Abstract
The aim of this work was to test innovative approach for enhancing ascorbyl palmitate stability in microemulsions for topical application by addition of newly synthesized co-antioxidant 4-(tridecyloxy)benzaldehyde oxime (TDBO) and to investigate its antioxidant activity and finally to evaluate cytotoxicity of TDBO-loaded microemulsions on keratinocyte cells. TDBO significantly increased ascorbyl palmitate stability in oil-dispersed-in-water (o/w) microemulsions, most presumably due to reduction of ascorbyl palmitate radical back to ascorbyl palmitate, since TDBO free-radical scavenging activity was confirmed. Cytotoxicity experiments demonstrated no significant change in cell viability or morphology in the presence of TDBO-loaded microemulsions regarding unloaded microemulsions, although greater cytotoxicity was observed with increased microemulsion concentrations. Therefore, the incorporation of TDBO as non-cytotoxic co-antioxidant in o/w microemulsions is a promising new strategy for enhancing ascorbyl palmitate stability that could be used to support antioxidant network in the skin.
Key words: ascorbyl palmitate, keratinocyte, lipophilic oxime, microemulsions, stability
INTRODUCTION
The use of l-ascorbic acid (vitamin C) for topical application is not a new concept. It has been used in pharmaceutical and cosmetic preparations on the basis of its many favourable effects on the skin for a long time (1). As an antioxidant, it can scavenge and eliminate reactive oxygen species (ROS) especially free radicals (2,3). Indeed, the skin possesses a wide range of interlinked antioxidant defence mechanisms to protect itself from damage caused by ROS which are produced in physiological and in exogenously induced processes. They have a crucial role in photoaging, inflammation and skin carcinogenesis. However, the capacity of this antioxidant network in skin is limited. Supporting the cutaneous antioxidant defence systems with exogenous antioxidants could thus prevent or reduce radical and other reactive-species-mediated damages (4).
Vitamin C also improves the elasticity of the skin and reduces wrinkles by stimulating collagen synthesis (5,6). Since it suppresses pigmentation and decomposes melanin, it is used as a skin-whitening agent (7,8). However, applied ascorbic acid is extremely reactive and therefore unstable in dispersions due to the fast oxidation and further irreversible chemical transformation. Therefore, the use of less reactive derivatives like ascorbyl phosphate salts (pro-antioxidant) or lipophilic ascorbyl esters (9) is an attempt to prolong their stability. Moreover, ascorbyl palmitate (AP) due to its amphiphilic character is able to penetrate the skin better and has increased stability. However, it is still not adequate. The main problem of AP is its oxidation mediated by transition metal ions presented in traces.
It is well known that the stability of all ascorbyl fatty acid esters is influenced also by structural properties of the formulations (9). Colloidal carriers such as microemulsions (ME), liposomes and solid lipid nanoparticles have been investigated; however, the long-term stability of AP in such colloidal carries was still not adequate (10,11). ME are nevertheless of special interest because of their high solubilisation capacity and thermodynamic stability and the simple technology of preparation (12). The structures of ME are in nanometer range, they can be formed as elongated, rod-like micelles, water-dispersed-in-oil (w/o) or oil-dispersed-in-water (o/w) spherical droplet and bicontinuous or lamellar structures. In the water-rich region, o/w droplets are the most frequent form while opposite, in ME where water content is low, w/o structures are formed (13). Additionally, ME may increase the chemical stability of some active compounds, and they are especially convenient for cutaneous application, where the objective is better skin penetration or prolonged activity (14). Because formulation alone has a limited contribution to drug stability, we have investigated an alternative approach to increase AP stability in ME by simultaneous incorporation of additional antioxidant.
Among different lipophilic oximes, we have selected 4-(tridecyloxy)benzaldehyde oxime (TDBO). Its structure is similar to AP as both compounds have lipophilic alkyl residue and a polar moiety. The polar character is, in the case of AP, more expressed because enol hydroxyl group of AP is acidic. Due to the amphiphilic similarity, we assume similar partitioning and similar incorporation into formulation for both compounds. So far, oximes have been studied as inhibitors of low-density lipoprotein oxidation to prevent atherosclerotic diseases and as inhibitors of lipid peroxidation induced by different oxidant agents (15,16). Up to now, their antioxidant properties and capacities are not well understood, although their antioxidant activity is possibly a consequence of their scavenging activity toward different reactive species and/or their metal-chelating properties (17).
The aim of the present work was to evaluate the influence of TDBO on AP stability in ME. To the best of our knowledge, this was the first time that co-antioxidant of oxime type was introduced into ME to increase AP stability. We studied the AP stability in the presence of TDBO in o/w and w/o type of ME. The TDBO free-radical scavenging capacity was tested separately, and the cytotoxicity of TDBO-loaded ME was studied by evaluating the viability of the keratinocyte cell line (NCTC 2544) and by monitoring morphological changes of the cells by light and fluorescence microscopy.
MATERIALS AND METHODS
Materials
AP was provided by Fluka BioChemica. w/o and o/w ME were composed of the medium-chain triglyceride Miglyol 812® (Sasol, Germany) as lipophilic phase, caprylocaproyl macrogolglycerides Labrasol® (Gattefosse, France) as surfactant, polyglyceryl-6 dioleate Plurol Oleique® (Gattefosse, France) as cosurfactant and double-distilled water as hydrophilic phase. Other chemicals were ethylenediaminetetraacetic acid (EDTA; Merck, Germany) and FeSO4·7H2O (Sigma-Aldrich, Germany).
Synthesis of 4-(Tridecyloxy)benzaldehyde Oxime
Synthesis of 4-(Tridecyloxy)benzaldehyde
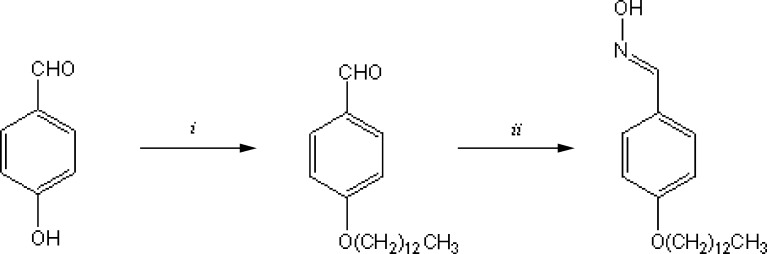
4-Hydroxybenzaldehyde (6.00 g, 49.1 mmol), bromotridecane (24.3 g; 92.1 mmol), K2CO3 (13.6 g; 98.3 mmol) and two drops of trimethyloctylammonium bromide were heated for 3 h at 120°C. After cooling, 50 ml water and 20 ml dichloromethane were added and the phases separated. The aqueous phase was extracted with diethyl ether (3 × 20 ml). The combined organic phases were washed with brine (25 ml) and dried over Na2SO4. The solvent was removed under reduced pressure, and the crude product used in the next reaction step without further purification (Fig. 1).
Fig. 1.
Synthesis of TDBO. i: bromotridecane, trimethyloctylammonium bromide, K2CO3; ii: hydroxylamine hydrochloride, K2CO3, EtOH
Yield, 66%; yellow viscous liquid; TLC: Rf (silica gel, chloroform) = 0.58; 1H NMR (300 MHz, DMSO-d6): δ 9.86 (s, 1H, CHO), 7.09–8.36 (AA′BB′, 4H, J = 8.7 Hz, Δν = 375 Hz, Ar-H), 4.07 (t, 2H, J = 6.6 Hz, CH2), 1.32 (m, 22H, CH2), 0.84 (t, 3H, J = 6.6 Hz, CH3) ppm; MS(EI+): m/z (%) 305 (M+, 33), 73(100); IR (KBr): ν 2925, 2854, 1699, 1602, 1466, 1258, 1159 cm−1.
Synthesis of 4-(Tridecyloxy)benzaldehyde oxime
A mixture of 4-(tridecyloxy)benzaldehyde (7.21 g; 23.6 mmol), hydroxylamine hydrochloride (2.47 g; 35.5 mmol) and K2CO3 (2.70 g; 19.5 mmol) in absolute ethanol (50 ml) was refluxed overnight. The solvent was removed under reduced pressure, and 1 M HCl (25 ml) was added. The aqueous phase was extracted with diethyl ether (4 × 25 ml), the combined organic phases washed with brine (25 ml) and dried over Na2SO4. The solvent was removed in vacuum and the crude product purified by column chromatography (silica gel; chloroform).
Yield, 38%; white solid; m.p. 47–52°C; TLC: Rf (silica gel, chloroform) = 0.10; 1H NMR (300 MHz, DMSO-d6): δ 10.92 (s, 1H, OH), 8.05 (s, 1H, Ar-CH), 6.93–7.51 (AA′BB′, 4H, J = 8.7 Hz, Δν = 168 Hz, Ar-H), 3.48 (t, 2H, J = 6.4 Hz, CH2), 1.20 (m, 22H, CH2), 0.86 (t, 3H, J = 6.4 Hz, CH3) ppm; MS(EI+): m/z (%) 319 (M+, 42), 137(100); IR (KBr): ν 3413, 2916, 2847, 1608, 1468, 1251, 1175, 940, 828, 719 cm−1; Anal. calc. for C20H33NO2: C 75.19%, H 10.41%, N 4.38%. Found: C 75.25%, H 10.33%, N 4.36%.
Methods
Preparation and Physical Characterization of ME
AP was incorporated into ME at 1% w/w concentration. TDBO addition was preliminary tested at AP/TDBO molar ratios of 10:1; 10:2 and 10:4, but only ME with AP/TDBO molar ratio of 10:1 were further evaluated. AP and TDBO were dissolved in Labrasol, within 60 min with ultrasonic agitation. The other three components were added afterwards. ME were formed spontaneously on gentle hand mixing. The compositions of both types of ME are shown in Table I.
Table I.
The Composition of w/o and o/w ME (% w/w) (10)
| Component | w/o | o/w |
|---|---|---|
| Miglyol® | 24.75 | 7.43 |
| Labrasol® | 47.53 | 38.02 |
| Plurol Oleique® | 11.88 | 9.50 |
| Water | 15.84 | 45.05 |
Dynamic light scattering measurements (PCS) of the hydrodynamic radius of the ME structure were performed at 24°C using a Nano ZS, Malvern Instruments. Samples were thermostated 2 min before measurements. The viscosity of the samples was determined with a SV-10 Vibro Viscosimeter (A&D Company, Japan).
Stability Studies
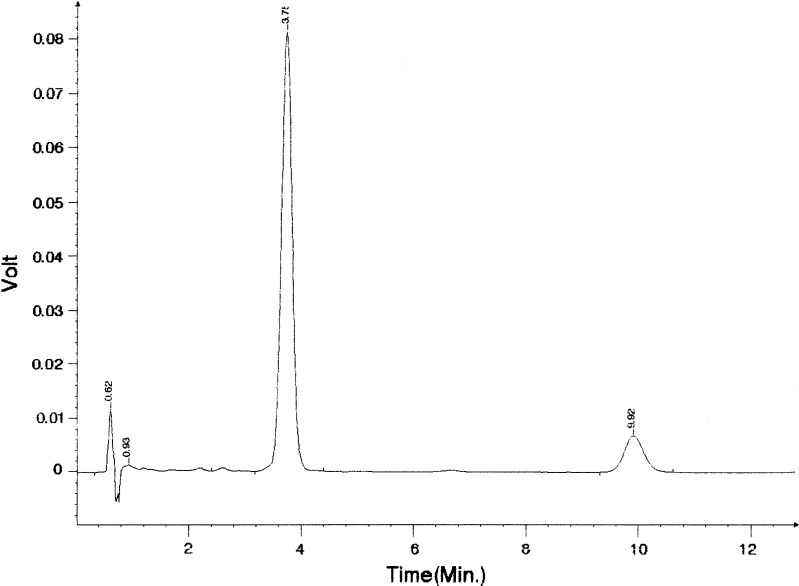
All samples were stored in closed 25 ml glass flasks at room temperature (22 ± 1°C) in the dark over 3 months. The amount of AP and TDBO in ME was determined quantitatively on days 1, 7, 14, 28, 42, 56 and 84 using high-performance liquid chromatography (HPLC; Knauer HPLC pump K-1001, sample injector with a 20-μl sample loop (Midas, Holland Spark) and a Knauer K-2501 variable wavelength detector). The HPLC analysis was adopted according to (10) and (11); the conditions for HPLC analysis were the same for both compounds. The stationary phase was a 125 × 4 mm ID column packed with 5 μm Nucleosil C18, and the mobile phase methanol–acetonitrile–0.02 M phosphate buffer pH 3.5 (75:10:15). The flow rate was 1.5 ml min−1, and detection was at 254 nm. One hundred microlitres of microemulsion was diluted 1:100 (v/v) with methanol prior to HPLC assay. All analyses were performed in triplicate at ambient temperature. The resolution was adequate, with retention time of 3.7 min for AP and 9.9 min for TDBO, without interfering peak from ME components during the analysis time (Fig. 2).
Fig. 2.
HPLC chromatogram of ME with AP (RT = 3.7 min) and TDBO (RT = 9.9 min) under conditions described in “MATERIALS AND METHODS”
Free-Radical Scavenging
The free-radical scavenging capacity of the antioxidant was assayed by the 2,2-diphenyl-1-picrylhydrazyl (DPPH•) method (18). A methanolic solution (0.063 mM) of DPPH• was prepared daily and protected from light. Absorbance was recorded to check the stability of the radical throughout the time of analysis. Five different concentrations of DPPH•, comprising from 15 to 63 μM, were used to establish a linear relationship between DPPH• concentration and absorbance.
The scavenging ability is the ability of TDBO to reduce DPPH radicals. The degree of DPPH• reduction is followed by monitoring the decrease in its absorbance at 516 nm during the reaction (19). One millilitre of TDBO solution in methanol at four different concentrations was added to 2 ml of methanolic DPPH• solution. Absorbance at 516 nm was recorded (Hewlett Packard UV/VIS 8453 spectrophotometer (Germany)) each minute in the first 10 min and every 5 min afterwards until the reaction reached a plateau. The blank reference cuvette contained only methanol. All measurements were performed in triplicate. Reaction kinetics of TDBO with DPPH• were recorded for each antioxidant concentration tested. From these plots, the percentage of DPPH• remaining at the final steady state (DPPH• rem) was determined using Eq. 1:
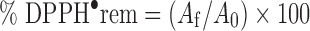 |
1 |
where A0 and Af correspond to the absorbance at 516 nm of the radical at the beginning and final state.
Cell Culture
Normal human undifferentiated keratinocytes, NCTC 2544, were from Interlab Cell line collection (Genoa, Italy). They were grown in 75-cm2 culture flasks using Eagle’s minimum essential medium with Earle’s balanced salt solution (Sigma, Germany), supplemented with 10% fetal bovine serum (Gibco, Invitrogen, USA), 1% penicillin/streptomycin mixture (Sigma, Germany), 1% 2 mM l-glutamine (Sigma, Germany) and 1% non-essential amino acids (Sigma, Germany). They were incubated at 37°C in a humidified atmosphere of 5% CO2. Cells were subcultured with trypsin/EDTA (Sigma, Germany) when they reached 80–90% confluence.
MTT Assay
The effect of unloaded and TDBO-loaded ME on cell viability was evaluated by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay according to the method of Mosmann (20) with slight modifications. MTT assay is an economical, rapid, sensitive and specific colourimetric method for assessing cell viability and in vitro cytotoxicity. This method is based on the reduction of the yellow MTT, a tetrazole, by succinate-tetrazolium reductase system of mitochondrial respiratory chain, active only in viable cells, to purple formazan that absorbs at 540 nm. Since the amount of formed formazan is directly proportional to the number of living cells in culture, the intensity of produced colour is an indication of cell viability: reduction of this intensity thus indicates cellular death. NCTC 2544 cells were seeded in 96-well plates (2.5 × 104 cells per well, 100 μl of cell culture medium), and when appropriate confluence was reached, 10 μl of test formulation was added and left for 4 h. Test formulations were prepared by diluting ME containing 0.1% w/w TDBO in phosphate-buffered saline (PBS) to final concentrations of 0.45, 0.90, 2.25 and 4.50 mg/ml. Cells were treated with PBS (negative control) and sodium dodecyl sulphate (SDS) solution (positive control) with final concentrations of 0.45 and 4.50 mg/ml. After 1 h, 11 μl of MTT in the assay medium (5 mg/ml) was added to each well and incubated at 37°C and 5% CO2 for 3 h. The insoluble purple formazan product was extracted from the cells by acidic isopropanol. The absorbance of the coloured solution was quantified by measuring at 540 nm using a Safire2™ microplate reader (Tecan, Switzerland). Average cell viability of treated cells was calculated according to Eq. 2:
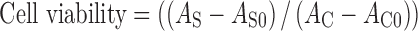 |
2 |
where AS is the absorbance of the treated cells, AC the absorbance of untreated cells (control), AS0 the absorbance of ME diluted in medium without cells and AC0 the absorbance of the medium alone (21). All tests were done in sextuplicate in three independent assays.
Morphological Examination of NCTC 2544 Cells
The morphology of NCTC 2544 cells was examined by inverted light (Olympus CKX41, Japan) and fluorescence (Olympus IX81, Japan) microscopes. Cells were plated on square glass coverslips in supplemented medium (“Cell Culture”) and incubated in six-well plates overnight. Following incubation with TDBO-loaded ME (final concentrations of 0.45, 0.90, 2.25 and 4.50 mg/ml of cell culture medium), cells were examined under inverted microscope.
For fluorescence microscopy, cells were fixed with ice-cold 4% paraformaldehyde in PBS pH 7.4 for 10 min and permeabilized for 10 min in 0.1% Triton X-100 (both Sigma, Germany). Cell nuclei were visualized by staining with DNA intercalating dye Hoechst 33342 (Riedel de Haën, Germany, 5 μg/ml) for 30 min in the dark. Actin fibres were stained with phalloidin TRITC (Sigma, USA, 1:40). After staining, the coverslips were removed from the wells, mounted on a slide and viewed using 360/420 nm (Hoechst) and 535/635 nm (phalloidin TRITC) excitation/emission filter sets.
Statistical Analysis
The results are expressed as means ± SD. Statistical analysis was carried out using independent-samples Student’s t test. Significance was tested at the 0.05 level of probability.
RESULTS AND DISCUSSION
Influence of TDBO on AP Stability in ME
AP and TDBO at a molar ratio of 10:1 were incorporated in o/w and w/o ME. Both types of ME were composed of the same ingredients; they differ only in quantitative composition (Table I); consequently, oily droplets are formed in the water-rich region and water droplets in oil-rich region (22). Nevertheless, interfacial film of surfactant and cosurfactant molecules, where AP and TDBO molecules are located, is formed in both types.
The physical characterization of ME with and without AP or AP/TDBO at a molar ratio of 10:1 was done by determination of absolute viscosity and droplet size (Table II). Concerning viscosity, w/o ME exhibited higher viscosity as expected with lipophilic phase being external, yet the addition of AP or AP/TDBO resulted in practically unchanged viscosity for both types of ME. Furthermore, unloaded and AP- or AP/TDBO-loaded ME demonstrated no evident difference in droplet size. So droplet size analysis together with viscosity results indicates no impairment of ME structure due to AP or TDBO.
Table II.
Absolute Viscosities and Droplet Size of Tested ME at 24°C
| Sample | Viscosity (mPa s) | Size (nm) |
|---|---|---|
| o/w | 54.7 | 118.90 ± 1.20 |
| o/w AP | 55.5 | 99.88 ± 0.32 |
| o/w AP + TDBO | 55.6 | 126.90 ± 7.57 |
| w/o | 81.4 | 651.10 ± 8.20a |
| w/o AP | 82.8 | 594.90 ± 0.85a |
| w/o AP + TDBO | 84.4 | 541.13 ± 0.18a |
aDue to continuously changing internal structure, values of droplet size are not absolute and are primarily used for comparison after incorporation of AP and AP/TDBO
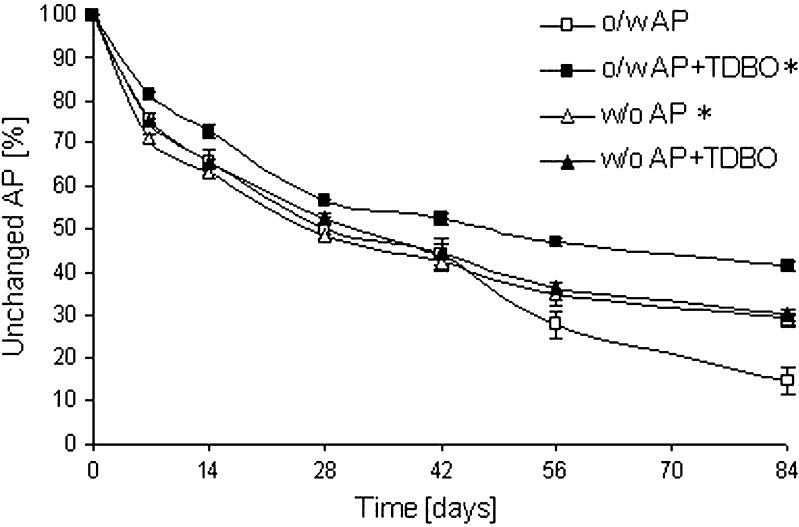
AP was substantially oxidized in both types of ME after 84 days; however, the amount of unchanged AP was higher in w/o ME, being 29.3% in comparison to 14.5% in o/w type (Fig. 3). These results are in accordance with literature data; the higher stability of AP in w/o ME was explained in terms of its different partition patterns in two microemulsion types (10). Due to their amphiphilic structure, the molecules of AP have their palmitic residue in the lipophilic phase while the polar ascorbyl moiety is exposed to the aqueous phase (23). Only the polar part is sensitive to oxidation, and in w/o ME, this part is exposed to the internal aqueous phase, while in o/w ME, it is exposed to the external water phase. Although oxygen is more soluble in the external oil phase of w/o ME than in the external aqueous phase of o/w ME, the oxidation of AP is greater in the latter because the w/o interface acts as a barrier for the diffusion of oxygen to the internal aqueous phase (24).
Fig. 3.
Influence of ME type and TDBO incorporation on AP transformation at AP/TDBO molar ratio of 10:1. *p < 0.05 compared to o/w ME with AP
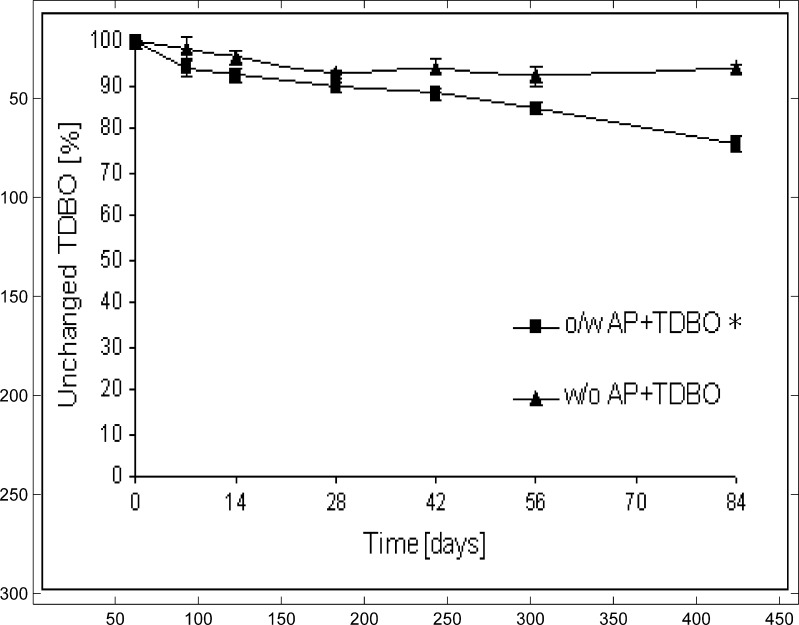
TDBO in an AP/TDBO molar ratio of 10:1 improved the stability of AP in o/w ME; the fraction of unchanged AP was 41.5% after 84 days. Surprisingly, this effect was not observed in w/o ME, where TDBO did not show any influence on AP stability (Fig.3). In the same time intervals, the concentration of TDBO was also determined in both types of ME. The amount of TDBO was progressively reduced in o/w ME to 76.6%, while in w/o ME its amount is reduced only slightly (to 93.7% in 84 days; Fig.4). With respect to these results, only o/w ME was included in further experiments. The effect of TDBO in higher molar ratios, AP/TDBO of 10:2 and 10:4 in o/w ME, was also evaluated. After 84 days, the amount of AP was slightly lower than at a molar ratio of 10:1 (Table III); therefore, the latter was taken as a reasonably effective molar ratio. Consequently, our further work comprised of o/w ME with AP/TDBO molar ratio of 10:1.
Fig. 4.
Influence of ME type on TDBO transformation at AP/TDBO molar ratio of 10:1. *p < 0.05 compared to w/o ME with AP/TDBO = 10:1
Table III.
Unchanged AP (%) in o/w ME with Different Incorporated Components after 84 Days
| Sample (o/w ME) | Unchanged AP (%) |
|---|---|
| AP | 14.5 ± 8.4 |
| AP/TDBO = 10:1 | 41.5 ± 2.4* |
| AP/TDBO = 10:2 | 35.8 ± 5.5* |
| AP/TDBO = 10:4 | 37.2 ± 5.3* |
| AP + Fe2+ | 0.4 ± 0.1 |
| AP + TDBO + Fe2+ | 0.5 ± 0.1 |
| AP + EDTA | 30.5 ± 2.3** |
| AP + EDTA + Fe2+ | 4.5 ± 1.1 |
| AP + TDBO + Fe2+ + EDTA | 11.3 ± 5.0 |
| AP + TDBO + EDTA | 27.5 ± 7.2 |
*p < 0.05 compared to o/w ME with AP; **p < 0.05 compared to o/w ME with AP/TDBO = 10:1
We observed higher stability of AP in o/w ME in the presence of TDBO while in w/o system the AP is more stable in the absence of TDBO. The influence of ME and TDBO on AP stability seems to be rather complex and could be explained by taking into account the partition pattern of AP and TDBO in different types of ME. Amphiphilic AP is soluble in the lipophilic phase and surfactant/cosurfactant mixture but not in the hydrophilic phase. Because of the smaller proportion of lipophilic phase in o/w ME, the local concentration of AP is higher. Assuming a similar TDBO structure, we can also anticipate its higher local concentration in the inner phase of o/w ME and consequently better protection and increased AP stability.
Moreover, some very early steps to determine TDBO mechanism for increasing AP stability were done. Since ferrous ions are important in the oxidative process (25) and therefore participate in AP transformation, the stability of the latter was monitored in the presence of TDBO and metal ions together, to determine whether TDBO could chelate metal ions. Double-distilled water was therefore replaced either with 0.001 mM water solution of FeSO4·7H2O or 0.003 mM water solution of chelating agent EDTA. Results are presented in Table III.
Both TDBO and EDTA reduce the extent of AP transformation whereas, as expected, almost all of the AP was eliminated in the presence of metal ions. Moreover, the same effect was observed with metal ions and TDBO incorporated simultaneously, indicating that TDBO at 0.024 mmol/10 g ME does not chelate metal ions. Also, the amount of unchanged AP was higher in the presence of TDBO than in water solution of EDTA. Thus, TDBO molecules increased AP stability in o/w ME, probably due to its scavenging activity towards different reactive species rather than to its chelating properties. Further work on the evaluation of TDBO antioxidant activity has to be done to evaluate this hypothesis.
TDBO Scavenging Ability on DPPH Radicals
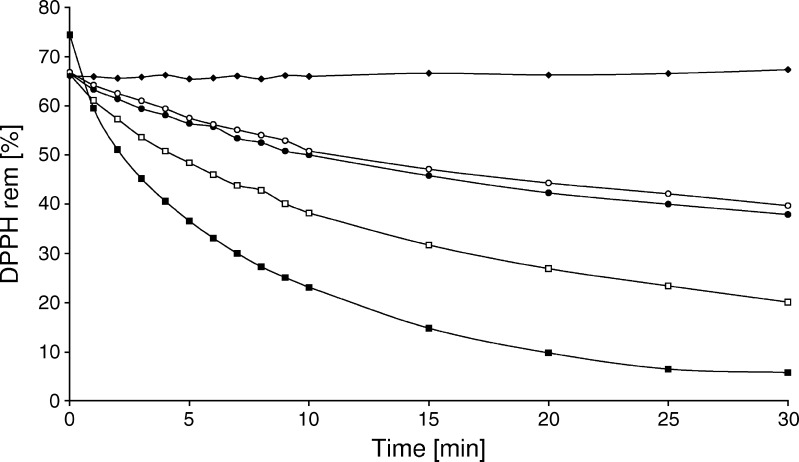
The reduction of DPPH• is shown in Fig. 5. The higher the TDBO concentration (1.6 to 11.0 mM), the smaller was the amount of remaining DPPH• and the higher was the free-radical scavenging activity. Even though the concentration of TDBO is evidently higher compared to DPPH• in all samples tested, the results confirmed TDBO hydrogen donating ability. At 1.6 and 2.2 mM, the TDBO reacted similarly with DPPH•, indicating its minimal effective concentration for its antioxidant activity being 1.6 mM. Moreover, TDBO antioxidant activity when incorporated in w/o and o/w ME was also tested, and DPPH• reduction was observed, indicating no loss of its antioxidant activity in delivery system (data not shown). Additionally, the rate of the reaction depended on the nature of the antioxidant being tested. TDBO reacted slowly with the DPPH•, and this slower rate resulted in hyperbolic curves taking approximately 30 min to reach a steady state. For comparison, reaction with vitamin E, a standard antioxidant, was performed. As expected, it demonstrated better antioxidant capacity: it reacted rapidly with the DPPH radical, reaching a steady state in approximately 3 min, and also concentrations were 100× lower when compared to TDBO (data not shown). While our idea was to utilize an antioxidant that could protect AP during longer period of time, the use of TDBO is substantiated.
Fig. 5.
Influence of different TDBO concentrations; 11 mM (filled squares); 5.5 mM (empty squares); 2.2 mM (filled circles) and 1.6 mM (empty circles) towards DPPH• (filled triangles)
The instability of AP is a result of its oxidative degradation leading to intermediate formation of ascorbyl radical. The higher stability of AP has already been proven when ME were flooded with argon (10), yet, at normal storage conditions, oxygen influence cannot be avoid. The reaction of TDBO with DPPH• was therefore intended to provide the link with the reaction taking place in ME samples, that is at ambient conditions, but protected from light. Despite slow kinetics, the results confirm the TDBO ability to react with DPPH• that indicates the possible reduction of AP radical back to AP, resulting in increased AP stability in o/w ME.
Cytotoxicity of TDBO-Loaded ME on NCTC 2544 Cells
Topical formulations have to be evaluated in terms of their dermal tolerability and toxicity (26,27). The potential skin irritation activity of ME should be especially considered as they are typically composed of large amounts of surfactant that are not innocuous and may cause skin irritation, especially at higher concentrations (28,29). Moreover, the possible cytotoxicity of the newly synthesized TDBO was determined by its influence on the viability of keratinocyte cell line (NCTC 2544). This cell line was chosen since it is simple to grow, is economic and is used to evaluate skin irritation (30).
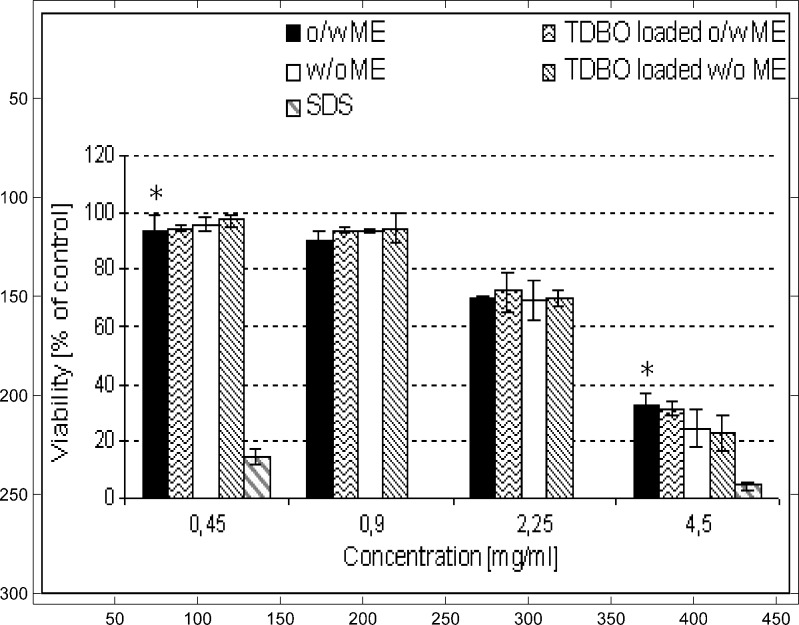
The MTT test was used to investigate the effect of TDBO-loaded o/w and w/o ME. It is well known that AP, a known antioxidant, can directly reduce MTT solution to formazan, so only ME with TDBO incorporated were tested. However, AP is generally recognized as safe and therefore acceptable for dermal delivery (31). Non-treated cell cultures were tested at time zero to represent 100% viability. All other results are expressed as percentages of cell viability compared to non-treated control (Fig. 6).
Fig. 6.
Influence of TDBO on the viability of NCTC 2544 cells at different ME concentrations. Values are means ± SD of three independent experiments, each performed in sextuplicate. *p < 0.05 compared to SDS solution of the same concentration
TDBO was incorporated in both types of ME at 0.1% w/w, since this was confirmed as an effective concentration for AP protection. In order to obtain complete information regarding the acceptability of the delivery system, different concentrations of ME were tested. Since there is no standard protocol for cell viability test on NCTC 2544 cells, concentrations of 0.45, 0.90, 2.25 and 4.50 mg/ml were chosen to cover a wide concentration range. ME showed increased cytotoxicity in correlation with increased concentrations; concentrations 0.45 and 0.90 mg/ml showed minimum effect on cell viability, whereas at higher concentrations (2.25 and 4.50 mg/ml), viability was decreased (Fig. 6). No evident difference between o/w and w/o ME was observed in this respect. All formulations performed considerably better than SDS solution, a standard irritant, tested at two different final concentrations, i.e. 0.45 and 4.5 mg/ml, in order to compare results with border concentrations of ME, where 0.45 mg/ml was the lowest and 4.5 mg/ml the highest concentration tested. SDS solution expressed only 4.4% of cell viability at 4.5 mg/ml and 14.5% at 0.45 mg/ml concentration (Fig. 6).
Most importantly, TDBO was confirmed as non-cytotoxic, since no significant impact on viability of NCTC 2544 cells has been observed.
Cell Morphology
Direct microscopic observation of the cells under the inverted light microscope documented the effect of increasing ME concentration on the cells and correlated with the MTT results. Since TDBO was proven to increase AP stability only in o/w ME and also no significant difference between o/w and w/o ME was detected by the MTT assay, only o/w ME micrographs are presented (Fig. 7). In accordance with the MTT results, no differences between the formulations at lower concentrations (0.45 and 0.9 mg/ml) were observed. The cell morphology was the same as for the non-treated cells, both with and without incorporated TDBO (Fig. 7b, c). Cells treated with formulations at 2.25 mg/ml assumed more rounded shape; however, they were not detached or reduced in number (Fig. 7d, e). On the contrary, at a concentration of 4.5 mg/ml, cells were round and progressively detached and cell clusters appeared (Fig. 7f, g). Again, no evident difference in cell shape or number with TDBO-loaded ME was observed at any concentration used.
Fig. 7.
Representative live-cell light transmission micrographs of NCTC 2544 morphology (phase annulus of ×10); a PBS (control); b, c TDBO-loaded and unloaded o/w ME at 0.9 mg/ml; d, e TDBO-loaded and unloaded o/w ME at 2.25 mg/ml; f, g TDBO-loaded and unloaded o/w ME at 4.5 mg/ml. Micrographs were taken after 4-h incubation with test samples
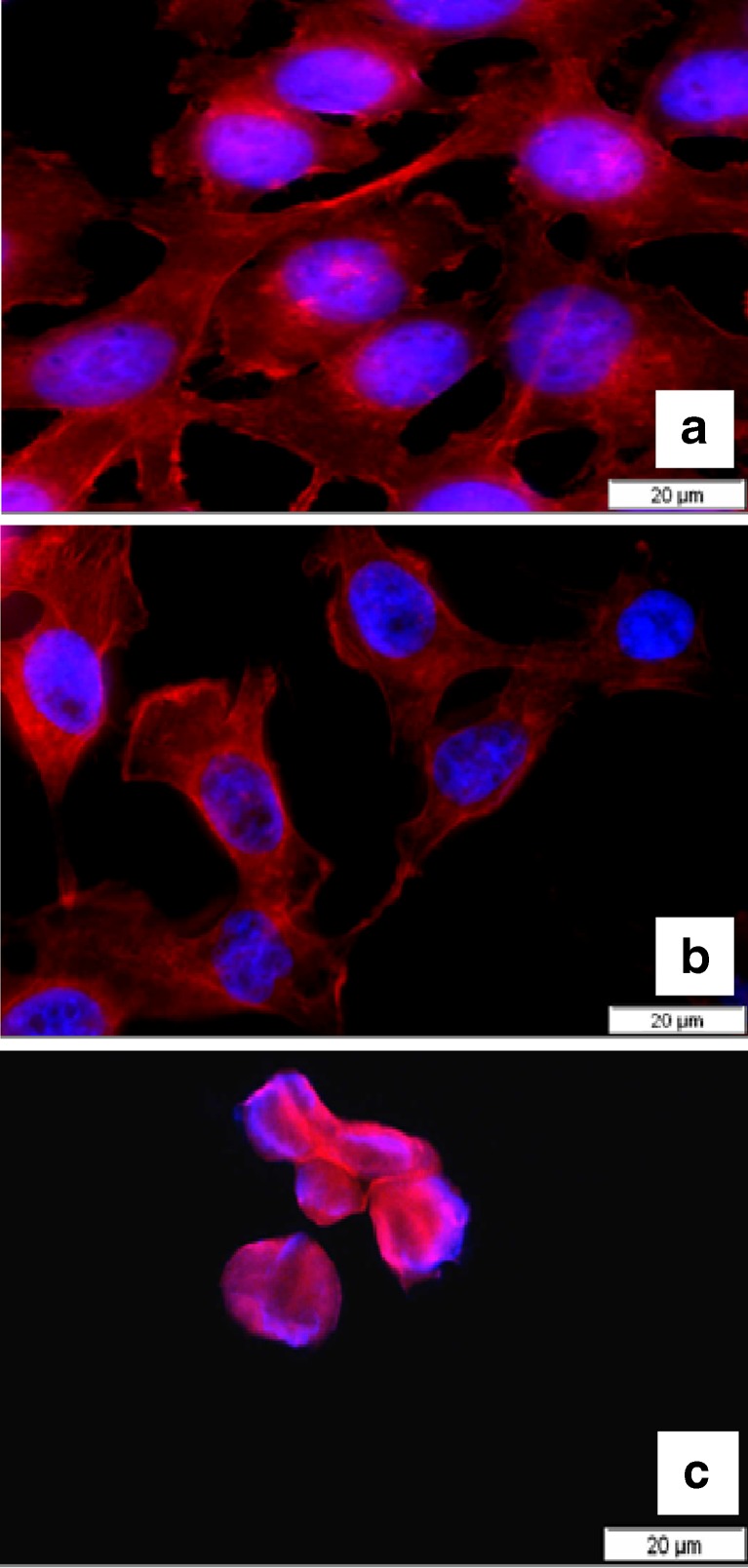
Fluorescence micrographs were taken to further evaluate the effects of TDBO in ME on cell morphology. Alterations in nuclear morphology were visualized by staining DNA with intercalating dye Hoechst 33342, and actin fibres were examined by staining with phalloidin TRITC (Fig. 8). For the previously stated reasons, only fluorescence micrographs of TDBO-loaded o/w ME at concentrations of 0.9 mg/ml (Fig. 8b) and 4.5 mg/ml (Fig. 8c) are presented, due to obvious cell alteration with concentration. With fluorescence microscopy, we observed fine structural alternations of actin fibres at 0.9 mg/ml, which were not detectable by the inverted light microscope; however, no considerable differences from control cells (Fig. 8a) either in number or shape and size of cell nuclei were observed, whereas, as expected, treating cells with formulation at 4.45 mg/ml resulted in their destruction. The cells detached and those remained on the slip during fixation were associated. Actin fibres were totally demolished while cell nuclei became smaller, indicating the cytotoxicity of ME in higher concentrations but again no cytotoxicity of TDBO.
Fig. 8.
Fluorescence micrographs of NCTC 2544 cells grown on a coverslip with blue-stained nuclei and red-stained actin cytoskeleton; a PBS (control); b cells treated with TDBO-loaded o/w ME at 0.9 mg/ml; c cells treated with TDBO-loaded o/w ME at 4.45 mg/ml. No differences were observed when compared to unloaded ME
CONCLUSIONS
The lipophilic oxime TDBO was evaluated for antioxidant properties and cytotoxicity. TDBO molecules have been shown to significantly enhance AP stability in o/w ME for topical application, which has not been reported up to now. Metal chelation by TDBO is marginal due to high degradation of AP when metal ions were added, irrespective of the presence of TDBO. Radical scavenging activity was proposed as the possible mechanism and confirmed by the DPPH method.
Additionally, cytotoxicity experiments on NCTC 2544 cells demonstrated that TDBO is a safe compound, since it showed no significant change in cell viability or morphology regarding unloaded ME, and therefore it can be a potential candidate for use in topical application.
The incorporation of TDBO as additional antioxidant in o/w ME may thus prove a novel and promising strategy for enhancing AP stability, which could therefore be used to support antioxidant defence systems in the skin.
ACKNOWLEDGMENT
Authors would like to thank Prof. Roger H. Pain for proofreading the manuscript. We acknowledge Dr. Branka Rozman and assistant Karmen Teskač for their help with cell culture and fluorescence microscope.
REFERENCES
- 1.Silva GM, Maia Campos PM. Ascorbic acid and its derivatives in cosmetic formulations. Cosmet Toil. 2000;115:59–62. [Google Scholar]
- 2.Colven RM, Pinnell SR. Topical vitamin C in aging. Clin Dermatol. 1996;14:227–234. doi: 10.1016/0738-081X(95)00158-C. [DOI] [PubMed] [Google Scholar]
- 3.Keller KL, Fenske NA. Uses of vitamins A, C and E and related compounds dermatology: a review. J Am Acad Dermatol. 1998;39:611–625. doi: 10.1016/S0190-9622(98)70011-8. [DOI] [PubMed] [Google Scholar]
- 4.Špiclin P, Homar M, Zupančič-Valant A, Gašperlin M. Sodium ascorbyl palmitate in topical microemulsions. Int J Pharm. 2003;256:65–73. doi: 10.1016/S0378-5173(03)00063-2. [DOI] [PubMed] [Google Scholar]
- 5.Fox C. Advances in the cosmetic science and technology of topical bioactive materials. Cosmet Toil. 1997;112:67–84. [Google Scholar]
- 6.Burke KE. Prevention and treatment of aging skin with topical antioxidants. In: Dayan N, editor. Skin aging handbook: an integrated approach to biochemistry and product development. Portland: William Andrew; 2008. pp. 149–176. [Google Scholar]
- 7.Kameyama K, Sakai C, Kondoh S. Inhibitory effect of magnesium l-ascorbyl-2-phosphate (VC-PMG) on melanogenesis in vitro and in vivo. J Am Acad Dermatol. 1996;34:29–33. doi: 10.1016/S0190-9622(96)90830-0. [DOI] [PubMed] [Google Scholar]
- 8.Zhai HZ, Mainbach HI. Skin whitening agents. Cosmet Toilet. 2001;116(1):20–25. [Google Scholar]
- 9.Austria R, Semenzato A, Bettero A. Stability of vitamin C derivatives in solution and topical formulations. J Pharm Sci. 1997;92:58–66. doi: 10.1016/s0731-7085(96)01904-8. [DOI] [PubMed] [Google Scholar]
- 10.Špiclin P, Gašperlin M, Kmetec V. Stability of ascorbyl palmitate in topical microemulsions. Int J Pharm. 2001;222:271–279. doi: 10.1016/S0378-5173(01)00715-3. [DOI] [PubMed] [Google Scholar]
- 11.Kristl J, Volk B, Gašperlin M, Šentjurc M, Jurkovič P. Effect of colloidal carriers on ascorbyl palmitate stability. Eur J Pharm Sci. 2003;19:181–189. doi: 10.1016/S0928-0987(03)00104-0. [DOI] [PubMed] [Google Scholar]
- 12.Terjarla S. Microemulsions: an overview and pharmaceutical application. Crit Rev Ther Drug Carrier Syst. 1999;16:461–521. [PubMed] [Google Scholar]
- 13.Gašperlin M, Bester-Rogač M. Physical characterization of pharmaceutically applicable microemulsions: Tween 40/Imwitor 308/isopropyl myristate/water. In: Fanun M, editor. Microemulsions—properties and applications. Boca Raton: CRC; 2008. pp. 293–313. [Google Scholar]
- 14.Schmalfuss U, Neubert R, Wohlrab W. Modification of drug penetration into human skin using microemulsions. J Cont Release. 1997;46:279–285. doi: 10.1016/S0168-3659(96)01609-4. [DOI] [Google Scholar]
- 15.Worek F, Thiermann H, Szinicz L, Eyer P. Kinetic analysis of interactions between human acetylcholinesterase, structurally different organophosphorus compounds and oximes. Biochem Pharmacol. 2004;68:2237–2248. doi: 10.1016/j.bcp.2004.07.038. [DOI] [PubMed] [Google Scholar]
- 16.De Lima Portella R, Barcelos RP, de Bem AF, Carratu VS, Bresolin L, da Rocha JBT, et al. Oximes as inhibitors of low density lipoprotein oxidation. Life Sci. 2008;83:878–885. doi: 10.1016/j.lfs.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Puntel GO, de Carvalho NR, Gubert P, Schwertner Palma A, Dorte CLD, Avila DS, et al. Chem Biol Interact. 2009;177:153–160. doi: 10.1016/j.cbi.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 18.Sanchez-Moreno C, Larrauri JA, Saura-Calixto F. Free radical scavenging capacity and inhibition of lipid oxidation of wines, grape juices and related polyphenolic constituents. Food Res Int. 1999;32:407–412. doi: 10.1016/S0963-9969(99)00097-6. [DOI] [Google Scholar]
- 19.Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. Lebensm-Wiss u Technol. 1997;28:25–30. [Google Scholar]
- 20.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 21.Kristl J, Teskač K, Milek M, Mlinarič-Raščan I. Surface active stabilizer tyloxapol in colloidal dispersions exerts cytostatic effects and apoptotic dismissal of cells. Toxicol Appl Pharmacol. 2008;363:183–191. doi: 10.1016/j.taap.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 22.Zvonar A, Rozman B, Bešter Rogač M, Gašperlin M. The influence of microstructure on celecoxib release from a pharmaceutically applicable system. Acta Chim Slov. 2009;56:131–138. [Google Scholar]
- 23.Jurkovič P, Šentjurc M, Gašperlin M, Kristl J, Pečar S. Skin protection against ultraviolet induced free radicals with ascorbyl palmitate in microemulsions. Eur J Pharm Biopharm. 2003;56:59–66. doi: 10.1016/S0939-6411(03)00062-6. [DOI] [PubMed] [Google Scholar]
- 24.Gallarate M, Carlotti ME, Trotta M, Bovo S. On the stability of ascorbic acid in emulsified systems for topical and cosmetic use. Int J Pharm. 1999;188:233–241. doi: 10.1016/S0378-5173(99)00228-8. [DOI] [PubMed] [Google Scholar]
- 25.Liang C-H, Syu J-L, Mau J-L. Antioxidant properties of solid-state fermented adlay and rice by Phellinus linteus. Food Chem. 2009;116:841–845. doi: 10.1016/j.foodchem.2009.03.032. [DOI] [Google Scholar]
- 26.Kreilgaard M. Influence of microemulsions on cutaneous drug delivery. Adv Drug Deliv Rev. 2002;54:77–98. doi: 10.1016/S0169-409X(02)00116-3. [DOI] [PubMed] [Google Scholar]
- 27.Yuan JS, Ansari M, Samaan M, Acosta EJ. Linker-based lecithin microemulsions for transdermal delivery of lidocaine. Int J Pharm. 2008;349:130–143. doi: 10.1016/j.ijpharm.2007.07.047. [DOI] [PubMed] [Google Scholar]
- 28.Paolino D, Ventura CA, Nistico S, Puglisi G, Fresta M. Lecithin microemulsions for the topical administration of ketoprofen: percutaneous adsorption through human skin and in vivo human skin tolerability. Int J Pharm. 2002;244:21–31. doi: 10.1016/S0378-5173(02)00295-8. [DOI] [PubMed] [Google Scholar]
- 29.Date AA, Patravale VB. Microemulsions: applications in transdermal and dermal delivery. Crit Rev Ther Drug Carrier Syst. 2007;24(6):547–596. doi: 10.1615/critrevtherdrugcarriersyst.v24.i6.20. [DOI] [PubMed] [Google Scholar]
- 30.Burlando B, Parodi A, Volante A, Bassi AM. Comparison of the irritation potentials of Boswellia serrata gum resin and of acetyl-11-keto-beta-boswellic acid by in vitro cytotoxicity tests on human skin-derived cell lines. Toxicol Lett. 2008;177:144–149. doi: 10.1016/j.toxlet.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 31.Andersen FA. Final report on the safety assessment of ascorbyl palmitate, ascorbyl dipalmitate, ascorbyl stearate, erythorbic acid, and sodium erythorbate. Int J Toxicol. 1999;18(3):1–26. doi: 10.1177/109158189901800303. [DOI] [Google Scholar]