholar]
- 16.Fidock DA, Rosenthal PJ, Croft SL, Brun RS, Waka N. Anti-malarial drug discovery: efficacy models for compound screening. Nat Rev Drug Discov. 2004;3:509–20. doi: 10.1038/nrd1416. [DOI] [PubMed] [Google Scholar]
- 17.Azhgikhin S. Determination of the hardness of suppository bases using Kaminskii’s device. Aptechn Delo. 1965;14:14–9. [PubMed] [Google Scholar]
- 18.Grove M, Müllertz A, Nielsen JL, Gitte PP. Bioavailability of seocalcitol II: development and characterization of self-microemulsifying drug delivery systems (SMEDDS) for oral administration containing medium and long chain triglycerides. Eur J Pharm Sci. 2006;28:233–42. doi: 10.1016/j.ejps.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 19.Belal HMD, Rashid M, Motahar AKMH. Effect of different waxy materials on the release of Ibuprofen from polyethylene Glycol based suppositories. Pak J Biol Sci. 2004;7(12):2082–85. doi: 10.3923/pjbs.2004.2082.2085. [DOI] [Google Scholar]
- 20.Xiang L, Shu-fang N, Jun K, Ning L, Cheng-yi J, Wei-san P. A controlled-release ocular delivery system for ibuprofen based on nanostructured lipid carriers. Int J Pharm. 2008;363:177–82. doi: 10.1016/j.ijpharm.2008.07.017. [DOI] [PubMed] [Google Scholar]