Abstract
To develop a gentamicin-loaded wound dressing, cross-linked hydrogel films were prepared with polyvinyl alcohol (PVA) and dextran using the freezing–thawing method. Their gel properties such as gel fraction, swelling, water vapor transmission test, morphology, tensile strength, and thermal property were investigated. In vitro protein adsorption test, in vivo wound healing test, and histopathology were performed. Dextran decreased the gel fraction, maximum strength, and thermal stability of hydrogels. However, it increased the swelling ability, water vapor transmission rate, elasticity, porosity, and protein adsorption. The drug gave a little positive effect on the gel properties of hydrogels. The gentamicin-loaded wound dressing composed of 2.5% PVA, 1.13% dextran, and 0.1% drug was more swellable, flexible, and elastic than that with only PVA because of its cross-linking interaction with PVA. In particular, it could provide an adequate level of moisture and build up the exudates on the wound area. From the in vivo wound healing and histological results, this gentamicin-loaded wound dressing enhanced the healing effect more compared to conventional product because of the potential healing effect of gentamicin. Thus, this gentamicin-loaded wound dressing would be used as a potential wound dressing with excellent forming and improved healing effect in wound care.
KEY WORDS: dextran, gentamicin, histological examination, wound dressing, wound healing effect
INTRODUCTION
Ideal wound dressing maintains a moist environment around the wound and absorbs the exudates from the wound surface (1). Acute and partial wounds showed a significant increase in re-epithelialization rates when they were maintained in a moist local environment (2,3). Hydrogels, three-dimensional cross-linked hydrophilic polymers with a very high intrinsic content of water, can provide a moist environment to the wound area and absorb the exudates (4–8).
Many hydrogels are prepared by physical methods such as repeated freezing and thawing, chemical methods using a covalent cross-linking agent including boric acid, glutaraldehyde, and formaldehyde, or radiation methods using electron beam or γ-irradiation (9–11). Polyvinyl alcohol (PVA) hydrogels prepared with a freeze–thawing method have been studied for biomedical and pharmaceutical applications because of their non-toxicity and good biocompatibility (12,13).
In our previous reports, nitrofurazone-loaded PVA/sodium alginate (14) and clindamycin-loaded PVA/sodium alginate hydrogels (15) could not improve the healing effect compared to conventional products. Thus, in this study, to develop an effective gentamicin-loaded wound dressing with an enhanced healing effect, dextran and gentamicin were used instead of sodium alginate and other drugs. The cross-linked hydrogel films were prepared with PVA and dextran using the freezing–thawing method. These PVA/dextran hydrogels formed a matrix of physically cross-linked polymeric chains containing uncross-linked polymer and water. Their gel properties such as gel fraction, swelling, water vapor transmission test, morphology, tensile strength, and thermal property were investigated. In vitro protein adsorption test, in vivo wound healing test, and histological examination in rat dorsum were carried out.
Dextran is a polysaccharide consisting of glucose molecules coupled into long branched chains, mainly through 1,6-glucosidic and some through 1,3-glucosidic linkages. This material is colloidal, hydrophilic, water-soluble, and inert in biological systems and hardly affects the cell viability. Because of these properties, dextran has been studied for use as a carrier system for a variety of therapeutic agents including antidiabetic, antibiotic, anticancer, peptide, and enzyme (16). PVA is a semi-crystalline copolymer of vinyl acetate and vinyl alcohol that has been widely utilized in the chemical and medical industries because of its biocompatibility, non-toxicity, hydrophilicity, fiber/film-forming ability, chemical resistance, and protein adsorption (17,18). Gentamicin has been used topically in the treatment of superficial infections of the skin since it is effective against many aerobic Gram-negative and some aerobic Gram-positive bacteria (19).
MATERIALS AND METHODS
Materials
PVA (typical average Mw = 146,000–186,000; +99% hydrolyzed) and dextran (typical average Mw = 60,000–90,000) were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Human serum albumin (HSA; Mw = 66 kDa; albumin, 97.31%) and human plasma fibrinogen (HPF; Mw = 341 kDa; clottable proteins, >95%) were purchased from Fluka Co. (Buchs, Switzerland) and Calbiochem Co. (San Diego, CA, USA), respectively. Gentamicin sulfate was kindly supplied from Han-Mi Pharm. Co. (Yong-in, South Korea). Conventional wound dressing product (Medifoam™) was purchased from Ildong Pharm. Co. (Seoul, South Korea). All other chemicals were used without any further purification.
Animals
Ten male Sprague–Dawley rats weighting 250–280 g purchased from Charles River Company Korea (Orient, Seoul, South Korea) were used to evaluate the in vivo wound healing effect and histopathology of hydrogels. All animal care and procedures were conducted according to the Guiding Principles in the Use of Animals in Toxicology, as adopted in 1989, revised in 1999, and amended in 2008 by the Society of Toxicology (20). Furthermore, the protocols for the animal studies were approved by the Institute of Laboratory Animal Resources of Yeungnam University. The rats were allowed free access to food and water at a temperature of 20–23°C and a relative humidity of 50 ± 5% for 24 h prior to the experiments.
Preparation of Hydrogels
PVA/dextran-based hydrogels were obtained by the freezing–thawing (F–T) cycle. In brief, 100 g PVA and 15 g dextran were dissolved in 1 L distilled water, respectively. Then, 10% PVA solution and 1.5% dextran solution were mixed at the volume ratio of 20:0, 15:5, 12.5:7.5, 10:10, 7.5:12.5, and 5:15, respectively. Gentamicin (0 or 20 mg) was mixed to these PVA/dextran blended solutions, vortexed for 1 h, and poured into Petri dishes. The compositions of PVA/dextran solutions for hydrogels are given in Table I. They were frozen at −20°C for 18 h and then thawed at 25°C for 6 h. This procedure was repeated for three consecutive cycles, leading to PVA/dextran-based hydrogels (21,22).
Table I.
Compositions of PVA/Dextran-Based Hydrogels
| Ingredient | I | II | III | IV | V | VI |
|---|---|---|---|---|---|---|
| PVA (g) | 10 | 7.5 | 6.25 | 5.0 | 3.75 | 2.5 |
| Dextran (g) | – | 0.38 | 0.56 | 0.75 | 0.94 | 1.13 |
| Water (mL) | 100 | 100 | 100 | 100 | 100 | 100 |
Each composition contained no drug or 0.1% (w/v) gentamycin
Determination of Gel Fraction
The pieces of hydrogel samples (2 × 2 cm) were dried for 6 h at 50°C under vacuum (Wo). They were soaked in 10 mL distilled water into Petri dishes for 24 h up to a constant weight and taken out from Petri dishes in order to remove the soluble parts. The gels were dried again at 50°C under vacuum (We). The gel fraction percentage was calculated by the following equation (23,24):
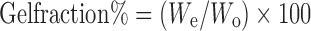 |
1 |
where Wo and We are the weights of hydrogel samples dried for 6 h at 50°C before and after soaking, respectively.
Determination of Swelling Ratio
The pieces of hydrogel samples (2 × 2 cm) were dried at 60°C under vacuum for 12 h (Wa). They were then soaked in pH 7.4 phosphate buffer solution (PBS) at 37°C (Ws). The swelling ratio (SR) was calculated using the following equation (14,23):
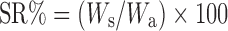 |
2 |
where Wa and Ws are the weights of hydrogel samples dried for 12 h at 60°C and soaked in PBS at 37°C, respectively.
Water Vapor Transmission Test
The water vapor transmission tests were performed using the JIS 1099A standard method (22). A round piece of hydrogel was mounted on the mouth of a cup (7-cm diameter) containing 50 g of CaCl2 and placed in an incubator of 90% RH at 40°C. The water vapor transmission rate (WVTR) was determined as follows:
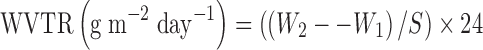 |
3 |
where W1 and W2 are the weights of the whole cup at the first and second hours, respectively, and S is the transmitting area of the sample.
Morphology Observation
The internal structure of the samples was investigated by scanning electron microscopy (SEM, S-4100, Hitachi, Japan). The hydrogels were dehydrated by a freeze dryer and coated with platinum using an ion sputter (E-1030, Hitachi, Ltd.) (21,25).
Mechanical Properties
The tensile strength and breaking elongation of hydrogels were determined using a tensile test machine (Instron 4464, UK). After three F–T cycles, the hydrogels were cut into a specific dog bone shape (6 cm long and 2 cm wide at the ends and 1 cm wide in the middle). The mechanical analysis was performed at a stretching rate of 20 mm/min with a pre-load of 0.5 N to determine the maximum load for each matrix. The thickness of the hydrogel was measured with a digimatic caliper (CD-15CPX, Mitutoyo Co., Japan) before examination (26).
Thermal Analysis Properties
The thermal characteristics of hydrogels were investigated using a differential scanning calorimeter (TA instruments Co., USA) in order to measure their crystallization temperature (TC) and melting temperature (Tm). The analysis was performed at a heating rate of 20°C/min, from 30°C to 200°C, under a nitrogen gas with a flow rate of 10 mL/min.
For the determination of the thermostability of the hydrogels, thermogravimetric analysis was conducted under 50 mL/min nitrogen gas with a heating rate of 10°C/min in the range from 30°C to 500°C by a TGA 2950 (TA instruments Co.). The weight changes of the hydrogel preparations were observed (14,27,28).
Adsorption of Protein onto Hydrogel Surface
The pieces of hydrogels cut into 2 × 2 cm were immersed in 4 mL of pH 7.4 PBS containing HSA and HPF at 37°C. After shaking at 100 rpm for 24 h, they were mildly taken out and rinsed five times with PBS. They were placed in six wells containing an aqueous solution of 1% sodium dodecyl sulfate and shaken for 1 h to remove the protein adsorbed on the surface. The protein contents of each sample were measured using the BCA reagents. The absorbance at 562 nm was measured using a microplate reader (Molecular Devices Versa MAX Sunnyvale, CA, USA) (14,22).
In Vivo Wound Healing Test
The rats were anesthetized by i.p. injection of Zoletil 50® (tiletamine/zolazepam), and the dorsal hair of each animal was shaved with an electric razor. After creating two full-thickness wound areas (1.5 × 1.5 cm) by excising the dorsum, 70% ethanol was used for sterilization. Each wound was covered with sterile gauze (control), the hydrogel without drug, the hydrogel with drug, and the commercial wound dressing product, respectively. All materials were fixed with an elastic adhesive bandage (Soft Cloth Tape®, 3M, USA). All rats were separately kept in individual cages. At the predetermined intervals, each wound size was measured using a digital camera. The relative wound size reduction was calculated as follows:
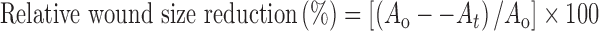 |
4 |
where Ao and At are the wound size at initial time and time “t”, respectively. The wound size was surveyed using the Adobe® Acrobat® 7 Program (9,14).
Histopathology
Histological Process
After wound healing test of 15 days post-operation, the rats were killed. The wounded area of skin containing the dermis and hypodermis was then sampled and carefully trimmed with a cutter. All trimmed skins were fixed in 10% neutral buffered formalin. After paraffin embedding, 3- to 4-μm sections were prepared. Representative sections were stained with hematoxylin and eosin (H&E) and Masson’s trichrome. The light microscopic examination on histological profiles of individual skin was performed (29–31).
Histomorphometry
The desquamated epithelium regions (mm), numbers of microvessels in granulation tissues (vessels/mm2 of field), numbers of infiltrated inflammatory cells in granulation tissues (cells/mm2 of field), percentages of collagen-occupied regions in granulation tissues (%/mm2 of field), and thicknesses of central regions of granulation tissues (millimeter from epidermis to dermis) were measured on the histological skin samples using a digital image analyser (DMI-300, DMI, South Korea), respectively (32). Furthermore, the re-epithelialization was calculated as follows (33):
 |
5 |
Statistical Analysis
Multiple comparison tests for different dose groups were conducted. Variance homogeneity was examined using the Levene test (34). If the Levene test indicated no significant deviations from variance homogeneity, the data were analyzed by one-way ANOVA test and the least significant differences multi-comparison test. In case of significant deviations from the variance, homogeneity was observed using the Levene test, a non-parametric comparison test, and the Kruskal–Wallis H test. A P value ≤0.05 was considered to be statistically significant using SPSS for Windows (Release 14.0, SPSS Inc., USA) (35). Moreover, to evaluate the comparable efficacy of gauze control and test materials, their changes were calculated as follows:
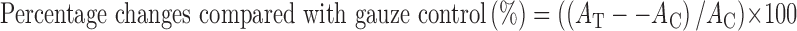 |
6 |
where AT and AC are data of the test groups and gauze control, respectively.
RESULTS AND DISCUSSION
Gel Fraction
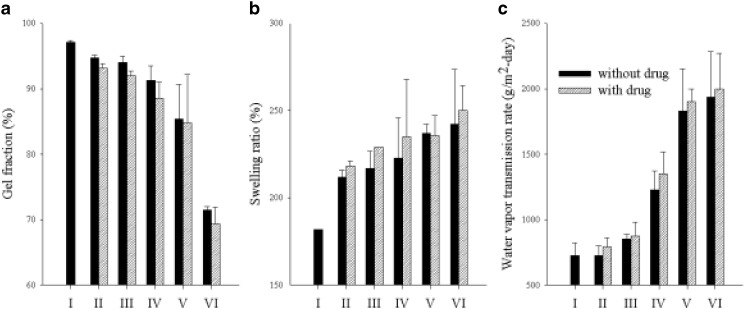
Three consecutive cycles of freezing and thawing formed an insoluble and entangled hydrogel. The influence of dextran and drugs on the gel fraction percentage is shown in Fig. 1a. Similar to other polymers, increasing dextran content in the hydrogel resulted in lower gel fraction (14). The gel fraction of hydrogel with only PVA and no dextran was about 97% and relatively high, suggesting that PVA was almost completely cross-linked (36). The gel fraction decreased to <65% at 1.13% dextran concentration. During F–T cycles, the cross-linking strength of dextran was weaker than that of PVA, even though dextran formed a cross-linking bond with PVA in the gel. Generally, as the gel fraction decreased, the strength of the gel was weakened but the flexibility was increased (14,23). Therefore, dextran could be used to control the strength and flexibility of hydrogel because it reduced the cross-linking reaction and, consequently, the gelation process. Furthermore, the hydrogel with drug gave lower gel fraction than did the hydrogel without drug. The drugs reduced the cross-linking interaction between polymers.
Fig. 1.
Effect of dextran and drugs on the a gel fraction, b swelling ratio, and c WVTR. Each value represents the mean ± SD (n = 3)
Hydrophilicity
The swelling ability increased to more than 250% at 1.13% dextran with increased dextran concentration (Fig. 1b). This could produce a less cross-linked hydrogel than PVA did. Lesser cross-linked hydrogels showed a higher water uptake because the highly cross-linked structure could not sustain much water within the gel structure (9,37). The hydrogel with drug improved the swelling ability compared to the hydrogel without drug.
The effect of dextran and drugs on the WVTR of the hydrogel was given in Fig. 1c. Queen et al. (38) reported that a water vapor transmission rate of 2,000–2,500 g m−2 day−1 would provide an adequate level of moisture to prevent excessive dehydration and build up the exudates on the wound area. A higher WVTR dried the wound more quickly and produced the scars. Moreover, a lower WVTR accumulated exudates, which might retard the healing process and result in increased risk of bacterial growth (38). Dextran increased the WVTR of hydrogels. The WVTR of the hydrogel with 1.13% dextran was 1,900–2,000 g m−2 day−1, which was close to the ideal value for wound dressing. Thus, it might provide an adequate level of moisture and build up the exudates on the wound area. Similarly, the hydrogel with drug improved the WVTR than did the hydrogel without drug. The drugs had a little influence on WVTR as gentamicin could interfere with the cross-linking of PVA and dextran.
Scanning Electron Microscopy
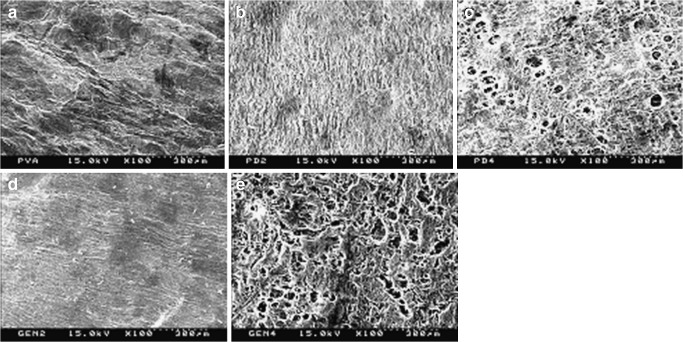
The internal structures of hydrogels prepared by the freezing–thawing method were shown in Fig. 2. As shown in Figs. 2a–c, dextran produced more homogeneous gels. Dextran might favor the crystallization process of PVA in this gel, resulting in the formation of a more ordered and homogeneous structure (21). However, as shown in Fig. 2bversus d and c versus e, the drug incorporated in the hydrogel did not affect the structure of the hydrogel.
Fig. 2.
SEM micrographs of the cross-section of hydrogels: a hydrogel with only PVA; b hydrogel with 0.56% dextran and no drug; c hydrogel with 1.13% dextran and no drug; d hydrogel with 0.56% dextran and 0.1% drug; e hydrogel with 1.13% dextran and 0.1% drug
Mechanical Properties
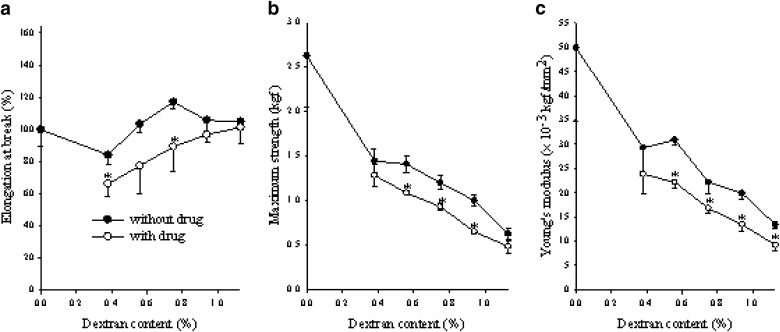
To investigate the influence of dextran on the mechanical properties of the hydrogels, their tensile strength, elongation at break, and Young’s modulus were evaluated (Fig. 3). Dextran hardly affected the elongation at break (Fig. 3a). However, it decreased the tensile strength (Fig. 3b) and reduced the Young’s modulus of the hydrogel (Fig. 3c). As dextran was blended with PVA, the cross-linking density of the gel was decreased (10). Thus, dextran produced less stiff and more elastic hydrogels (14,17). Furthermore, gentamycin in the hydrogel significantly decreased the tensile strength, elongation at break, and Young’s modulus of the hydrogels (Fig. 3). This might be due to the formation of hydrogen bonding or intermolecular interaction between the drug and polymer molecules, resulting in its relatively reduced cross-linking density (15,39).
Fig. 3.
Effect of dextran and drug on the elongation at a break, b maximum strength, and c Young’s modulus. Each value represents the mean ± SD (n = 3). *P < 0.05 compared with the hydrogel without drug
Thermal Analysis
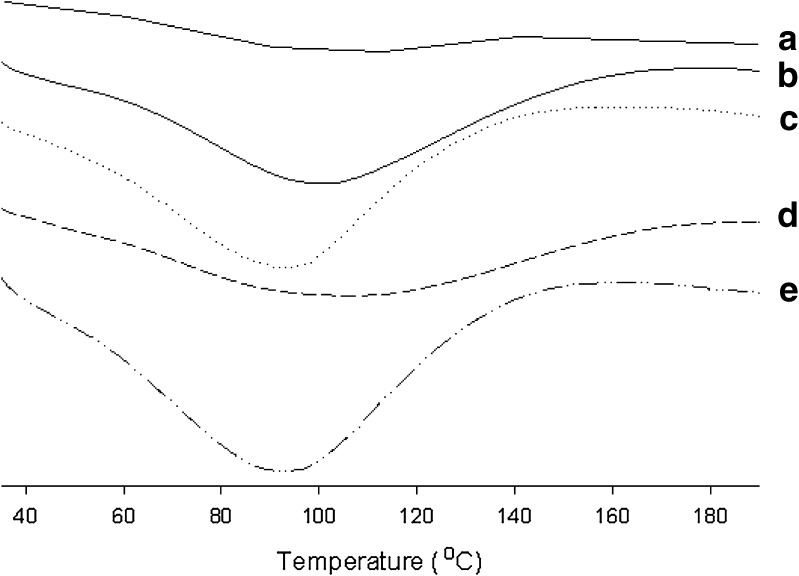
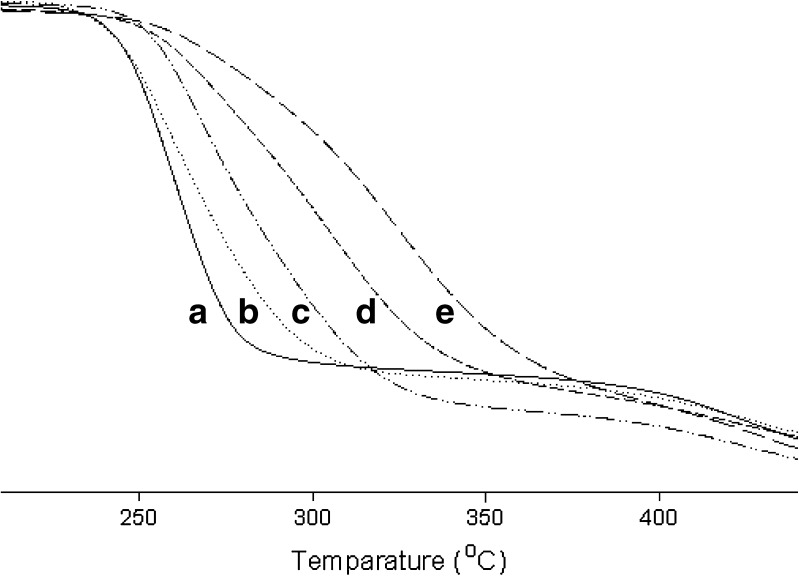
The differential scanning calorimetry (DSC) thermograms of the hydrogels are illustrated in Fig. 4. The hydrogel with PVA gave no intrinsic peak (Fig. 4a). However, the broad endothermic peak was observed at about 80–90°C in the hydrogels with PVA and dextran. However, these peaks showed a similar pattern, suggesting that substantial modification did not occur in the nature of PVA crystallites because of their biological components (21). Moreover, the hydrogel with drug gave a similar pattern of endothermic curve compared to the hydrogel without drug. Furthermore, the effect of dextran and drugs on the thermal stability of hydrogels was shown in Fig. 5. As both dextran and drugs increased, the weight loss increased. Thus, they improved the thermal stability of gels because the hydroxyl groups of dextran and drugs might form hydrogen bonding with the hydroxyl groups of PVA (26).
Fig. 4.
DSC thermogram of PVA and PVA/dextran mixed hydrogels: a hydrogel with only PVA; b hydrogel with 0.56% dextran and no drug; c hydrogel with 1.13% dextran and no drug; d hydrogel with 0.56% dextran and 0.1% drug; e hydrogel with 1.13% dextran and 0.1% drug
Fig. 5.
TGA thermogram of PVA and PVA/dextran mixed hydrogels: a hydrogel with only PVA; b hydrogel with 0.56% dextran and no drug; c hydrogel with 1.13% dextran and no drug; d hydrogel with 0.56% dextran and 0.1% drug; e hydrogel with 1.13% dextran and 0.1% drug
From these findings, the wound dressing developed with PVA and dextran was more swellable, flexible, and elastic than that with only PVA because of its cross-linking interaction with PVA. In particular, the hydrogel composed of 2.5% PVA, 1.13% dextran, and 0.1% drug could provide an adequate level of moisture and build up the exudates on the wound area. Thus, it was selected as a formulation of gentamicin-loaded wound dressing for further study.
Adsorption of Proteins onto the Hydrogel Surfaces
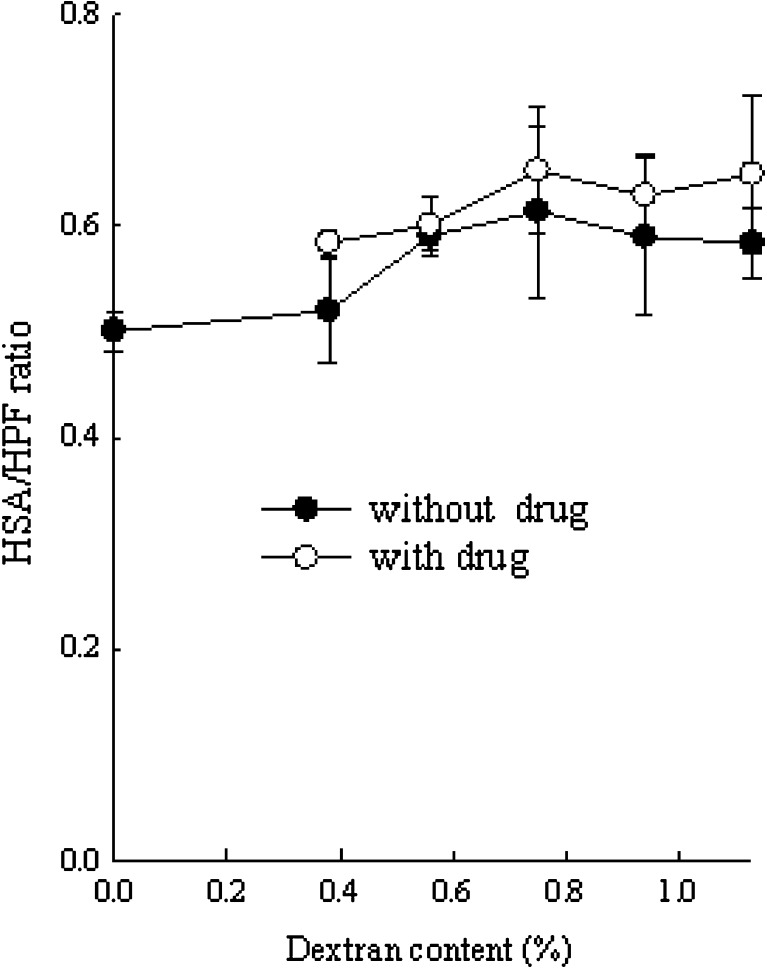
The blood compatibility of the hydrogel was evaluated by the amount of plasma protein adsorbed onto the hydrogel surface. When foreign material was placed in contact with blood, the adsorption of protein onto the surface occurred, leading to platelet adhesion and activation (29,40). Because the albumin adsorption on the synthetic surfaces could inhibit platelet activation, it did not promote clot formation.
All the hydrogels with PVA and dextran gave higher HSA/HPF ratio than did the hydrogel with only PVA (Fig. 6). The other hydrogels gave about 1.2-fold higher HSA/HPF ratio than that with 0.38% dextran. However, they showed no significant changes in HSA/HPF ratios. Furthermore, the hydrogels with drug gave a higher HSA/HPF ratio than did the hydrogel without drug. Generally, as the albumin/fibrinogen adsorption ratio was higher, the number of adhering platelets was lower (41). Thus, dextran and drug gave less adhesion of platelets to artificial surfaces.
Fig. 6.
Effect of dextran and drug on the HSA/HPF ratio. Each value represents mean ± SD (n = 3)
In Vivo Wound Healing
To estimate the wound healing ability of the hydrogel for the acceleration of wound repair, the PVA/dextran hydrogels were applied to wound spots in the rat dorsum. In the preliminary study, to investigate the location of the wound spots in the rat dorsum on the healing effects, four wound spots in different locations (see Fig. 7a) were covered with sterile gauze (control) followed by fixing with an elastic adhesive bandage. When they were observed for 15 days post-operation, their macroscopic appearances and relative size reductions and histological appearances were not significantly different. Thus, in this study, their healing effects were not dependent upon the location of the wound spots in the rat dorsum.
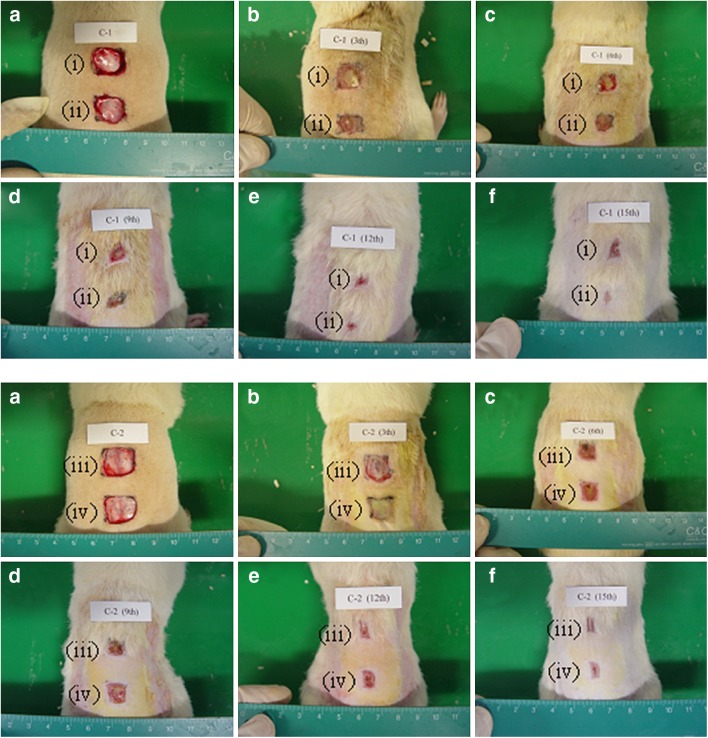
Fig. 7.
Photographs of macroscopic appearance of wounds treated with (i) sterile gauze, (ii) hydrogel with 1.13% dextran and no drug, (iii) conventional product, and (iv) hydrogel with 1.13% dextran and 0.1% drug at a 0 day, b 3 days, c 6 days, d 9 days, e 12 days and, f 15 days of post-operation
Figure 7 shows the macroscopic appearance of wounds treated with a sterile gauze, the hydrogel with drug, the hydrogel without drug, and the conventional products at various days of post-operation. In this experiment, a sterile gauze was used as a control. The hydrogel without or with drug were the hydrogel with 1.13% dextran and no drug and the hydrogel with 1.13% dextran and 0.1% drug, respectively. Each wound was observed for a period of 3, 6, 9, 12, and 15 days post-operation. All rats survived throughout the postoperative period until killing. There were no evidences of necrosis. At 3 days post-operation, little discrete inflammation was visibly observed in all rats. The conventional product and the hydrogels with and without drug induced no infection or contraction of the wound, whereas the control group showed a scabbed wound spot. At 6 days post-operation, the gauze control showed hemorrhage by second damage. However, the hydrogels hardly induced hemorrhage. From 9 days post-operation, the majority of the wounds appeared to be healed and almost completely sealed.
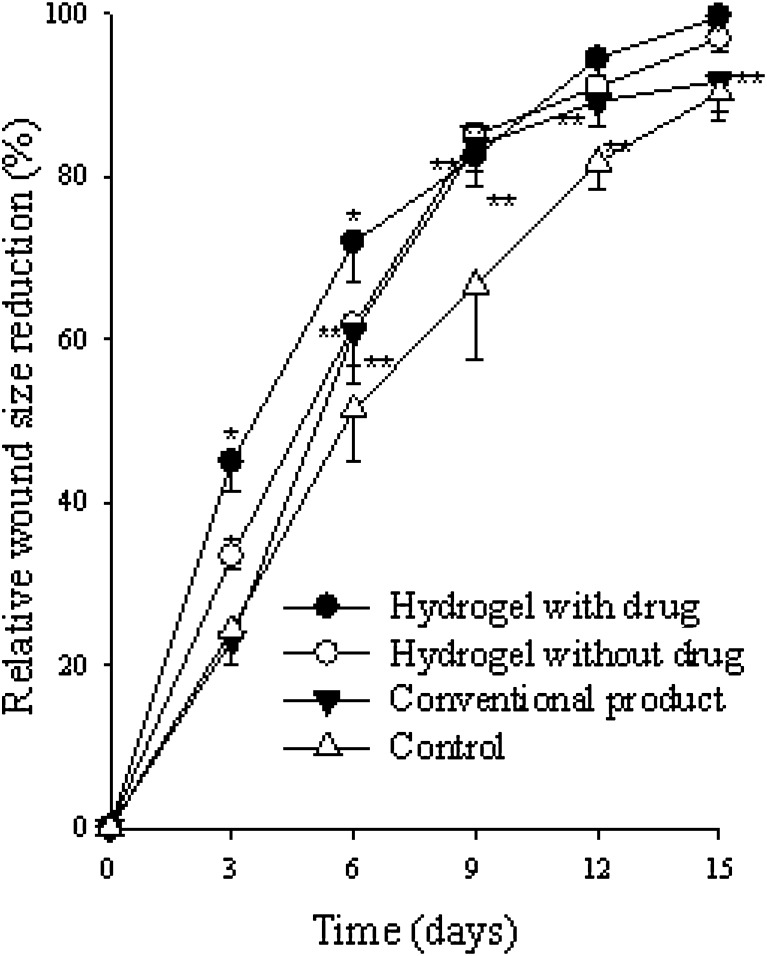
The relative size reduction of the wounds treated with various materials is illustrated in Fig. 8. At 3 days post-operation, the hydrogel with drug showed a significantly greater wound size reduction compared with the other materials. At 6 days, the wound size reduction was significantly greater in the order of the hydrogel with drug > the hydrogel without drug = conventional product > gauze control. Furthermore, from 9 days, the hydrogels and conventional product significantly reduced the wound size compared to control group. In particular, the hydrogels and conventional product gave about a 95–100% wound size reduction at 15 days of post-operation. Our results suggested that the hydrogel with drug significantly improved wound healing compared with conventional product.
Fig. 8.
Relative size reduction of wound treated with sterile gauze, conventional product, hydrogel with 1.13% dextran and no drug, and hydrogel with 1.13% dextran and 0.1% drug in rat dorsum. Each value represents the mean ± SD (n = 5). *P < 0.05 compared with the sterile gauze, conventional product, and the hydrogel with 1.13% dextran and no drug. **P < 0.05 compared with the sterile gauze and the conventional product. ***P < 0.05 compared with the sterile gauze
Histological Examination
Wound healing is a fundamental response to tissue injury. It involves overlapping steps of inflammation, cell migration and proliferation, angiogenesis, neovascularization, extracellular matrix production, and remodeling (42,43). Inflammation followed by tissue repair is a complex physiological process of epithelialization, the formation of granulation tissue, and tissue remodeling (42–45). Moreover, the collagen is a major component of the extracellular matrix (43).
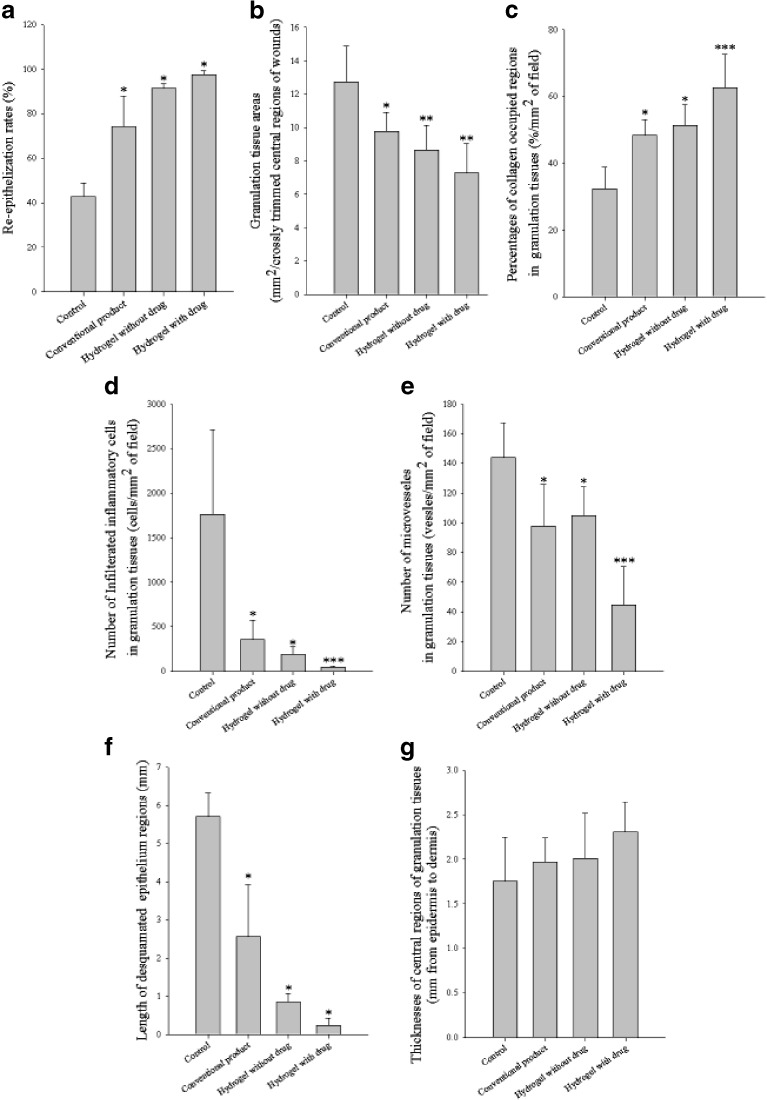
As shown in Fig. 9a, the re-epithelialization rate (%) of the control group was 43 ± 6%. The conventional product and hydrogels gave significantly higher re-epithelialization rate than did the gauze control. The re-epithelialization rate was significantly higher in the order of the conventional product (74 ± 14%) < hydrogel without drug (91 ± 2%) < hydrogel with drug (98 ± 2%). Thus, the hydrogel with drug greatly enhanced the re-epithelialization rates.
Fig. 9.
Histomorphometrical values: a Re-epithelialization rates. All data are P < 0.05 versus other groups. b Granulation tissue areas in central regions of prepared crossly trimmed wounds. c Percentages of collagen occupied regions in granulation tissues. d Number of infiltrated inflammatory cells. e Numbers of microvessels in granulation tissues. f Desquamated epithelium regions. All data are P < 0.05 versus other groups. g Thicknesses of central regions of granulation tissues. After wound healing test of 15 days post-operation, the wounded area of skin containing dermis and hypodermis was sampled. Each value represents mean ± SD (n = 5). The control, conventional product, and the hydrogel without drug and with drug were the group treated with gauze control and conventional product, hydrogel with 1.13% dextran and no drug, and hydrogel with 1.13% dextran and 0.1% drug, respectively. *P < 0.05 compared with the gauze control. **P < 0.05 compared with the gauze control and the conventional product. ***P < 0.05 compared with the gauze control, conventional product, and the hydrogel with no drug
The hydrogels and conventional product significantly decreased the granulation tissue areas compared with the gauze control (Fig. 9b). Moreover, the hydrogels significantly decreased the granulation tissue areas compared with the conventional product.
The percentages of collagen-occupied regions in granulation tissues, number of infiltrated inflammatory cells, numbers of microvessels, and length of desquamated epithelium regions in granulation tissues detected in this study are shown in Fig. 9c–f, respectively. The hydrogels and conventional product significantly increased collagen fibers, but dramatically decreased the number of infiltrated inflammatory cells, numbers of microvessels, and length of desquamated epithelium regions in granulation tissues compared with the gauze control. Furthermore, the hydrogel with drug significantly increased collagen fibers, but decreased the others compared with the conventional product and the hydrogel with drug. Thus, the hydrogels facilitated the disappearances of granulation tissues and changed the wound to normal skin tissues (46,47).
The thicknesses of central regions of granulation tissues were increased in the hydrogels and conventional product (Fig. 9g). However, compared with the gauze control, there was no significant difference.
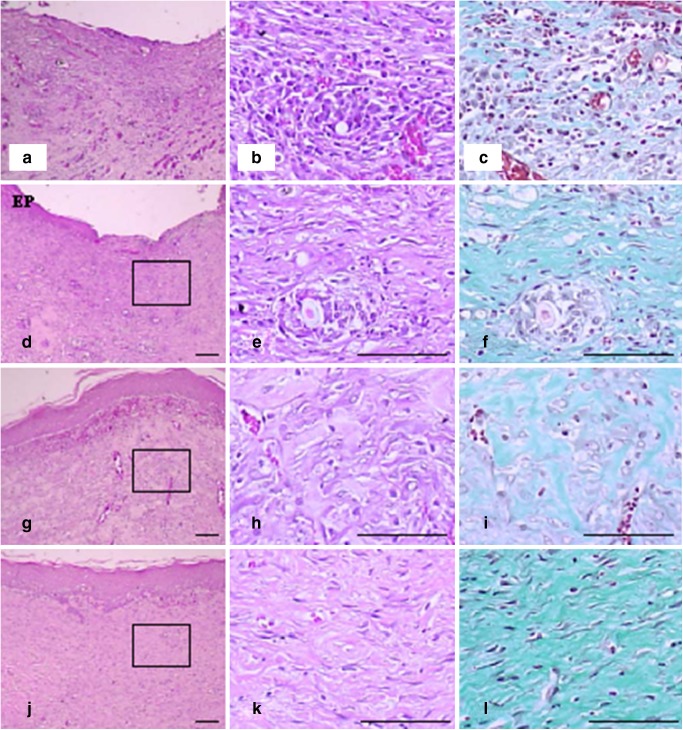
The representative histological profiles of groups were given in Fig. 10. Re-epithelialization of desquamated epithelial regions (panels a, d, g, and j; asterisk) was more extensively observed in the hydrogel and conventional product compared with the gauze control. The hydrogel and conventional product gave less inflammatory cell infiltrations and neovascularization (arrows) in granulation tissues (panels b, e, h, and k) and more numerous collagen proliferations (panels c, f, i, and l; green colors) than did the gauze control.
Fig. 10.
Representative histopathological profiles of skin wounds (granulation tissues) of a–c gauze control, d–f conventional product, g–i hydrogel with 1.13% dextran and no drug, and j–l hydrogel with 1.13% dextran and 0.1% drug treated groups. After wound healing test of 15 days post-operation, the wounded area of skin containing dermis and hypodermis was sampled. DE dermis, EP regenerated epithelium, squares mean the enlarged areas in right columns. a, b, d, e, f, h, j, and k H&E stain. c, f, i, and l Masson’s trichrome stain. Scale bars = 80 μm
The hydrogel without and with drug more favorably facilitated the re-epithelialization and reconstruction of skin tissues on the full-thickness wounds than did the conventional product. They contained more collagen tissues and less inflammatory cells compared with the conventional product. Especially, the hydrogel with drug gave more rapid regeneration of wounds compared to the hydrogel without drug. Furthermore, the hydrogel with drug significantly improved wound healing compared with the hydrogel without drug because of the potential healing effect of gentamicin (20). Further study on its microbial inhibition after microbial infection on the wound spots in the rat dorsum will be carried out.
PVA is a biodegradable and nontoxic polymer. As PVA and dextran gave a high aqueous solubility, they needed cross-linking to be applied to the human body as a wound dressing system. The PVA hydrogels prepared by the F–T method formed physically cross-linked polymer chains containing uncross-linked polymer and water. These gels were non-toxic and biocompatible (12, 13). In this work, the cross-linked hydrogels were prepared at various proportions of dextran to PVA. Dextran decreased the gel fraction, maximum strength, and thermal stability of hydrogels. However, it increased the swelling ability, water vapor transmission rate, elasticity, porosity, and protein adsorption. The drug gave little positive effect on the gel properties of hydrogels. Thus, the gentamicin-loaded wound dressing with PVA and dextran was more swellable, flexible, and elastic than that with only PVA because of its cross-linking interaction with PVA. Furthermore, it gave a greater healing effect than did the hydrogel without drug and conventional product because of the potential healing effect of gentamicin. Compared to the conventional product, the gentamicin-loaded wound dressing could provide an adequate level of moisture and build up the exudates on the wound area. It gave excellent elasticity and enhanced wound healing.
CONCLUSION
In conclusion, the gentamicin-loaded wound dressing developed using the freezing–thawing method with PVA and dextran was more swellable, flexible, and elastic because of its cross-linking interaction with PVA. The hydrogel composed of 2.5% PVA, 1.13% dextran, and 0.1% drug significantly improved the wound healing effect compared with the gauze control, the hydrogel without drug, and the conventional product. Thus, it is a potential wound dressing with excellent forming and improved healing effect in wound care.
Acknowledgments
This research was supported by the Regional R&D Cluster Project designated by the Ministry of Science and Technology & the Ministry of Commerce, Industry, and Energy (2009) and a grant from the Korean Health Technology R&D Project, Ministry for Health, Welfare and Family Affairs, Republic of Korea (A092018).
Contributor Information
Chul Soon Yong, Phone: +82-53-8102812, FAX: +82-53-8104654, Email: csyong@yumail.ac.kr.
Han-Gon Choi, Phone: +82-53-8102813, FAX: +82-53-8104654, Email: hangon@yumail.ac.kr.
References
- 1.Turner TD. Hospital usage of absorbent dressings. Pharmaceut J. 1979;222:421–4. [Google Scholar]
- 2.Hinman CD, Maibach H. Effect of air exposure and occlusion on experimental human skin wounds. Nature. 1963;200:377–8. doi: 10.1038/200377a0. [DOI] [PubMed] [Google Scholar]
- 3.Winter GD. Formation of the scab and the rate of epithelization of superficial wounds in the skin of the young domestic pig. Nature. 1962;193:293–4. doi: 10.1038/193293a0. [DOI] [PubMed] [Google Scholar]
- 4.Morin RJ, Tomaselli NL. Interactive dressings and topical agents. Clin Plast Surg. 2007;34:643–58. doi: 10.1016/j.cps.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 5.Ajji Z, Mirjalili G, Alkhatab A, Dada H. Use of electron beam for the production of hydrogel dressings. Radiat Phys Chem. 2008;77:200–2. doi: 10.1016/j.radphyschem.2007.05.016. [DOI] [Google Scholar]
- 6.Hoffman AS. Hydrogels for biomedical applications. Adv Drug Deliv Rev. 2002;43:3–12. doi: 10.1016/S0169-409X(01)00239-3. [DOI] [PubMed] [Google Scholar]
- 7.Stashak TS, Farstvedt E, Othic A. Update on wound dressings: indications and best use. Clin Tec Equine Pract. 2004;3:148–63. doi: 10.1053/j.ctep.2004.08.006. [DOI] [Google Scholar]
- 8.Valenta C, Auner BG. The use of polymers for dermal and transdermal delivery. Eur J Pharm Biopharm. 2004;58:279–89. doi: 10.1016/j.ejpb.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 9.Hassan CM, Stewart JE, Peppas NA. Diffusional characteristics of freeze/thawed poly(vinyl alcohol) hydrogels: applications to protein controlled release from multilaminate devices. Eur J Pharm Biopharm. 2000;49:161–5. doi: 10.1016/S0939-6411(99)00056-9. [DOI] [PubMed] [Google Scholar]
- 10.Razzak MT, Darmawan D, Zainuddin S. Irradiation of polyvinyl alcohol and polyvinyl pyrrolidone blended hydrogel for wound dressing. Radiat Phys Chem. 2001;62:107–13. doi: 10.1016/S0969-806X(01)00427-3. [DOI] [Google Scholar]
- 11.Yang JM, Su WY, Leu TL, Yang MC. Evaluation of chitosan/PVA blended hydrogel membranes. J Membr Sci. 2004;236:39–51. doi: 10.1016/j.memsci.2004.02.005. [DOI] [Google Scholar]
- 12.Peppas NA. Turbidimetric studies of aqueous poly(vinyl alcohol) solutions. Makromol Chem. 1975;176:3433–40. doi: 10.1002/macp.1975.021761125. [DOI] [Google Scholar]
- 13.Stauffer SR, Peppas NA. Poly(vinyl alcohol) hydrogels prepared by freezing–thawing cyclic processing. Polymer. 1992;33:3932–6. doi: 10.1016/0032-3861(92)90385-A. [DOI] [Google Scholar]
- 14.Kim JO, Park JK, Kim JH, Jin SG, Yong CS, Li DX, Choi JY, Woo JS, Yoo BK, Lyoo WS, Kim JA, Choi HG. Development of polyvinyl alcohol–sodium alginate gel-matrix-based wound dressing system containing nitrofurazone. Int J Pharm. 2008;359:79–86. doi: 10.1016/j.ijpharm.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 15.Kim JO, Choi JY, Park JK, Kim JH, Jin SG, Chang SW, Li DX, Hwang MR, Woo JS, Kim JA, Lyoo WS, Yong CS, Choi HG. Development of clindamycin-loaded wound dressing with polyvinyl alcohol and sodium alginate. Biol Pharm Bull. 2008;31:2277–82. doi: 10.1248/bpb.31.2277. [DOI] [PubMed] [Google Scholar]
- 16.Kim IS, Jeong YI, Kim SH. Self-assembled hydrogel nanoparticles composed of dextran and poly(ethylene glycol) macromer. Int J Pharm. 2000;205:109–16. doi: 10.1016/S0378-5173(00)00486-5. [DOI] [PubMed] [Google Scholar]
- 17.Cascone MG, Sim B, Downes S. Blends of synthetic and natural polymers as drug delivery systems for growth hormone. Biomaterials. 1995;16:569–74. doi: 10.1016/0142-9612(95)91131-H. [DOI] [PubMed] [Google Scholar]
- 18.Yeo JH, Lee KG, Kim HC, Oh YL, Kim AJ, Kim SY. The effects of PVA/chitosan/fibroin (PCF)-blended spongy sheets on wound healing in rats. Biol Pharm Bull. 2000;23:1220–3. doi: 10.1248/bpb.23.1220. [DOI] [PubMed] [Google Scholar]
- 19.Gehrig KA, Warshaw EM. Allergic contact dermatitis to topical antibiotics: epidemiology, responsible allergens, and management. J Am Acad Dermatol. 2008;58:1–21. doi: 10.1016/j.jaad.2007.07.050. [DOI] [PubMed] [Google Scholar]
- 20.Society of Toxicology (SOT) (2008) Guiding principles in the use of animals in toxicology. www.toxicology.org/AI/FA/guidingprinciples.pdf.
- 21.Cascone MG, Maltinti S, Barbani N. Effect of chitosan and dextran on the properties of poly(vinyl alcohol) hydrogels. J Mater Sci Mater Med. 1999;10:431–5. doi: 10.1023/A:1008983215833. [DOI] [PubMed] [Google Scholar]
- 22.Huang MH, Yang MC. Evaluation of glucan/poly(vinyl alcohol) blend wound dressing using rat models. Int J Pharm. 2008;346:38–46. doi: 10.1016/j.ijpharm.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 23.Ajji Z, Othman I, Rosiak JM. Production of hydrogel wound dressing using gamma radiation. Nucl Instrum Methods Phys Res: Sect B. 2005;229:375–80. doi: 10.1016/j.nimb.2004.12.135. [DOI] [Google Scholar]
- 24.Yoshii F, Zhanshan Y, Isobe K, Shinozaki K, Makuuchi K. Electron beam cross-linked PEO and PEO/PVA hydrogels for wound dressing. Radiat Phys Chem. 1999;55:133–8. doi: 10.1016/S0969-806X(98)00318-1. [DOI] [Google Scholar]
- 25.Yang X, Liu Q, Chen X, Yu F, Zhu Z. Investigation of PVA/ws-chitosan hydrogels prepared by combined γ-irradiation and freeze–thawing. Carbohydr Polym. 2008;73:401–8. doi: 10.1016/j.carbpol.2007.12.008. [DOI] [Google Scholar]
- 26.Lin WC, Yu DG, Yang MC. Blood compatibility of novel PGA (poly glutamic acid)/poly vinyl alcohol hydrogels. Colloids Surf B. 2006;47:43–9. doi: 10.1016/j.colsurfb.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 27.Loh XJ, Sng KBC, Li J. Synthesis and water-swelling of thermo-responsive poly(ester urethane)s containing poly(ε-caprolactone), poly(ethylene glycol) and poly(propylene glycol) Biomaterials. 2008;29:3185–94. doi: 10.1016/j.biomaterials.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 28.Yoo MK, Kweon HY, Lee KG, Lee HC, Cho CS. Preparation of semi-interpenetrating polymer networks composed of silk fibroin and poloxamer macromer. Int J Biol Macromol. 2004;34:263–70. doi: 10.1016/j.ijbiomac.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 29.Burkatovskaya M, Tegos GP, Swietlik E, Demidova TN, Castano A, Hamblin MR. Use of chitosan bandage to prevent fatal infections developing from highly contaminated wounds in mice. Biomaterials. 2006;27:4157–64. doi: 10.1016/j.biomaterials.2006.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim HJ, Choi EY, Oh JS, Lee HC, Park SS, Cho CS. Possibility of wound dressing using poly(l-leucine)/poly(ethylene glycol)/poly(l-leucine)triblock copolymer. Biomaterials. 2000;21:131–41. doi: 10.1016/S0142-9612(99)00140-4. [DOI] [PubMed] [Google Scholar]
- 31.Park SN, Kim JK, Suh H. Evaluation of antibiotic-loaded collagen–hyaluronic acid matrix as a skin substitute. Biomaterials. 2004;25:3689–98. doi: 10.1016/j.biomaterials.2003.10.072. [DOI] [PubMed] [Google Scholar]
- 32.Quintanilha Ribeiro Fde A, Borges Jde P, Guaraldo L, Vianna MR. Study of wound healing in rats treated with topical and injected mitomycin-C. Rev Bras Otorrinolaringol. 2008;74:328–30. doi: 10.1016/S1808-8694(15)30563-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hirose K, Onishi H, Sasatsu M, Takeshita K, Kouzuma K, Isowa K, Machida Y. In vivo evaluation of Kumazasa extract and chitosan films containing the extract against deep skin ulcer model in rats. Biol Pharm Bull. 2007;30:2406–11. doi: 10.1248/bpb.30.2406. [DOI] [PubMed] [Google Scholar]
- 34.Levene A. Pathological factors influencing excision of tumours in the head and neck. Part I. Clin Otalary. 1981;6:145–51. doi: 10.1111/j.1365-2273.1981.tb01800.x. [DOI] [PubMed] [Google Scholar]
- 35.Ludbrook J. Update: microcomputer statistics packages. A personal view. Clin Exp Pharmacol Physiol. 1997;24:294–6. doi: 10.1111/j.1440-1681.1997.tb01823.x. [DOI] [PubMed] [Google Scholar]
- 36.Yokoyama F, Masada I, Shimamura K, Ikawa T, Monobe K. Morphology and structure of highly elastic poly(vinyl alcohol) hydrogel prepared by repeated freezing-and-melting. Colloid Polym Sci. 1986;264:595–601. doi: 10.1007/BF01412597. [DOI] [Google Scholar]
- 37.Choi YS, Hong SR, Lee YM, Song KW, Park MH, Nam YS. Study on gelatin-containing artificial skin: I. Preparation and characteristics of novel gelatin–alginate sponge. Biomaterials. 1999;20:409–17. doi: 10.1016/S0142-9612(98)00180-X. [DOI] [PubMed] [Google Scholar]
- 38.Queen D, Gaylor JDS, Evans JH, Courtney JM, Reid WH. The preclinical evaluation of the water vapor transmission rate through burn wound dressings. Biomaterials. 1987;8:367–71. doi: 10.1016/0142-9612(87)90007-X. [DOI] [PubMed] [Google Scholar]
- 39.Kim IY, Yoo MK, Kim BC, Kim SK, Lee HC, Cho CS. Preparation of semi-interpenetrating polymer networks composed of chitosan and poloxamer. Int J Biol Macromol. 2006;38:51–8. doi: 10.1016/j.ijbiomac.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 40.Coleman DL, Gregonis DE, Andrade JD. Blood–materials interactions: the minimum interfacial free energy and the optimum polar/apolar ratio hypothesise. J Biomed Mater Res. 1982;16:381–98. doi: 10.1002/jbm.820160407. [DOI] [PubMed] [Google Scholar]
- 41.Dion I, Baquey C, Havlik P, Monties JR. A new model to test platelet adhesion under dynamic conditions. Application to the evaluation of a titanium nitride coating. Int J Artif Organs. 1993;6:545–50. [PubMed] [Google Scholar]
- 42.Evans P. The healing process at cellular level: a review. Physiotherapy. 1980;66:256–9. [PubMed] [Google Scholar]
- 43.Froget S, Barthelemy E, Guillot F, Soler C, Coudert MC, Benbunan M, Dosquet C. Wound healing mediator production by human dermal fibroblasts grown within a collagen–GAG matrix for skin repair in humans. Eur Cytokine Netw. 2003;14:60–4. [PubMed] [Google Scholar]
- 44.Martin P. Wound healing—aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 45.Risbud M, Hardikar A, Bhonde R. Growth modulation of fibroblasts by chitosan–polyvinyl pyrrolidone hydrogel: implications for wound management. J Biosci Bioeng. 2000;25:25–31. doi: 10.1007/BF02985178. [DOI] [PubMed] [Google Scholar]
- 46.Sugihara A, Sugiura K, Morita H, Ninagawa T, Tubouchi K, Tobe R, Izumiya M, Horio T, Abraham NG, Ikehara S. Promotive effects of a silk film on epidermal recovery from full-thickness skin wounds. Proc Soc Exp Biol Med. 2000;225:58–64. doi: 10.1046/j.1525-1373.2000.22507.x. [DOI] [PubMed] [Google Scholar]
- 47.Halper J, Leshin LS, Lewis SJ, Li WI. Wound healing and angiogenic properties of supernatants from Lactobacillus cultures. Exp Biol Med. 2003;228:1329–37. doi: 10.1177/153537020322801111. [DOI] [PubMed] [Google Scholar]